Abstract
The green synthesis of zinc oxide nanoparticles (ZnO-NPs) mediated fruit peel extract is gaining importance due to its cost-effectiveness and ecofriendly nature. Herein, ZnO-NPs were synthesized using pomegranate peel extract as a reducing and stabilizing agent. The synthesized ZnO-NPs were characterized using SEM, TEM-SAID, FT-IR, XRD, and particle size analysis. According to the findings, the ZnO-NPs were agglomerated into spherical and hexagonal shapes with an average diameter of 20 to 40 nm and crystallinity formed. The antimicrobial activity of ZnO-NPs against pathogenic microbes was significant in multiple applications, with 62.5 and 31.25 μg/ml of MIC for both Gram-positive and Gram-negative bacteria, respectively, and 125 and 250 μg/ml of MIC for Aspergillus niger and Aspergillus flavus, respectively. In addition, ZnO-NPs showed antioxidant activity with IC50 = 240 and 250 μg/ml by DPPH and ABTS, respectively. All concentrations of ZnO-NPs significantly improved the germination of barley seed and shoot height, with the optimum concentration reaching 2 and 12 ppm of ZnO-NPs for both seed germination (90%) and shoot height (6.5), respectively, while the greatest root extension (6 cm) was observed at 2 ppm of ZnO-NPs. The mitotic index increased at lower nanoparticle concentrations and exposure times but declined considerably as the nanoparticle dose and exposure duration increased, until most concentrations reached 100% suppression after 12 h with various chromosomal abnormalities. The researchers were able to create efficient, eco-friendly, and simple multifunctional ZnO-NPs using a green synthetic strategy and, in the process, obtain a better understanding of the cytotoxicity and genotoxicity of ZnO-NPs in plant cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology enables the development of unique nanostructures, the study of their novel features, and the application of these structures in a variety of domains of application [1]. Nanomaterials have made significant contributions to basic and applied sciences. Several approaches can be used to synthesis nanoparticles such as chemical, physical, and green approach. However, green synthesis has gained popularity in recent years [2]. Green synthesis has several advantages over standard chemical or physical techniques, including the requirement for mild reaction conditions, the use of less harmful ingredients, low cost, and ecofriendly [3]. Biological agents such as plant extracts, fruit extracts, and microorganisms are all employed in green synthesis [4,5,6].
Plant extracts allow the biological production of many metallic nanoparticles, which is more environmentally friendly and allows the regulated synthesis of nanoparticles with well-defined size and form [7,8,9]. The term “power fruit” refers to the pomegranate (Punica granatum), a fruit known for its distinctive medicinal properties consumer health benefits [10]. The peel of Punica granatum (P. granatum) accounts for approximately one-third of the fruit’s weight and is scraped off after harvest. Agro-waste from fruits has recently received significant attention due to its abundant availability and inexpensive cost [11]. The high concentration of phenolic compounds in the peel of P. granatum has long been recognized as a natural source of antioxidants [12]. In addition to punicalagin and the gallic and ellagic acids present in pomegranate peels, there are also chlorogenic acids, caffeic acids, punicalins, and other phenolic compounds such as apigenin, quercetin, pelargonidin, and granatin A and B [12]. Due to the general importance of using natural, renewable, and low-cost materials to avoid the effects of toxic compounds and solvents, P. granatum was used as a green synthesis of metal oxide nanoparticles in an aqueous phase [13,14,15].
On a global basis, zinc oxide nanoparticles (ZnO-NPs) are one of the most widely produced nanomaterials. These NPs are utilized in a wide range of industrial products, including sunscreens, cosmetics, and paints [16, 17]. In various plant species (including crops), ZnO-NPs at optimal concentrations are used to improve seed germination and seedling growth [18, 19]. Additionally, ZnO-NPs have been proposed as a source of zinc (Zn) for plants [20]. Since metallic Zn is involved in a range of enzymatic and physiological activities, it is essential for plant growth and development [21]. Zn is required for protein, carbohydrate, and nucleic acid synthesis, as well as chlorophyll biosynthesis, energy production, and macromolecule metabolism, where it acts as an enzyme component, a catalyst, or a structural cofactor [22]. Zn increases seed germination, stimulates radical growth, regulates water absorption and transport capacity, and protects plants from the damaging effects of heat, drought, and salt stress. Additionally, Zn is necessary for the synthesis of plant hormones such as auxins and gibberellins [23,24,25].
Due to their propensity to absorb and gather nanoparticles from the soil, water, and air, plants are especially vulnerable to nanotoxicity. As a result, plants are frequently used as a model organism for assessing the potential toxicity of various nanomaterials [26, 27]. Barley (Hordeum vulgare L.) is the world’s fourth most significant cereal crop and one of the most widely cultivated cereals for human and animal consumption [28, 29]. Due to its diploid nature and high genetic diversity, barley has been effectively utilized as a model organism for genetic studies [30, 31].
The rise in antimicrobial resistance (AMR) has become a global concern. Apoptosis, a cell death caused by the oxidative stress caused by ZnO-NPs, is an effective method inhibiting bacterial growth and biofilm formation [32]. ZnO-NPs and conventional antibiotics can work synergistically against Acinetobacter baumannii resistance, according to [33]. Faisal et al. [34] revealed that biosynthesized ZnO-NPs are biocompatible and effective antioxidant nanomaterials. Moreover, Yazhiniprabha et al. released evidence indicating that ZnO-NP nanoparticles could be utilized as nanomedicine against microbial disease [35]. ZnO-NPs were found to improve the absorption of antibiotics by enhancing bacteria’s and fungi’s ability to metabolize them [32, 36, 37].
This study aimed to conduct a green synthesis of ZnO-NPs using pomegranate peels extract and their physicochemical characterizations. The ZnO-NPs were evaluated for their antimicrobial and antioxidant activities, as well as seed germination, root, shoot length, and cytogenetics effects of ZnO-NPs were investigated in vitro. According to our knowledge, this is the first report for multiple applications of green synthesized ZnO-NPs as well as the effect of higher concentrations of ZnO-NPs on the genotoxicity of barley seeds.
2 Material and methods
2.1 Materials
Zinc nitrate [Zn(NO3)2·6H2O] as a source of Zn2+, sodium hydroxide and ethanol were purchased from Sigma, whereas barley seeds were obtained from Egypt’s Ministry of Agriculture.
2.2 Methods
2.2.1 Pomegranate peel (POP) extraction
Pomegranate (P. granatum) fruit was obtained from the local market in Egypt, and then washed well with clean tap water to remove extraneous materials and the peels and seeds were separated manually. The POP extract was prepared by the method described by [38] with slight modifications. The fresh POP (100 gm) was cut into small pieces and ground in 500 ml distilled water using a high-speed blender (800ES blender, 230 V, 50 HZ, 330 Wt, model BB90E, USA) for 10 min at room temperature. The dispersed POP mixture was boiled for 45 min under steered conditions. Then, the mixture was then centrifuged at 15,000 rpm for 15 min at 4 °C, and the supernatant of POP extracts were filtered, collected, and stored at 4 °C for further use [38].
2.2.2 Green synthesis of ZnO-NPs
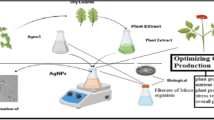
Zinc nitrate hexahydrate [Zn(NO3)2·6H2O] and aqueous extract of POP were used in a 1:20 ratio for the green route production of ZnO-NPs. 1 M NaOH was added drop by drop to adjust pH from 8 to 12 and stirred for 2 h at 100 °C, yielding a yellowish precipitate. To separate this precipitate, the solution was cooled and centrifuged at 10,000 rpm, then washed several times with distilled water and ethanol to remove dust particles. Afterword, a solid powder was obtained and dried in a 60 °C oven. The product was calcined for 4 h at 450 °C in a muffle furnace (Fig. 1).
2.2.3 Characterization of ZnO-NPs
The Fourier transform infrared spectroscopy (FT-IR) was measured using a spectrum 100 spectrophotometer (Perkin Elmer Inc., USA) in a total-reflection mode. The measurements were performed from 4000 to 250 cm−1 at a resolution of 4 cm−1. Scanning electron microscope (SEM) (Philips, model Quanta Feg 250) was used to monitor the fracture surface of the films. The samples for SEM analysis were coated with a layer of gold and were imaged at an operating voltage of 20 kV under vacuum. Samples were examined using transmission electron microscopy (TEM) on JEOL (GEM-1010) at a voltage of 76 kV. X-ray diffractometer (XRD) was measured by (PANalytical, Netherlands) at 25 °C with Cu Kα a monochromatic radiation source (λ = 0.154 nm, 2θ = 5°: 80°, and scanning time 5 min). The biosynthesis of ZnO-NPs was recorded using UV–vis spectra (JENWAY 6305 Spectrophotometer) at wavelengths of 300–800 nm to detect an intense absorption peak which was related to surface plasmon excitation.
2.2.4 Antimicrobial activity
The antimicrobial activities of biosynthesized ZnO-NPs against pathogenic microbial strains, Gram-positive bacteria Bacillus cereus ATCC 10,987 (B. cereus) and Staphylococcus aureus ATCC 6538 (S. aureus), Gram-negative bacteria Escherichia coli ATCC 8739 (E. coli) and Klebsiella pneumoniae ATCC 13,883 (K. pneumonia), and multicellular fungi Aspergillus niger (A. niger) and Aspergillus flavus (A. flavus) was evaluated under aseptic conditions, using Muller Hinton agar for bacteria and Potato dextrose agar for fungi by agar well-diffusion technique. All bacteria used were obtained from American Type Culture Collection (ATCC). At a concentration of 1000 μg/ml, about 100 μl of base fluid (glycerol ammonium citrate solution) was used as a negative control according to Saline et al. [39]. The same amount of biosynthesized ZnO-NPs nanofluid was used. This nanofluid was made by dissolving ZnO-NPs in base fluid, which makes nanofluid suspensions stable for several months. In addition, azithromycin (15 ug/ml) was used as antibiotic control for the bacteria, whereas fluconazole powder (25 ug/ml) was used as antifungal control. All plates were placed in a refrigerator for 2 h to inhibit the microbial used and allow the tested compounds to diffuse. Bacteria were then incubated at 37 °C for 24 h and fungi at 28 °C for 72–96 h. At the end of the incubation, the zone of the inhibition by mm was measured [40].
The minimum inhibitory concentration (MIC) of the biosynthesized ZnO-NP nanofluid was determined, using concentrations ranging from 500 μg/ml and diluted double-fold to 7.8 ug/m [41]. Each trial was repeated three times.
2.2.5 Antioxidant activity
DPPH assay
Antioxidant activity of ZnO-NPs was carried out using DPPH (2,2-diphenyl-1-picrylhydrazyl) method according to [42]. The DPPH method was modified to evaluate the scavenging activity of ZnO-NPs on free radicals. DPPH reagent was prepared by dissolving 8 mg of DPPH in 100 ml of MeOH. The scavenging activity of ZnO-NPs was done by mixing 100 μL DPPH reagent with 100 μL of ZnO-NPs in a well of the micro-plate then incubated for 30 min at room temperature. Subsequently, the plate was measured at 490 nm using an ELISA plate reader (TECAN, Austria). The scavenging activity for free radicals by ZnO-NPs was calculated as follows:
Different concentrations of ZnO-NPs (1000, 500, 250, 125, 62.5, 31.25, 15.6, and 7.8 μg/ml) were used to determine their DPPH scavenging activity. The antioxidant activity of ascorbic acid as standard and ZnO-NPs was estimated. The IC50 values (the concentration of tested material required to inhibit 50% of DPPH radicals).
ABTS assay
Another assay used to evaluate the antioxidant activity was ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) according to the modified method used by Lee [43].
2.2.6 Evaluation of ZnO-NPs for seed germination and length of root and shoot
Barley seeds (Hordeum vulgare) were obtained from Egypt’s Ministry of Agriculture. To assure the sterility of the surface, healthy and uniform barley seeds were immersed in 1% sodium hypochlorite solution for 5 min and then rinsed three times with distilled water to ensure the sterility of the surface. At 25 °C, seeds were germinated on filter sheets soaked with ZnO-NPs (1, 2, 4, 8, and 12 mg/L) and distilled water as a negative control. Each treatment consisted of five duplicates of ten seeds. The number of seedlings was counted after 4 days of treatment to determine the germination percentage, once seeds germinate, a 2-mm radicle grows from them. The percentage of germinated seeds equals the number of germinated seeds divided by the total number of seeds. Regular shoot height and root length measurements were taken until the tenth day.
2.2.7 Evaluation of ZnO-NPs for cytotoxicity and genotoxicity
Seeds of barley were germinated on moistened filter sheets with distilled water until the roots attained a length of approximately 1 cm. Roots were subjected to previously determined concentrations for 3, 6, 9, and 12 h, with three duplicates for each treatment. After 24 h of fixation in ethanol:acetic acid (3:1), the roots were hydrolyzed for 10 min in a water bath at 60 °C with 1 N HCl. After that, the root tips were dyed using the Feulgen squash process [44]. To determine the mitotic index (MI) and chromosomal aberrations, a minimum of 2000 cells from the control and all treated groups were scored using an optical microscope at a magnification of 40. The MI % is computed by dividing the total number of dividing cells by the total number of cells counted. The percentage of phase index is computed by dividing the total number of dividing cells seen by the number of cells in each phase. The overall chromosomal abnormality percentage (CA, the number of cells with chromosomal abnormalities/number of cells in division 100) was calculated by counting cells at various stages of mitosis. The researchers counted micronuclei (MN) that were smaller than a third of the diameter of the major nucleus. The frequency of MN was calculated using the number of cells with micronuclei per 1000 scored interphase cells [45].
3 Results and discussion
3.1 Characterization of green synthesized ZnO-NPs
3.1.1 SEM and TEM analysis
SEM measurement was carried out to analyze the morphology of ZnO-NPs. The SEM images (Fig. 2A) demonstrate that, the produced ZnO-NPs are nearly spherical in shape with agglomeration due to their water interaction and the intramolecular Van der Waals, magnetic and electrostatic interactions. TEM images reveal the nanoscale and uniformity of ZnO-NPs. The shape of the nanoparticles produced is spherical, with an average size of 20 to 40 nm, as shown in Fig. 2B. The SAED pattern in Fig. 2C indicates that the biosynthesized ZnO-NPs were polycrystalline in nature. The particle size distributions for the sample are depicted in Fig. 2D. Low polydispersity index values indicate that the particles are homogeneous with direct spherical forms (confirmed by the SEM analysis). Particle size distributions cover a small range of particle diameters, and the smallest particles in the sample had diameters ranging from 1 to 550 nm, with an average size diameter of 122.6 nm [46].
3.1.2 FT-IR, XRD, and UV analysis
Figure 3A shows the FT-IR spectrum of ZnO-NPs. The presence of organic compounds in the sample is associated with absorption peaks in the region of 800–1600 cm−1. The stretching vibration band of C = O of the amide group, as well as the presence of carboxyl (COOH) and hydroxyl (OH) groups, are shown by the absorption peaks at 1630.12 cm−1 and 3437.05 cm−1, respectively [47], while the -CH2- and C–C in the aromatic ring are responsible for the peaks at 2920.46 cm−1 and 1411 cm−1, respectively. Stretching vibrations of C = O can also be attributed to the absorption peaks at 1630 cm−1. The presence of phenolic groups, alcohols, and aliphatic amines is also indicated by a peak at 1039.81 cm−1. The phenols in the capping agent bond to the surface of ZnO and cause ZnO-NPs to form, whereas the C = O, C = O–C and C = C groups of heterocyclic compounds may act as a stabilizer [48, 49]. Then, FT-IR spectrum absorbs the peak at 875.38 cm−1 indicating the stretching vibrations of ZnO because the peaks in the range from 500–900 cm−1 are attributed to metal oxide bonds [50]. Using XRD analysis is a valuable tool to determine ZnO-NPs phase and crystalline nature. In the current study, XRD pattern of ZnO-NPs (Fig. 3B) showed different peaks at (2ϴ) = 31.75 (1 0 0), 34.46 (0 0 2), 36.32 (1 0 1), 47.46 (1 0 2), 56.63 (1 1 0), 62.75 (1 0 3), 66.36 (2 0 0), 68.13 (1 1 2), and 69.10 (2 0 1) which agree with the JCPDS card (NO 36–1451) for a typical hexagonal phase of wurtzite form crystalline material [39, 51]. The presence of (1 0 0), (0 0 2), (1 0 1) planes in XRD designates the formation of the hexagonal phase of the wurtzite structure of ZnO-NPs. No impurity peaks were noted indicating the formation of pure ZnO-NPs. The prominent sharp peak at (101) represents the polycrystalline structure of ZnO-NPs. The average crystalline size was found to be 22 nm which was further confirmed by TEM analysis and SAED. These results are in accordance with previous studies on ZnO-NPs synthesized using leaf extract of Tecoma castanifolia [50] and Conyza canadensis [52]. The UV–visible absorption investigation of pure ZnO-NPs is shown in Fig. 3C, at the wavelength ranges from 300 to 800 nm. The absorbance value is exclusively dependent on various factors like the size of nanoparticles and deformities or defects in the grain structure. The UV–Vis spectrum of ZnO-NPs shows the edge absorption peak at 386 nm due to exciton recombination at room temperature [53].
3.2 Antimicrobial activity of ZnO-NPs
The antimicrobial effect of biosynthesized ZnO-NPs can be interpreted as different actions such as (1) the generation of reactive oxygen species (ROS); (2) ZnO-NPs with microbe cell walls, which results in oxidative stress and cell death; (3) electrostatic attraction the microbial cell; and (4) entry of Zn2+ ions into the cell that released from ZnO-NPs leads to direct interactions with the functional groups of nucleic acids and protein (e.g., (–SH), (–NH), and (–COOH) groups) [54,55,56].
The ZnO-NPs nanofluid inhibits the growth of all tested pathogenic microorganisms, with antimicrobial activities by diameters ranging from 19.7 ± 0.29 to 24.6 ± 0.32 mm at a concentration of 1000 μg/ml as shown in Table 1 and Fig. 4. We can observe that the ZnO-NPs exhibit inhibition effects against Gram-negative bacteria more than Gram-positive bacteria and fungi. On the other hand, there was no effect of base fluid against any of the tested microorganisms, which proved the antimicrobial activity of ZnO-NPs. Different inhibition zone results for antibiotic controls were recorded, which were less than those obtained from ZnO-NPs.
The detection of MIC values of biomolecules against pathogenic microorganisms especially if these biomolecules are integrated into medical applications had a significant value. The effect of different concentrations of ZnO-NPs (500, 250, 125, 62.5, 31.25, 15.6 and 7.8 μg/ml) against tested microbes showed that, the MIC value for Gram-positive bacteria (B. cereus and S. aureus) were 62.5 μg/ml; Gram-negative bacteria (E. coli and K. pneumonia) were 31.25 μg/ml, while MIC value for A. niger and A. flavus were 125 and 250 μg/ml, respectively.
In the current study, Gram-negative bacteria (E. coli) was the most sensitive microbes toward biosynthesized ZnO-NPs, which can be due to differences in cell wall structures between Gram-negative and Gram-positive bacteria. The Gram-negative bacterial cell wall was distinguished by a thin layer of peptidoglycan and lipopolysaccharides (LPS). The positive charge of ZnO-NPs is hardly adhesive to the negative charge of LPS, and hence, it is the adsorbed on the Gram-negative bacterial cell membrane that ultimately disrupts selective permeability [56]. Moreover, the inhibition effect of ZnO-NPs on fungi significantly deforms conidial formation and conidiophores of Penicillium expansum and Botrytis cinerea, displaying antifungal properties [55, 57].
3.3 Antioxidant activity of ZnO-NPs
Antioxidant activity has been found in a wide range of natural and synthetic substances [58]. In the current study, antioxidant activity was assessed using DPPH radical-scavenging activity and ABTS radical-scavenging activity tests. Antioxidants are chemicals that neutralize ROS, which is formed as a byproduct of biological events [59]. Several antioxidant qualities, including anti-atherosclerosis, anti-inflammatory, anti-tumor, anticancer, anti-mutagenesis, anti-carcinogenesis and anti-microbiosis, have led to their use as therapeutic agents (Fig. 5). In the current study, the antioxidant activity of ZnO-NPs was evaluated using DPPH and ABTS assays, as shown in Fig. 5. Results revealed that the antioxidant activity of ZnO-NPs with IC50 = 240 μg/ml compared to 16.2 μg/ml for ascorbic acid in the case of DPPH, while IC50 of ZnO-NPs and ascorbic acid using ABTS assay were 250 and 1.6 μg/ml, respectively.
3.4 Evaluation of ZnO-NPs for seed germination
Short-term phytotoxicity of emerging contaminants, such as designed NPs, can be evaluated using seed germination and root/shoot elongation experiments [60]. In this study, germination was defined as the radicle or plumule emerging from the seed coat. The germination of barley seeds exposed to ZnO-NPs was assessed after 4 days of treatment. ZnO-NP treatment demonstrated a positive effect on barley seeds compared to the control group. The seed germination of barley was significantly increased at all ZnO-NPs doses examined. The seedlings germinated with the addition of ZnO-NPs at 2 mg/l had the highest germination rate (90%), while the control seeds had a substantially lower germination rate (63%). At 1, 4, 8, and 12 PPM of ZnO-NPs, the seed germination rate was recorded 83, 67, 77, and 83%, respectively (Fig. 6a). Germination of the seeds began after 1 day, with a proportion ranging from 60% under 1 and 2 PPM ZnO-NPs conditions to 50% under control. Zn is an essential plant micronutrient frequently supplemented with zinc sulfate in agricultural practices to prevent Zn deficiency. Our findings are consistent with those of Plaksenkova et al., who reported that low ZnO-NPs concentrations of 1, 2, and 4 PPM significantly increased barley seed germination [61]. In addition, Pand et al. recorded an increase in the germination of rice seeds treated with Zn NPs, which is consistent with our findings [62]. In contrast to Xiang et al., ZnO-NPs at concentrations of 1–80 mg/l did not influence on Chinese cabbage seed germination compared to the control [60]. Raliya et al. also reported that ZnO-NPs had no effect on tomato seed germination at concentrations up to 750 mg/kg [63]. According to Zhang et al., ZnO-NPs at concentrations of 10, 100, and 1,000 mg/l had no statistically significant effect on maize or cucumber germination, suggesting that germination rate is species- and concentration-dependent [64].
3.5 Assessment of ZnO-NPs for root and shoot length
In plants, Zn acts as a cofactor for RNA polymerases and other plant enzymes, influencing their activity. Phosphorus mobilization enzymes such as phosphatase and phytases are stimulated by ZnO-NPs in the rhizosphere, increasing the amount of phosphorus available to plants [65, 66]. ZnO-NPs have a dual role as essential nutrients and native phosphorous mobilizers are supported by improved physiological and biochemical responses [63].
Both root and shoot lengths were influenced by ZnO-NPs as demonstrated in (Fig. 6B). In addition, the difference in root elongation between control and all ZnO-NPs concentrations was substantial (Fig. 6B). The maximum root elongation at 2 PPM ZnO-NPs concentration was 6 ± 1.2 cm, while the control plants’ root length was 3.6 ± 1.2 cm. At a concentration of 12 PPM ZnO-NPs, the minimum root length was found to be 3.1 ± 1.3 cm. According to previous controlled studies, the toxicity of NPs during the early stages of plant growth is most likely due to the following factors: (i) chemical and physical properties that affect the release of ions or the aggregation of particles into more stable forms; and (ii) particle size and shape, which determine the specific surface area of NPs [67, 68]. A quick and commonly used acute phytotoxicity test method, seed germination and root elongation, have various advantages, including sensitivity, simplicity, cheap cost, and applicability for unstable substances or samples [69, 70]. Figure 6B demonstrates the effects of suspended ZnO-NPs on young shoots. In shoots, ZnO-NPs at all concentrations induced significant increases in shoot length, which progressively increased as concentrations increased. At 12 PPM of ZnO-NP concentration, the highest shoot elongation was 6.5 ± 1.2 cm, compared to 3.2 ± 0.8 cm for the control shoot. In contrast, most concentrations of ZnO-NPs (1, 2, 4, and 8 mg/l) in our investigation encouraged root and shoot growth compared to control, except for 12 PPM, where root growth compared to control (Fig. 7). ZnO-NPs at a concentration of 10 mg/l significantly increased the root length of germinated maize, according to (Zhang et al., 2015). Our results align with those of Plaksenkova et al., who found that low ZnO-NPs concentrations of 1, 2, and 4 PPM significantly improved barley root and shoot lengths [61].
Root and shoot length in tomato (Lycopersicon esculentum L.) plants treated with ZnO-NPs at doses of 2, 4, 8, or 16 mg/l had a longer length than control plants [68]. Furthermore, lower ZnO-NP concentrations (10 and 20 mg/l) resulted in a significant increase in onion seedling shoot and root lengths, but higher concentrations (30 and 40 mg/l) resulted in a significant decrease in onion seedling shoot and root lengths [67]. In contrast to Munzuroglu and Geckil., a wide range of ZnO-NPs concentrations, from low (50 mg/l) to very high (3200 mg/l), had no effect on the length of three-week-old onion plantlets grown in vitro [70].
On the contrary, Wang et al. reported that, the ZnO-NPs suppressed garlic root growth as concentration increased. At a concentration of 50 mg/l, ZnO-NPs completely inhibited root development [69]. Low ZnO-NP concentrations cause an increase in root and shoot length, which could be a nutritional benefit of nano zinc oxide. The significant concentration of Zn in seeds plays an important physiological role during seed germination and early seedling growth [71]. Zn contributes to aids elongation and cell division by stabilizing indole-3-acetic acid, the most common auxin in plants when collected in significant concentrations. However, it is hazardous due to its episodic binding to proteins and subsequent displacement of other metal ions, particularly Fe [72, 73].
Finally, variations in plant responsiveness to ZnO-NPs application may be attributed to genotype, plant part/organ (treatment of seeds, roots, or leaves), experiment environment (in vitro or in vivo), NP features (size, shape, and concentration), and exposure time [74].
3.6 Effect of ZnO-NPs on MI
In genotoxicity studies, cytogenetic analysis of root meristems with an optical microscope is a quick and effective way to determine the MI, chromosome breakages and anomalies, micronuclei [75], spindle failure, and polyploidy and aneuploidy occurrence [76, 77]. The cytotoxicity and genotoxicity capacity of ZnO-NPs on barley seedlings was estimated using cytological indicators such as the MI, and the number and percentage of chromosomal abnormalities. Tables 1 and 2 illustrate the cytological and chromosomal abnormalities seen in the root tip cells of barley treated with varying doses of ZnO-NPs.
The control sample had a MI of 5.67 ± 0.04 and normal divisional phases, except for a minor number of aberrant cells at prophase (3.53 ± 0.01). The percentage of dividing cells increased significantly until 8 mg/l ZnO-NPs was added, after which the value decreased significantly. The value of the MI (1.25 ± 0.07%) was lowered by nearly 4% at a dose concentration of 12 PPM compared to the control. This higher rise in MI at lower concentrations (1 and 2 PPM) was limited by short-time exposure (3 and 6 h), but subsequent increases in nanoparticle dosage and time resulted in a significant decrease in MI until total suppression was achieved at 12 h with 4, 8, and 12 PPM concentrations. MI levels at all concentrations decreased with time as a result of long-term exposure as presented in Table 2 and Fig. 8. These results are consistent with those reported by Plaksenkova et al., where the cytotoxicity generated by ZnO-NPs depends on their concentration and time exposure [61]. Furthermore, there was a concentration-dependent decrease of MI in V. faba, indicating that ZnO-NPs have cytotoxic potential, according to [78]. According to our findings, exposing barley roots to ZnO-NPs produces cytotoxicity and genotoxicity. Table 2 summarizes the mitotic parameters recorded before and after nanoparticle treatment. According to the tests, ZnO-NPs caused significant alterations in MI and a rise in chromosomal aberrations (Fig. 8) as a clear dose response impact, even when plants were exposed to low concentrations of them over a short period of time. CAs revealed that mitotic inhibition was linked to the gradual production of several chromosomal abnormalities.
The highest value of MI (8.930.06) was recorded after 3 h of treatment with 1 PPM of ZnO-NPs, and the lowest (1.230.01) was reported after 9 h of treatment with 12 PPM of ZnO-NPs. The decline in MI was statistically highly significant for a 12-h treatment at two doses with a minimum value of 1.73 ± 0.06 and 1.25 ± 0.07% for (1 and 2 PPM ZnO-NPs, respectively) and complete inhibition for the other dosages. We can conclude that, the increased exposure period, ZnO-NP concentration, and lower mitotic activity are all linked. Prophase was the most critical mitotic stage for all exposure doses examined, except when 2 PPM was applied for 9 h and metaphase was the most abundant at 90 ± 0.07 (Table 2 and Fig. 8). The obtained results agree with Giorgetti et al., who used different ZnO-NPs treatments induce distinct increases in MI values in the V. faba root cell, with the lowest dosage (10 mg/l) causing most significant rise [78]. Previous research showed that the MI values in the root tips of A. cepa and V. faba declined when the concentration of Zn or ZnO-NPs and exposure length increased [79,80,81]. MI was suppressed in a concentration-dependent manner indicating that ZnO-NPs have cytotoxic potential in barley. Several studies described similar effects on MI, with a decrease in MI as nanoparticle concentration increased [82, 83]. Since ZnO-NPs appear to have a mitodepressive effect, this suggests that they may interfere with mitosis’ natural development, preventing some cells from entering prophase and stopping the mitotic cycle in the interphase, thus limiting DNA/protein synthesis [84, 85].
3.7 Effect of ZnO-NPs on chromosomal aberration
Changes in chromosome structure caused by a break or exchange of chromosomal material are known as chromosomal aberrations. According to the findings, with varied quantities of ZnO-NP suspensions, multiple chromosomal abnormalities were found to be caused at all phases of the cell cycle, as well as changes in mitotic stages and nuclear membrane damage.
As the exposure length or ZnO-NP concentration rose, the rate of chromosomal abnormalities increased. For all concentrations tested, the percentage chromosomal aberration values were stated to be higher. At higher nanoparticle dosages of 12 PPM with all-time exposure and at lesser nanoparticle dosages with extended time exposure of 12 h, aberrant chromosomes were observed with their maximum percentage abnormality of 100% (Table 3). Increases in the percentage of abnormalities in root meristems indicate that the test substances are genotoxic [27]. A variety of reasons can cause increased chromosomal abnormalities. The most significant is due to chemical interference during DNA repair. The clastogenicity of chemicals/nanoparticles is represented by many forms of chromosomal abnormalities.
Bridges, lagging, fragmented, disordered, micronucleus, and sticky chromosomes were found in the root meristem of barley that had been minimally exposed to various concentrations of ZnO-NPs as the most common abnormal cells (Table 3 and Fig. 9). When the total number of CAs was considered, the most common anomaly was irregular prophase, which had a high incidence in cell division stages, followed by stickiness and C-mitosis as the most common anomaly. The higher percentage of these CAs was recorded at the 2 PPM concentration as (88.18 ± 0.03 and 88.99 ± 0.04, respectively) (Table 3 and Fig. 9).
Sticky chromosomes are one of the most frequently detected abnormalities in root tips of plants treated with ZnO-NPs during metaphase, anaphase, and telophase stages of mitosis (Fig. 9). This finding contradicts the findings of [82, 86], who demonstrated that chromosomal stickiness was the most common CA, and it supports the significant DNA fragmentation observed in the comet assays conducted by [27, 86] using A. cepa specimens. Previously, researchers revealed that nanoparticles could demonstrate various clastogenic effects, including stickiness, aberrant metaphase, and cell wall dissociation, at various exposure dosages [87,88,89]. Chromosomal stickiness and other chromosomal anomalies recorded in this study could be due to the ZnO-NP binding to DNA and proteins and altering their physico-chemical properties, leading to harmful changes in their chromatin structure, and nucleus condensation, or the formation of inter- and intra-chromatid cross-links. This hypothesis is supported by (i) the strong binding affinity of ZnO-NPs for DNA [90], (ii) the formation of ZnO-NPs complexes with the ring N atom or NH site in nucleobases of DNA [91], and (iii) the interaction, as well as the development of a bioconjugate between protein and ZnO-NPs, reported by Bhunia et al., who observed that the ZnO-NPs induced structural changes/unfolding of protein [92]. Other researcher, suggested that stickiness indicates high chemical toxicity leads to abnormal protein–protein interactions [93]. Sticky chromosomes are considered a kind of chromatid aberration [94]. Sticky chromosomes are defined by the loss of function of one or two types of particular nonhistone proteins that control chromatid separation and segregation, according to [95]. According to Darlington and McLENH (1951), stability could be caused by chromosomal DNA degradation or depolymerization [96]. In addition, the stickiness caused a disruption in the enzyme system, which may have resulted in a reduction in the rate of cell division [97]. The high values of C-metaphases (Figs. 8, 9 and Table 3) show that Zn compounds are aneugenic, probably due to the disturbance of calmodulin, a tiny Ca2+-binding protein involved in chromosomal mobility via microtubule polymerization/depolymerization regulation [98]. In addition, very high C-mitosis frequencies could imply partial inhibition of the mitotic apparatus due to oxidative stress caused by higher ZnO-NPs concentrations. There was evidence of structural chromosomal rearrangement and a possible clastogenic character to ZnO-NPs, since chromosomal breakage, ring chromosomes, and chromatin bridges were found. During the anaphase stage, chromosomal breakage, and disruption were also reported (Fig. 10). The meristematic cells that were exposed to the ZnO-NPs had bridges during anaphase and telophase. The frequency of micronuclei was seen as a marker for two doses of ZnO-NPs, 4 and 8 PPM, at all times of exposure except at 12 h, when there were no dividing cells, but there were no micronucleated cells in the distilled water as a control. At certain concentrations, ZnO-NPs are clearly clastogenic/genotoxic and cytotoxic agents in barley root cells, as shown by micronucleus. ZnO-NPs demonstrated similar genotoxic effects on A. cepa and V. faba, according to the findings of [27]. In V. faba root tip cells, the micronucleus test was utilized by Manzo et al. to investigate the toxicity of ZnO-NPs polluting soil [99]. It has been suggested that micronuclei are formed by chromosomal fragments that do not integrate into either of the daughter nuclei during mitotic telophase, according to [100]. Many different metaphase abnormalities and ana-telophase chromosomal aberrations suggest Zn has genotoxic potential. Chromosome abnormalities indicate that Zn interferes with nucleic acids and has a clastogenic potential.
Different treatments of ZnO-NPs caused abnormalities in barley root meristems: A–C C-metaphase; D, E irregular, granulated, and vacuolated prophase; F laggard at metaphase; G–K sticky metaphase; L metaphase with 2 lagging chromosomes; M, O sticky telophase; N laggard at anaphase; P–R sticky anaphase; S vacuolated nucleus at interphase and disturbed anaphase; T, U star anaphase; V single bridge at anaphase; W micronucleus, and X Metaphase with fragment and laggard
4 Conclusion
Fruit peel extract was used as a reducing, chelating, and capping agent to stabilize ZnO-NPs using environmentally acceptable and low-cost green synthesis methods. The nanoscale formation of ZnO-NPs was confirmed by morphological structure, particle size, main groups, and crystalline nature. The biological activities of the biosynthesized ZnO-NPs were evaluated, including antimicrobial, antioxidant, seed germination, root length, shoot height, cytotoxicity, and genotoxicity. The ZnO-NPs appeared to have spherical and hexagonal forms with an average diameter of 20 to 40 nm and crystallinity, as determined by the outcomes of characterization procedures. Data showed that the activities of biosynthesized ZnO-NPs were dose- and time-dependent. The ZnO-NPs demonstrated a wide range of activities against the human pathogens used as well as the higher concentrations of ZnO-NPs brought higher shoot length, while the rooting length was more significant at 2 PPM of ZnO-NPs. The antioxidant activity of ZnO-NPs was determined by IC50 = 240 and 250 μg/ml by DPPH and ABTS, respectively. The current study demonstrated the high efficacy of POP extract as a biocatalyst for the green synthesis of ZnO-NPs, which was used as an antimicrobial agent as well as promoting the growth of barley in terms of enhanced seed germination, roots, and shoot length, thus promoting the development of sustainable agriculture and improving the overall yield.
Data availability
The dataset and analyzed during the current study are available from the corresponding authors on realistic demand.
References
Saratale RG, Saratale GD, Shin HS, Jacob JM, Pugazhendhi A, Bhaisare M, Kumar G (2018) New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res 25(11):10164–10183
Sithara R, Selvakumar P, Arun C, Anandan S, Sivashanmugam P (2017) Economical synthesis of silver nanoparticles using leaf extract of Acalypha hispida and its application in the detection of Mn (II) ions. J Adv Res 8(6):561–568
Padalia H, Chanda S (2017) Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol 45(8):1751–1761
Parveen K, Banse V, Ledwani L (2016) Green synthesis of nanoparticles: their advantages and disadvantages, AIP conference proceedings. AIP Publishing LLC, New York, p 020048
Pandey S, Do JY, Kim J, Kang M (2020) Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr Polym 230:115597
Pandey S, Ramontja J (2016) Sodium alginate stabilized silver nanoparticles–silica nanohybrid and their antibacterial characteristics. Int J Biol Macromol 93:712–723
Salam HA, Sivaraj R, Venckatesh R (2014) Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae leaf extract. Mater Lett 131:16–18
Azizi S, Ahmad MB, Namvar F, Mohamad R (2014) Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett 116:275–277
Yuvakkumar R, Suresh J, Nathanael AJ, Sundrarajan M, Hong S (2014) Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater Sci Eng C 41:17–27
Chaudhary A, Rahul SN (2017) Antibacterial activity of Punica granatum (pomegranate) fruit peel extract against pathogenic and drug resistance bacterial strains. Int J Curr Microbiol App Sci 6(12):3802–3807
Adelere IA, Lateef A (2016) A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol Rev 5(6):567–587
Singh B, Singh JP, Kaur A, Singh N (2018) Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem 261:75–86
Phongtongpasuk S, Poadang S (2016) Green synthesis of silver nanoparticles using pomegranate peel extract. Adv Mater Res 1131:227–230
Fuku X, Diallo A, Maaza M (2016) Nanoscaled electrocatalytic optically modulated ZnO nanoparticles through green process of Punica granatum L and their antibacterial activities. Int J Electrochem 2016:1–10
Nasiriboroumand M, Montazer M, Barani H (2018) Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J Photochem Photobiol, B 179:98–104
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D, Hull MS (2015) Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6(1):1769–1780
Hussain A, Ali S, Rizwan M, ur Rehman MZ, Javed MR, Imran M, Chatha SAS, Nazir R (2018) Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ Pollut 242:1518–1526
de la Rosa G, López-Moreno ML, de Haro D, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: root development and X-ray absorption spectroscopy studies. Pure Appl Chem 85(12):2161–2174
Jayarambabu N, Kumari BS, Rao KV, Prabhu Y (2014) Germination and growth characteristics of mungbean seeds (Vigna radiata L.) affected by synthesized zinc oxide nanoparticles. Int J Curr Eng Technol 4:2347–5161
Rizwan M, Ali S, Qayyum MF, Ok YS, Adrees M, Ibrahim M, Zia-ur-Rehman M, Farid M, Abbas F (2017) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16
Misra A, Srivastava A, Srivastava N, Khan A (2005) Zn-acquisition and its role in growth, photosynthesis, photosynthetic pigments, and biochemical changes in essential monoterpene oil (s) of Pelargonium graveolens. Photosynthetica 43(1):153–155
Eisvand H, Kamaei H, Nazarian F (2018) Chlorophyll fluorescence, yield and yield components of bread wheat affected by phosphate bio-fertilizer, zinc and boron under late-season heat stress. Photosynthetica 56(4):1287–1296
Tsonev T, Cebola Lidon FJ (2012) Zinc in plants-an overview. Emirates J Food Agricult (EJFA) 24(4):322–333
Sedghi M, Hadi M, Toluie SG (2013) Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann West Univ Timisoara Ser Biol 16(2):73
Szőllősi R, Molnár Á, Kondak S, Kolbert Z (2020) Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 9(12):1745
Kaveh R, Li Y-S, Ranjbar S, Tehrani R, Brueck CL, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol 47(18):10637–10644
Ghosh M, Ghosh I, Godderis L, Hoet P, Mukherjee A (2019) Genotoxicity of engineered nanoparticles in higher plants, Mutation Research/Genetic Toxicology and Environmental. Mutagenesis 842:132–145
Long NV, Dolstra O, Malosetti M, Kilian B, Graner A, Visser RG, van der Linden CG (2013) Association mapping of salt tolerance in barley (Hordeum vulgare L). Theor Appl Genet 126(9):2335–2351
Mattiello A, Filippi A, Pošćić F, Musetti R, Salvatici MC, Giordano C, Vischi M, Bertolini A, Marchiol L (2015) Evidence of phytotoxicity and genotoxicity in Hordeum vulgare L. exposed to CeO2 and TiO2 nanoparticles. Front Plant Sci 6:1043
Ferdous J, Li Y, Reid N, Langridge P, Shi B-J, Tricker PJ (2015) Identification of reference genes for quantitative expression analysis of microRNAs and mRNAs in barley under various stress conditions. PLoS One 10(3):e0118503
Bian J, Deng P, Zhan H, Wu X, Nishantha MD, Yan Z, Du X, Nie X, Song W (2019) Transcriptional dynamics of grain development in barley (Hordeum vulgare L.). Int J Mol Sci 20(4):962
Dwivedi S, Wahab R, Khan F, Mishra YK, Musarrat J, Al-Khedhairy AA (2014) Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS One 9(11):e111289
Ghasemi F, Jalal R (2016) Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multidrug-resistant Acinetobacter baumannii. J Glob Antimicrob Resist 6:118–122
Faisal S, Jan H, Shah SA, Shah S, Khan A, Akbar MT, Rizwan M, Jan F, Wajidullah N. Akhtar (2021) Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: their characterizations and biological and environmental applications. ACS Omega 6(14):9709–9722
Yazhiniprabha M, Banu S, Ishwarya R, Vinotha V, Govindarajan M, Wadaan MA, Mahboob S, Nicoletti M, Vaseeharan B (2022) Biomimetically synthesized Physalis minima fruit extract-based zinc oxide nanoparticles as eco-friendly biomaterials for biological applications. J Drug Deliv Sci Technol 73:103475
Chamaraja N, Mahesh B, Rekha N (2022) Green synthesis of Zn/Cu oxide nanoparticles by Vernicia fordii seed extract: their photocatalytic activity toward industrial dye degradation and their biological activity. Inorg Nano-Metal Chem. https://doi.org/10.1080/24701556.2022.2069123
El-Kattan N, Emam AN, Mansour AS, Ibrahim MA, Abd El-Razik AB, Allam KA, Riad NY, Ibrahim SA (2022) Curcumin assisted green synthesis of silver and zinc oxide nanostructures and their antibacterial activity against some clinical pathogenic multi-drug resistant bacteria. RSC Adv 12(28):18022–18038
Patel M, Siddiqi NJ, Sharma P, Alhomida AS, Khan HA (2019) Reproductive toxicity of pomegranate peel extract synthesized gold nanoparticles: a multigeneration study in C. elegans. J Nanomater 2019:e8767943
Saliani M, Jalal R, Goharshadi EK (2015) Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J Microbiol 8(2):e17115
Humphries R, Bobenchik AM, Hindler JA, Schuetz AN (2021) Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100. J Clin Microbiol 59(12):e00213-e221
Magaldi S, Mata-Essayag S, De Capriles CH, Pérez C, Colella M, Olaizola C, Ontiveros Y (2004) Well diffusion for antifungal susceptibility testing. Int J Infect Dis 8(1):39–45
Sharaf MH, Abdelaziz AM, Kalaba MH, Radwan AA, Hashem AH (2022) Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Appl Biochem Biotechnol 194(3):1271–1289
Lee KJ, Oh YC, Cho WK, Ma JY (2015) Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid Based Complement Alternat Med 2015:e165457
Özkara A, Akyıl D, Erdoğmuş SF, Konuk M (2011) Evaluation of germination, root growth and cytological effects of wastewater of sugar factory (Afyonkarahisar) using Hordeum vulgare bioassays. Environ Monit Assess 183(1):517–524
Hu Y, Tan L, Zhang S-H, Zuo Y-T, Han X, Liu N, Lu W-Q, Liu A-L (2017) Detection of genotoxic effects of drinking water disinfection by-products using Vicia faba bioassay. Environ Sci Pollut Res 24(2):1509–1517
Kołodziejczak-Radzimska A, Markiewicz E, Jesionowski T (2012) Structural characterisation of ZnO particles obtained by the emulsion precipitation method. J Nanomater. https://doi.org/10.1155/2012/656353
Shabaani M, Rahaiee S, Zare M, Jafari SM (2020) Green synthesis of ZnO nanoparticles using loquat seed extract; Biological functions and photocatalytic degradation properties. Lwt 134:110133
Awwad AM, Salem NM, Abdeen AO (2012) Biosynthesis of silver nanoparticles using Olea europaea leaves extract and its antibacterial activity. Nanosci Nanotechnol 2(6):164–170
Mukherjee S, Sushma V, Patra S, Barui AK, Bhadra MP, Sreedhar B, Patra CR (2012) Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 23(45):455103
Sharmila G, Thirumarimurugan M, Muthukumaran C (2019) Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem J 145:578–587
Tiwari V, Mishra N, Gadani K, Solanki PS, Shah N, Tiwari M (2018) Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front Microbiol 9:1218
Ali J, Irshad R, Li B, Tahir K, Ahmad A, Shakeel M, Khan NU, Khan ZUH (2018) Synthesis and characterization of phytochemical fabricated zinc oxide nanoparticles with enhanced antibacterial and catalytic applications. J Photochem Photobiol, B 183:349–356
Degefa A, Bekele B, Jule LT, Fikadu B, Ramaswamy S, Dwarampudi LP, Nagaprasad N, Ramaswamy K (2021) Green synthesis, characterization of zinc oxide nanoparticles, and examination of properties for dye-sensitive solar cells using various vegetable extracts. J Nanomater 2021:e3941923
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed 12:1227–1249
Kalaba MH, Moghannem SA, El-Hawary AS, Radwan AA, Sharaf MH, Shaban AS (2021) Green synthesized ZnO nanoparticles mediated by Streptomyces plicatus: Characterizations, antimicrobial and nematicidal activities and cytogenetic effects. Plants 10(9):1760
Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, Choi I (2019) Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int J Mol Sci 20(10):2468
He L, Liu Y, Mustapha A, Lin M (2011) Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res 166(3):207–215
Lee JH, Lee BW, Kim B, Kim HT, Ko JM, Baek I-Y, Seo WT, Kang YM, Cho KM (2013) Changes in phenolic compounds (isoflavones and phenolic acids) and antioxidant properties in high-protein soybean (Glycine max L., cv. Saedanbaek) for different roasting conditions. J Korean Soc Appl Biol Chem 56(5):605–612
Kurutas EB (2015) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15(1):1–22
Xiang L, Zhao H-M, Li Y-W, Huang X-P, Wu X-L, Zhai T, Yuan Y, Cai Q-Y, Mo C-H (2015) Effects of the size and morphology of zinc oxide nanoparticles on the germination of Chinese cabbage seeds. Environ Sci Pollut Res 22(14):10452–10462
Plaksenkova I, Kokina I, Petrova A, Jermaļonoka M, Gerbreders V, Krasovska M (2020) The impact of zinc oxide nanoparticles on cytotoxicity, genotoxicity, and miRNA expression in barley (Hordeum vulgare L.) seedlings. Sci World J 2020:e6649746
Panda S (2017) Physiological impact of Zinc nanoparticle on germination of rice (Oryza sativa L) seed. J Plant Sci Phytopathol 1:062–070
Raliya R, Nair R, Chavalmane S, Wang W-N, Biswas P (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7(12):1584–1594
Zhang R, Zhang H, Tu C, Hu X, Li L, Luo Y, Christie P (2015) Phytotoxicity of ZnO nanoparticles and the released Zn (II) ion to corn (Zea mays L.) and cucumber (Cucumis sativus L.) during germination. Environ Sci Pollut Res 22(14):11109–11117
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agricult Res 2(1):48–57
Adhikari S, Adhikari A, Ghosh S, Roy D, Azahar I, Basuli D, Hossain Z (2020) Assessment of ZnO-NPs toxicity in maize: An integrative microRNAomic approach. Chemosphere 249:126197
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150(2):243–250
Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett 158(2):122–132
Wang X, Sun C, Gao S, Wang L, Shuokui H (2001) Validation of germination rate and root elongation as indicator to assess phytotoxicity with Cucumis sativus. Chemosphere 44(8):1711–1721
Munzuroglu O, Geckil H (2002) Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Arch Environ Contam Toxicol 43(2):203–213
Faizan M, Faraz A, Yusuf M, Khan S, Hayat S (2018) Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56(2):678–686
Linnainmaa K, Meretoja T, Sorsa M, Vainio H (1978) Cytogenetic effects of styrene and styrene oxide. Mutat Res Genet Toxicol 58(2–3):277–286
Tymoszuk A, Wojnarowicz J (2020) Zinc oxide and zinc oxide nanoparticles impact on in vitro germination and seedling growth in Allium cepa L. Materials 13(12):2784
Shaymurat T, Gu J, Xu C, Yang Z, Zhao Q, Liu Y, Liu Y (2012) Phytotoxic and genotoxic effects of ZnO nanoparticles on garlic (Allium sativum L.): a morphological study. Nanotoxicology 6(3):241–248
Prasad T, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad T, Sajanlal P, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35(6):905–927
Lin Y-F, Aarts MG (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69(19):3187–3206
Nalci OB, Nadaroglu H, Pour AH, Gungor AA, Haliloglu K (2019) Effects of ZnO, CuO and γ-Fe3O4 nanoparticles on mature embryo culture of wheat (Triticum aestivum L.). Plant Cell Tissue Organ Cult (PCTOC) 136(2):269–277
Giorgetti L, Talouizte H, Merzouki M, Caltavuturo L, Geri C, Frassinetti S (2011) Genotoxicity evaluation of effluents from textile industries of the region Fez-Boulmane, Morocco: a case study. Ecotoxicol Environ Saf 74(8):2275–2283
Ruffini Castiglione M, Giorgetti L, Cremonini R, Bottega S, Spanò C (2014) Impact of TiO2 nanoparticles on Vicia narbonensis L.: potential toxicity effects. Protoplasma 251(6):1471–1479
Ruffini Castiglione M, Giorgetti L, Becarelli S, Siracusa G, Lorenzi R, Di Gregorio S (2016) Polycyclic aromatic hydrocarbon-contaminated soils: bioaugmentation of autochthonous bacteria and toxicological assessment of the bioremediation process by means of Vicia faba L. Environ Sci Pollut Res 23(8):7930–7941
Barbafieri M, Giorgetti L (2016) Contaminant bioavailability in soil and phytotoxicity/genotoxicity tests in Vicia faba L.: a case study of boron contamination. Environ Sci Pollut Res 23(23):24327–24336
Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater 190(1–3):613–621
Youssef MS, Elamawi RM (2020) Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia faba. Environ Sci Pollut Res 27(16):18972–18984
Aa E-G, Ai E-N, Mm M (2000) The action of atrazine herbicide as an inhibitor of cell division on chromosomes and nucleic acids content in root meristems of Allium cepa and Vicia faba. Cytologia 65(3):277–287
Taranath T, Patil BN, Santosh T, Sharath B (2015) Cytotoxicity of zinc nanoparticles fabricated by Justicia adhatoda L. on root tips of Allium cepa L.—a model approach. Environ Sci Pollut Res 22(11):8611–8617
Sun Z, Xiong T, Zhang T, Wang N, Chen D, Li S (2019) Influences of zinc oxide nanoparticles on Allium cepa root cells and the primary cause of phytotoxicity. Ecotoxicology 28(2):175–188
Ahmed B, Shahid M, Khan MS, Musarrat J (2018) Chromosomal aberrations, cell suppression and oxidative stress generation induced by metal oxide nanoparticles in onion (Allium cepa) bulb. Metallomics 10(9):1315–1327
Krishnamoorthy K, Moon JY, Hyun HB, Cho SK, Kim S-J (2012) Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J Mater Chem 22(47):24610–24617
Bakand S, Hayes A (2016) Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int J Mol Sci 17(6):929
Das S, Chatterjee S, Pramanik S, Devi PS, Kumar GS (2018) A new insight into the interaction of ZnO with calf thymus DNA through surface defects. J Photochem Photobiol, B 178:339–347
Saha S, Sarkar P (2014) Understanding the interaction of DNA–RNA nucleobases with different ZnO nanomaterials. Phys Chem Chem Phys 16(29):15355–15366
Bhunia A, Samanta P, Saha S, Kamilya T (2013) ZnO nanoparticle-protein interaction: Corona formation with associated unfolding. Appl Phys Lett 103(14):143701
Nwakanma NMC, Okoli BE (2010) Cytological effects of the root extracts of Boerhaavia diffusa on root tips of Crinum jagus. EurAsian J BioSci 4(1):105–111
Badr A (1986) Effects of the s-triazine herbicide turbutryn on mitosis, chromosomes and nucleic acids in root tips of Vicia faba. Cytologia 51(3):571–577
Gaulden ME (1987) Hypothesis: some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosome stickiness, which causes chromosome aberrations. Mutagenesis 2(5):357–365
Darlington C, McLeish J (1951) Action of maleic hydrazide on the cell. Nature 167(4245):407–408
Mahakhode R, Somkuwar S (2013) Mitotic abnormalities induced by glyphosate in Psoralea corylifolia L. Inter J Curr Pharmaceut Res 5:46–48
Zou J, Wang M, Jiang W, Liu D (2006) Effects of hexavalent chromium (VI) on root growth and cell division in root tip cells of Amaranthus viridis L. Pak J Bot 38(3):673
Manzo S, Rocco A, Carotenuto R, De Luca Picione F, Miglietta ML, Rametta G, Di Francia G (2011) Investigation of ZnO nanoparticles’ ecotoxicological effects towards different soil organisms. Environ Sci Pollut Res 18(5):756–763
Krishna G, Hayashi M (2000) In vivo rodent micronucleus assay: protocol, conduct and data interpretation. Mutat Res Fundam Mol Mech Mutagen 455(1–2):155–166
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Abdelghany S. Shaban was in charge of concept development, methodology, writing—review and editing. The ZnO-NP was synthesized and characterized by M. E. Owda and Ahmed K. Saleh. The cytogenetics investigation was conducted by Mostafa M. Basyoni and M.A.Mousa. Antimicrobial screening was conducted by Ahmed A. Radwan. Each author contributed to the inquiry and statistics and data analysis in accordance with their role in the methodology. All authors contributed equally to the writing—preparation of the initial draught. Supervision was exercised by Abdelghany S. Shaban and Ahmed K. Saleh. The published version of the manuscript has been read and approved by all authors.
Corresponding authors
Ethics declarations
Ethical approval
There is no ethical approval due to this study not including human or animal studies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaban, A.S., Owda, M.E., Basuoni, M.M. et al. Punica granatum peel extract mediated green synthesis of zinc oxide nanoparticles: structure and evaluation of their biological applications. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03185-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03185-7