Abstract
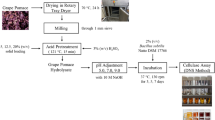
Pomegranate peels, which are normally considered waste, are used for the synthesis of cellulase in this study. Through submerged state fermentation, Trichoderma reesei filamentous fungus was employed to manufacture cellulase from pomegranate peels. Response Surface Methodology was used to screen the nutritional medium and improve the media composition for cellulase production using statistical experimental methods (RSM). Nine nutrients were screened using the Plackett–Burman Design (PBD) technique and the most impacting nutrient mediums were chosen. Central Composite design (CCD) identified four nutrients as being more critical for cellulase production, and their compositions were optimized as well. The best medium compositions for submerged fermentation of cellulase using pomegranate peel were Avicel—24.812 g/L, KH2PO4—4.626 g/L, soybean cake flour—20.7 g/L, and MnSO4.H2O—1.036 g/L. Cellulase production was determined to be 9.3 IU/mL under optimum medium conditions. Furthermore, Box–Behnken Design improved process parameters such as pH (5.5), temperature (36 °C), initial substrate concentration (3.2%), inoculum concentration (7%), and fermentation length (5 days). Finally, under the optimum medium and process conditions, the maximal cellulase production was 12.3 IU/mL. The Monod model (R2:0.9316), Luedeking–Piret model-substrate consumption (R2:0.9217), Michaelis–Menten kinetics (R2:0.94), Lineweaver–Burk plot (R2:0.9264), Hanes–Woolf plot (R2:0.0642), and Eadie–Hofstee plot (R2:0.0642) were used to model the cellulase production process. Furthermore, pomegranate peels, a cheap waste raw material, would be the best carbon source for a high production of cellulase enzyme.







Similar content being viewed by others
References
Gielen D, Boshell F, Saygin D, Bazilian MD, Wagner N, Gorini R (2019) The role of renewable energy in the global energy transformation. Energy Strateg Rev 24:38–50
Halkos GE, Gkampoura EC (2020) Reviewing usage, potentials, and limitations of renewable energy sources. Energies 13(11):2906
Kumar A, Bhattacharya T, Hasnain SM, Nayak AK, Hasnain S (2020) Applications of biomass-derived materials for energy production, conversion, and storage. Mater Sci Energy Technol 3:905–920
Gupta VK, Kubicek CP, Berri JG, Wilson DW, Couturier M, Berlin A, Ezeji T (2016) Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem Sci 41(7):633–645
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37(1):19–27
Mohanty SK, Swain MR (2019) Bioethanol production from corn and wheat: food, fuel, and future. In Bioethanol production from food crops (pp. 45–59). Academic Press.
Yang H, Shi Z, Xu G, Qin Y, Deng J, Yang J (2019) Bioethanol production from bamboo with alkali-catalyzed liquid hot water pretreatment. Bioresour Technol 274:261–266
Limayem A, Ricke SC (2012) Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci 38(4):449–467
Zhou Z, Lei F, Li P, Jiang J (2018) Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolvpretreatment technology. Biotechnol Bioeng 115(11):2683–2702
Mussatto SI, Teixeira JA (2010) Lignocellulose as raw material in fermentation processes. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology (book chapter), 897–907.
Goukanapalle PKR, Kanderi DK, Rajoji G, Shanthi Kumari BS, Bontha RR (2020) Optimization of cellulase production by a novel endophytic fungus Pestalotiopsismicrospora TKBRR isolated from Thalakona forest. Cellulose 27:6299–6316
Demiray E, Karatay SE, Dönmez G (2019) Improvement of bioethanol production from pomegranate peels via acidic pretreatment and enzymatic hydrolysis. Environ Sci Pollut Res 26(28):29366–29378
Adsul M, Sandhu SK, Singhania RR, Gupta R, Puri SK, Mathur A (2020) Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzyme Microb Technol 133:109442
Johnson E (2016) Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuel Bioprod Biorefin 10(2):164–174
Singhvi MS, Gokhale DV (2019) Lignocellulosic biomass: hurdles and challenges in its valorization. Appl Microbiol Biotechnol 103(23):9305–9320
Ge S, Duo L, Wang J, Yang J, Li Z, Tu Y (2021) A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J Ethnopharmacol 113877
Rosas-Burgos EC, Burgos-Hernández A, Noguera-Artiaga L, Kačániová M, Hernández-García F, Cárdenas-López JL, Carbonell-Barrachina ÁA (2017) Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J Sci Food Agric 97(3):802–810
FAO (2012) Statistical database. Food and Agriculture Organization of the United Nations. Codex Alimentarius Commission, Tunis http://www.fao.org. Accessed May 23, 2012
Saleem A, Hussain A, Chaudhary A, Iqtedar M, Javid A, Akram AM (2020) Acid hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Convers Biorefin 1–12
Derakhshan Z, Ferrante M, Tadi M, Ansari F, Heydari A, Hosseini MS, Sadrabad EK (2018) Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem Toxicol 114:108–111
Salgado JM, Ferreira TRB, de Oliveira BF, dos Santos Dias CT (2012) Increased antioxidant content in juice enriched with dried extract of pomegranate (Punica granatum) peel. Plant Foods Hum Nutr 67(1):39–43
Paul T, Sinharoy A, Baskaran D, Pakshirajan K, Pugazhenthi G, Lens PN (2020) Bio-oil production from oleaginous microorganisms using hydrothermal liquefaction: a biorefinery approach. Crit Rev Environ Sci Technol1–39
Vázquez-Montoya EL, Castro-Ochoa LD, Maldonado-Mendoza IE, Luna-Suárez S, Castro-Martínez C (2020) Moringa straw as cellulase production inducer and cellulolytic fungi source. Rev Argent Microbiol 52(1):4–12
Qian Y, Zhong L, Sun Y, Sun N, Zhang L, Liu W, Zhong Y (2019) Enhancement of cellulase production in Trichoderma reesei via disruption of multiple protease genes identified by comparative secretomics. Front Microbio 10:2784
Bajaj P, Mahajan R (2019) Cellulase and xylanase synergism in industrial biotechnology. Appl Microbiol Biotechnol 103(21):8711–8724
Sathendra ER, Baskar G, Praveenkumar R, Gnansounou E (2019) Bioethanol production from palm wood using Trichoderma reesei and Kluveromycesmarxianus. Bioresour Technol 271:345–352
Gooruee R, Hojjati M, Behbahani BA et al (2022) Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02626-7
Gao J, Qian Y, Wang Y, Qu Y, Zhong Y (2017) Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei. Biotechnol Biofuels 10:272. https://doi.org/10.1186/s13068-017-0963-1
Verma N, Bansal MC, Kumar V (2018) Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal Agricult Biotechnol 16:483–492. https://doi.org/10.1016/j.bcab.2018.09.021
Verma N, Kumar V (2022) Utilization of bottle gourd vegetable peel waste biomass in cellulase production by Trichoderma reesei and Neurospora crassa. Biomass Conv Bioref 12:1105–1114. https://doi.org/10.1007/s13399-020-00727-9
Askari H, Shahbazi S (2018) Improvement of cellulose degrading enzymes activity by mutagenesis in Trichoderma reesei fungi. Agric Biotechnol 9:41–50
Ellilä S, Fonseca L, Uchima C, Cota J, Goldman GH, Saloheimo M, Siika-Aho M (2017) Development of a low-cost cellulase production process using Trichoderma reesei for Brazilian biorefineries. Biotechnol Biofuels 10(1):1–17
Salim N, Santhiagu A, Joji K (2019) Process modeling and optimization of high yielding L-methioninase from a newly isolated Trichoderma harzianum using response surface methodology and artificial neural network coupled genetic algorithm. Biocatal Agric Biotechnol 17:299–308
Latha S, Sivaranjani G, Dhanasekaran D (2017) Response surface methodology: A non-conventional statistical tool to maximize the throughput of Streptomyces species biomass and their bioactive metabolites. Crit Rev Microbiol 43(5):567–582
Paul T, Baskaran D, Pakshiraja K, Pugazhenthi G (2020) Valorization of refinery wastewater for lipid-rich biomass production by Rhodococcusopacus in batch system: A kinetic approach. Biomass Bioenerg 143:105867
Sinharoy A, Baskaran D, Pakshirajan K (2019) Sustainable biohydrogen production by dark fermentation using carbon monoxide as the sole carbon and energy source. Int J Hydrog Energy 44(26):13114–13125
Baskaran D, Rajamanickam R (2019) Aerobic biodegradation of trichloroethylene by consortium microorganism from turkey litter compost. J Environ Chem Eng 7(4):103260
Saravanan P, Ramesh S, Jaya N, Jabasingh SA (2021) Prospective evaluation of xylitol production using Dabaryomyceshansenii var hansenii, Pachysolentannophilus, and Candida guillermondii with sustainable agricultural residues. Biomass Convers Biorefin 1–19
Ghosh TK (1987) Measurement of cellulase activities. Inter Union Pure Appl Chem 59(2):257–262
Chang XG, Yang J, Wang D (2011) Box-Behnken design: an alternative for the optimization of analytical methods. Chem Prod Process Model 6:14
Montesano D, Cossignani L, Giua L, Urbani E, Simonetti MS, Blasi F (2016) A simple HPLC-ELSD method for sugar analysis in goji berry. J Chem. https://doi.org/10.1155/2016/6271808
Brijwani K, Oberoi HS, Vadlani PV (2010) Production of a cellulolytic enzyme system in mixed-culture solid-state fermentation of soybean hulls supplemented with wheat bran. Process Biochem 45(1):120–128
Singh K, Richa K, Bose H, Karthik L, Kumar G, Rao KVB (2014) Statistical media optimization and cellulase production from marine Bacillus VITRKHB. 3 Biotech 4(6):591–598
Yahya S, Jahangir S, Shaukat SS, Sohail M, Khan SA (2016) Production optimization by using Plackett-Burman design and partial characterization of amylase from Aspergillus tubingensis SY 1. Pak J Bot 48(6):2557–2561
Naghipour D, Taghavi K, Jaafari J, Mahdavi Y, GhanbariGhozikali M, Ameri R, Hossein Mahvi A (2016) Statistical modeling and optimization of the phosphorus biosorption by modified Lemna minor from aqueous solution using response surface methodology (RSM). Desalin Water Treat 57(41):19431–19442
Keharom S, Mahachai R, Chanthai S (2016) The optimization study of α-amylase activity based on central composite design-response surface methodology by dinitrosalicylic acid method. Int Food Res J 23(1):10–17
Ciric A, Krajnc B, Heath D, Ogrinc N (2020) Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem Toxicol 135:110976
Vardhan MV, Sankaraiah G, Yohan M, Rao HJ (2017) Optimization of Parameters in CNC milling of P20 steel using Response Surface methodology and Taguchi Method. Mater Today Proc 4(8):9163–9169
Singhania RR, Patel AK, Sukumaran RK, Larroche C, Pandey A (2013) Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour Technol 127:500–507
Badhan AK, Chadha BS, Sonia KG, Saini HS, Bhat MK (2004) Functionally diverse multiple xylanases of thermophilic fungus Myceliophthora sp. IMI 387099. Enzyme Microb Technol 35(5):460–466
Mondala AH (2015) Direct fungal fermentation of lignocellulosic biomass into itaconic, fumaric, and malic acids: current and future prospects. J Ind Microbiol Biotechnol 42(4):487–506
Ouedraogo N, Savadogo A, Somda MK, Tapsoba F, Zongo C, Traore AS (2017) Effect of mineral salts and nitrogen source on yeast (Candida utilis NOY1) biomass production using tubers wastes. Afr J Biotechnol 16(8):359–365
Rajeswari P, Jose PA, Amiya R, Jebakumar SRD (2015) Characterization of saltern based Streptomyces sp. and statistical media optimization for its improved antibacterial activity. Front Microbiol 5: 753
Zhang K, Yu C, Yang ST (2015) Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Process Biochem 50(2):173–179
Alemawor F, Dzogbefi VP, Oddoye EO, Oldham JH (2009) Effect of Pleurotusostreatus fermentation on cocoa pod husk composition: Influence of fermentation period and Mn2+ supplementation on the fermentation process. Afr J Biotechnol 8(9):1950–1958
Sangian HF (2016) Analysis of retention time and substances released enzymatically from lignocellulose, coconut coir treated by alkaline, ionic liquid [mmim][dmp] and combined method by observing the HPLC-RI spectra. Int J ChemTech Res 9(12):715–724
Jaradat Z, Dawagreh A, Ababneh Q, Saadoun I (2008) Influence of culture conditions on cellulase production by Streptomyces sp. (strain J2). Jordan J Biol Sci 1(4):141–146
Liming X, Xueliang S (2004) High-yield cellulase production by Trichoderma reesei ZU-02 on corn cob residue. Bioresour Technol 91(3):259–262
Kashyap P, Sabu A, Pandey A, Szakacs G, Soccol CR (2002) Extra-cellular L-glutaminase production by Zygosaccharomyces rouxii under solid-state fermentation. Process Biochem 38(3):307–312
Pachauri P, More S, Aranganathan V, Sullia SB, Deshmukh S (2018) Kinetic study and characterization of cellulase enzyme from isolated Aspergillus niger subsp. awamori for cellulosic biofuels. J Sci Ind Res 77:55–60
Membrillo I, Sánchez C, Meneses M, Favela E, Loera O (2008) Effect of substrate particle size and additional nitrogen source on production of lignocellulolytic enzymes by Pleurotusostreatus strains. Bioresour Technol 99(16):7842–7847
Badhan AK, Chadha BS, Kaur J, Saini HS, Bhat MK (2007) Production of multiple xylanolytic and cellulolytic enzymes by thermophilic fungus Myceliophthora sp IMI 387099. Bioresour Technol 98(3):504–510
Kalogeris E, Christakopoulos P, Katapodis P, Alexiou A, Vlachou S, Kekos D, Macris BJ (2003) Production and characterization of cellulolytic enzymes from the thermophilic fungus Thermoascusaurantiacus under solid state cultivation of agricultural wastes. Process Biochem 38(7):1099–1104
Manikandan K, Saravanan V, Viruthagiri T (2008) Kinetics studies on ethanol production from banana peel waste using mutant strain of Saccharomyces cerevisiae. Indian J Biotechnol 83–88
Tomczak JM, Węglarz-Tomczak E (2019) Estimating kinetic constants in the Michaelis-Menten model from one enzymatic assay using Approximate Bayesian Computation. FEBS Lett 593(19):2742–2750
Rodriguez JMG, Hux NP, Philips SJ, Towns MH (2019) Michaelis-Menten graphs, Lineweaver-Burk plots, and reaction schemes: investigating introductory biochemistry students’ conceptions of representations in enzyme kinetics. J Chem Educ 96(9):1833–1845
Tracy TS (2008) Enzyme kinetics. Drug Metab Drug Des Develop: Basic Concepts Pract 4:89–112
Guerra NP (2017) Enzyme kinetics experiment with the multi enzyme complex viscozyme L and two substrates for the accurate determination of michaelian parameters. J Chem Educ 94(6):795–799
Manikandan K, Saravanan V, Viruthagiri T (2008) Kinetics studies on bioethanol production from banana peel waste using mutant strain of saccharomyces cerevisiae. Indian J Biotechnol 7:83–88
Bezerra RMF, Dias AA, Fraga I, Pereira AN (2011) Cellulose hydrolysis by cellobiohydrolase Cel7A shows mixed hyperbolic product inhibition. Appl Biochem Biotechnol 165:178–189. https://doi.org/10.1007/s12010-011-9242-y
Khalseh R (2016) Evaluation of Different Kinetics for Bioethanol Production withEmphasis to Analytical Solution of Substrate Equation. Theor Found Chem Eng 50(4):392–397. https://doi.org/10.1134/S0040579516040357
Monod J (1949) The growth of bacterial cultures. Ann Rev Microbiol 3:371–394
Jin H, Liu R, He Y (2011) Kinetics for batch fermentations for ethanol production withimmobilized Saccharomyces cerevisiae growing on sweet sorghum stalk juice. Procedia Environ Sci 12:137–145. https://doi.org/10.1016/j.proenv.2012.01
Sivarathnakumara S, Jayamuthunagai J, Baskar G, Praveenkumar R, Aberna Ebenezer Selvakumari I, Bharathiraja B (2019) Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour Technol 293:122060
Mansouri A, Rihani R, Laoufi AN, Özkan M (2016) Production of bioethanol from amixture of agricultural feedstocks: Biofuels characterization. Fuel 185:612–621. https://doi.org/10.1016/j.fuel.2016.08.008
Shuler ML, Kargi F, Kargi F (2002) Bioprocess engineering: basic concepts. Prentice Hall, Upper Saddle River
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals. McGraw-Hill, New York
Verma N, Kumar V (2020) Impact of process parameters and plant polysaccharide hydrolysates in cellulase production by Trichoderma reesei and Neurospora crassa under wheat bran based solid state fermentation. Biotechnology Reports 25:e00416
Nitin V, Vivek MC (2011) Pea peel waste: a lignocellulosic waste and its utility in cellulase production by Trichoderma reesei under solid state fermentation. BioResources 6(2):1505–1519
Gautam SP, Bundela PS, Pandey AK, Khan J, Awasthi MK, Sarsaiya S (2011) Optimization for the production of cellulase enzyme from municipal solid waste residue by two novel cellulolytic fungi. Biotechnol Res Int 2011:1–8
Gordillo-Fuenzalida F, Echeverria-Vega A, Cuadros-Orellana S, Faundez C, K¨ahne T, Morales-Vera R (2019) Cellulases production by a Trichoderma sp. using food manufacturing wastes. Appl. Sci. 9(20) https://doi.org/10.3390/app9204419.
Silva AFV, Santos LA, Valença RB, Porto TS, Da Motta Sobrinho MA, Gomes GJC, Santos AFMS (2019) Cellulase production to obtain biogas from passion fruit (Passiflora edulis) peel waste hydrolysate. J Environ Chem Eng 7(6):103510. https://doi.org/10.1016/j.jece.2019.103510
Sinjaroonsak S, Chaiyaso T, H-Kittikun A (2020) Optimization of cellulase and xylanase productions by streptomyces thermocoprophilus TC13W using low cost pretreated oil palm empty fruit bunch. Waste Biomass Valori 11(8):3925–3936. https://doi.org/10.1007/s12649-019-00720-y
Nehad EA, Yoness MF, Reem AA (2019) Optimization and purification of cellulase produced by Penicillium decumbens and its application. Egypt Pharm J 18:391–402
Srivastava N, Srivastava M, Manikanta A, Singh P, Ramteke PW, Mishra PK, Malhotra BD (2017) Production and optimization of physicochemical parameters of cellulase using untreated orange waste by newly isolated Emericella variecolor NS3. Appl Biochem Biotechnol 183(2):601–612. https://doi.org/10.1007/s12010-017-2561-x
Umikalsom MS, Ariff AB, Zulkifli HS, Tong CC, Hassan MA, Karim MIA (1997) The treatment of oil palm empty fruit bunch fibre for subsequent use as substrate for cellulase production by Chaetomium globosum Kunze. Bioresour Technol 62(1):1–9. https://doi.org/10.1016/S0960-8524(97)00132-6
Krishna C (1999) Production of bacterial cellulases by solid state bioprocessing of banana wastes. Bioresour Technol 69(3):231–239. https://doi.org/10.1016/S0960-8524(98)00193-X
Acknowledgements
The authors are thankful to Bharathidasan Institute of Technology (BIT) Campus, Anna University, Tiruchirappalli, and Annamalai University, Annamalainagar, Chidambaram, India, for the technical support provided.
Author information
Authors and Affiliations
Contributions
Panchamoorthy Saravanan had the idea for the article. All authors contributed to the literature search and data analysis. The first draft of the manuscript was written by Panchamoorthy Saravanan and all authors commented and critically revised the work. All authors read and approved the final manuscript. R.Muthuvelayudham contributed to supervision.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baskaran, D., Saravanan, P., Saravanan, V. et al. Pomegranate peel utilization by an indigenous fungal strain of Trichoderma reesei NCIM 1186: Optimization and Kinetics studies on production of cellulase. Biomass Conv. Bioref. 14, 6435–6453 (2024). https://doi.org/10.1007/s13399-022-02901-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02901-7