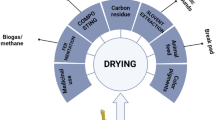
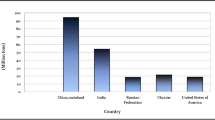
Abstract
Enormous waste has been generated from the vegetable and fruit processing industries, which are a good source of carbohydrates. Such unused remnant imposes huge disposal and severe pollution problems. Due to the presence of cellulose, hemicellulose, pectin, minerals, and vitamins, these waste materials have a great prospective for its bioconversion into useful products, viz., acids, enzymes, fuels, and value-added products. To reveal their possible potential, separate sets of experiments have been conducted by using bottle gourd peel waste biomass as a carbon source for cellulase production. It was observed from experimental findings that 30 °C temperature and 0.56 g/l of inoculum dosages are the most promising situations for cellulase production by both the fungal strains. FPase and CMCase activity considerably increases by the inclusion of whey as well as starch hydrolysates in the media used in the production study. The present study portrays the utility of bottle gourd peel waste, whey, and starch-based hydrolysates in cellulase production by Trichoderma reesei and Neurospora crassa. The exploitation of cost-effective, cheap, bottle gourd vegetable peel waste for cellulase production could be an innovative, effective, sustainable, and green approach in cellulase production.




Similar content being viewed by others
References
Ahmed D, Abid H, Riaz A (2018) Lagenaria siceraria peel biomass as a potential biosorbent for the removal of toxic metals from industrial wastewaters. Int J Environ Stud 75(5):63–773. https://doi.org/10.1080/00207233.2018.1457285
Ahmed S, Ru W, Han H, Cheng L, Bian X, Li G, Jin L, Wu P, Bao J (2019) Fine molecular structure and its effects on physicochemical properties of starches in potatoes grown in two locations. Food Hydrocoll 97(29)
Alvani K, Qi X, Tester RF (2011) Use of carbohydrates, including dextrins, for oral delivery. Starch-Special issue. Clinl Nutr-Appl Alpha-Glucans 63(7):424–431. https://doi.org/10.1002/star.201000110
An C, Ma SJ, Chang F, Xue WJ (2017) Efficient production of pullulan by Aureobasidium pullulans grown on mixtures of potato starch hydrolysate and sucrose. Braz J Microbiol 48(1):80–185. https://doi.org/10.1016/j.bjm.2016.11.001
Anekar S, Rao CR (2009) Ultra filtration – tool to recover valuable constituent from dairy waste water. J Appl Sci Environ Sanit 4(2):125–132
Behera S, Gupta N (2015) Utilization of vegetable waste for biomass production of some wild edible mushroom cultures. Tropic Plant Res 2(1):05–09
Cai J, He Y, Yu X, Banks SW, Yang Y, Zhang X, Yu Y, Liu R, Bridgewater AV (2017) Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sust Energ Rev 76:309–322. https://doi.org/10.1016/j.rser.2017.03.072
Carioca JOB, Arora HL, Panir Selvam PV, Tavares FCA, Kennedy JF, Knill CJ (1996) Industrial utilisation of starch and its derived products in Brazil. Starch 48(9):322–326. https://doi.org/10.1002/star.19960480904
Chen S, Wayman M (1992) Novel inducers derived from starch for cellulase production by Trichoderma reesei. Process Biochem 27:327–334. https://doi.org/10.1016/0032-9592(92)87010-E
Chen S, Wayman M (1993) Use of sorbose to enhance cellobiose activity in Trichoderma reesei cellulase system produced on wheat hydrolysates. Biotechnol Tech 7:345–350. https://doi.org/10.3389/fmicb.2016.00620
Dhillon SS, Gill RK, Gill SS, Singh M (2004) Studies on the utilization of citrus peel for pectinase production using fungus Aspergillus niger. Intl J Env Studies 61(2):199–210. https://doi.org/10.1080/0020723032000143346
Foletto EL, Weber CT, Paz DS, Mazutti M, Meili L, Basacco MM, Colazzo GC (2012) Adsorption of leather dye onto activated carbon prepared from bottle gourd: equilibrium, kinetic and mechanism studies. Water Sci Technol 67(1):201–209. https://doi.org/10.2166/wst.2012.555
Gajera RR, Joshi DC, Ravani A (2017) Processing potential of bottle gourd (L. siceraria). Fruits 5(4):106–109
Gao J, Qian Y, Wang Y, Qu Y, Zhong Y (2017) Production of the versatile cellulase for cellulose bioconversion and cellulase inducer synthesis by genetic improvement of Trichoderma reesei. Biotechnol Biofuels 10:272. https://doi.org/10.1186/s13068-017-0963-1
Gerits LR, Jay BP, Delcour A (2015) Wheat starch swelling, gelatinization and pasting: effects of enzymatic modification of wheat endogenous lipids. LWT Food Sci Technol 63(1):361–366
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. https://doi.org/10.1351/pac198759020257
Guo GL, Hsu DC, Chen WH, Chen WH, Hwong WS (2009) Charecterisation of enzymatic saccharification for acid pretreated lignocellulosic materials with different lignin composition. Enzym Microb Technol 45(2):80–87. https://doi.org/10.1155/2012/276278
Ibrahim MM, Dufresne A, El-Zawawy WK, Agblevor FA (2010a) Banana fibers and microfibrils as lignocellulosic reinforcements in polymer composites. Carbohydr Polym 81:811–819. https://doi.org/10.1016/j.carbpol.2010.03.057
Ibrahim MNM, Ahmed-Haras MR, Sipaut CS, Aboul-Encin HY, Mohamed AA (2010b) Preparation and characterization of a newly water soluble lignin graft copolymer from oil palm lignocellulosic waste. Carbohydr Polym 80(4):1102–1110
Jiang LQ, Fang Z, Li X, Luo J (2013) Production of 2,3-butanediol from cellulose and jatropha hulls after ionic liquid pretreatment and dilute acid hydrolysis. AMB Express,3(1):48,1–8. https://doi.org/10.1186/2191-0855-3-48
Jin Q, Kirk MF (2018) pH as a primary control in environmental microbiology: 1. Thermodynamic Perspective, Front. Environ. Sci. https://doi.org/10.3389/fenvs.2018.00021
Kodagoda KHGK, Marapana RAUJ (2017) Utilization of fruit processing by-products for industrial applications: A review. Int J Food Sci Nutr 2(6):24–30. https://doi.org/10.22271/food
Kubde MS, Khadabadi SS, Farooqui IA, Deore SL (2010) Lagenaria siceraria: Phytochemistry,phamacognosy and pharmacological studies. Report Opin 2(3):91–98
Laboratory Manual. 2001. Laboratory manual of central pulp and paper research institute, analysis of fibrous raw materials, proximate chemical analysis, Saharanpur, 247001(U.P.), India, “Estimation of acid insoluble lignin in wood/nonwood, TM1A-7; Estimation of acid soluble lignin in wood/nonwood, TM1A-8; Estimation of holocellulose in wood/nonwood; TM1-A9
Leeman AM, Karlsson ME, Eliasson AC, Björck IME (2006) Resistant starch formation in temperature treated potato starches varying in amylose/amylopectin ratio. Carbohydr Polym 65(3):306–313
Mandhania S, Jain V, Malhotra SP (2010) Culture optimization for enhanced production of microbial pectin methylesterase under submerged conditions. Asian J Biochem 5:12–22. https://doi.org/10.3923/ajb.2010.12.22
Marwaha SS, Kennedy JF (2007) Whey pollution problem and potential utilization. Int J Food Sci Technol 23:323–336. https://doi.org/10.1111/j.1365-2621.1988.tb00586.x
Morikawa Y, Ohashi T, Mantani O, Okada H (1995) Cellulase induction by lactose in Trichoderma reesei PC-3-7. Appl Microbiol Biotechnol 44(1–2):106–111. https://doi.org/10.1007/BF00164488
Nedwell DB (1999) Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol 30(2):101–111. https://doi.org/10.1111/j.1574-6941.1999.tb00639.x
Palamthodi S, Lele SS (2016) Optimization and evaluation of reactive dye adsorption on bottle gourd peel. J Environ Chem Eng 4(4, Part A):4299–4309. https://doi.org/10.1016/j.jece.2016.09.032
Pérez S, Baldwin PM, Gallant DJ (2009) Structural features of starch granules I., Starch,3rd edition., Chemistry and Technology.,149–192
Prajapati RP, Kalariya M, Parmar SK, Sheth NR (2010) Phytochemical and pharmacological review of Lagenaria sicereria. J Ayurveda Integr Med 1(4):266–272. https://doi.org/10.4103/0975-9476.74431
Prasad C, Karlapudi S, Rao CN, Venkateswarlu P, Bahadur I (2017) A highly resourceful magnetically separable magnetic nanoparticles from aqueous peel extract of Bottle gourds for organic dyes degradation. J Mol Liq 243:611–615
Qi B, Vu A, Wickramasinghe SR, Qian X (2018) Glucose production from lignocellulosic biomass using a membrane-based polymeric solid acid catalyst. Biomass Bioenergy 117:137–145. https://doi.org/10.1016/j.biombioe.2018.07.017
Roopan SM, Rajeswari VD, Kalpana VN, Elango G (2016) Biotechnology and pharmacological evaluation of Indian vegetable crop Lagenaria siceraria: an overview. Appl Microbiol Biotechnol 100(3):1153–1162. https://doi.org/10.1007/s00253-015-7190-0
Rosicka-Kaczmarek J.,Kwaśniewska-Karolak I.,Nebesny E., Komisarczyk A., 2018. The Functionality of wheat starch.,S tarch in Food., 2nd Edition, Woodhead Publishng Series in Food Science Technology and Nutrition., 325–352
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresources Bioprocess 5:1. https://doi.org/10.1186/s40643-017-0187-z
Silviya R, Macwan Bhumika K, Dabhi SC, Parmarand KD, Aparnathi (2016) Whey and its utilization. Int J Curr Microbiol AppSci 5(8):134–155. https://doi.org/10.20546/ijcmas.2016.508.016
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass--an overview. Bioresour Technol 199:76–82. https://doi.org/10.1016/j.biotech.2015.08.030
Słomińska L, Zielonka R, Jarosławski L (2013) The unconventional single stage hydrolysis of potato starch. Pol J Chem Technol 15(3):7–14
Spiance MAS, Lambert CS, Fermoselli KKG, De Paoli MA (2009) Characterization of lignocellulosic curaua fibers. Carbohydr Polym 77(1):47–53. https://doi.org/10.1016/j.carbpol.2008.12.005
Sternberg D, Mandels GR (1979) Induction of cellulolytic enzymes in T.reesei by sophrose. J. Bacteriol 139:761–769
Tripathi P, Singh PC, Mishra A, Chauhan P, Dwivedi S, Bais RT, Tripathi RD (2013) Trichoderma: a potential bioremediator for environmental cleanup. Clean Techn Environ Policy 15(4). https://doi.org/10.1007/s10098-012-0553-7
Tserki V, Matzinos P, Kokkou S, Panayiotou C (2005) Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part I, Surface chemical modification and caracterization of waste flour. Compos A: Appl Sci Manufact 36(7):965–974. https://doi.org/10.1016/j.compositesa.2004.11.010
Venkatraman K, Achi M (2004) To eliminate the disposal of salty whey from a dairy industry into the sewer in an environmental, social and economical way – a case study from dairy farmers, Toowoomba, Queensland, Australia. Int J Environ Technol Manag 4:365–374. https://doi.org/10.1504/IJETM.2004.005722
Verma, N., Kumar, V., Bansal, M. C., 2009. Various inducers involved in environmental viable cellulase biosynthesis. Proceedings of International Conference on Emerging Technologies in Environmental Science and Engineering, at Civil Engineering Department of Aligarh Muslim University (AMU) India in collaboration with Toledo University, U.S.A., Oct 26–28, 1690–1695
Verma N, Bansal MC, Kumar V (2011) Scanning electron microscopic analysis of Aspergillus niger pellets and biofilms under various process conditions. Int J Microbiol Res 2(1):08–11 http://idosi.org/ijmr/ijmr2(1)11/2.pdf
Verma N, Bansal MC, Kumar V (2018) Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal Agricult Biotechnol 16:483–492. https://doi.org/10.1016/j.bcab.2018.09.021
Virgilio S, Cupertino FB, Bernardes NE, Freitas FZ, Takeda AAS, Fontes MRM (2016) Molecular components of the Neurospora crassa pH signaling pathway and their regulation by pH and the PAC-3 transcription factor. PLoS ONE 11(8). https://doi.org/10.1371/journal.pone.0161659
Vriesekoop F, Rathband A, MacKinlay J, Bryce JH (2010) The Evolution of dextrins during the mashing and fermentation of all-malt whisky production. J Inst Brew 116(3):230–238. https://doi.org/10.1002/j.2050-0416.2010.tb00425.x
Wang CH, Hseu TH, Huang CM (1988) Induction of cellulases by cellooligosaccharides in Trichoderma koninghii G-39. J Biotechnol 9:47–60
Widowati E, Utami R, Mahadjoeno E, Saputro GP (2017) Effect of temperature and pH onpolygalacturonase production by pectinolyticbacteria Bacillus licheniformis strain GD2a insubmerged medium from Raja Nangka (Musaparadisiaca var. formatypica) banana peel waste. IOP Conf. Ser.: Mater. Sci. Eng. https://doi.org/10.1088/1757-899X/193/1/012018
Xiong Y, Wu VW, Lubbe A, Qin L, Deng S, Kennedy M, Bauer D, Singan VR, Barry K, Northen TR, Grigoriev IV, Glass NL (2017) A fungal transcription factor essential for starch degradation affects integration of carbon and nitrogen metabolism. PLoS Genet 13(5). https://doi.org/10.1371/journal.pgen.1006737
Yang S, Li J, Zheng Z, Meng Z (2009) Lignocellulosic structural changes of Spartina alterniflora after anaerobic mono and co digestion. Int Biodeterior Biodegrad 63:569–575. https://doi.org/10.1016/j.ibiod.2009.02.007
Zhang X, Zhang B, Miao R, Zhou J, Ye L, Jia D, Peng W, Yan L, Tan W, Li X (2018) Influence of temperature on the bacterial community in substrate and extracellular enzyme activity of Auricularia corne. Mycobiology 46(3):224–235. https://doi.org/10.1080/12298093.2018.1497795
Zhu F (2018) Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci Technol 78:234–242. https://doi.org/10.1016/j.tifs.2018.05.024
Acknowledgments
Authors gratefully acknowledged the ministry of human resource and development, India, for providing fellowship to carry out present research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, N., Kumar, V. Utilization of bottle gourd vegetable peel waste biomass in cellulase production by Trichoderma reesei and Neurospora crassa. Biomass Conv. Bioref. 12, 1105–1114 (2022). https://doi.org/10.1007/s13399-020-00727-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00727-9