Abstract
This article is the ninth in the series of Fungal Diversity Notes, where 107 taxa distributed in three phyla, nine classes, 31 orders and 57 families are described and illustrated. Taxa described in the present study include 12 new genera, 74 new species, three new combinations, two reference specimens, a re-circumscription of the epitype, and 15 records of sexual-asexual morph connections, new hosts and new geographical distributions. Twelve new genera comprise Brunneofusispora, Brunneomurispora, Liua, Lonicericola, Neoeutypella, Paratrimmatostroma, Parazalerion, Proliferophorum, Pseudoastrosphaeriellopsis, Septomelanconiella, Velebitea and Vicosamyces. Seventy-four new species are Agaricus memnonius, A. langensis, Aleurodiscus patagonicus, Amanita flavoalba, A. subtropicana, Amphisphaeria mangrovei, Baorangia major, Bartalinia kunmingensis, Brunneofusispora sinensis, Brunneomurispora lonicerae, Capronia camelliae-yunnanensis, Clavulina thindii, Coniochaeta simbalensis, Conlarium thailandense, Coprinus trigonosporus, Liua muriformis, Cyphellophora filicis, Cytospora ulmicola, Dacrymyces invisibilis, Dictyocheirospora metroxylonis, Distoseptispora thysanolaenae, Emericellopsis koreana, Galiicola baoshanensis, Hygrocybe lucida, Hypoxylon teeravasati, Hyweljonesia indica, Keissleriella caraganae, Lactarius olivaceopallidus, Lactifluus midnapurensis, Lembosia brigadeirensis, Leptosphaeria urticae, Lonicericola hyaloseptispora, Lophiotrema mucilaginosis, Marasmiellus bicoloripes, Marasmius indojasminodorus, Micropeltis phetchaburiensis, Mucor orantomantidis, Murilentithecium lonicerae, Neobambusicola brunnea, Neoeutypella baoshanensis, Neoroussoella heveae, Neosetophoma lonicerae, Ophiobolus malleolus, Parabambusicola thysanolaenae, Paratrimmatostroma kunmingensis, Parazalerion indica, Penicillium dokdoense, Peroneutypa mangrovei, Phaeosphaeria cycadis, Phanerochaete australosanguinea, Plectosphaerella kunmingensis, Plenodomus artemisiae, P. lijiangensis, Proliferophorum thailandicum, Pseudoastrosphaeriellopsis kaveriana, Pseudohelicomyces menglunicus, Pseudoplagiostoma mangiferae, Robillarda mangiferae, Roussoella elaeicola, Russula choptae, R. uttarakhandia, Septomelanconiella thailandica, Spencermartinsia acericola, Sphaerellopsis isthmospora, Thozetella lithocarpi, Trechispora echinospora, Tremellochaete atlantica, Trichoderma koreanum, T. pinicola, T. rugulosum, Velebitea chrysotexta, Vicosamyces venturisporus, Wojnowiciella kunmingensis and Zopfiella indica. Three new combinations are Baorangia rufomaculata, Lanmaoa pallidorosea and Wojnowiciella rosicola. The reference specimens of Canalisporium kenyense and Tamsiniella labiosa are designated. The epitype of Sarcopeziza sicula is re-circumscribed based on cyto- and histochemical analyses. The sexual-asexual morph connection of Plenodomus sinensis is reported from ferns and Cirsium for the first time. In addition, the new host records and country records are Amanita altipes, A. melleialba, Amarenomyces dactylidis, Chaetosphaeria panamensis, Coniella vitis, Coprinopsis kubickae, Dothiorella sarmentorum, Leptobacillium leptobactrum var. calidus, Muyocopron lithocarpi, Neoroussoella solani, Periconia cortaderiae, Phragmocamarosporium hederae, Sphaerellopsis paraphysata and Sphaeropsis eucalypticola.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Table of contents
Ascomycota R.H. Whittaker
Dothideomycetes O.E. Erikss. & Winka
Dothideomycetidae P.M. Kirk et al.
Capnodiales Woron.
Teratosphaeriaceae Crous & U. Braun
929.Hyweljonesia indica P.N. Singh & S.K. Singh, sp. nov.
Pleosporomycetidae C.L. Schoch et al.
Pleosporales Luttr. ex M.E. Barr
Dictyosporiaceae Boonmee & K.D. Hyde
930.Dictyocheirospora metroxylonis Konta & K.D. Hyde, sp. nov.
Didymosphaeriaceae Munk
931.Vicosamyces Firmino, A.R. Machado & O.L. Pereira, gen. nov.
932.Vicosamyces venturisporus Firmino, A.R. Machado & O.L. Pereira, sp. nov.
Lentitheciaceae Yin. Zhang et al.
933.Keissleriella caraganae Chaiwan, Phookamsak, Wanas. & K.D. Hyde, sp. nov.
934.Murilentithecium lonicerae Phookamsak, Chaiwan, Wanas. & K.D. Hyde, sp. nov.
935. Phragmocamarosporium hederae Wijayaw., R.K. Schumach. & K.D. Hyde, Index Fungorum 370: 1 (2018), new host record
Leptosphaeriaceae M.E. Barr
936.Leptosphaeria urticae D. Pem, E.B.G. Jones & K.D. Hyde, sp. nov.
937.Plenodomus artemisiae A. Karunarathna, Phookamsak & K.D. Hyde, sp. nov.
938.Plenodomus lijiangensis Phookamsak, A. Karunarathna & K.D. Hyde, sp. nov.
939.Plenodomus sinensis Tennakoon, Phookamsak & K.D. Hyde, in Tennakoon et al., Phytotaxa 324(1): 76 (2017), new hosts and asexual morph records
940.Sphaerellopsis isthmospora A. Karunarathna, Phookamsak & K.D. Hyde, sp. nov.
941.Sphaerellopsis paraphysata Crous & Alfenas, in Trakunyingcharoen et al., IMA Fungus 5(2): 411 (2014), new host record from Yunnan, China
Lophiotremataceae K. Hiray. & Kaz. Tanaka
942.Lophiotrema mucilaginosis M. Raza & L. Cai, sp. nov.
Occultibambusaceae D.Q. Dai & K.D. Hyde
943.Brunneofusispora S.K. Huang & K.D. Hyde, gen. nov.
944.Brunneofusispora sinensis S.K. Huang & K.D. Hyde, sp. nov.
Parabambusicolaceae Kaz. Tanaka & K. Hiray.
945.Lonicericola Phookamsak, Jayasiri & K.D. Hyde, gen. nov.
946.Lonicericola hyaloseptispora Phookamsak, Jayasiri & K.D. Hyde, sp. nov.
947.Parabambusicola thysanolaenae Goonas., Jayasiri, Phookamsak & K.D. Hyde, sp. nov.
948.Paratrimmatostroma Jayasiri, Phookamsak, D.J. Bhat & K.D. Hyde, gen. nov.
949.Paratrimmatostroma kunmingensis Jayasiri, Phookamsak, D.J. Bhat & K.D. Hyde, sp. nov.
Periconiaceae (Sacc.) Nann.
950.Periconia cortaderiae Thambug. & K.D. Hyde, in Thambugala et al., Mycosphere 8(4): 734 (2017), new host record from Yunnan, China
Phaeosphaeriaceae M.E. Barr
951.Amarenomyces dactylidis Mapook, Camporesi & K.D. Hyde, in Hyde et al., Fungal Divers 87: 78 (2017), new host record from Yunnan, China
952.Brunneomurispora Phookamsak, Konta, Wanas. & K.D. Hyde, gen. nov.
953.Brunneomurispora lonicerae Konta, Phookamsak, Wanas. & K.D. Hyde, sp. nov.
954.Galiicola baoshanensis Phookamsak, Wanas. & K.D. Hyde, sp. nov.
955.Neosetophoma lonicerae Phookamsak, Wanas. & K.D. Hyde, sp. nov.
956.Ophiobolus malleolus S.K. Huang, Bulgakov & K.D. Hyde, sp. nov.
957.Phaeosphaeria cycadis Wanas., Phookamsak & K.D. Hyde, sp. nov.
958.Wojnowiciella kunmingensis Phookamsak, Wanas. & K.D. Hyde, sp. nov.
959.Wojnowiciella rosicola (W.J. Li et al.) Wanas., Phookamsak & K.D. Hyde, comb. nov.
Pseudoastrosphaeriellaceae Phookamsak & K.D. Hyde
960.Pseudoastrosphaeriellopsis Devadatha, Wanas., Jeewon & V.V. Sarma, gen. nov.
961.Pseudoastrosphaeriellopsis kaveriana Devadatha, Wanas., Jeewon & V.V. Sarma, sp. nov.
Roussoellaceae J.K. Liu et al.
962.Neoroussoella heveae Senwanna, Phookamsak & K.D. Hyde, sp. nov.
963.Neoroussoella leucaenae Jayasiri, E.B.G. Jones & K.D. Hyde, Mycosphere 10(1): 1–186 (2019), new host record from Yunnan, China
964.Roussoella elaeicola Konta & K.D. Hyde, sp. nov.
Sulcatisporaceae Kaz. Tanaka & K. Hiray.
965. Neobambusicola brunnea Y. Chen & Norphanphoun, sp. nov.
Thyridariaceae Q. Tian & K.D. Hyde
966.Liua Phookamsak & K.D. Hyde, gen. nov.
967.Liua muriformis Phookamsak, H.B. Jiang & K.D. Hyde, sp. nov.
Dothideomycetes, orders incertae sedis
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Asterinaceae Hansf.
968.Lembosia brigadeirensis Firmino, A.R. Machado & O.L. Pereira, sp. nov.
Botryosphaeriales C.L. Schoch et al.
Botryosphaeriaceae Theiss. & P. Syd.
969. Dothiorella acericola Phookamsak, Tennakoon & K.D. Hyde, sp. nov.
970.Dothiorella sarmentorum (Fr.) A.J.L. Phillips, A. Alves & J. Luque, Mycologia 97(2): 522 (2005), new host record from Russia
971.Sphaeropsis eucalypticola A.J.L. Phillips, in Phillips et al., Stud Mycol 76: 158 (2013), new host record
Microthyriales G. Arnaud
Microthyriales, genera incertae sedis
972.Parazalerion Madrid, Gené & Cano, gen. nov.
973.Parazalerion indica Madrid, Gené, & Cano, sp. nov.
Muyocopronales Mapook et al.
Muyocopronaceae K.D. Hyde
974.Muyocopron lithocarpi Mapook, Boonmee & K.D. Hyde, in Mapook et al., Phytotaxa 265(3): 235 (2016), new host record from Yunnan, China
Tubeufiales Boonmee & K.D. Hyde
Tubeufiaceae M.E. Barr
975.Pseudohelicomyces menglunicus J.F. Li, Phookamsak & K.D. Hyde, sp. nov.
Eurotiomycetes O.E. Erikss. & Winka
Chaetothyriomycetidae Doweld
Chaetothyriales M.E. Barr
Cyphellophoraceae Réblová & Unter.
976.Cyphellophora filicis Hongsanan, Phookamsak & K.D. Hyde, sp. nov.
Herpotrichiellaceae Munk
977.Capronia camelliae-yunnanensis M. Raza, Z.F. Zhang & L. Cai, sp. nov.
Eurotiomycetidae Geiser & Lutzoni
Eurotiales G.W. Martin ex Benny & Kimbr.
Trichocomaceae E. Fisch.
978.Penicillium dokdoense Hyang B. Lee & T.T.T. Nguyen, sp. nov.
Lecanoromycetes, O.E. Erikss. & Winka
Lecanoromycetes, families incertae sedis
Micropeltidaceae Clem. & Shear
979.Micropeltis phetchaburiensis Dayarathne, Hongsanan & K.D. Hyde, sp. nov.
Leotiomycetes O.E. Erikss. & Winka
Helotiales Nannf. ex Korf & Lizoň
Lachnaceae Raitv.
980.Velebitea I. Kušan, Matočec & Jadan, gen. nov.
981.Velebitea chrysotexta I. Kušan, Matočec & Jadan, sp. nov.
Pezizomycetes O.E. Erikss. & Winka
Pezizales J. Schröt.
Pezizaceae Dumort.
982.Sarcopeziza sicula (Inzenga) Agnello, Loizides & P. Alvarado, Ascomycete.org 10(4): 179 (2018), re-circumscription of the epitype
Sordariomycetes O.E. Erikss. & Winka
Diaporthomycetidae Senan. et al.
Atractosporales H. Zhang et al.
Conlariaceae H. Zhang et al.
983.Conlarium thailandense X.D. Yu, H. Zhang & K.D. Hyde, sp.nov.
Diaporthales Nannf.
Cytosporaceae Fr.
984.Cytospora ulmicola Norphanphoun, Bulgakov, T.C. Wen & K.D. Hyde, sp. nov.
Melanconiellaceae Senan. et al.
985.Septomelanconiella Samarak. & K.D. Hyde, gen. nov.
986.Septomelanconiella thailandica Samarak. & K.D. Hyde, sp. nov.
Pseudoplagiostomataceae Cheew. et al.
987.Pseudoplagiostoma mangiferae Dayarathne, Phookamsak & K.D. Hyde, sp. nov.
Schizoparmaceae Rossman
988.Coniella vitis Chethana, J.Y. Yan, X.H. Li & K.D. Hyde, Pl Dis 101: 2129 (2017), new host record from Russia
Diaporthomycetidae, families incertae sedis
Distoseptisporaceae K.D. Hyde & McKenzie
989.Distoseptispora thysanolaenae Goonas., Dayarathne, Phookamsak & K.D. Hyde, sp. nov.
Diaporthomycetidae, genera incertae sedis
990.Proliferophorum G.N. Wang, H. Zhang & Senan., gen. nov.
991.Proliferophorum thailandicum G.N. Wang, H. Zhang & Senan., sp. nov.
Hypocreomycetidae O.E. Erikss. & Winka
Glomerellales Chadef. ex Réblová et al.
Plectosphaerellaceae W. Gams et al.
992.Plectosphaerella kunmingensis Phookamsak, J.F. Li & K.D. Hyde, sp. nov.
Hypocreales Lindau
Cordycipitaceae Kreisel ex G.H. Sung et al.
993.Leptobacillium leptobactrumvar.calidus (W. Gams) Zare & W. Gams, Mycol Prog 15: 1003 (2016), new record for India
Hypocreaceae De Not.
994.Trichoderma koreanum S-Y. Oh, M.S. Park & Y.W. Lim, sp. nov.
995.Trichoderma pinicola S-Y. Oh, M.S. Park & Y.W. Lim, sp. nov.
996.Trichoderma rugulosum M.S. Park, S-Y. Oh & Y.W. Lim, sp. nov.
Hypocreales, genera incertae sedis
997.Emericellopsis koreana Hyang B. Lee, S.J. Jeon & T.T.T. Nguyen, sp. nov.
Savoryellomycetidae Hongsanan et al.
Savoryellales Boonyuen et al.
Savoryellaceae Jaklitsch & Réblová
998.Canalisporium kenyense Goh, W.H. Ho & K.D. Hyde, Can J Bot 76: 148 (1998), reference specimen
Sordariomycetidae O.E. Erikss. & Winka
Chaetosphaeriales Huhndorf et al.
Chaetosphaeriaceae Réblová et al.
999.Chaetosphaeria panamensis Huhndorf & F.A. Fernández, Fungal Divers 19: 33 (2005), new host record from Taiwan
1000.Thozetella lithocarpi R.H. Perera & K.D. Hyde, sp. nov.
Coniochaetales Huhndorf et al.
Coniochaetaceae Malloch & Cain
1001.Coniochaeta simbalensis S. Rana & S.K. Singh, sp. nov.
Phyllachorales M.E. Barr
Phyllachoraceae Theiss. & H. Syd.
1002.Tamsiniella labiosa S.W. Wong, K.D. Hyde, W.H. Ho & S.J. Stanley, Can J Bot 76(2): 334 (1998), reference specimen
Sordariales Chadef. ex D. Hawksw. & O.E. Erikss.
Lasiosphaeriaceae Nannf.
1003.Zopfiella indica Devadatha, Jeewon & V.V. Sarma, sp. nov.
Xylariomycetidae O.E. Erikss. & Winka
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Amphisphaeriaceae G. Winter
1004.Amphisphaeria mangrovei Devadatha & V.V. Sarma, sp. nov.
Sporocadaceae Corda
1005.Bartalinia kunmingensis Thiyag., Wanas., Phookamsak & K.D. Hyde, sp. nov.
1006.Robillarda mangiferae Thiyag., Wanas., Phookamsak & K.D. Hyde, sp. nov.
Xylariales Nannf.
Diatrypaceae Nitschke
1007.Neoeutypella M. Raza, Q.J. Shang, Phookamsak & L. Cai, gen. nov.
1008. Neoeutypella baoshanensis M. Raza, Q.J. Shang, Phookamsak & L. Cai, sp. nov.
1009. Peroneutypa mangrovei Devadatha & V.V. Sarma, sp. nov.
Hypoxylaceae DC.
1010.Hypoxylon teeravasati Devadatha, V.V. Sarma & E.B.G. Jones, sp. nov.
Basidiomycota R.T. Moore
Agaricomycetes Doweld
Agaricomycetidae Parmasto
Agaricales Underw.
Agaricaceae Chevall.
1011.Agaricus memnonius M.Q. He & R.L. Zhao, sp. nov.
1012.Agaricus langensis M.Q. He & R.L. Zhao, sp. nov.
1013.Coprinus trigonosporus Tkalčec & Mešić, sp. nov.
Amanitaceae E.-J. Gilbert
1014.Amanita altipes Zhu L. Yang, M. Weiss & Oberw., Mycologia 96(3): 636 (2004), new record from Thailand
1015.Amanita flavoalba Mehmood & R.P. Bhatt, sp. nov.
1016.Amanita melleialba Zhu L. Yang, Qing Cai & Yang Y. Cui, in Ariyawansa et al., Fungal Divers: https://doi.org/10.1007/s13225-015-0346-5, [163] (2015), new record from Thailand
1017.Amanita subtropicana Mehmood & R.P. Bhatt, sp. nov.
Hygrophoraceae Lotsy
1018.Hygrocybe lucida K. Acharya & A.K. Dutta, sp. nov.
Marasmiaceae Roze ex Kühner
1019.Marasmius indojasminodorus A.K. Dutta, K. Acharya & K. Das, sp. nov.
Omphalotaceae Bresinsky
1020.Marasmiellus bicoloripes K.P.D. Latha, K.N.A Raj & Manim., sp. nov.
Psathyrellaceae Vilgalys et al.
1021.Coprinopsis kubickae (Pilát & Svrček) Redhead et al., in Redhead et al., Taxon 50(1): 229 (2001), new record for Croatia
Boletales E.-J. Gilbert
Boletaceae Chevall.
1022.Baorangia major Raspé & Vadthanarat, sp. nov.
1023.Baorangia rufomaculata (Both) Raspé & Vadthanarat, comb. nov.
1024.Lanmaoa pallidorosea (Both) Raspé & Vadthanarat, comb. nov.
Cantharellales Gäum.
Clavulinaceae Donk
1025.Clavulina thindii U. Singh, sp. nov.
Polyporales Gӓum.
Phanerochaetaceae Jülich
1026.Phanerochaete australosanguinea Telleria, M. Dueñas & M.P. Martín, sp. nov.
Russulales Kreisel ex P.M. Kirk et al.
Russulaceae Lotsy
1027.Lactarius olivaceopallidus Uniyal, sp. nov.
1028.Lactifluus midnapurensis S. Paloi & K. Acharya, sp.nov.
1029.Russula choptae A. Ghosh & K. Das, sp.nov.
1030. Russula uttarakhandia A. Ghosh & K. Das, sp.nov.
Stereaceae Pilát
1031.Aleurodiscus patagonicus Nogal, Telleria, M. Dueñas & M.P. Martín, sp. nov.
Trechisporales K.H. Larss.
Hydnodontaceae Jülich
1032.Trechispora echinospora Telleria, M. Dueñas, I. Melo & M.P. Martín, sp. nov.
Auriculariomycetidae Jülich
Auriculariales J. Schröt.
Auriculariaceae Fr. ex Lindau
1033.Tremellochaete atlantica Alvarenga, sp. nov.
Dacrymycetes Doweld
Dacrymycetales Henn.
Dacrymycetaceae J. Schröt.
1034.Dacrymyces invisibilis M. Dueñas, Telleria & M.P. Martín, sp. nov.
Mucoromycota Doweld
Mucoromycetes Doweld
Mucorales Fr.
Mucoraceae Dumort.
1035.Mucor orantomantidis Hyang B. Lee, P.M. Kirk & T.T.T. Nguyen, sp. nov.
Introduction
Fungi are well-known as a large and diverse group of microorganisms that play important functional roles from agricultural, ecological and economic perspectives. They are crucial to natural ecosystems as decomposers degrading dead organic materials, accelerating rock weathering and response to plant growth, nutrient cycling, as well as maintaining plant diversity (Kendrick 2000; Finlay 2008; Zechmeister-Boltenstern et al. 2015; Drinkwater et al. 2017; Horwath 2017; Hyde et al. 2018a, b; Willis 2018). They are heterotrophic and may change their lifestyles from endophytic to pathogenic to saprobic on plants or other organisms as well as other fungi depending on the environmental circumstances (Hyde et al. 2007, 2018a; Promputtha et al. 2007, 2010; Slippers and Wingfield 2007; Ghimire and Hyde 2008; Hyde and Soytong 2008; Gomes et al. 2013; Zhan et al. 2016; Ariyawansa et al. 2018; Haelewaters et al. 2018; Liyanage et al. 2018; Lofgren et al. 2018; Wang et al. 2018; Sun et al. 2019). Sixteen phyla are accepted in the Kingdom Fungi (Tedersoo et al. 2018; Wijayawardene et al. 2018b).
Hawksworth (1991, 2001) estimated 1.5 million species of fungi worldwide, with fewer than 5–10% having been described. Hawksworth and Lücking (2017) attempted to derive an updated estimate of global fungal diversity based on scientific evidence such as the extrapolations of plant/fungus ratios, including molecular and fieldwork data from the same sites. They concluded that there is an estimated 2.2–3.8 million undescribed species with taxa awaiting discovery in biodiversity hot spots, with only 120,000 species described and accepted (Hawksworth and Lücking 2017).
In our ongoing research compiling notes on new fungal taxa, reference specimens, new data, and other taxonomic contributions, more than 900 species have been introduced, re-circumscribed and illustrated worldwide based up on morphological characteristics and phylogenetic analyses. This is the ninth paper in the fungal diversity series with more than 100 species contributions which were mainly collected from China, some other Asian countries, as well as other parts of the world.
Materials and methods
Materials and methods follow the previous fungal diversity notes (Hyde et al. 2016; Tibpromma et al. 2017). Fresh and dried specimens in this study were collected from Australia, Brazil, Chile, China, Croatia, Equatorial Guinea, India, Italy, Korea, New Zealand, Russia, Saudi Arabia, Taiwan, Thailand, UK and the USA. Media agar used to cultivated fungi is shown in Table 1. The genes and primers used in this study are shown in the Table 2. Phylogenetic analyses were performed based on Bayesian inference (BI), maximum likelihood (ML) and maximum parsimony (MP) (see Table 2).
Phylum Ascomycota R.H. Whittaker
We follow the latest treatments and updated accounts of Ascomycota in Wijayawardene et al. (2017a, 2018a).
Class Dothideomycetes O.E. Erikss. & Winka
The Classification of families in Dothideomycetes follow Hyde et al. (2013), Liu et al. (2017a) and Wijayawardene et al. (2018a). The subclasses, orders and families of Dothideomycetes are listed in alphabetical order.
Subclass Dothideomycetidae P.M. Kirk
Capnodiales Woron.
Teratosphaeriaceae Crous & U. Braun
Teratosphaeriaceae was introduced by Crous et al. (2007a) and is typified by Teratosphaeria Syd. & P. Syd. The family was introduced to accommodate several important leaf spot and extremotolerant species initially included in the genera Teratosphaeria, Mycosphaerella and related asexual morph genera. Recently, 59 genera were listed in this family (Wijayawardene et al. 2018a). The latest treatments of genera in Teratosphaeriaceae were outlined in Quaedvlieg et al. (2014), Wäli et al. (2014) and Hyde et al. (2017).
Hyweljonesia R.G. Shivas et al.
A monotypic genus, Hyweljonesia was introduced in Teratosphaeriaceae by Shivas et al. (2016) to accommodate H. queenslandica R.G. Shivas et al. (as the type species) isolated from a cocoon of an unidentified microlepidoptera parasitized by a chalcidoid wasp (Hymenoptera: Chalcoidea), collected from tropical forests of northern Queensland, Australia. The genus is characterized by white, septate, smooth-walled, hyaline to subhyaline mycelial hyphae often form hyphal tufts from which straight, unbranched, light brown, smooth-walled, and septate conidiophores arise laterally. Subhyaline, cuneiform, smooth-walled conidia are produced on characteristic integrated, pale brown and minutely verruculose conidiogenous cells forming apical whorls (1–5) of conidiogenous cells with inconspicuous conidial scars (Shivas et al. 2016). In this study, a new species, H. indica is introduced, which was collected as a saprobe associated with leaves of Shorea robusta Roth colonized by black moulds in India. Phylogenetic analysis from maximum likelihood based on a combined LSU and ITS sequence dataset (Fig. 1) is provided to clarify its phylogenetic affinities within Teratosphaeriaceae.
Phylogram generated from maximum likelihood analysis based on the combined ITS and LSU sequences of representative species in Teratosphaeriaceae. Bootstrap support value for maximum likelihood equal to or greater than 50% are indicated at the nodes. The novel species is shown in blue. The ex-type strains are indicated in bold. The tree is rooted to Harknessia ellipsoidea (CPC 13077)
Hyweljonesia indica P.N. Singh & S.K. Singh, sp. nov.
MycoBank number: MB821804; Facesoffungi number: FoF03526, Fig. 2
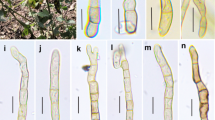
Hyweljonesia indica (AMH 9889, holotype). a Lower surface of Shorea robusta leaf showing patches of black moulds. b Colony characteristics on PDA (front view). c Enlarged view of single colony on PDA showing mycelial tufts. d Conidiophores bearing conidiogenous cells and whorls of conidia arising from tuft of mycelial hyphae. e Tufts of white vegetative mycelial hyphae in stereoscopic view. f Numerous conidiophores arising laterally from loose and tufted mycelial hyphae. g Enlarged view of single conidiophore bearing whorl of conidia. h Conidiophore branched at base. i Conidiophore bearing two conidiogenous cells and attached conidia. j Obovoid to pyriform hyaline conidia with refractive conidial scars. Scale barsd, f–j = 10 µm
Etymology: The specific epithet “indica” refers to the country of origin.
Holotype: AMH 9889
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Saprobic on leaves of Shorea robusta (Dipterocarpaceae) forests in terrestrial habitats. Sexual morph Undetermined. Asexual morph Vegetative hyphae smooth-walled, septate, subhyaline to light olivaceous, up to 4 µm wide. Conidiophores arising from loose to compact hyphal tufts, macronematous, lateral, unbranched to rarely branched at base, 0–1-septate, straight, smooth-walled, light olivaceous, 4.5–24.5 µm long (\( \bar{x} \) = 9.6 µm, n = 30); base flared, 3.5–8.5 µm wide (\( \bar{x} \) = 5.87 µm, n = 30); apex narrow, cylindrical, 1.5–4.5 µm wide (\( \bar{x} \) = 2.74 µm, n = 30). Conidiogenous cells terminal, 1(–2), straight, smooth-walled, subhyaline to olivaceous, cylindrical to clavate, scars inconspicuous, 5.5–12.8 × 2–5 μm (\( \bar{x} \) = 10.3 × 3.28 µm, n = 30). Conidia acrogenous to rarely acropleurogenous, produced in apical whorl of 1–12 conidia, simple, aseptate, obovoid to pyriform, smooth-walled, hyaline, apex rounded, base truncate, hilum refractive, 2.4–6.8 × 1.5–2.6 µm (\( \bar{x} \) = 4.6 × 2 µm, n = 30).
Culture characteristics: Colonies on PDA reaching average 12.5 mm diam. in 12 days, after 2 weeks of incubation at 25 °C, colonies were circular, margin regular, smooth, and orange white (6A2). Later turning to grey (2C1), mucoid, centre raised, umbonate, periphery white (6A1), with abundant hyphal tufts, sulcate, up to 7500 × 132–220 µm. Reverse brownish orange (5C4), margin smooth-walled, wrinkled.
Material examined: INDIA, Uttar Pradesh, Gorakhpur District, on Shorea robusta (leaf infested with black mold), 5 May 2016, P.N. Singh, AMH 9889 (holotype), ex-type living culture, NFCCI 4146 (National Fungal Culture Collection of India-WDCM 932).
GenBank numbers: ITS = MF322773, LSU = MF322775.
Notes: Detail study of in vitro cultural characteristics and morphology revealed a few morphological similarities with Hyweljonesia queenslandica. However, H. indica is distinct in having obovoid to pyriform conidia which are significantly larger when compared to the cuneiform conidia of H. queenslandica (Fig. 2). Conidiogenous cells of H. indica mostly arise singly from the conidiophores, while they are produced in 1–5 whorls of H. queenslandica (Shivas et al. 2016).
Sequence analysis of ITS and LSU positions Hyweljonesia indica in the genus Hyweljonesia closely related to H. queenslandica with strong bootstrap support (100% ML; Fig. 1). The BLASTn search of ITS sequence shows 95% similarity (468/491) with H. queenslandica (BRIP 61322b) and same similarity was recorded for LSU sequence with 98% similarity (838/851). Thus following the guidelines of Jeewon and Hyde (2016) this is a new species. To our understanding this genus and species is isolated and reported for the first time from India as a saprobic black mold associated with leaves of Shorea robusta.
Subclass Pleosporomycetidae C.L. Schoch et al.
Pleosporales Luttr. ex M.E. Barr
Dictyosporiaceae Boonmee & K.D. Hyde
We follow the latest treatments and updated accounts of Dictyosporiaceae in Boonmee et al. (2016), Wang et al. (2016), Hyde et al. (2017), Tibpromma et al. (2018) and Yang et al. (2018b). Recently, 12 genera were listed in this family (Wijayawardene et al. 2018a).
Dictyocheirospora M.J. D’souza et al.
Dictyocheirospora was introduced by Boonmee et al. (2016) with D. rotunda M.J. D’souza et al. as the type species. Boonmee et al. (2016) included Dictyocheirospora in the new family Dictyosporiaceae based on the fact that Dictyocheirospora species have dark sporodochial colonies, and produce aeroaquatic cheiroid dictyospores. Many species were subsequently accommodated in this genus (Wang et al. 2016; Hyde et al. 2017; Tibpromma et al. 2018; Yang et al. 2018b) and 17 species are listed in Index Fungorum (2019). In this study, Dictyocheirospora metroxylonis Konta & K.D. Hyde, sp. nov. is introduced from dead Metroxylon sagu (Arecaceae) in Thailand based on morphological and multigene phylogenetic support.
Dictyocheirospora metroxylonis Konta & K.D. Hyde, sp. nov.
Index Fungorum number: IF555290; Facesoffungi number: FoF04833, Fig. 4
Etymology: Name reflects the host genus Metroxylon.
Holotype: MFLU 15-0028
Saprobic on dead Metroxylon sagu. Sexual morph Undetermined. Asexual morph Hyphomycetous. Sporodochia on natural substrate in small groups, punctiform, 100–200 μm diam. (\( \bar{x} \) = 130 μm, n = 10), velvety, greyish to dark brown. Mycelium immersed, composed of brown, smooth, thin-walled, septate, branched hyphae. Conidiophores micronematous, pale brown, smooth, thin-walled. Conidiogenous cells 3–8 × 3–5 μm (\( \bar{x} \) = 5.2 × 4.6 μm, n = 10), holoblastic, integrated, terminal, determinate, pale brown, smooth-walled. Conidia 45–69 × 15–29 μm (\( \bar{x} \) = 61 × 20 μm, n = 20), solitary, monoblastic, acrogenous, cheiroid, pale brown, consisting of 4–6 rows of cells, rows digitate, cylindrical, inwardly curved at the tip, arising from a basal cell, each arm composed of 9–14 cells, distoseptate, constricted at thr septa, large guttule in each central cell. Conidial arm 29–58 × 5–7 μm (\( \bar{x} \) = 47 × 6 μm, n = 10) (Fig. 3).
Maximum likelihood majority rule consensus tree for the analysed Dictyosporiaceae isolates based on a dataset of combined ITS, LSU and TEF1-α sequence data. Bootstrap support values for maximum likelihood (ML) and maximum parsimony (MP) greater than 75% and Bayesian posterior probabilities greater than 0.95 are indicated above the nodes as ML/MP/PP. Branches with 100% ML, 100% MP and 1.00 BYPP are shown as black circle at the nodes. The tree is rooted with Periconia igniaria (CBS 379.86, CBS 845.96). The new taxon is in red and ex-type strains are in black bold
Culture characteristics: Conidia germinated on MEA within 24 h and germ tubes produced from the basal cells of the conidium. Colonies on MEA reaching 7–7.5 cm diam. after 2 weeks, at 25–28 °C, initially white, becoming grey-light brown, not sporulating on media.
Material examined: THAILAND, Krabi Province, on dead Metroxylon sagu Rottb. (Arecaceae), 8 December 2014, S. Konta, KBR04d (MFLU 15-0028, holotype), ex-type living culture, MFLUCC 15-0282.
GenBank numbers: ITS = MH742321, LSU = MH742313, SSU = MH742317, (MFLUCC 15-0282a); ITS = MH742322, LSU = MH742314, SSU = MH742318, TEF1-α = MH764301 (MFLUCC 15-0282b); ITS = MH742323, LSU = MH742315, SSU = MH742319, TEF1-α = MH764302 (MFLUCC 15-0282c); ITS = MH742324, LSU = MH742316, SSU = MH742320, TEF1-α = MH764303 (MFLUCC 15-0282d).
Notes: Dictyocheirospora metroxylonis differs from other Dictyocheirospora species by its conidial size, and number of rows and cell numbers in each row. Phylogenetic analyses of a combined ITS, LSU, SSU and TEF1-α sequence dataset (Fig. 3) show that D. metroxylonis forms a distinct lineage, clustered with other Dictyocheirospora species with moderate support in ML analysis (84% ML) and high support in BI analysis (0.99 BYPP). Since Dictyocheirospora has been introduced in Dictyosporiaceae (Dothideomycetes), many species were subsequently introduced to this genus with morphological and phylogenetic evidence. Interestingly, D. metroxylonis strain MFLUCC 150282d formed a clear zone against contaminated fungi on MEA during our experiment (Fig. 4, r).
Dictyocheirospora metroxylonis (MFLU 15-0028, holotype). a Sporodochia on the substrate. b–c Close up sporodochia on the substrate. d–f Immature conidia. g–m Mature conidia. n–p Germinating conidium. q Colony on MEA. r Colony on MEA with clear zone against contaminated fungi. Scale barsa = 500 μm, b, c = 100 μm, g–p = 20 μm, d–f = 10 μm
Didymosphaeriaceae Munk
We follow the latest treatment and updated accounts of Didymosphaeriaceae in Ariyawansa et al. (2014), Wanasinghe et al. (2018) and Tibpromma et al. (2018). There are 26 genera accepted in Didymosphaeriaceae (Wijayawardene et al. 2018a). Here we introduce a monotypic genus Vicosamyces.
Vicosamyces Firmino, A.R. Machado & O.L. Pereira, gen. nov.
MycoBank number: MB822577; Facesoffungi number: FoF03786
Etymology: The generic epithet “Vicosamyces” refers to the city “Viçosa”, where the type was collected.
Biotrophic or necrotrophic associated with plant disease on living leaves, forming a large, irregular, slightly raised, rough, orange brown wound, with orange margin. Sexual morphAscomata immersed in orange brown wound tissue, solitary, brown, globose to pyriform, ostiolate. Peridium thin-walled, composed of dark brown, pseudoparenchymatous cells, of textura angularis to textura prismatica. Hamathecium comprising numerous, cylindrical, filiform, septate, unbranched, hyaline pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, subsessile to short pedicellate, with furcate pedicel, apically rounded with well-developed ocular chamber. Ascospores overlapping 1–2-seriate, brown, 2-celled, apiosporous, smaller at the lower cell, subfusoid to clavate, or obovoid, narrower towards the lower cell. Asexual morph Undetermined.
Type species: Vicosamyces venturisporus Firmino, A.R. Machado & O.L. Pereira
Notes: Vicosamyces is introduced as a new genus based on morphology and phylogenetic support (LSU and ITS sequence dataset). Phylogenetic analysis of a combined LSU and ITS sequence dataset (Fig. 5) shows the fungus belongs to Didymosphaeriaceae, clustering with the genus Austropleospora R.G. Shivas & L. Morin. Vicosamyces has 2-celled, apiospores, while, Austropleospora has muriform ascospores (Morin et al. 2010; Thambugala et al. 2014; Ariyawansa et al. 2015a). Both genera have been found as biotrophic or necrotrophic pathogens associated with plant disease on living leaves, or stems. However, these two genera are associated with different symptoms on the host tissue. Austropleospora forms subglobose ascomata, solitary or in groups, immersed in small, brown, raised necrotic spots on Chrysanthemoides monilifera ssp. rotundata (Asteraceae) (Morin et al. 2010; Thambugala et al. 2014). Vicosamyces forms globose to pyriform ascomata, solitary, immersed in large, orange-brown wound, with orange margin on leaves of Eugenia sp. (Myrtaceae). In this study, the phylogenetic relationship of Austropleospora and Vicosamyces was not well-resolved. Phylogenetic analysis obtained from more informative genes will provide a better phylogenetic relationship of these genera.
Bayesian inference tree obtained from the concatenated ITS and LSU sequences including 83 taxa of representative genera in Didymosphaeriaceae. Taxa of Pleosporaceae (Pleosporales) were selected as the outgroup. Bayesian posterior probabilities (BYPP) represented by percentage equal or greater than 50% are shown above the nodes. The new isolates are in blue, ex-type strains are in bold
Vicosamyces venturisporus Firmino, A.R. Machado & O.L. Pereira, sp. nov.
MycoBank number: MB822578; Facesoffungi number: FoF03787, Fig. 6
Etymology: The specific epithet “venturisporus” refers to the ascospores which are similar in shape to the ascospores of the genus Venturia.
Holotype: VIC 44320
Biotrophic or necrotrophic associated with plant disease on living leaves, forming a large, irregular, slightly raised, rough, orange brown wound, with orange margin. Sexual morphAscomata 240–340 × 250–310 μm, immersed in orange brown wound, solitary, brown, globose to pyriform, ostiolate. Peridium thin-walled, composed of dark brown, pseudoparenchymatous cells, of textura angularis to textura prismatica. Hamathecium comprising 2–2.5 μm wide, numerous, cylindrical, filiform, septate, unbranched, hyaline pseudoparaphyses. Asci 125–152.5 × 14–15 µm, 8-spored, bitunicate, fissitunicate, cylindrical, subsessile to short pedicellate, with furcate pedicel, apically rounded with well-developed ocular chamber. Ascospores 22.5–30 × 6–8 µm, overlapping 1–2-seriate, upper cell brown with reddish tint, lower cell pale brown with a reddish tint, 2-celled, apiosporous, smaller at the lower cell, subfusoid to clavate, or obovoid, narrower towards the lower cell, with rounded to acute ends, slightly constricted at the septum, guttulate, smooth-walled. Asexual morph Undetermined.
Material examined: BRAZIL, Minas Gerais, Viçosa, Recanto das Cigarras, on leaves of Eugenia sp. (Myrtaceae), 10 September 2015, A.R. Machado (VIC 44320, holotype).
GenBank numbers: ITS = MF802825, LSU = MF802828 (CDA1494); ITS = MF802826, LSU = MF802829 (CDA1495); ITS = MF802827, LSU = MF802830 (CDA495).
Lentitheciaceae Y. Zhang ter et al.
The family Lentitheciaceae was introduced by Zhang et al. (2009a) with L. fluviatile (Aptroot & Van Ryck.) K.D. Hyde as the type species. Thirteen genera are included in this family (Wanasinghe et al. 2014a, 2018; Knapp et al. 2015; Phookamsak et al. 2015a; Tanaka et al. 2015; Wijayawardene et al. 2015, 2018a; Dayarathne et al. 2018). We follow the latest treatment and updated accounts of Lentitheciaceae in Wanasinghe et al. (2014a), Wijayawardene et al. (2015), Tibpromma et al. (2017) and Dayarathne et al. (2018). Based on phylogenetic analysis of a combined LSU, SSU, ITS and TEF1-α sequence dataset, two novel species, Keissleriella caraganae and Murilentithecium lonicerae are introduced. In addition, Phragmocamarosporium hederae Wijayaw. et al. associated with leaf spots on Cycas sp. (Cycadaceae) is reported in Yunnan, China for the first time.
Keissleriella Höhn
We follow the latest treatment and updated accounts of Keissleriella in Wanasinghe et al. (2018). Although 43 epithets of Keissleriella are listed in Index Fungorum (2018), only 19 species have been confirmed in Lentitheciaceae based on molecular data (Fig. 7).
Phylogram generated from maximum likelihood analysis based on the combined LSU, SSU, ITS and TEF1-α sequence dataset for taxa in Lentitheciaceae. Related sequences were obtained from Wanasinghe et al. (2018). Eighty-three strains are included in the combined sequence analyses, which comprise 3419 characters with gaps. Single gene analyses were also performed and topology and clade stability compared from combined gene analyses. Massarina cisti (CBS 266.62) and M. eburnea (CBS 473.64, H3953) were used as the outgroup taxa. Bootstrap support value for ML equal to or greater than 60% and Bayesian posterior probabilities equal to or greater than 0.95 BYPP are given above the nodes. Newly generated sequences are in blue. Type strains are in bold
Keissleriella caraganae Chaiwan, Phookamsak, Wanas. & K.D. Hyde, sp. nov.
Index Fungorum number: IF555523; Facesoffungi number: FoF04965, Fig. 8
Keissleriella caraganae (KUN-HKAS 102236, holotype). a Appearance of ascomata on host surface. b, c Section through ascomata. d, e Section through peridium. f, g Asci embedded in cellular pseudoparaphyses (g = stained in Indian ink). h, i Asci. j–l Ascospores. m Ascospores stained in Indian ink. n, o Culture on PDA after one week (n = from above, o = from below). Scale barsa = 200 µm, b, c = 50 μm, d–i = 20 μm, j–m = 5 μm
Etymology: The specific epithet “caraganae” refers to the host genus Caragana, from which the holotype was collected.
Holotype: KUN-HKAS 102236
Saprobic on Caragana arborescens (Fabaceae). Sexual morphAscomata 140–175 μm high, 170–235 μm diam., scattered, solitary or in groups, semi-immersed, visible as raised, black dots on host surface, globose to subglobose, glabrous, ostiolate at centre, with minute papilla, filled with short, brown, aseptate periphyses. Peridium 15–25 μm wide, thin-walled, of equal thickness, composed of several layers of small, flattened, brown to dark brown pseudoparenchymatous cells, arranged in a textura angularis to textura prismatica, intermixed with the host cells. Hamathecium composed of dense, 2–3 μm wide, broad filamentous, distinctly septate, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci 39–75 × 10–12 μm (\( \bar{x} \) = 60.1 × 11.1 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with well-developed ocular chamber. Ascospores 14–20 × 3–7 μm (\( \bar{x} \) = 16.9 × 5.1 μm, n = 20), overlapping 1–2-seriate, pale yellowish, fusiform to ellipsoidal, with rounded ends, (1–)3(–4)-septate, slightly constricted at the central septum, smooth-walled, with small guttules, surrunded by a distinct mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 22–29 mm diam. after 1 week at 20–25 °C, colony from above, white to cream at the margin, greenish grey in the centre; from below, white to cream at the margin, greenish grey in the centre; medium dense, circular, slightly raised, surface smooth, with edge entire, floccose to velvety, not producing pigmentation in agar.
Material examined: CHINA, Yunnan Province, Kunming Institute of Botany, on dead hanging branch of Caragana arborescens Lam. (Fabaceae), 2 November 2017, R. Phookamsak, KIB018 (KUN-HKAS 102236, holotype), ex-type living culture, KUMCC 18-0163 = MFLUCC 18-0682 (KIB018A), KUMCC 18-0164 (KIB018B).
GenBank numbers: ITS = MK214368, LSU = MK214371, SSU = MK214374, TEF1-α = MK214377 (KUMCC 18-0163); ITS = MK359434, LSU = MK359439, SSU = MK359444 TEF1-α = MK359073 (KUMCC 18-0164).
Notes: Keissleriella caraganae is similar to other Keissleriella species in having ascomata with an ostiolar neck, filled with short, brown, aseptate periphyses, bitunicate, broadly cylindrical to cylindric-clavate asci and septate ascospores, surrounded by distinct mucilaginous sheath (Tanaka et al. 2015; Wanasinghe et al. 2018). Multigene phylogenetic analyses (Fig. 7) show that K. caraganae is sister to K. yonaguniensis Kaz. Tanaka & K. Hiray. (KT2604). Although it clusters with other species of Keissleriella and Pleurophoma Höhn. the clade is not well-resolved agreeing with previous studies (Tibpromma et al. 2017; Hyde et al. 2018b; Wanasinghe et al. 2018). Keissleriella caraganae has ellipsoidal to fusiform, pale yellowish, 3-septate ascospores, whereas K. yonaguniensis has cylindrical, yellowish, 5-septate ascospores, with rounded ends (Tanaka et al. 2015). Both K. caraganae and K. rosacearum Phukhams. et al. (MFLU 15-1044) have fusiform, pale yellowish, 3-septate ascospores, but K. rosacearum was collected from Rosa canina L. (Rosaceae) in Italy (Wanasinghe et al. 2018). Multigene phylogenetic analysis (Fig. 7) shows that these two species form distinct lineages in different clades.
Murilentithecium Wanas. et al.
We follow the latest treatment and updated accounts of Murilentithecium in Wanasinghe et al. (2018). Generic notes were also provided by Wanasinghe et al. (2014a). Three species (including our new species) are presently included in this genus viz. M. clematidis Wanas. et al., M. lonicerae (in this study) and M. rosae Phukhams. et al. (Index Fungorum 2019). These three species were collected from Clematis vitalba L. (Italy), Lonicera maackii (Rupr.) Maxim (Yunnan, China) and Rosa canina L. (Italy).
Murilentithecium lonicerae Phookamsak, Chaiwan, Wanas. & K.D. Hyde, sp. nov.
Index Fungorum number: IF555524; Facesoffungi number: FoF04966, Fig. 9
Murilentithecium lonicerae (KUN-HKAS 102238, holotype). a Appearance of conidiomata on host surface. b Section through conidioma. c Section through conidioma wall. d–g Conidiogenous cells and conidia. h–k Conidia. l Germinating of conidium. m, n Culture on PDA after 1 week (m = from above, n = from below). Scale barsa = 200 µm, b = 50 μm, c, l = 20 μm, d–k = 10 μm
Etymology: The specific epithet “lonicerae” refers to the host genus Lonicera, from which the holotype was collected.
Holotype: KUN-HKAS 102238
Saprobic on Lonicera maackii. Sexual morph Undetermined. Asexual morphConidiomata 95–150 μm high, 110–170 μm diam., pycnidial, semi-immersed, visible as raised, black dots on host surface, solitary, globose to subglobose, glabrous, uni-loculate, ostiolate at centre, with minute papilla, lacking periphyses. Conidiomata walls 5–15 μm diam., thin-walled, of unequal thickness, slightly thickened at the base, composed of 5–7 layers, of flattened, brown pseudoparenchymatous cells, slightly dark at the apex, arranged in textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 8–15 × (3–)4–8 µm (\( \bar{x} \) = 11 × 5.5 μm, n = 35), enteroblastic, phialidic, rarely annellidic, discrete, determinate, hyaline, smooth, aseptate, cylindrical to doliiform, with narrow channel, minute collarette and periclinal wall thickening, arising from the inner cavity of pycnidial wall. Conidia (13.5–)14–17(–18.5) × 7–10(–12) µm (\( \bar{x} \) = 15.6 × 9.4 μm, n = 50), initially light brown to pale yellowish, aseptate, becoming reddish brown to dark brown, muriform, subglobose to obovoid, or turbinate, with truncate base, (1–)2–4 transverse septa, with several longitudinal sectors, not constricted at the septa, smooth-walled with minute guttules.
Culture characteristics: Colonies on PDA reaching 30–35 mm diam. after 3 weeks at 20–25 °C; colony from above, white-grey at the margin, grey at the centre; from below, white-grey at the margin, grey to dark grey at the centre, slightly radiated outwards colony; dense, circular, slightly raised to umbonate, surface smooth, with edge entire, floccose; not producing pigmentation in agar.
Material examined: CHINA, Yunnan Province, Kunming Institute of Botany, Lonicera maackii (Rupr.) Maxim. (Caprifoliaceae), 20 April 2017, R. Phookamsak, KIB035 (KUN-HKAS 102238, holotype), ex-type living culture, MFLUCC 18-0675 = KUMCC 18-0167 (KIB035IA), KUMCC 18-0168 (KIB035IB), KUMCC 18-0169 (KIB035IIA), KUMCC 18-0170 (KIB035IIB).
GenBank numbers: ITS = MK214370, LSU = MK214373, SSU = MK214376, TEF1-α = MK214379 (KUMCC 18-0167); ITS = MK359436, LSU = MK359441, SSU = MK359446, TEF1-α = MK359075 (KUMCC 18-0168); ITS = MK359437, LSU = MK359442, SSU = MK359447, TEF1-α = MK359076 (KUMCC 18-0169); ITS = MK359438, LSU = MK359443, SSU = MK359448, TEF1-α = MK359077 (KUMCC 18-0170).
Notes: Murilentithecium lonicerae can be distinguished from M. clematidis and M. rosae in having reddish brown to dark brown, subglobose to obovoid, or turbinate conidia, with truncate base, (1–)2–4 transverse septa, with several longitudinal sectors. Murilentithecium clematidis has pale brown to brown, oblong to clavate conidia, with 3–5 transverse septa, and 2–5 longitudinal septa (Wanasinghe et al. 2014a; Wijayawardene et al. 2016). Murilentithecium rosae has yellowish brown to dark brown, ovoid conidia, with 3 transverse septa, and 1–2 longitudinal septa (Wanasinghe et al. 2018). Multigene phylogenetic analyses (Fig. 7) show that M. lonicerae forms a distinct lineage basal to Murilentithecium.
Phragmocamarosporium Wijayaw. et al.
We follow the latest treatment and updated accounts of Phragmocamarosporium in Wanasinghe et al. (2018). There are only three species in this genus, P. hederae, P. platani Wijayaw. et al. and P. rosae Wanas. et al. Phragmocamarosporium hederae and P. rosae were collected from Hedera helix L. and Rosa canina in Europe (Germany and Great Britain respectively). Whereas, P. platani was found on Platanus sp. in Asia (Guizhou, China). In this study, P. hederae is reported from China on a different host.
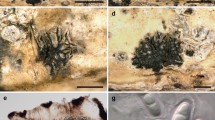
Phragmocamarosporium hederae Wijayaw., R.K. Schumach. & K.D. Hyde, Index Fungorum 370: 1 (2018), Fig. 10
Holotype: GERMANY, near Berlin, park, on a twig of Hedera helix L. (Araliaceae), 18 May 2013, Rene Klaus Schumacher, NNW GER 014/8 (MFLU 15-0165), living cultures MFLUCC 13-0552, GUCC 8.
Associated with leaf spots on Cycas (Cycadaceae). Sexual morph Undetermined. Asexual morphConidiomata 130–170 μm high, 180–270 μm diam., pycnidial, semi-immersed, visible as raised, black dots on host surface, scattered, solitary to gregarious, globose to subglobose, glabrous, uni-loculate, ostiolate at centre, with minute papilla, lacking periphyses. Conidiomata walls 10–20 μm, thin-walled, of equal thickness, composed of 3–5 layers, of flattened, brown to dark brown, pseudoparenchymatous cells, with blackened cells at the papilla, arranged in textura angularis to textura prismatica, difficult to distinguish from conidiogenous cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells (2.5–)3–5(–8) × (1.5–)2–5(–7) µm (\( \bar{x} \) = 4.3 × 3.7 μm, n = 30), holoblastic, phialidic, hyaline, smooth, aseptate, ampulliform, arising from the inner cavity of the conidioma wall. Conidia (8–)10–13(–14) × 3–4 µm (\( \bar{x} \) = 12 × 4.2 μm, n = 50), initially light brown, becoming reddish-brown to brown, oblong to ellipsoidal, or subclavate with truncate base, 3-septate, not constricted at the septa, smooth-walled.
Culture characteristics: Colonies on PDA reaching 10–15 mm diam. after 10 days at 25–30 °C; from above, white to cream at the margin, grey at the centre; from below, white to cream at the margin, black at the centre; medium dense, circular, slightly raised, surface slightly smooth, with edge entire, fluffy to feathery; not producing pigmentation in agar.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, associated with leaf spots on Cycas (Cycadaceae), 5 April 2017, R. Phookamsak, KIB020 (KUN-HKAS 102237), living culture, KUMCC 18-0165 (KIB020A), MFLUCC 18-0677 = KUMCC 18-0166 (KIB020B).
Known hosts and distribution: Hedera helix L. (Araliaceae; Germany) and associated with leaf spots on Cycas (Yunnan Province, China) (Wijayawardene et al. 2015 and this study).
GenBank numbers: ITS = MK214369, LSU = MK214372, SSU = MK214375, TEF1-α = MK214378 (KUMCC 18-0165); ITS = MK359434, LSU = MK359439, SSU = MK359444, TEF1-α = MK359073 (KUMCC 18-0166).
Notes: Multigene phylogenetic analyses (Fig. 7) show that the strain MFLUCC 18-0677 grouped with Phragmocamarosporium hederae and P. platani in Lentitheciaceae. A BLASTn search of LSU and SSU sequence data indicates that MFLUCC 18-0677 is identical to P. hederae (100% and 99% similarities, respectively). We therefore, identify our isolate as P. hederae and this species was collected from Cycas in China for the first time. Our new isolate is similar to P. hederae in having phragmosporous conidia. Whearas, P. platani has phragmosporous and muriform conidia at maturity (Wijayawardene et al. 2015). Compared to the type of P. hederae our new isolate has shorter and broader conidiogenous cells (8–10 × 1.5–2.5 μm in the type collection) and longer conidia (9–11 × 3–4.5 µm in the type collection). Phragmocamarosporium platani has smaller conidiogenous cells (1.5–3 × 1.5–2.5 μm) and narrower conidia (12–13 × 5–7.5 μm) (Wijayawardene et al. 2015). Only LSU and SSU sequence data for P. hederae and P. platani are available in GenBank, and sequences of more informative genes are needed to clarify species in this genus.
Leptosphaeriaceae M.E. Barr
Leptosphaeriaceae was introduced by Barr (1987) and is typified by Leptosphaeria Ces. & De Not. to accommodate species having immersed, subglobose, thick-walled ascomata containing interascal filamentous pseudoparaphyses, with bitunicate, broad asci bearing fusiform, transversely septate, hyaline to yellow-brown ascospores and coelomycetous asexual morphs in the order Pleosporales (Ariyawansa et al. 2015b). Ariyawansa et al. (2015b) re-circumscribed the genera in Leptosphaeriaceae based on morphological characteristics and multigene phylogenetic analyses, and accepted ten genera with more than 140 species. This is in agreement of the taxonomic outline of Ascomycota, provided by Wijayawardene et al. (2018a) and the notes of each genus in this family were provided by Ariyawansa et al. (2015b) and Wijayawardene et al. (2017a).
We follow the latest treatment of Leptosphaeriaceae in Ariyawansa et al. (2015b) and updated accounts of taxa in Leptosphaeriaceae in Hyde et al. (2016, 2017), Tennakoon et al. (2017) and Tibpromma et al. (2017). In this paper, we introduce four new species, Leptosphaeria urticae, Plenodomus artemisiae, P. lijiangensis and Sphaerellopsis isthmospora in Leptosphaeriaceae. The asexual morph of Plenodomus sinensis is also introduced from a fern in China and a new host record of Sphaerellopsis paraphysata associated with rust on living leaves of Liriope spicata (Thunb.) Lour (Asparagaceae) is reported.
Leptosphaeria Ces. & De Not.
Leptosphaeria was introduced by Cesati and De Notaris (1863) and is typified by L. doliolum (Pers.) Ces. & De Not. (lectotype designated by Shearer et al. 1990). The genus is characterized by semi-immersed to erumpent, coriaceous ascomata, which become superficial, a thick-walled peridium composed of scleroplectenchymatous cells, cylindrical to cylindric-clavate asci, reddish to yellowish brown, ellipsoidal to fusiform, septate ascospores and coelomycetous coniothyrium-like and phoma-like asexual morphs (Ariyawansa et al. 2015b; Dayarathne et al. 2015). Taxonomic revision of the genus was discussed in Ariyawansa et al. (2015b). Over 1600 epithets are listed for Leptosphaeria (Index Fungorum 2019), but few species have been confirmed by phylogenetic analysis. Most Leptosphaeria species lack molecular data to clarify their phylogenetic placements. Some other Leptosphaeria sensu lato species have been treated in different genera in Leptosphaeriaceae and other related families (de Gruyter et al. 2013; Ariyawansa et al. 2015b).
Leptosphaeria urticae D. Pem, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF555597; Facesoffungi number: FoF04370, Fig. 11
Leptosphaeria urticae (MFLU 18-0591, holotype). a–c Appearance of ascomata on host surface. d Section through an ascoma. e Peridium. f Pseudoparaphyses. g–i Asci. j–m Ascospores. n Spore germination on MEA after 24 h. o Culture from above and below. Scale barsa, b = 500 µm, c = 200 μm, d = 100 μm, e, g–i = 50 μm, j–m = 10 μm, f = 5 μm
Etymology: Name reflects the host from which the fungus was isolated.
Holotype: MFLU 18-0591
Saprobic on dead branches of Urtica dioica. Sexual morphAscomata 100–130 high, 70–110 μm diam., solitary, scattered or in small groups, erumpent through host epidermis to superficial, conical to mammiform, dark brown to black, coriaceous, smooth, easily removed from the host substrate, ostiolate with minute papilla. Ostioles 50–70 μm diam., papillate, black, shiny, smooth. Peridium 25–50 μm wide, comprising two cell types, outer layer composed of small, thick-walled cells of textura angularis to textura globulosa, surface heavily pigmented termed as scleroplectenchyma, thinner at the apex, wide at sides, inner layer composed of subhyaline or light brown relatively thin-walled cells of textura angularis, cells near the base comparatively larger. Hamathecium comprising numerous, dense, 1.5–2 μm wide, filamentous, septate, cellular pseudoparaphyses, branched and anastomosing, embedded in gelatinous matrix. Asci 60–140 × 9–11 μm (\( \bar{x} \) = 104.5 × 10 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, rounded at the apex, pedicellate, numerous, with ocular chamber. Ascospores 35–40 × 4–6 μm (\( \bar{x} \) = 38.3 × 5.2 μm, n = 20), overlapping 1–2-seriate, initially hyaline, becoming yellowish brown at maturity, long fusiform, (8–)9-septate, constricted at the septa, narrowly rounded at both ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on MEA within 24 h. Colonies growing on MEA reaching 2 cm diam. in one week at 16 °C. Mycelium superficial, surface smooth, irregular, slightly raised, edge crenate, velutinous, from above white to pale yellow, reverse yellow.
Material examined: UK, Sussex, Singleton, on dead stem of Urtica dioica L. (Urticaceae), 5 April 2017, E.B Gareth Jones, 353 UK (MFLU 18-0591, holotype), ex-type living culture MFLUCC 17-2302.
GenBank numbers: ITS = MK123333, LSU = MK123332, SSU = MK123329, TEF1-α = MH028391.
Notes: Phylogenetic analyses of a combined LSU, SSU and ITS sequence dataset (Fig. 12) reveal that Leptosphaeria urticae (MFLU 18-0591) is sister to L. italica Dayar. et al. (MFLU 15-0174). Leptosphaeria urticae differs from L. italica in having longer asci (L. urticae, 60–140 × 9–11 μm versus 60–112 × 7–12 μm, L. italica), longer ascospores (L. urticae, 35–40 × 4–6 μm versus 12–18 × 4–6 μm, L. italica) and more ascospore septation (L. urticae, (8–)9-septate versus 3-septate, L. italica) (Dayarathne et al. 2015). Based on the NCBI BLASTn search of ITS sequence data, L. urticae has 96% similarity with L. sclerotioides (Preuss ex Sacc.) Gruyter et al. However, the two species cannot be compared as L. urticae is represented only by its sexual morph, whereas, L. sclerotioides is known only by its asexual morph (de Gruyter et al. 2013). Phylogenetic analysis indicates that these two species are not conspecific. Hence, we introduce L. urticae as a new species based on both morphological and molecular data.
Phylogram generated from maximum likelihood analysis based on a combined LSU, SSU and ITS sequence dataset of taxa in Leptosphaeriaceae. The updated sequence data was derived from Tennakoon et al. (2017). Seventy strains are included in the combined sequence analyses. Single gene analyses were also performed and topology and clade stability compared from combined gene analyses. Phaeosphaeria oryzae (CBS 110110) and Phaeosphaeriopsis glauco-punctata (MFLUCC 13-0265) and Paraphoma radicina (CBS 111.79) were used as the outgroup taxa. Bootstrap support values for ML equal to or greater than 60% and Bayesian posterior probabilities equal to or greater than 0.80 BYPP are indicated at the nodes. Newly generated sequences are in blue and ex-type strains are in bold
Five Leptosphaeria species have been reported from Urtica: L. acuta (Fuckel) P. Karst., L. acutiuscula Berl., L. atropurpurea Petr., L. doliolum (Pers.) Ces. & De Not. and L. ogilviensis (Berk. & Broome) Ces. & De Not. (Shoemaker 1984; Farr and Rossman 2018). These species can be distinguished from each other based on ascospore septation.
Plenodomus Preuss
Plenodomus was introduced by Preuss (1851) and is typified by P. rabenhorstii. Subsequently, Boerema and Kesteren (1964) designated P. lingam (Tode) Höhn. as the type combination over P. rabenhorstii because the type material of P. rabenhorstii was lost during the World War II (de Gruyter et al. 2013; Ariyawansa et al. 2015b; Tennakoon et al. 2017). Based on molecular phylogeny, de Gruyter et al. (2013) reclassified Phoma section Plenodomus and synonymized species in Phoma section Plenodomus under the genus Plenodomus in Leptosphaeriaceae. The genus was re-circumscribed by Ariyawansa et al. (2015b) based on study of type and representative specimens coupled with molecular data. Marin-Felix et al. (2017) and Tennakoon et al. (2017) updated the accounts of Plenodomus based on molecular data. There are 97 epithets available in Index Fungorum (2019).
Plenodomus artemisiae A. Karunarathna, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF556118; Facesoffungi number: FoF05696, Fig. 13
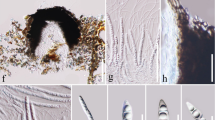
Plenodomus artemisiae (KUN-HKAS 102226, holotype). a Ascomata on host. b, c Vertical section of ascomata. d Ostiole. e Section through peridium. f Pseudoparaphyses. g–i Asci. j–m Ascospores. n Ascospore germination. o, p Culture characteristics (o = from above, p = from below). Scale barsa = 500 μm, b, c = 100 µm, d, e = 50 µm, f–i, n = 20 µm, j–m = 10 µm
Etymology: The specific epithet “artemisiae” refers to the host genus Artemisia, on which the type species was collected.
Holotype: KUN-HKAS 102226.
Saprobic on dead branches and stems of Artemisia sp. Sexual morphAscomata 140–280 μm high, 300–450 μm diam., black, shiny on the host surface, solitary to aggregated, immersed at the base, becoming superficial, uni-loculate, subglobose to irregular in shape, with truncate base, glabrous, ostiolate, papillate. Ostioles central, dark brown, beak-like papilla, ostiolar canal filled with periphyses. Peridium 10–85 μm wide, thick-walled of unequal thickness, thickened at the based, slightly thin at the apex, composed of several cell layers of dark brown scleroplectenchymatous cells, arranged in a textura angularis to textura globulosa. Hamathecium composed of hyaline, filamentous, 2–4 μm wide, distinctly septate pseudoparaphyses, anastomosing, embedded in a hyaline gelatinous matrix. Asci (64–)70–90(–100) × (9.5–)10–13 μm (\( \bar{x} \) = 82.7 × 11.2 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical, short, pedicellate, apically rounded with a distinct ocular chamber. Ascospores (28–)30–40 × (4.5–)5–6 μm (\( \bar{x} \) = 34.4 × 5.5 μm, n = 30), overlapping 2–3-seriate, pale brown, fusiform, 5-septate, slightly constricted at the septa, enlarge at the third cell from above, lacking a mucilaginous sheath and appendages. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 30–33 mm diam. after 4 weeks. Colony dense, circular, low convex, surface smooth, with edge entire, floccose; from above white; from below, yellowish-grey at the edge, with white to cream margin, dark yellowish at the centre, slightly radiating outwards colony; not produced pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead branches and stems of Artemisia sp., 20 December 2015, R. Phookamsak, AS003 (KUN-HKAS 102226, holotype). ex-type living culture, KUMCC 18-0151.
GenBank numbers: ITS = MK387920, LSU = MK387958, SSU = MK387928, TEF1-α = MK435600, RPB2 = MK435607 (KUMCC 18-0151).
Notes: Plenodomus artemisiae forms a distinct lineage and is sister to P. salviae Thambug. et al. (MFLUCC 13-0219) with high support (99% ML and 0.99 BYPP; Fig. 12). Plenodomus artemisiae can be distinguished from P. salviae in having shorter and broader, pale brown, fusiform ascospores, enlarged at the third cell from above. Plenodomus salviae has longer and thinner, yellowish brown, cylindric-fusiform ascospores (30–48 × 3.1–4.3 μm), and without the enlarged cell (Ariyawansa et al. 2015b). Plenodomus artemisiae is also similar to Leptosphaeria artemisiae (Fuckel) Auersw. in having 5-septate ascospores, with the enlarged third cell and occurring on Artemisia. However, L. artemisiae has larger ascomata and narrower ascospores (32–37 × 8.5–10; Shoemaker 1984). Furthermore, P. artemisiae has pale brown, fusiform ascospores, whereas, L. artemisiae has light reddish brown, broadly elliptical ascospores (Shoemaker 1984). Phylogenetic affinity of L. artemisiae could not be resolved due to lack of molecular data.
Plenodomus lijiangensis Phookamsak, A. Karunarathna & K.D. Hyde, sp. nov.
Index Fungorum number: IF556137; Facesoffungi number: FoF05697, Fig. 14
Plenodomus lijiangensis (KUN-HKAS 102249, holotype). a–i Morphological characteristics on natural substrate. l–u Morphological characteristics in vitro. a Conidiomata on host. b Vertical section of conidioma. c Section through conidioma wall. d–f Conidiogenous cells. g–i Conidia. j, k Culture characteristics on PDA (j = from above, k = from below). l–n Conidiomata forming on PDA after three months. o Squash mount of conidioma. p Vertical section of conidioma. q Section through conidioma wall stained with congo red. r, s Conidiogenous cells. t Conidiogenous cells stained with congo red. u Conidia. Scale barsa = 200 μm, b, n, o = 100 µm, c, p = 50 µm, u = 10 µm, g, q–t = 5 µm, h, i = 2 µm
Etymology: The specific epithet “lijiangensis” refers to Lijiang prefecture-level city, of Yunnan Province, China where the holotype was collected.
Holotype: KUN-HKAS 102249
Saprobic on dead fronds of fern. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 140–290 µm high, 135–240 µm diam., pycnidial, black, scattered, gregarious, superficial, uni-loculate, varied in shape, subconical to ovoid, or subglobose, with truncate base, widest at the base, glabrous, with indistinct ostiole. Conidiomata walls 17–100 µm wide, thick-walled, of unequal thickness, thickened at the apex, comprising several cell layers, outer layer composed of broad, dark brown to black, scleroplectenchymatous cells of textura angularis to textura globulosa, inner layer composed of broad, hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–9 × 5–8 µm (\( \bar{x} \) = 6.4 × 6.5 μm, n = 40), enteroblastic, phialidic, discrete, determinate, ampulliform to doliiform, hyaline, smooth, with minute collarette, with 1–2 apertures, and periclinal wall thickening, arising from the inner cavity of the conidioma wall. Conidia 3–5 × 1.7–2.3 µm (\( \bar{x} \) = 4.3 × 2 µm, n = 50), hyaline, oblong to obovoid, aseptate, smooth-walled, with 1–2 guttules.
Culture characteristics: Colonies on PDA, reaching 57–58 mm diam. after 3 weeks. Colony dense, circular, flattened, slightly raised, surface smooth, with edge entire, floccose; from above white at the margin, cream at the centre, with pale grey concentric ring near the margin; from below, yellowish brown at the edge, with paler margin, dark brown to black at the centre, colony slightly radiating outwards; not producing pigmentation on agar medium. Sporulation on PDA after three months. Conidiomata 120–250 µm high, 130–230 µm diam., scattered, solitary to gregarious, semi-immersed in culture colony, or embedded in agar medium, perithecial, pycnidial, with short stipe (19–49 µm long), black, glabrous, globose to subglobose, lacking ostioles. Conidiomata walls 3–8 µm wide, thin-walled, equally thick, comprising 1–2 cell layers of dark brown to black pseudoparenchymatous cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2–3 × 1.5–3 µm (\( \bar{x} \) = 2.5 × 2.2 μm, n = 20), enteroblastic, phialidic, discrete, determinate, oblong to pyriform, hyaline, with minute collarette, and periclinal wall thickening, arising from the inner cavity of the conidioma wall, difficult to distinguish from the conidioma wall. Conidia 3–4(–4.5) × 1.5–2.5 µm (\( \bar{x} \) = 3.9 × 2, n = 50), hyaline, oblong to ellipsoidal, or obovoid, aseptate, smooth-walled, with 1–2 small guttules.
Material examined: CHINA, Yunnan Province, Lijiang, Yulong, on dead fronds of fern, 1 August 2015, R. Phookamsak, LJ003 (KUN-HKAS 102249, holotype), ex-type living culture, KUMCC 18-0186.
GenBank numbers: ITS = MK387921, LSU = MK387959, SSU = MK387929, TEF1-α = MK435601 (KUMCC 18-0186).
Notes: Based on the NCBI BLASTn search of ITS sequence data, Plenodomus lijiangensis closest match is P. deqinensis Qian Chen & L. Cai (CGMCC 3.18221; 98% similarity). Phylogenetic analyses of a concatenated LSU, SSU and ITS sequence dataset (Fig. 12) reveal that P. lijiangensis forms a sister lineage with P. deqinensis and groups with P. agnitus (Desm.) Gruyter et al., P. fallaciosus (Berl.) Gruyter et al. and P. lupini (Ellis & Everh.) Gruyter et al. Plenodomus lijiangensis shares a size range of conidia and conidiogenous cells with P. deqinensis and was also collected from Yunnan, China (Marin-Felix et al. 2017). However, P. lijiangensis was isolated from dead fronds of fern, while P. deqinensis was isolated from soil. In vitro, P. lijiangensis forms a globose to subglobose conidiomata, inconspicuous ostiole, with a short stipe which is similar to the asexual morph of P. sinensis Tennakoon et al. (Fig. 16). While, P. deqinensis forms globose to subglobose, slightly papillate ostiole with a narrow pore or opening via a rupture (Marin-Felix et al. 2017). A comparison of ITS sequence shows that P. lijiangensis differs from P. deqinensis in eight base positions (1.55%/517 bp). According to the guidelines in Jeewon and Hyde (2016), we introduce P. lijiangensis as a new species.
Plenodomus sinensis Tennakoon, Phookamsak & K.D. Hyde, in Tennakoon et al., Phytotaxa 324(1): 76 (2017), Figs. 15, 16
Plenodomus sinensis (KUN-HKAS 102228, asexual morph). a Conidiomata on host. b Squash mount of conidioma. c Squash mount of conidioma showing ostiole. d Section through conidioma. e Section through conidioma wall. f Stalk of conidioma. g–i Conidiogenous cells. j–n Conidia. Scale barsa = 500 µm, c = 50 µm, b, d = 20 µm, e, f, j = 10 µm, g–i = 5 µm, k–n = 2 µm
Holotype: CHINA, Yunnan Province, Xishuangbanna, Nabanhe, dead branch of Tamarindus indica (Fabaceae), 25 November 2015, D.S. Tennakoon, DXH 015 (MFLU 17-0767).
Saprobic on dead fronds of ferns and dead stems of Cirsium sp. Sexual morphAscomata 250–290 µm high, 300–360 µm diam., black, shiny, scattered, gregarious, semi-immersed to erumpent through host epidermis, subglobose to subconical, uni-loculate, glabrous, ostiolate. Peridium thick-walled of unequal thickness, thickened at base, thinner toward sides and apex, composed of three type cell layers, inner layer 5–20 µm wide, comprising 2–3 strata of flattened, pale brown, thin-walled, pseudoparenchymatous cells, arranged in textura angularis to textura prismatica, middle layer 25–100 µm wide, comprising several strata, of hyaline, thick-walled, scleroplectenchymatous cells of textura angularis to textura globulosa, outer layer thin-walled, comprising 1 stratum, of black, coriaceous cells of textura angularis. Hamathecium comprising filamentous, septate, 2–4 µm wide, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci (75–)80–95(–107) × (8.5–)9–11(–12) µm (\( \bar{x} \) = 88.1 × 10.3, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, subsessile to short pedicellate, with knob-like to truncate pedicel, apically rounded, with well-developed ocular chamber. Ascospores 30–39 × 4–6(–6.5) µm (\( \bar{x} \) = 34.1 × 5.3, n = 40), overlapping 2–3-seriate, fusiform, initially hyaline, becoming pale brown to pale yellowish at maturity, 6-septate, widest at the third cell, slightly constricted at the septa, deeply constricted at the third septum from above, smooth-walled, inconspicuous minute appendages at both end cells. Asexual morph Coelomycetous. Conidiomata 50–120 µm high, 50–110 µm diam., pycnidial, black, shiny, scattered, gregarious, superficial, uni-loculate, globose to subglobose, with short stipe (5–10 × 7–11 µm), glabrous, ostiole central, with pore-like opening, apapillate. Conidiomata walls 5–10 µm wide, thin-walled, of equal thickness, comprising 2–3 cell layers, of dark brown pseudoparenchymatous cells, of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells (3–)4–7(–8) × 4–6(–8) µm (\( \bar{x} \) = 5.6 × 5.1 μm, n = 30), enteroblastic, phialidic, discrete, determinate, ampulliform to doliiform, hyaline, collarette, and periclinal wall thickening, arising from the inner cavity of the conidioma wall. Conidia (2.7–)3–4 × 1–2 µm (\( \bar{x} \) = 3.8 × 1.4, n = 100), hyaline, oblong, slightly curved, aseptate, smooth-walled.
Culture characteristics: Colonies on PDA reaching 28–30 mm diam. after 4 weeks at room temperature. Colony dense, irregular in shape, slightly raised to low convex, surface smooth, edge undulate, with margin well-defined; from above dark grey; from below, black; not produced pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Lijiang, Yulong, on dead fronds of fern, 29 July 2015, R. Phookamsak, LJ001 (KUN-HKAS 102229, sexual morph), living culture, KUMCC 18-0153; ibid., Baoshan, Shuizai, Dawazi mountain, on dead fronds of fern, 22 October 2015, I.D. Goonasekara, BS010 (KUN-HKAS 102228, asexual morph), living culture, KUMCC 18-0152; Baoshan, Shuizai, Dawazi mountain, on dead stems of Cirsium sp. (Asteraceae), 22 October 2015, R. Phookamsak, BS023 (KUN-HKAS 102227).
Known hosts and distribution: Plukenetia volubilis L. (Euphorbiaceae) Tamarindus indica L. (Fabaceae) (Xishuangbanna, China) (Tennakoon et al. 2017).
GenBank numbers: ITS = MK387922, LSU = MK387960, SSU = MK387930, TEF1-α = MK435602, RPB2 = MK435608 (KUMCC 18-0153); ITS = MK387923, LSU = MK387961, SSU = MK387931, TEF1-α = MK435603 (KUMCC 18-0152); ITS = MK387924, LSU = MK387962, SSU = MK387932 (KUN-HKAS 102227).
Notes: Based on the NCBI BLASTn search of ITS sequences, our isolates (KUMCC 18-0152, KUMCC 18-0153 and KUN-HKAS 102227) match with Plenodomus sinensis Tennakoon et al. (MFLU 17-0757), with 99% similarity. The sexual morph of KUMCC 18-0153 and KUN-HKAS 102227 share similar size of ascomata, asci and ascospores with the type, as well as sharing similar ascospore characters with fusiform, 6-septate ascospores (Tennakoon et al. 2017). Phylogenetic analyses of a concatenated LSU, SSU and ITS sequence dataset (Fig. 12) reveal that our isolates cluster with P. sinensis (MFLU17-0757) with moderate support (86% ML and 0.90 BYPP).
The asexual morph of P. sinensis, which is reported for the first time in this study, is similar to the asexual morph of P. lijiangensis in having globose to subglobose conidiomata with a short stipe. However, these two species are phylogenetically distinct.
Tennakoon et al. (2017) introduced Plenodomus sinensis as a saprobic species occurring on Plukenetia volubilis and Tamarindus indica from Xishuangbanna, Yunnan, China (tropical rain forest climate). In this study, P. sinensis was found on ferns and Cirsium sp. from Baoshan (mild subtropical highland climate) and Lijiang (a mild, with abundant rainfall and plenty of sunshine climate), Yunnan, China. This indicates that P. sinensis may occur on a wide range of hosts and in different climatic regions.
Sphaerellopsis Cooke
Sphaerellopsis was introduced by Sutton (1977) to accommodate mycoparasitic taxa occurring on a wide range of rusts and is typified with S. filum (Biv.) B. Sutton. Sphaerellopsis was re-circumscribed by Trakunyingcharoen et al. (2014) and Ariyawansa et al. (2015b) based on molecular phylogeny. The link between the sexual genus Eudarluca Speg. and the asexual genus Sphaerellopsis is still debated. Eudarluca was synonymized under Sphaerellopsis by Rossman et al. (2015) based on holomorphic characters of Eudarluca caricis (Fr.) O.E. Erikss. However, Phookamsak et al. (2014b) re-examined the isotype specimen of Eudarluca australis Speg. and treated Eudarluca in Phaeosphaeriaceae according to the generic type, E. australis is not congeneric with E. caricis. Eudarluca australis is typical of Phaeosphaeriaceae in having uni-loculate ascomata, a thin-walled peridium, comprising 1–2 layers of brown, pseudoparenchymatous cells, cylindrical asci, with pale brown, ellipsoidal to fusiform, (1–)2-septate ascospores and this concurs with the iconotype of E. australis, established by Spegazzini (1908) (Phookamsak et al. 2014b). Whereas, E. caricis forms black, multi-loculate ascostroma, with thick-walled peridium (Yuan et al. 1998; confirming the connection of E. caricis and Sphaerellopsis filum). Phylogenetic affinity of Eudarluca australis has not been proved yet.
Sphaerellopsis isthmospora A. Karunarathna, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF556138; Facesoffungi number: FoF05698, Fig. 17
Etymology: The specific epithet “isthmospora” refers to the fungus having isthmospores.
Holotype: KUN-HKAS 102225
Saprobic on dead branches of herbaceous plant. Sexual morphAscomata 230–330 μm high, 260–510 μm diam., black, shiny, scattered, solitary to gregarious, erumpent through host epidermis, becoming semi-immersed to superficial, varied in shape, subglobose to mammiform, with flattened, quadrilateral, truncate base, uni-loculate, glabrous, ostiolate, minutely papillate. Ostioles central, with obtuse, minute papilla, dark brown to black, ostiolar canal filled with periphyses. Peridium 18–60 μm wide, thick-walled of unequal thickness, thicker at the sides towards the apex, with flattened base, comprising several cell layers of black, coriaceous, pseudoparenchymatous cells, arranged in textura angularis to textura prismatica. Hamathecium initially comprising 2–5 µm wide, hyaline, filamentous distinctly septate pseudoparaphyses, laterally becoming, 6–18 µm wide, broadly cellular, hyaline, septate catenophyses, deeply constricted at the septa. Asci (120–)130–150(–165) × (15–)19–23(–28) µm (\( \bar{x} \) = 141.4 × 21.6 µm, n = 20), 8-spored, bitunicate, fissitunicate, subcylindric-clavate, subsessile to short pedicellate, with truncate pedicel, apically rounded with well-developed ocular chamber. Ascospores (65–)75–95(–118) × 4–7 µm (\( \bar{x} \) = 87.1 × 5.9 µm, n = 30), isthmosporous, overlapping 2–3-seriate, hyaline to yellowish, elongate cylindrical to subcylindric-clavate, bent at the 8th septum, 10–12-septate, slightly constricted at the septa, deeply constricted at the 8th septum, split into two part-spores; upper part 40–70(–82) µm long, 5–7-septate, cylindrical, with rounded end; lower part 28–35(–50) µm long, 2–3-septate, subcylindric-clavate, with acute end, guttulate, lacking a mucilaginous sheath. Asexual morph Undetermined.
Material examined: CHINA, Yunnan Province, Baoshan, Shuizai, Dawazi mountain, on dead branches of herbaceous plant, 23 October 2015, R. Phookamsak, BS012 (KUN-HKAS 102225, holotype).
GenBank numbers: ITS = MK387925, LSU = MK387963, SSU = MK387933, TEF1-α = MK435604 (HKAS 102225A); ITS = MK387926, LSU = MK387964, SSU = MK387934, TEF1-α = MK435605 (HKAS 102225B).
Notes: Sphaerellopsis isthmospora forms a phylogenetically distinct lineage, but clusters with other Sphaerellopsis species in Leptosphaeriaceae (Fig. 12). Sphaerellopsis isthmospora can be distinguished from other Sphaerellopsis species in its sexual morph having isthmosporous ascospores, with 10–12-septate, deeply constricted and bent at the 8th septum. Sphaerellopsis filum (sexual morph: Eudarluca caricis) has spindle-shaped, slightly inequilateral, 2–3-septate ascospores (Yuan et al. 1998). Based on morphological difference and phylogenetic affinity, we therefore, introduce a new species S. isthmospora from herbaceous plant in Baoshan, China.
Sphaerellopsis paraphysata Crous & Alfenas, in Trakunyingcharoen et al., IMA Fungus 5(2): 411 (2014)
Facesoffungi number: FoF04968, Fig. 18
Sphaerellopsis paraphysata (KUN-HKAS 101483). aLiriope spicata. b–d Appearance of conidiomata associated with rust on host substrate. e Section through conidioma wall. f Section through conidioma. g, h Culture on PDA after 2 weeks (g = from above, h = from below). i–r in vitro (OA). i Sporulation on OA after 4 weeks. j Section through conidioma. k Section through conidioma wall. l, m Conidiogenous cells stained in congo red. n–r Conidia. Scale barsj = 100 µm, c, d, f = 50 µm, k = 20 µm, e, l, m = 10 µm, n = 5 µm, o–r = 2 µm
Holotype: BRAZIL, Minas Gerais, Viçosa, Universidade Federal de Viçosa campus, on rust on Pennisetum sp., 18 November 2012, A.C. Alfenas, CBS H-21848, ex-type living culture, CPC 21841 = CBS138579.
Associated with rust on living leaves of Liriope spicata (Thunb.) Lour. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 105–160 µm high, 90–150 µm diam., black, pycnidial, solitary, associated with rust stromatic along the leaf veins, semi-immersed to superficial on stromata, globose to subglobose, uni-loculate, glabrous, ostiole central, with pore-like opening. Conidiomata walls 12–30 µm wide, composed of 2–5 layers, of dark brown pseudoparenchymatous cells of textura angularis. Conidiophores 15–21 × 2.4–3 µm (\( \bar{x} \) = 18 × 2.7 µm, n = 20), arising from the basal cavity, 1–2-celled, hyaline, curved, cylindrical, or reduced to conidiogenous cells. Conidiogenous cells 3.5–6 × 2.5–4.5 µm (\( \bar{x} \) = 4.7 × 3.5 µm, n = 20), enteroblastic, phialidic, discrete, determinate, cylindrical to ampulliform to doliiform, hyaline, 0–1-septate, smooth, thin-walled, minute collarette, with 1–2 apertures, and periclinal wall thickening. Conidia 14–17 × 3–5 µm (\( \bar{x} \) = 15.5 × 4.5 µm, n = 20), hyaline, fusiform to ellipsoidal, mostly 1–3-septate, constricted at the central septum, smooth-walled.
Culture characteristics: Colonies on PDA reaching 25–30 mm diam. after 4 weeks at room temperature. Colony dense, irregular in shape, flattened, slightly raised, surface slightly rough, heaped and folded at the centre, with small granular and black, stromatic, edge undulate, with margin well-defined, felted at the centre, fluffy at the edge; from above white at the margin, with yellowish grey to greenish grey at the centre; from below, white to cream at the margin, black at the centre; not producing pigmentation on agar medium, sporulating on PDA after 3 weeks.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, associated with rust on living leaves of Liriope spicata (Thunb.) Lour (Asparagaceae), 6 December 2017, R. Phookamsak, KIB044 (KUN-HKAS 101483), living culture, KUMCC 18-0195.
Known hosts and distribution: Associated with rust on Pennisetum sp. (Brazil), on Ravenelia macowania on Vachellia karroo (South Africa), on leaves of Phragmites sp. (Australia), and associated with rust on living leaves of Liriope spicata (Yunnan, China) (Trakunyingcharoen et al. 2014; Crous et al. 2018; this study).
GenBank numbers: ITS = MK387927, LSU = MK387965, SSU = MK387935, TEF1-α = MK435606.
Notes: Sphaerellopsis paraphysata was introduced by Trakunyingcharoen et al. (2014) based on morphological comparisons and phylogenetic analysis. We made a new collection from China associated with a rust on living leaves of Liriope spicata. The new isolate (KUN-HKAS 101483) is similar in morphology with S. paraphysata but differs from the type of S. paraphysata in having smaller conidiomata, presence of conidiophores and lacking paraphyses. Phylogenetic analyses of a combined LSU, SSU and ITS sequence dataset show that our strain (KUMCC 18-0195) forms a sister lineage with S. paraphysata (CPC 21841) with high support (100% ML and 1.00 BYPP). A comparison of ITS nucleotide base shows that our new isolate has same base pairs with the type strain of S. paraphysata. Thus we identify the new isolate as S. paraphysata and our new collection is a new host record in China.
Lophiotremataceae K. Hiray. & Kaz. Tanaka
Lophiotremataceae was introduced by Hirayama and Tanaka (2011) to accommodate the type genus Lophiotrema Sacc. and is typified by L. nucula (Fr.) Sacc. Lophiotrema shares morphological characters with Lophiostoma due to its compressed carbonaceous ascomata with crest-like apex but is distinguished by peridial structure and shape of asci (Zhang et al. 2009b, 2012; Hirayama and Tanaka 2011; Hyde et al. 2013). We follow the latest treatment and the updated accounts of Lophiotremataceae in Hyde et al. (2016) and Hashimoto et al. (2017). Lophiotremataceae comprises Atrocalyx A. Hashim. & Kaz. Tanaka, Crassimassarina A. Hashim. & Kaz. Tanaka, Cryptoclypeus A. Hashim. & Kaz. Tanaka, Galeaticarpa A. Hashim. & Kaz. Tanaka, Lophiotrema and Pseudocryptoclypeus A. Hashim. & Kaz. Tanaka (Hashimoto et al. 2017; Wijayawardene et al. 2018a). In the present study, a new species, Lophiotrema mucilaginosis collected on dead wood in China, is introduced.
Lophiotrema Sacc.
Lophiotrema was introduced by Saccardo (1878) and is typified by L. nucula (Fr.) Ces. & De Not. The genus was established to accommodate taxa in Pleosporales, characterized by ascomata with a slit-like ostioles, a peridium of uniform thickness, cylindrical to cylindric-clavate asci with a short stipe, hyaline, ellipsoidal to fusiform, septate ascospores and pycnidial coelomycetous asexual morphs (Hirayama and Tanaka 2011; Hirayama et al. 2014; Hashimoto et al. 2017). The genus has a long taxonomic history and has always been confused with Lophiostoma Ces. & De Not. and Massarina Sacc. in previous studies (Zhang et al. 2009b, 2012; Hirayama and Tanaka 2011; Hyde et al. 2013; Hirayama et al. 2014). However, Lophiotrema was re-classified based on molecular data by Hashimoto et al. (2017) and many species of Lophiotrema sensu lato were treated as new genera in Lophiotremataceae.
Lophiotrema mucilaginosis M. Raza & L. Cai, sp. nov.
Index Fungorum number: IF555333; Facesoffungi number: FoF04941, Fig. 19
Lophiotrema mucilaginosis (HMAS 255437, holotype). a Blackish ascomata on dead wood. b Vertical section of ascoma. c Peridial structure. d Pseudoparaphyses. e, f Immature asci. g, h Mature asci. i Apical ring stained with cotton blue. j Immature ascospores. k–o Mature ascospores. p Ascospores with mucilaginous sheath. q Germination of ascospore. r, s Culture characteristics on PDA (r = from above, s = from below). Scale barsb = 100 μm, c = 50 μm, d, p, q = 20 μm, e–i = 10 μm, o = 5, j–n = 2 μm
Etymology: In reference to the mucilaginous sheath around spores.
Holotype: HMAS 255437
Saprobic on dead wood. Sexual morphAscomata 220–340 μm high, 240–400 μm diam., black, scattered, solitary, semi-immersed to erumpent through host surface, conical to mammiform, with flattened base, uni-loculate, glabrous, ostiolate, papillate. Ostioles 50–90 × 25–60 μm, apically with crest-like papilla, filled with periphyses, carbonaceous, with beak-like opening. Peridium 55–80 μm wide, outer layer thick, composed of dark, coriaceous, pseudoparenchymatous cells of textura epidermoidea, inner layer comprising light pigmented to hyaline cells of textura angularis. Hamathecium composed of branched, 1.5–2 μm wide, filamentous, indistinct septate, anastomosed pseudoparaphyses, embedded in a hyaline, gelatinous matrix. Asci (83.5–)102–144(–210) × (10.5–)13–15(–21.5) μm (\( \bar{x} \) = 127.5 × 14.5 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate with furcate to truncate pedicel, apically rounded, with well-developed ocular chamber. Ascospores (31.5–)34–45.5(–48) × (5.5–)6–11(–12.5) μm (\( \bar{x} \) = 39.1 × 8.6 μm, n = 40), overlapping 2-seriate, hyaline, subfusoid to fusiform, with rounded or obtuse ends, 1(–3)-septate, smooth-walled, guttulate when young, with an entire mucilaginous sheath (9–20.5 μm wide at sides). Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 2.5–3 mm diam. after 1 week at 25 ± 2 °C, circular, convex or dome-shaped, rough with entire edge, mucoid, smooth at the margin; from above, green earth at the fruiting zone, grey at the productive zone and light grey at ageing zone, dome, shining black mucoid colony; from below, light grey at the fruiting zone, light green to blackish at the productive zone, dark grey at the ageing zone; cracking and not producing pigmentation in PDA agar medium.
Material examined: CHINA, Yunnan Province, Baoshan City, Longling County, on wood litter, October 2015, M. Raza, BAP 119 (HMAS 255437, holotype), ex-type living culture, LC12112.
GenBank numbers: ITS = MH822889, LSU = MH822890, SSU = MH822891, RPB2 = MH822892, TEF1-α = MH822893.
Notes: Multi-loci phylogenetic analyses based on a concatenated ITS, LSU, SSU, TEF1-α and RPB2 sequence dataset show that Lophiotrema mucilaginosis forms a well-supported lineage (100% ML and 1.00 BYPP; Fig. 20), sister to L. hydei J.F. Zhang et al. and clusters with L. neohysterioides M.E. Barr. Lophiotrema mucilaginosis and L. hydei were collected on wood litter and herbaceous plant from Yunnan and Guizhou Provinces in China respectively. Lophiotrema mucilaginosis can be distinguished from L. hydei in having larger asci (78–89(–99) × 6.9–8.8 μm, L. hydei) and larger ascospores (23–28 × 3–4 μm, L. hydei) (Zhang et al. 2018). In addition, L. mucilaginosis has conical to mammiform ascomata, with flattened base and 1(–3)-septate ascospores. Whereas, L. hydei has globose to subglobose ascomata and inconspicuously 0–1-septate ascospores. Lophiotrema mucilaginosis differs from L. neohysterioides by its larger asci (70–96 × 7–10 μm, L. neohysterioides) and larger ascospores (17–25 × 3–5 μm, L. neohysterioides) and presence of mucilaginous sheath surrounding the ascospores, a feature not observed in L. neohysterioides (Tanaka and Harada 2003a).
Phylogenetic tree generated from maximum likelihood analysis (RAxML) based on a combined ITS, LSU, SSU, TEF1-α and RPB2 sequence dataset of genera in Lophiotremataceae. Maximum likelihood bootstrap support values greater than 70% and Bayesian posterior probabilities greater than 0.95 BYPP are indicated on the branches. The new isolate is in blue. The type strains are in bold. The tree is rooted with Cryptocoryneum akitaense (KT 3019)
Occultibambusaceae D.Q. Dai & K.D. Hyde
Occultibambusaceae was introduced by Dai et al. (2017a) and is typified by Occultibambusa D.Q. Dai & K.D. Hyde with O. bambusae D.Q. Dai & K.D. Hyde being the type species. The family was introduced to accommodate bambusicola-like taxa, mainly occurring on bamboo (Dai et al. 2017a; Doilom et al. 2017). Dai et al. (2017a) accepted four genera in Occultibambusaceae viz. Neooccultibambusa Doilom & K.D. Hyde., Occultibambusa, Seriascoma Phookamsak et al. and Versicolorisporium Sat. Hatak. et al. and this is in agreement of Wijayawardene et al. (2018a). Subsequent authors introduced new taxa in this family (Hyde et al. 2016, 2018b; Zhang et al. 2017b; Tibpromma et al. 2018). We follow the latest treatment of Occultibambusaceae in Dai et al. (2017a) and introduce the new genus, Brunneofusispora S.K. Huang & K.D. Hyde to accommodate Brunneofusispora sinensis and other isolates of Massarina rubi. The updated sequence data were retrieved from Zhang et al. (2017b).
Brunneofusispora S.K. Huang & K.D. Hyde, gen. nov.
Index Fungorum number: IF555599; Facesoffungi number: FoF04862
Etymology: The generic epithet “Brunneofusispora” refers to the taxon having brown, fusiform ascospores.
Saprobic on dead wood. Sexual morphAscomata solitary to scattered, immersed, eventually erumpent, globose to subglobose, uni-loculate, glabrous, dark brown to black, ostiolate, with long beak. Peridium composed of brown to hyaline pseudoparenchymatous cells of textura angularis. Hamathecium composed of numerous, filamentous, septate pseudoparaphyses, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical to clavate, short pedicellate, rounded at the apex, with an ocular chamber. Ascospores overlapping 2-seriate, hyaline to brown, broadly fusiform, 1-septate, constricted at the septum, smooth-walled, with guttules, surrounded by a mucilaginous sheath. Asexual morph Undetermined.
Type species: Brunneofusispora sinensis S.K. Huang & K.D. Hyde
Notes: Multigene phylogenetic analyses reveal that our new taxon forms a distinct lineage, but clusters with three isolates of Massarina rubi (Fuckel) Sacc. (current name: Lophiotrema rubi (Fuckel) Y. Zhang ter et al.) in Occultibambusaceae (Fig. 21). We introduce a new genus Brunneofusispora to accommodate B. sinensis, which was collected from a woody plant in Yunnan, China. Brunneofusispora resembles Neooccultibambusa in occurring on woody plants and forming globose to subglobose ascomata, but Brunneofusispora differs from Neooccultibambusa in having a long prominent neck (Doilom et al. 2017). Brunneofusispora resembles Lophiotrema in having cylindrical, cylindric-clavate asci and fusiform ascospores, but it differs from Lophiotrema in having globose to subglobose ascomata, with a long prominent neck. Lophiotrema species have minute papilla, with crest-like or slit-like openings (Zhang et al. 2009b; Hashimoto et al. 2017).
Maximum likelihood phylogenetic tree generated from analysis of a combined LSU, SSU, ITS, RPB2 and TEF1-α sequence dataset for 77 taxa of representative families in Pleosporales. Melanomma pulvispyrius (CBS 124080) was selected as the outgroup taxon. ML and MP support values greater than 60% and Bayesian posterior probabilities greater than 0.90 BYPP are indicated above the nodes as ML/PP/MP. The strain numbers are noted before the species names. Isolates from this study are indicated in blue. Ex-type strains are indicated in bold
Lophiotrema rubi (≡ Masarina rubi) is an orphan species in Lophiotremataceae, which was transferred from Massarina based on phylogenetic analysis by Zhang et al. (2009b). It is characterized by immersed ascomata with broadly fusiform ascospores (Saccardo 1883b; Aptroot 1998). In this study, L. rubi strains CBS 691.95, MUT 4323 and MUT 4887 clustered with our strain (KUMCC 17-0030) and they form an independent clade within Occultibambusaceae (Fig. 21). However, the morphological characteristics of these strains have never been described (Zhang et al. 2009a; Gnavi et al. 2017). We therefore, tentatively include these strains of Massarina rubi until they are clarified base on evidence from morphology and phylogeny.
Brunneofusispora sinensis S.K. Huang & K.D. Hyde, sp. nov.
Index Fungorum number: IF555600; Facesoffungi number: FoF04863, Fig. 22
Brunneofusispora sinensis (KUN-HKAS 97451, holotype). a Habitat. b, c Appearance of ascomata on dead wood. d Ascoma in vertical section. e Peridium. f Asci with pseudoparaphyses stained in congo red. g–i Developing stages of asci. j–m Ascospores (note: l, m stained in congo red). Scale barsc = 500 µm, d = 200 µm, f = 50 µm, g–i = 20 µm, e, j–m = 10 µm
Etymology: The specific epithet “sinensis” refers to the country, China, where the taxon was collected.
Holotype: KUN-HKAS 97451.
Saprobic on dead wood. Sexual morphAscomata 325–370 μm diam., perithecial, solitary to scattered, immersed, eventually erumpent, globose to subglobose, uni-loculate, glabrous, dark brown to black, ostiole, papillate with long beak. Ostioles central, lined with periphyses. Peridium 20–45 μm wide, equally thick-walled, composed of 5–8 strata, of blackened pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium composed of numerous, 1.5–3.5 μm wide, filamentous, septate pseudoparaphyses, embedded in a gelatinous matrix. Asci 53–110 × 9–18 μm (\( \bar{x} \) = 75 × 14 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindric-clavate to clavate, short pedicellate, rounded at the apex, with an ocular chamber. Ascospores 18–22 × 5–8.5 μm (\( \bar{x} \) = 20 × 7 μm, n = 50), overlapping 2-seriate, initially hyaline, becoming light brown to brown at maturity, broadly fusiform, 1-septate, constricted at the septum, smooth-walled, with guttules, surrounded by mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinated on PDA within 2 weeks at 23 °C, colony on PDA reaching 1 cm diam. after 4 weeks, irregular in shape, surface rough, with edge umbonate and well-defined margin, velvety to floccose; from above brown; from below cream, not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Songming, Niulan river, on dead wood, 1 October 2016, S.K. Huang (KUN-HKAS 97451, holotype), ex-type living culture, KUMCC 17-0030.
GenBank numbers: ITS = MH393558, LSU = MH393557, SSU = MH393556, TEF1-α = MH395329.
Parabambusicolaceae Kaz. Tanaka & K. Hiray.
We follow the latest treatment and updated accounts of Parabambusicolaceae in Tanaka et al. (2015), Li et al. (2016), Phukhamsakda et al. (2016, 2018) and Wanasinghe et al. (2017). Two novel genera are introduced based on molecular phylogeny (Fig. 23) coupled with morphological characterization viz. Lonicericola and Paratrimmatostroma. In addition, a novel species Parabambusicola thysanolaenae is introduced.
Simplified phylogram showing the best RAxML tree obtained from a combined multigene (SSU, ITS, LSU and TEF1-α) analyses. Matrix of 42 taxa including related families of the family Parabambusicolaceae (Phukhamsakda et al. 2018). The matrix comprises 3580 characters with gaps. The best scoring RAxML tree with a final likelihood value of -14853.890740 is presented. MLBS above 70% and Bayesian posterior probabilities above 0.90 are given at each branch. The tree is rooted with Morosphaeria velatispora KH218 (Morosphaeriaceae). Type species are in bold and new isolates are in blue
Lonicericola Phookamsak, Jayasiri & K.D. Hyde, gen. nov.
Index Fungorum number: IF556139; Facesoffungi number: FoF04962
Etymology: The generic epithet “Lonicericola” refers to the host genus Lonicera, from which the type species was collected.
Saprobic o dead hanging branches of Lonicera maackii. Sexual morphAscomata black, scattered, solitary to gregarious, immersed under host epidermis, slightly raised, globose to subglobose, uni-loculate, glabrous, ostiolate, papillate. Peridium of equal thickness, composed of several layers, of flattened to broad, brown to dark brown, pseudoparenchymatous cells, arranged in textura angularis to textura prismatica. Hamathecium composed of numerous, filamentous, septate, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, subsessile to short pedicellate, with furcate to obtuse pedicel, apically rounded, with ocular chamber. Ascospores overlapping 2–3-seriate, hyaline, fusiform to vermiform, with enlarged cell, septate, constricted at the septa, smooth-walled, surrounded by distinct mucilaginous sheath. Asexual morph Undetermined.
Type species: Lonicericola hyaloseptispora Phookamsak, Jayasiri & K.D. Hyde
Notes: Lonicericola is similar to the sexual genera Aquastroma Kaz. Tanaka & K. Hiray., Multiseptospora Phookamsak & K.D. Hyde, Neoaquastroma Wanas. et al. and Parabambusicola Kaz. Tanaka & K. Hiray in Parabambusicolaceae, in having hyaline, fusiform to vermiform, multi-septate ascospores, with an entire sheath (Liu et al. 2015a; Tanaka et al. 2015). Lonicericola can be distinguished from these related genera based on habitat, ascomal shape, ascospore septation and multigene phylogenetic evidence. Lonicericola can be distinguished from Aquastroma based on its terrestrial habitat, broadly cylindrical to cylindric-clavate asci, with a subsessile to short pedicel and 9-septate ascospores. Aquastroma was found on submerged woody plant from aquatic habitat and has clavate asci, with a longer pedicel and 6–8-septate ascospores (Tanaka et al. 2015). Lonicericola resembles Multiseptospora in having globose to subglobose ascomata immersed in the host tissue. However, Multiseptospora has 10–11-septate ascospores, the ascomata are covered by brown to dark brown vegetative hyphal tufts, lack papilla, and have pore-like openings (Liu et al. 2015a). Neoaquastroma has 3–7-septate ascospores in N. guttulatum, 4–7-septate in N. bauhiniae and 5–8-septate in N. krabiense (Wanasinghe et al. 2017; Phukhamsakda et al. 2018). The asexual morph of Neoaquastroma has been reported as coelomycetous; whereas an asexual morph is not yet known for Lonicericola. Parabambusicola differs from Lonicericola in having hemispherical to conical ascomata, with a flattened base, and 5–6-septate ascospores as well as occurring on bamboo and stout grasses (Tanaka et al. 2015).
Multigene phylogenetic analyses reveal that Lonicericola forms a distinct lineage with Aquastroma, Multiseptospora, Neoaquastroma and Parabambusicola and clusters with the hyphomycetous genera Pseudomonodictys Doilom et al. and Paratrimmatostroma Jayasiri et al. with moderate support (77% ML and 0.99 BYPP). A comparison of ITS, LSU, SSU and TEF1-α sequence data indicates that Lonicericola differs from Pseudomonodictys in 97/593 bp (16.4%, ITS), 14/888 bp (1.6%, LSU), 18/1033 bp (1.7%, SSU) and 36/959 bp (3.7%, TEF1-α). However, we could not compare the morphological characteristics of Lonicericola with Pseudomonodictys and Paratrimmatostroma as they are represented by different morphs.
Lonicericola hyaloseptispora Phookamsak, Jayasiri & K.D. Hyde, sp. nov.
Index Fungorum number: IF556140; Facesoffungi number: FoF04963, Fig. 24
Lonicericola hyaloseptispora (KUN-HKAS 102223, holotype). a, b Appearance of ascomata on host substrate. c Section through ascoma. d Ostiole with papilla immersed in the host. e Section through peridium. f Asci immersed in hyaline, cellular pseudoparaphyses. g, h Asci. i–k Ascospores. l Ascospore stained with Indian ink. m Germinating ascospore. Scale barsa, b = 500 µm, c = 100 µm, d, e = 50 µm, f–m = 20 µm
Etymology: The specific epithet “hyaloseptispora” refers to the fungus having hyaline, multi-septate ascospores.
Holotype: KUN-HKAS 102223
Saprobic dead hanging branches of Lonicera maackii. Sexual morphAscomata 170–240 µm high, 165–250 µm diam., black, scattered, solitary to gregarious, immersed under host epidermis, slightly raised, globose to subglobose, uni-loculate, glabrous, ostiolate, papillate. Ostioles centrally located, oblong, with minute papilla, with pore-like opening, filled with hyaline periphyses. Peridium 8–25 µm wide, of equal thickness, composed of several layers, of flattened to broad, brown to dark brown, pseudoparenchymatous cells, arranged in textura angularis to textura prismatica. Hamathecium composed of numerous, 2–3.5 µm wide, filamentous, septate, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci (90–)100–120(–145) × (24–)25–30(–33) μm (\( \bar{x} \) = 115.1 × 27 μm, n = 30), 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, subsessile to short pedicellate, with furcate to obtuse pedicel, apically rounded, with ocular chamber clearly visible when young. Ascospores overlapping 2–3-seriate, hyaline, becoming brown when release from the asci, fusiform to vermiform, enlarged at the 4th cell from the apex (4–10 × 9–12 μm, l/w), (8–)9-septate, constricted at the septa, smooth-walled, with small to large guttules, surrounded by entire mucilaginous sheath (3.5–13 μm wide). Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 50–55 mm diam. after 3 weeks at room temperature (20–30 °C). Colony dense, circular, flattened, slightly raised, surface smooth, with entire edge, floccose to fluffy; from above dark grey to brown; from below, black; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming, Kunming Institute of Botany, on dead hanging branches of Lonicera maackii, 20 April 2017, R. Phookamsak, KIB034 (KUN-HKAS 102223, holotype), ex-type living culture KUMCC 18-0149 (KIB034A), KUMCC 18-0150 (KIB034B).
GenBank numbers: ITS = MK098191, LSU = MK098197, SSU = MK098203, (KUMCC 18-0149); ITS = MK098194, LSU = MK098200, SSU = MK098206, TEF1-α = MK098210 (KUMCC 18-0150).
Parabambusicola Kaz. Tanaka & K. Hiray
We follow the latest treatment and updated accounts of Parabambusicola in Tanaka et al. (2015). Previously, only P. bambusina was accommodated in this genus (Index Fungorum 2019). We introduce the second species, P. thysanolaenae, collected from Thysanolaena maxima in Yunnan, China.
Parabambusicola thysanolaenae Goonas., Jayasiri, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF555596; Facesoffungi number: FoF04964, Fig. 25
Parabambusicola thysanolaenae (KUN-HKAS 102222, holotype). a Appearance of ascomata on host substrate. b Section through ascoma. c Section through peridium. d Ostiole. e Asci immersed in hyaline, cellular pseudoparaphyses, stained in Indian ink. f Ascus. g–i Ascospores. j, k Ascospores stained in Indian ink. Scale barsa = 500 µm, b = 100 µm, c, d = 50 µm, e–k = 20 µm
Etymology: Named after the host from which the fungus was isolated.
Holotype: KUN-HKAS 102222
Saprobic on dead stems of Thysanolaena maxima, appearing as raised, dome-shaped areas on host surface, covered by brown, vegetative hyphae. Sexual morphAscomata 130–170 µm high, 430–600 µm wide, mostly clustered together, sometimes solitary, immersed under host epidermis, raised, becoming semi-immersed, globose in surface view, hemispherical with a flattened base in cross section, uni-loculate, glabrous, ostiole central, with pore-like opening. Peridium 25–60 µm wide, lateral walls composed of numerous layers of inner, hyaline, flattened cells to outer, pale brown to brown, textura angularis cells and pale brown to brown, globular or polygonal cells showing no conspicuous layers at the base, intermixed with host tissue. Hamathecium composed of numerous, 1.5–3 µm wide, filamentous, septate pseudoparaphyses, anastomosing above the asci, embedded in a hyaline gelatinous matrix. Asci (50–)80–120 × (10–)25–33 µm (\( \bar{x} \) = 107 × 28 µm, n = 30), 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, subsessile, rounded at the apex, with inconspicuous ocular chamber, clearly visible when young. Ascospores 45–55 × 7.5–11 µm (\( \bar{x} \) = 46.5 × 9 µm, n = 35), overlapping 2–3-seriate, hyaline, fusiform to vermiform, narrower towards the lower cell, enlarged at the 4th cell from apex, slightly curved, 5–(6–7)-septate, primary septum mostly median, constricted at the septa, smooth-walled, with an entire sheath, large guttules present when immature. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 30–32 mm diam. after 3 weeks at room temperature (20–30 °C). Colony dense, circular, flattened, surface smooth, with entire edge, velvety to floccose; from above greenish grey to dark green, paler at the edge; from below, black; produced dark brown pigmentation around colony on agar medium. Colonies on MEA reaching 28–30 diam. after 3 weeks at room temperature (20–30 °C). Colony dense, circular, flattened, surface smooth, with entire edge, floccose to cottony; from above cream to pale yellowish; from below, yellowish brown, paler at the edge; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Mengla County, Xishuangbanna Tropical Botanical Garden (XTBG), on dead stems of Thysanolaena maxima (Roxb.) Kuntze (Poaceae), 22 April 2017, R. Phookamsak, IS003 (KUN-HKAS 102222, holotype), ex-type living culture, KUMCC 18-0147 (IS003A), KUMCC 18-0148 (IS003B).
GenBank numbers: ITS = MK098190, LSU = MK098199, SSU = MK098205, TEF1-α = MK098209 (KUMCC 18-0147); ITS = MK098193, LSU = MK098198, SSU = MK098202, TEF1-α = MK098211 (KUMCC 18-0148).
Notes: Parabambusicola thysanolaenae shares similar peridial and ascal characters with P. bambusina (Teng) Kaz. Tanaka & K. Hiray. but can be distinguished by having larger ascomata (300–500 × 150–300 µm in P. bambusina), absence of a beak-like structure and wider peridium (10–20 µm in P. bambusina). Ascospores of P. thysanolaenae are 5-septate and shorter than the 3–5-septate ascospores of P. bambusina (54.7 × 8.4 μm, Tanaka and Harada 2003b). Phylogenetically P. thysanolaenae clusters with P. bambusina forming a well-separated lineage (100% ML and 1.00 BYPP; Fig. 23).
Paratrimmatostroma Jayasiri, Phookamsak, D.J. Bhat & K.D. Hyde, gen. nov.
Index Fungorum number: IF556153; Facesoffungi number: FoF04960
Etymology: With reference to similar morphology of genus “Trimmatostroma”
Saprobic on dead fronds of a fern. Sexual morph Undetermined. Asexual morph Hyphomycetous. Sporodochia effuse or confluent, visible as black powdery, superficial mass on host substrate, flattened, light brown, with a membranous base, composed of pseudoparenchymatous cells of textura angularis. Mycelium immersed, composed of septate, pale brown, branched hyphae. Conidiophores macronematous, or semi-macronematous, mononematous, prostrate, or erect, usually short, oblong to cylindrical, straight or flexuous, arising as lateral branches from creeping hyphae, septate, branched or unbranched, slightly constricted at the septa so as to give a monilioid appearance, pale brown, smooth-walled. Conidiogenous cells holoblastic, mono- to polyblastic, integrated, terminal, brown, smooth-walled. Conidia solitary, acropleurogenous, dark brown, paler at the apical cell, branched, straight or flexuous, variable in shape, helicoid, cylindrical, sigmoid, or reniform, solitary, tapering near apex and base, rounded at tip, septate, constricted at the septa, smooth and thick-walled.
Type species: Paratrimmatostroma kunmingensis Jayasiri, Phookamsak, D.J. Bhat & K.D. Hyde
Notes: Paratrimmatostroma is similar to Trimmatostroma Corda in forming effuse to confluent sporodochia, semi-macronematous, mononematous conidiophores, integrated, terminal conidiogenous cells, and branched, straight or flexuous, septate, pigmented conidia, which are variable in shape (Ellis 1971; Crous et al. 2007a). However, they are phylogenetically distinct in that Paratrimmatostroma belongs to Parabambusicolaceae (Pleosporales, Dothideomycetes), whereas Trimmatostroma was recently treated in Mollisiaceae Rehm (Helotiales, Leotiomycetes) (Crous et al. 2007a; Wijayawardene et al. 2018a). Multigene phylogenetic analyses show that Paratrimmatostroma forms a well-resolved clade (74% ML and 1.00 BYPP; Fig. 23), and clusters with Pseudomonodictys and Lonicericola. Paratrimmatostroma is distinct from Pseudomonodictys in forming sporodochia on the host substrate and having branched, straight or flexuous conidia, with variable conidial shape. Pseudomonodictys has muriform, top-shaped to ellipsoidal conidia and does not form sporodochia on host substrate (Ariyawansa et al. 2015a). Paratrimmatostroma was found on a fern in Yunnan, China (nonflowering vascular plants in the low-latitude monsoon climate), whereas, Pseudomonodictys was collected from teak in Thailand (flowering plant in tropical climate). A comparison of ITS, LSU, SSU and TEF1-α sequence dataset indicates that Paratrimmatostroma differs from Pseudomonodictys in 64/540 bp (11.8%, ITS), 23/857 bp (2.7%, LSU), 22/1060 bp (2.1%, SSU) and 41/928 bp (4.4%, TEF1-α). Based on phylogenetic analysis and morphological distinctiveness, we introduce Paratrimmatostroma as a new genus to accommodate a single species, P. kunmingensis.
Paratrimmatostroma kunmingensis Jayasiri, Phookamsak, D.J. Bhat & K.D. Hyde, sp. nov.
Index Fungorum number: IF556152; Facesoffungi number: FoF04961, Fig. 26
Etymology: The specific epithet “kunmingensis” refers to Kunming City, Yunnan Province, China, where the type was collected.
Holotype: KUN-HKAS 102224
Saprobic on dead fronds of a fern. Sexual morph Undetermined. Asexual morph Hyphomycetous. Sporodochia effuse or confluent, visible as black powdery, superficial mass on host substrate, flattened, light brown, with a membranous base, composed of pseudoparenchymatous cells of textura angularis. Mycelium immersed, composed of septate, pale brown, branched hyphae. Conidiophores (6–)15–30(–50) × 2–4 µm (\( \bar{x} \) = 19.2 × 3.2 µm, n = 20), macronematous or semi-macronematous, mononematous, prostrate, or erect, usually short, oblong to cylindrical, straight or flexuous, arising as lateral branches from creeping hyphae, septate, branched or unbranched, slightly constricted at the septa so as to give a monilioid appearance, pale brown, smooth-walled. Conidiogenous cells 3–10(–15) × 2–4.5 µm (\( \bar{x} \) = 7.1 × 3.1 µm, n = 30), holoblastic, mono- to polyblastic, integrated, terminal, brown, smooth-walled. Conidia solitary, acropleurogenous, dark brown, paler at the apical cell, branched, straight or flexuous, variable in shape, helicoid [(8–)10–20(–27) × (6–)10–20 µm (\( \bar{x} \) = 16.8 × 13.7 µm, n = 40)], cylindrical to sigmoid, or reniform [(8.5–)15–50 × 6–8(–10) µm (\( \bar{x} \) = 29.7 × 7.7 µm, n = 50)], solitary, tapering near apex and base, rounded at tip, multi-septate, 6–13-septate at maturity, constricted at the septa, smooth and thick-walled.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead fronds of a fern, 1 April 2017, R. Phookamsak, KIB025 (KUN-HKAS 102224, holotype).
GenBank numbers: ITS = MK098192, LSU = MK098196, SSU = MK098204, TEF1-α = MK098208 (KUN-HKAS 102224A); ITS = MK098195, LSU = MK098201, SSU = MK098207 (KUN-HKAS 102224B).
Periconiaceae (Sacc.) Nann.
Periconiaceae has long been unused and placed as members of Massarinaceae Munk until Tanaka et al. (2015) revised Massarineae and placed it as a distinct family based on phylogenetic analysis (Tanaka et al. 2015; Hyde et al. 2017, 2018b). We follow the latest treatment and updated accounts of Periconiaceae in Tanaka et al. (2015), Hyde et al. (2017, 2018b), Liu et al. (2017b) and Thambugala et al. (2017). We report Periconia cortaderiae Thambug. & K.D. Hyde from Caragana arborescens Lam. (Fabaceae) in Yunnan, China for the first time.
Periconia Tode
Periconia was introduced by Tode (1791) to accommodate hyphomycetous species having macronematous conidiophores and 1-celled, pigmented, verruculose to echinulate conidia and is typified by P. lichenoides Tode (Tanaka et al. 2015; Thambugala et al. 2017). The genus was re-circumscribed by Tanaka et al. (2015) and this was followed by subsequent authors (Hyde et al. 2017, 2018b; Liu et al. 2017b; Thambugala et al. 2017). We follow the latest treatment of Periconia in Tanaka et al. (2015). The updated phylogenetic analyses were retrieved from Thambugala et al. (2017) and Hyde et al. (2018b) (Fig. 27).
Phylogram generated from the best scoring of the RAxML tree based on combined ITS, LSU and TEF1-α sequence dataset of taxa in Periconiaceae and other related families (Massarinaceae, Didymellaceae and Lentitheciaceae). Taxa in Morosphaeriaceae, Morosphaeria ramunculicola (KH 220) and M. velatispora (KH 221) were selected as the outgroup taxa. Bootstrap support values for maximum likelihood (green) equal to or greater than 70% and the Bayesian posterior probabilities (blue) equal or higher than 0.95 are indicated above the nodes. Ex-type and ex-epitype strains are in bold. Newly generated sequences are indicated in blue
Periconia cortaderiae Thambug. & K.D. Hyde, in Thambugala et al., Mycosphere 8(4): 734 (2017), Fig. 28
Holotype: THAILAND, Chiang Rai, Mae Fah Luang University, on dead stems and leaves of Cortaderia sp. (Poaceae), 21 December 2014, K.M. Thambugala, KM 035 (MFLU16–2579), ex-type living culture MFLUCC 15–0457, ICMP 21414
Saprobic on dead, hanging branches of Caragana arborescens. Sexual morph Undetermined. Asexual morphColonies on the substratum superficial, numerous, effuse, black, floccose. Mycelium immersed on the substrate, composed of septate, branched, smooth, dark hyphae. Conidiophores 100–225 × 8.5–11 μm, macronematous, mononematous, septate, branched, erect, mostly slightly flexuous to curved, dark brown, forming sphaerical heads at apex, arising from a stromatic base. Conidiogenous cells 4.5–5.2 × 4.1–5.6 μm (\( \bar{x} \) = 4.9 × 5.1 μm, n = 20), mono- to polyblastic, discrete, terminal, globose, light brown. Conidia (5.5–)6–9(–12) × (4.5–)5–8 μm (\( \bar{x} \) = 7.8 × 6.4 μm, n = 50), solitary or catenate, in acropetal chains, subglobose to globose, occasionally ellipsoidal to cylindrical, light brown to moderately brown, verruculose.
Culture characteristics: Colonies on PDA reaching 68–74 mm diam. after 3 weeks at room temperature (20–30 °C). Colony medium sparse, circular, flattened, surface smooth, edge entire, velvety to woolly; from above, sectoring, with a part of cream to pale yellowish and yellowish-green to dark grey, with small white tufts in another part; from below, pale yellowish at the margin, sectoring at the centre, with a part of yellowish to orange-yellow and dark brown in another part; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead hanging branches of Caragana arborescens (Fabaceae), 2 April 2017, R. Phookamsak, KIB017 (KUN-HKAS 102240), living culture, KUMCC 18-0174 = MFLUCC18-0667 (KIB017A), KUMCC 18-0175 = MFLUCC18-0668 (KIB017B).
Known hosts and distribution: On dead stems and leaves of Cortaderia sp. (Poaceae, Thailand) and Caragana arborescens (Fabaceae, Yunnan, China; in this study).
GenBank numbers: ITS = MH892348, LSU = MH892401 (MFLUCC 18-0667); ITS = MH892349, LSU = MH892402, TEF1-α = MH908964 (MFLUCC18-0668).
Notes: Phylogenetic analyses of a concatenated LSU, ITS and TEF1-α sequence datset show that our strains (MFLUCC 18-0667 and MFLUCC 18-0668) form a well-resolved clade, clustering with Periconia cortaderiae (100% ML and 1.00 BYPP; Fig. 27). A comparison of ITS and TEF1-α nucleotide base pair indicates that our strains are identical to the type strain of P. cortaderiae, which is not significantly different (2/520 bp in ITS and 4/830 bp in TEF1-α). We therefore, identify our isolates as P. cortaderiae and report its occurrence on Caragana arborescens in Yunnan, China for the first time. Our isolate has shorter conidiophores (400–800 μm high, holotype) and slightly larger conidia (4–6.6 × 4.1–7.1 μm, holotype) (Thambugala et al. 2017). Periconia species are morphologically variable in different hosts and geographic distributions, but the molecular data of informative genes can clarify their conspecificity. Therefore, more informative genes such as RPB2 and TUB2 are needed in further studies of this genus.
Phaeosphaeriaceae M.E. Barr
Phaeosphaeriaceae was introduced by Barr (1979) to accommodate fungal taxa which mainly occurr on monocotyledons, but are also found on some herbaceous plants (Phookamsak et al. 2014b, 2017). Many additional genera have been introduced in this family since Phookamsak et al. (2014b) re-circumscribed the genera in Phaeosphaeriaceae. Wijayawardene et al. (2018a) listed 52 genera in Phaeosphaeriaceae. Wanasinghe et al. (2018), Bakhshi et al. (2019) and Maharachchikumbura et al. (2019) introduced other seven genera in this family, and 59 genera are now accepted in Phaeosphaeriaceae based on molecular phylogeny coupled with morphological characteristics (Wanasinghe et al. 2018; Wijayawardene et al. 2018a). We follow the latest treatment and updated accounts of Phaeosphaeriaceae in Phookamsak et al. (2014b), Hyde et al. (2018b) and Wanasinghe et al. (2018). A new genus Brunneomurispora is introduced to accommodate a single species, B. lonicerae. Five new species are also introduced viz. Galiicola baoshanensis, Neosetophoma lonicerae, Ophiobolus malleolus, Phaeosphaeria cycadis and Wojnowiciella kunmingensis. Furthermore, Wojnowicia rosicola W.J Li et al. is transferred to Wojnowiciella. Amarenomyces dactylidis Mapook et al. is reported from a fern in China for the first time. An updated phylogenetic analysis (Fig. 29) was performed following previous phylogenies derived from Hyde et al. (2018b) and Wanasinghe et al. (2018).
Phylogram generated from maximum likelihood analysis based on a concatenated LSU, SSU, TEF1-α and ITS sequence dataset of Phaeosphaeriaceae. Updated sequence data were obtained from GenBank (http://www.ncbi.nlm.nih.gov/) and retrieved from Hyde et al. (2018b) and Wanasinghe et al. (2018). One hundred and fifty-two strains are included in the analysis. Staurosphaeria rhamnicola (MFLUCC 17-0813 and MFLUCC 17-0814) were selected as the outgroup taxon. Bootstrap support values for maximum likelihood (left) equal to or greater than 60% and Bayesian posterior probabilities (right) equal or higher than 0.95 are indicated above the nodes. Newly generated sequences are in blue. Type strains are indicated in bold
Amarenomyces O.E. Erikss.
Amarenomyces was introduced by Eriksson (1981) and is typified by A. ammophilae (Lasch) O.E. Erikss., occurring on marine grass (Ammophila arenaria L., Poaceae). The genus is characterized by immersed to erumpent, globose to subglobose ascomata, thin-walled peridium, multi-layered endotunica, broadly cylindrical asci, with subsessile, knob-like pedicel and large, pigmented, septate, thick-walled and sheathed ascospores (Phookamsak et al. 2014b). Eriksson (1981) placed the genus in Botryosphaeriaceae Theiss. & Syd.; however, Zhang et al. (2009a) treated the genus as a synonym of Phaeosphaeria I. Miyake in Phaeosphaeriaceae based on molecular phylogeny. Phookamsak et al. (2014b) re-circumscribed the genera in Phaeosphaeriaceae based on multigene phylogenetic analyses coupled with morphological studies and thus, Amarenomyces was re-instated. Hyde et al. (2017) introduced a second species, A. dactylidis Mapook et al., collected from dead aerial stems of Dactylis glomerata L. in Italy. Only two species are presently accommodated in this genus.
Amarenomyces dactylidis Mapook, Camporesi & K.D. Hyde, in Hyde et al., Fungal Divers 87: 78 (2017), Fig. 30
Amarenomyces dactylidis (KUN-HKAS 102230). a Appearance of ascomata on host. b Section through ascoma. c Section through peridium. d Cellular pseudoparaphyses. e–h Asci. i–n Ascospores. o Ascospore stained with Indian ink. p Ascospore germination. Scale barsa = 200 μm, b = 50 μm, c–h = 20 μm, i–p = 10 μm
Holotype: ITALY, Forlì-Cesena Province, Camposonaldo-Santa Sofia, on dead aerial stems of Dactylis glomerata (Poaceae), 10 January 2014, E. Camporesi, MFLU 17-0498, ex-type living culture MFLUCC 14-0207.
Saprobic on dead fronds of a fern. Sexual morphAscomata 170–230 μm high, 160–260 µm diam., scattered, solitary, semi-immersed to superficial, visible as raised, black dots on the host surface, globose to subglobose, uni-loculate, glabrous, ostiole central, with minute, papilla (20–45 × 25–55 μm, l/w), lacking periphyses. Peridium 13–23 μm wide, thin-walled of equal thickness, composed of 5–6 cell layers, of flattened to broad, pseudoparenchymatous cells; outer layer comprising brown to dark brown cells of textura angularis; inner layer comprising flattened, hyaline to pale brown cells, of textura angularis to textura prismatica. Hamathecium composed of numerous, 1.8–4.5 μm wide, filamentous, septate, pseudoparaphyses, anastomosing above the asci, embedded in a hyaline gelatinous matrix. Asci (70–)75–95(–113) × (9.5–)10–13(–14) μm (\( \bar{x} \) = 88.9 × 12 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, with furcate pedicel, apically rounded, with well-developed ocular chamber. Ascospores (19–)(23–)25–28(–32) × 4.5–6 μm (\( \bar{x} \) = 26.1 × 5.5 μm, n = 50), overlapping 1–2-seriate, yellowish brown to brown, fusiform to subcylindrical, slightly narrower towards the end cells, asymmetrical, 7–8-septate, slightly constricted at the septa, enlarged at the 5th or 6th cell from above, flattened at the 2nd to 4th, or 5th cells, smooth-walled, surrounded by a distinct mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 35–38 mm diam. after 3 weeks at room temperature (20–30 °C). Colony dense, irregular in shape, flattened to slightly raised, surface smooth, with edge undulate, velvety to floccose; from above, initially white, with pale grey at the centre, becoming greenish grey after 4 weeks; from below, white to pale yellowish at the margin, dark brown at the centre, becoming black after 4 weeks; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Baoshan, Shuizai, Dawazi mountain, on dead fronds of a fern, 22 October 2015, I.D. Goonasekara, BS008 (KUN-HKAS 102230), living culture, KUMCC 18-0154.
Known hosts and distribution: On dead aerial stems of Dactylis glomerata (Poaceae, Italy) and on dead fronds of a fern (Yunnan, China) (Hyde et al. 2017; this study).
GenBank numbers: ITS = MK356371, LSU = MK356345, SSU = MK356359.
Notes: Multigene phylogenetic analyses show that the new strain KUMCC 18-0154 is sister to Amarenomyces dactylidis (MFLUCC 14-0207) with high support (98% ML and 1.00 BYPP). A comparison of ITS nucleotide base pairs shows that KUMCC 18-0154 is identical (1/541 bp) to A. dactylidis and thus, we identify our new isolate as A. dactylidis. Our isolate (KUN-HKAS 102230) shares a size range of the ascomata, asci and ascospores as well as the ascospore septation with the type (MFLU 17-0498). Although, our isolate is slightly larger in ascomata, asci and ascospores, but ITS sequence data showed that they are conspecific (Hyde et al. 2017). Therefore, A. dactylidis is reported from a fern in Yunnan, China for the first time.
Brunneomurispora Phookamsak, Wanas. & K.D. Hyde, gen. nov.
Index Fungorum number: IF556165; Facesoffungi number: FoF05699
Etymology: The generic epithet “Brunneomurispora” refers to the fungus having brown, muriform ascospores.
Saprobic on Lonicera maackii. Sexual morphAscomata scattered, solitary or in groups, semi-immersed to erumpent, globose to subglobose, or irregular in shape, uni-loculate, glabrous, ostiolate, papillate. Peridium slightly thick, composed of several layers, of small, flattened to broad, dark brown, pseudoparenchymatous cells of textura angularis to textura prismatica. Hamathecium composed of numerous, broad, filamentous, septate, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, or clavate, short pedicellate, apically rounded, with inconspicuous ocular chamber. Ascospores overlapping 1–2-seriate, dark brown, muriform, fusiform to ellipsoidal, asymmetrical, slightly larger in the upper part, constricted at the central septum, smooth-walled, lacking mucilaginous sheath. Asexual morph Undetermined.
Type species: Brunneomurispora lonicerae Phookamsak, Konta, Wanas. & K.D. Hyde
Notes: Phylogenetic analyses of a concatenated LSU, SSU, TEF1-α and ITS sequence dataset (Fig. 29) show that our new strains (KUMCC 18-0157 and KUMCC 18-0158) form a well-separated lineage basal to Neosetophoma Gruyter et al. with high support (94% ML and 0.99 BYPP). Our new isolate is distinct from Neosetophoma in having dark brown muriform ascospores. While, the sexual morph of Neosetophoma has phragmosporous, brown, fusiform ascospores (Tibpromma et al. 2017; Hyde et al. 2018b). Hence, we introduce a new genus Brunneomurispora herein to accommodate B. lonicerae which was isolated from Lonicera maackii in Yunnan, China.
Brunneomurispora resembles Embarria Wanas. et al. and Hydeomyces Maharachch. et al. in having immersed to erumpent, globose or subglobose ascomata, with a minute papilla, clavate asci, with dark brown, muriform, asymmetrical ascospores and ascospores that are constricted at the central septum (Wanasinghe et al. 2018; Maharachchikumbura et al. 2019). However, Brunneomurispora can be distinguished from Embarria in its peridium structure comprising several layers of brown, small, flattened to broad pseudoparenchymatous cells and its ascospores being 4–6 transverse septa, with 1–2 longitudinal septa, sectored, and lacking a mucilaginous sheath. Embarria has a thin-walled peridium, comprising large, 2–3 cell layers of pseudoparenchymatous cells and its ascospores are 4–6 transverse septa, with a single longitudinal septum, surrounded by a thick mucilaginous sheath (Wanasinghe et al. 2018). Hydeomyces differs from Brunneomurispora in having thicker peridium (35–60 μm thick), smaller, cylindrical asci (70–85 × 9–17 μm) and smaller, 1-seriate, muriform ascospores, with 2–4 transverse septa and 1 longitudinal septum (10–15 × 5–6.5 μm) (Maharachchikumbura et al. 2019). Hawksworthiana lonicerae Wanas. et al. was also isolated from Lonicera in Italy. This species differs from Brunneomurispora lonicerae in having cylindrical to cylindric-clavate asci, with yellowish brown, ellipsoidal, muriform, 3 transverse septa, with 1 longitudinal septum ascospores (Wanasinghe et al. 2018).
Many genera in Phaeosphaeriaceae are characterized by dictyosporous ascospores viz. Allophaeosphaeria Ariyaw. et al., Dactylidina Wanas. et al., Dematiopleospora Wanas. et al., Embarria, Galiicola Tibpromma et al., Hawksworthiana Wanas. et al., Hydeomyces Maharachch. et al., Italica Wanas. et al., Muriphaeosphaeria Phukhams. et al., Populocrescentia Wanas. et al. and Yunnanensis Karun. et al. (Wanasinghe et al. 2014b, 2018; Ariyawansa et al. 2015a; Liu et al. 2015a; Phukhamsakda et al. 2015; Karunarathna et al. 2017; Maharachchikumbura et al. 2019). These genera are represented by a single or a few species and have very little morphological differences in their sexual morphs. However, they always form distinct clades, separate from each other, as well as the asexual morphs of some different genera. Furthermore, Poaceicola and Populocrescentia are heterogeneous, forming both phragmosporous and dictyosporous ascospores (Wanasinghe et al. 2018). More sampling of taxa in these genera are needed for a better understanding.
Brunneomurispora lonicerae Phookamsak, Konta, Wanas. & K.D. Hyde, sp. nov.
Index Fungorum number: IF556166; Facesoffungi number: FoF05700, Fig. 31
Brunneomurispora lonicerae (KUN-HKAS 102232, holotype). a Appearance of ascomata on host. b Close up of ascoma on host. c Section through ascoma. d Section through peridium. e Cellular pseudoparaphyses. f–i Asci. j–o Ascospores. Scale barsa = 500 μm, b = 200 μm, c = 100 μm, d = 50 μm, e–i = 20 μm, j–o = 10 μm
Etymology: The specific epithet “lonicerae” refers to the host genus Lonicera, from which the fungus was collected.
Holotype: KUN-HKAS 102232
Saprobic on dead hanging branches of Lonicera maackii. Sexual morphAscomata 170–280 μm high, 230–330 µm diam., scattered, solitary, or in groups, semi-immersed to erumpent, visible as raised, black dot on the host surface, globose to subglobose, occasionally irregular in shape, uni-loculate, glabrous, ostiole central, with minute, mammiform papilla, lacking periphyses. Peridium 15–40 μm wide, of unequal thickness, composed of several layers, of small, flattened to broad, pseudoparenchymatous cells; outer layer comprising brown to dark brown cells of textura angularis; inner layer comprising flattened, hyaline cells of textura angularis to textura prismatica. Hamathecium composed of numerous, 2–5.5 μm wide, filamentous, septate, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci (67–)80–110(–132) × (13–)16–20(–24) μm (\( \bar{x} \) = 98 × 18.8 μm, n = 25), 8-spored, bitunicate, fissitunicate, broadly cylindrical to cylindric-clavate, or clavate, short pedicellate, with truncate pedicel, apically rounded, with inconspicuous ocular chamber, clearly seen when immature. Ascospores (12–)14–18(–20)(–23) × (4–)5–8(–13) μm (\( \bar{x} \) = 17 × 7.7 μm, n = 50), overlapping 1–2-seriate, dark brown, muriform, fusiform to ellipsoidal, with acute or rounded ends, or acute at the upper cells, asymmetrical, slightly larger in the upper part, straight, sometimes bent, mostly 4–6 transverse septa, with 1–2 longitudinal septa in each cell, becoming many sectors, constricted at the central septum, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 28–30 mm diam. after 1 week at room temperature (20–30 °C). Colony medium dense, circular, flattened, surface smooth, with edge entire, velvety to woolly; from above, white to cream at the margin towards the centre, with sectored, greenish grey to dull green or light green at the centre; from below, cream to pale yellowish at the margin, yellowish at the middle, brown-green at the centre; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead hanging branches of Lonicera maackii, 20 April 2017, R. Phookamsak, KIB030 (KUN-HKAS 102232, holotype), ex-type living culture KUMCC 18-0157 (KIB030A), KUMCC 18-0158 (KIB030B).
GenBank numbers: ITS = MK356372, LSU = MK356346, SSU = MK356360, TEF1-α = MK359064, RPB2 = MK359079 (KUMCC 18-0157); ITS = MK356373, LSU = MK356347, SSU = MK356361, TEF1-α = MK359065 (KUMCC 18-0157).
Galiicola Tibpromma et al.
Galiicola was introduced by Ariyawansa et al. (2015a) to accommodate a single species G. pseudophaeosphaeria Tibpromma et al. which was found as a saprobe on Galium in Italy. The genus is characterized by semi-immersed to erumpent, globose to subglobose ascomata, fissitunicate, cylindric-clavate asci, orange-brown, elongate fusiform ascospores with 4–5 transverse septa, some with 1–2 longitudinal septa; its asexual morph has not been found (Ariyawansa et al. 2015a). We introduce a second species, G. baoshanensis which is represented by its asexual morph.
Galiicola baoshanensis Phookamsak, Wanas. & K.D. Hyde, sp. nov.
Index Fungorum number: IF556167; Facesoffungi number: FoF05701, Fig. 32
Galiicola baoshanensis (KUN-HKAS 102234, holotype). a Appearance of conidiomata on host. b Section through conidioma. c Ostiole with pore-like opening. d Section through conidioma wall. e–g Conidiogenous cells. h Conidium when immature. i–m Conidia. Scale barsa = 200 μm, b = 50 μm, d = 20 μm, c, g–m = 10 μm, e, f = 5 μm
Etymology: The specific epithet “baoshanensis” refers to Baoshan prefecture-level city of Yunnan Province, China, where the holotype was collected.
Holotype: KUN-HKAS 102234
Saprobic on dead branches of herbaceous plant. Sexual morph Undetermined. Asexual morph Coelomycetous, amarenographium-like. Conidiomata 90–125 μm high, 90–120 μm diam., pycnidial, scattered, solitary, immersed to semi-immersed, uni-loculate, globose to subglobose, glabrous, dark brown, visible as small black dot on the host surface, ostiolate, apapillate, with pore-like opening. Conidiomata walls 8–25 μm wide, thin-walled, composed of flattened to broad, brown to dark brown pseudoparenchymatous cells, arranged in a textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–7(–12) × (2–)3–6 μm (\( \bar{x} \) = 5.7 × 4.7 µm, n = 30), enteroblastic, phialidic, discrete, determinate, ampulliform to doliiform, occasionally cylindrical, unbranched, aseptate, hyaline, smooth, minute collarette with periclinal wall thickening, arising from the inner cavity of the conidioma wall, difficult to distinguish from the conidioma wall. Conidia (30–)35–45 × (10–)11–14(–15.5) μm (\( \bar{x} \) = 39.6 × 12.9 µm, n = 50), muriform, brown to dark brown, paler at the end cells, ellipsoidal to broadly fusiform, or subclavate, apex rounded, base truncate or obtuse, 8–9 transverse septa, with 2–4 longitudinal septa, not constricted at the septa, rough-walled, echinulate, lacking mucilaginous sheath surrounding conidia.
Material examined: CHINA, Yunnan Province, Baoshan, Shuizai, Dawazi mountain, on dead branches of herbaceous plant, 23 October 2015, R. Phookamsak, BS018 (KUN-HKAS 102234, holotype).
GenBank numbers: ITS = MK356374, LSU = MK356348, SSU = MK356362, TEF1-α = MK359066.
Notes: Multigene phylogenetic analyses of a combined LSU, SSU, TEF1-α and ITS sequence dataset show that Galiicola baoshanensis forms a sister lineage with the generic type of Galiicola, G. pseudophaeosphaeria with high support (100% ML and 1.00 BYPP; Fig. 29). A comparison of TEF1-α sequences indicates that G. baoshanensis differs from G. pseudophaeosphaeria in 14/730 bp (1.9%). However, we could not compare the morphological characters of G. baoshanensis with G. pseudophaeosphaeria as they are represented by different morphs. Galiicola baoshanensis is introduced as the asexual species in Galiicola for the first time.
Galiicola baoshanensis is similar to Amarenographium ammophilae Wanas. et al. in having conidia that are muriform, clavate, ellipsoidal, ovoid or fusoid conidia, with rounded apex, acute or truncate base, and 7–9 transverse septa (Wijayawardene et al. 2016). However, G. baoshanensis can be distinguished from A. ammophilae in having brown to dark brown conidia, with 2–4 longitudinal septa and lacking appendages at the apex and the base. Whereas, A. ammophilae has yellowish brown to brown conidia, with 1–3 longitudinal septa and appendages at the apex and the base (Wijayawardene et al. 2016). A comparison of ITS and TEF1-α sequences indicates that G. baoshanensis differs from A. ammophilae in 71/570 bp (12.5%) and 58/899 bp (6.5%), respectively. Muti-gene phylogenetic analyses also supported their distinctiveness (Fig. 29).
Neosetophoma Gruyter et al.
We follow the latest treatment and updated accounts of Neosetophoma in Hyde et al. (2018b) and Wanasinghe et al. (2018). Seventeen species are known in this genus (Hyde et al. 2018b; Index Fungorum 2019).
Neosetophoma lonicerae Phookamsak, Wanas. & K.D. Hyde, sp. nov.
Index Fungorum number: IF556168; Facesoffungi number: FoF05702, Fig. 33
Neosetophoma lonicerae (KUN-HKAS 102231, holotype). a Appearance of conidiomata on host. b Section through conidioma. c, d Section through conidioma wall. e–h Conidiogenous cells. i–n Conidia. o Germinated conidium. p, q Culture characteristics on PDA (p = from above, q = from below). Scale barsa = 200 μm, b = 50 μm, c–h, o = 10 μm, i–n = 5 μm
Etymology: The specific epithet “lonicerae” refers to the host genus Lonicera, from which the holotype was collected.
Holotype: KUN-HKAS 102231
Saprobic on Lonicera maackii. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 110–160 μm high, 80–160 μm diam., pycnidial, scattered, solitary to gregarious, immersed to semi-immersed, uni-loculate, globose to subglobose, glabrous, dark brown to black, visible as small black dot on the host surface, associating with other fungal taxa, ostiole central, occasionally near the centre, minutely mammiform papilla. Conidiomata walls 5–12 μm wide, equally thin-walled, composed of 2–3 cell layers, of broad, brown to dark brown pseudoparenchymatous cells, arranged in a textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3.5–7 × (3–)5–10 μm (\( \bar{x} \) = 6.2 × 6.7 µm, n = 20), enteroblastic, phialidic, discrete, determinate, ampulliform to doliiform, unbranched, aseptate, hyaline, smooth, minute collarette with periclinal wall thickening, arising from the inner cavity of the conidioma wall. Conidia (8.5–)9–12(–14) × 4–5 μm (\( \bar{x} \) = 11 × 4.8 µm, n = 50), yellowish brown, ellipsoidal, 1–3-septate, not constricted at the septa, smooth-walled, lacking mucilaginous sheath surrounding conidia.
Culture characteristics: Colonies on PDA reaching 33–35 mm diam. after 3 weeks at room temperature (20–30 °C). Colony medium dense, circular, flattened to raised, surface slightly rough with hyphal tufts, edge entire, velvety to fluffy; from above, white to white yellowish at the margin, light green to yellowish green at the centre; from below, radiating outwards colony, white to cream at the margin, dark green to black at the middle, orangish brown at the centre; producing yellowish pigment on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead hanging branches of Lonicera maackii, 20 April 2017, R. Phookamsak, KIB033 (KUN-HKAS 102231, holotype), ex-type living culture, KUMCC 18-0155 (KIB033A), KUMCC 18-0156 (KIB033B).
GenBank numbers: ITS = MK356375, LSU = MK356349, SSU = MK356363, TEF1-α = MK359067 (KUMCC 18-0155); ITS = MK356376, LSU = MK356350, SSU = MK356364, TEF1-α = MK359068 (KUMCC 18-0156).
Notes: In the NCBI BLASTn search of ITS sequence, Neosetophoma lonicerae has a closest match with fungal endophyte species (M16-3161) with 100% similarity and is closely related to N. italica W.J. Li et al., N. rosarum R.H. Perera et al., N. samarorum (Desm.) Gruyter et al. and N. rosigena Wanas. et al. with 98% similarity. Multigene phylogenetic analyses based on a combined LSU, SSU, TEF1-α and ITS sequence dataset show that N. lonicerae forms a separate lineage, clustering with N. rosigena, N. samarorum and N. garethjonesii Tibpromma et al. with moderate support (76% ML and 0.98 BYPP; Fig. 29). A comparison of ITS nucleotide base pairs shows that N. lonicerae differs from N. rosigena, N. samarorum and N. garethjonesii in 14/555 bp (2.5%), 15/555 bp (2.7%) and 16/522 bp (3%), respectively. We therefore, introduce N. lonicerae as a new species following the guidelines of Jeewon and Hyde (2016).
Neosetophoma lonicerae is similar to some other Neosetophoma species in having pale brown to brown, oblong to ellipsoidal, or subfusoid, 1–3-septate conidia; such as in N. iranianum Papizadeh et al., N. italica, N. rosae R.H. Perera et al., N. rosarum and N. shoemakeri Senwanna et al. However, they can be distinguished based on their conidial size, host occurrence and phylogenetic distance (Liu et al. 2015a; Karunarathna et al. 2017; Hyde et al. 2018b; Wanasinghe et al. 2018).
Ophiobolus Riess
We follow the latest treatment and updated accounts of Ophiobolus in Phookamsak et al. (2017).
Ophiobolus malleolus S.K. Huang, Bulgakov & K.D. Hyde, sp. nov.
Index Fungorum number: IF554782; Facesoffungi number: FoF04686, Fig. 34
Ophiobolus malleolus (MFLU 15-2230, holotype) a Herbarium label. b Appearance of ascomata on Cirsium arvense stems. c Ascomata on host. d Ascoma in vertical section. e Peridium. f Pseudoparaphyses. g–i Developing stages of the asci. j–m Ascospores (note: m stained in Indian ink). Scale barsc = 500 µm, d = 100 µm, e = 50 µm, f–m = 20 µm
Etymology: The specific name “malleolus” refers to the hammer-shaped at top of the ascospores.
Holotype: MFLU 15-2230.
Saprobic on dead stems of Cirsium arvense (Asteraceae). Sexual morphAscomata 230–270 μm high, 265–310 μm diam., scattered, solitary to gregarious, immersed, eventually erumpent, uni-loculate, globose to subglobose, dark brown to black, papillate. Ostiole central, short papilla, filled with periphyses. Peridium 25–60 μm wide, thick-walled, composed of several cell layers of brown to dark brown cells, paler to hyaline towards the inner layers, outer layer comprising black, coriaceous stratum, arranged in a textura angularis. Hamathecium composed of numerous, 1–3 μm wide, filamentous, septate pseudoparaphyses, anastomosing at the apex, embedded in a gelatinous matrix. Asci 125–155 × 12–15 μm (\( \bar{x} \) = 140 × 12.5 μm, n = 20), 8-spored, bitunicate, cylindric-clavate, subsessile to short pedicellate, with knob-like or truncate pedicel, rounded at the apex, with a distinct ocular chamber. Ascospores 100–112 × 2.5–4.5 μm (\( \bar{x} \) = 107.5 × 3.5 μm, n = 50), fasciculate, in parallel or spiral, initially hyaline, becoming brown at maturity, guttulate, filiform, enlarged at the first cell with hammer-like, tapering towards the end cell, up to 15 septa, curved, slightly constricted at the septa at maturity, smooth-walled, with apical mucilaginous cap. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on PDA, colony reaching 10 mm diam. after 2 weeks at room temperature (20–30 °C). Colony dense, irregular in shape, flattened to umbonate, surface smooth, with edge erose, velvety to floccose; from above, cream; from below, cream to pale yellowish; not producing pigmentation on agar medium.
Material examined: RUSSIA, Rostov region, Krasnosulinsky District, Donskoye forestry, arboretum (47.8547249˚N, 40.2318907˚E), on dead stems of Cirsium arvense (Asteraceae), 28 June 2015, T.S. Bulgakov, T-526 (MFLU 15-2230, holotype), ex-type living culture, MFLUCC 15-1077.
GenBank numbers: ITS = MH399730, LSU = MH399731, SSU = MH399729.
Notes: Multigene phylogenetic analyses reveal a close phylogenetic affinity between Ophiobolus malleolus and O. disseminans (Fig. 29, 68% ML and 1.00 BYPP). Ophiobolus malleolus is distinct from O. disseminans in its ascospores being filiform with an enlarged at the apical cell similar to a hammer-like, with apical mucilaginous cap, and not splitting into two part spores. Ophiobolus disseminans has filiform ascospores with two swollen cells near the centre, lacks an apical mucilaginous cap, and splits into two part spores at the central septum (Phookamsak et al. 2014b, 2017).
Ophiobolus anguillides (Cooke) Sacc. also has filiform ascospores enlarged hammer-like at the apical cell and an apical mucilaginous cap. However, O. anguillides has larger ascomata and ascospores (500–600 μm diam., and 120–130 × 2.5–3 μm; Shoemaker 1976). Ophiobolus anguillides has been reported from many hosts, mainly on Artemesia in Europe and North America (Shoemaker 1976; Farr and Rossman 2018). However, the species has never been reported from Cirsium arvense. Unfortunately, there is no molecular data available for O. anguillides.
Phaeosphaeria I. Miyake
We follow the latest treatment and updated accounts of Phaeosphaeria in Phookamsak et al. (2014b), Hyde et al. (2017) and Tibpromma et al. (2017). More than 200 epithets are listed under Phaeosphaeria in Index Fungorum (2019); however, the phylogenetic affinities of few species have been confirmed based on molecular data. Some species listed under Phaeosphaeria have already been transferred to other related genera in Phaeosphaeriaceae and other related families (Phookamsak et al. 2014b; Ariyawansa et al. 2015a; Tennakoon et al. 2016).
Phaeosphaeria cycadis Wanas., Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF556169; Facesoffungi number: FoF05703, Fig. 35
Etymology: The specific name “cycadis” refers to the host family Cycadaceae, of which the holotype was collected.
Holotype: KUN-HKAS 102235
Associated with leaf spots on Cycas sp. (Cycadaceae). Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata (60–)100–160 μm high, (60–)135–200 μm diam., pycnidial, visible as black dot on host surface, scattered to clustered, solitary to gregarious, semi-immersed to erumpent, uni-loculate, subglobose to ampulliform, or irregular in shape, ostiolate, with minute papilla. Conidiomata walls 10–20(–30) μm wide, composed of 4–5 cell layers, of brown to dark brown pseudoparenchymatous cells, of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells (3.5–)4.5–8 × 4–8(–10) μm (\( \bar{x} \) = 6.4 × 5.7 μm, n = 30), ampulliform to broadly conical, gradually tapering toward the apex, holoblastic, phialidic, hyaline, smooth-walled, with periclinal wall thickening, arising from the inner cavity of the conidioma wall. Conidia (10–)12–14(– 16) × 3–5 μm (\( \bar{x} \) = 12.9 × 3.9 μm, n = 50), pale brown to light yellowish, oblong to ellipsoidal, or subcylindrical, (0–)1–2-septate, truncate to obtuse base, with obtuse apex, not constricted at the septa, smooth-walled.
Culture characteristics: Colonies on PDA reaching 50–52 mm diam. after 3 weeks at room temperature (20–30 °C). Colony dense, irregular in shape, flattened, surface slightly smooth, edge undulate, with entire margin, cottony to floccose, slightly sparse near the margin; from above, white to cream, slightly radiated outwards colony; from below, slightly radiating, cream at the margin, pale brown to yellowish-brown at the centre, sectering with golden brown; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, associated with leaf spots on Cycas sp. (Cycadaceae), 5 April 2017, R. Phookamsak, KIB022 (KUN-HKAS 102235, holotype), ex-type living culture, KUMCC 18-0161 (KIB022A), KUMCC 18-0162 (KIB022B).
GenBank numbers: ITS = MK356378, LSU = MK356352, SSU = MK356366, TEF1-α = MK359069 (KUMCC 18-0161); ITS = MK356379, LSU = MK356353, SSU = MK356367, TEF1-α = MK359070 (KUMCC 18-0162).
Notes: In the NCBI BLASTn search of ITS sequences, Phaeosphaeria cycadis most closely matches P. acaciae Tennakoon et al. with 99% similarity. Multigene phylogenetic analyses based on a combined LSU, SSU, TEF1-α and ITS sequence dataset show that P. cycadis forms a sister lineage with P. acaciae with high support (100% ML and 1.00 BYPP; Fig. 29). The ITS gene could not clarify the novelty of P. cycadis in this study as the species is not significantly different from P. acaciae in a comparison of ITS sequences (5/452 bp). Phookamsak et al. (2014b) mentioned that Phaeosphaeria contains species complexes that cannot be resolved based only on the ITS gene and that a combination of protein coding genes, such as TEF1-α and RPB2, is necessary to clarify species in this genus. Based on morphological characteristics, P. cycadis differs from P. acaciae in having larger conidia (P. cycadis, (10–)12–14(– 16) × 3–5 μm versus 8–12 × 2.4–3.5 μm, P. acaciae; Hyde et al. 2017), with (0–)1–2 conidial septa and is associated with leaf spots on Cycas sp. Phaeosphaeria acaciae has 1–3 conidial septa and occurs on dead stems of Acacia sp. as a saprobe (Hyde et al. 2017). Furthermore, P. acaciae produced a pink pigment on PDA, but this is absent in P. cycadis. We therefore, introduce P. cycadis as a new species in this study based on its morphological distinctiveness.
Wojnowiciella Crous et al.
Wojnowiciella was introduced by Crous et al. (2015b) and is typified by W. eucalypti Crous et al. Crous et al. (2015a) treated Wojnowicia as a synonym of Septoriella Oudem. based on nomenclature study and this is in agreement of Wijayawardene et al. (2017a). Subsequently, all identified Wojnowicia species in Phaeosphaeriaceae were synonymized under Wojnowiciella (Crous et al. 2015b; Hernandez-Restrepo et al. 2016). Wojnowiciella can be distinguished from Wojnowicia Sacc. in having apapillate, glabrous conidiomata and dark brown conidia (Crous et al. 2015b). Wojnowicia was introduced by Saccardo (1892) and is characterized by setose conidiomata (Crous et al. 2015a; Wijayawardene et al. 2016). Seven species are accommodated in this genus (Index Fungorum 2019).
Wojnowiciella kunmingensis Phookamsak, Wanas. & K.D. Hyde, sp. nov.
Index Fungorum number: IF556170; Facesoffungi number: FoF05704, Fig. 36
Etymology: The specific epithet “kunmingensis” refers to Kunming Institute of Botany, Kunming City, Yunnan Province, China, where the holotype was collected.
Holotype: KUN-HKAS 102233
Saprobic on Lonicera maackii. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 110–190 μm high, 110–190 μm diam., pycnidial, scattered, solitary to gregarious, immersed, slightly raised, visible as small black dot on host surface, uni-loculate, globose to subglobose, glabrous, dark brown to black, ostioles central, apapillate, with pore-like opening. Conidiomata walls 5–12 μm wide, thin-walled, of equal thickness, composed of 1–3 cell layers, of flattened, dark brown, pseudoparenchymatous cells, arranged in a textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells (3–)4–6.5(–9) × (2.5–)3.5–6(–8) μm l/w (\( \bar{x} \) = 5 × 5.3 µm, n = 40), holoblastic, phialidic, discrete, determinate, ampulliform to doliiform, unbranched, aseptate, hyaline, smooth, arising from the inner cavity of the conidioma wall. Conidia (16–)18–24(–27.5) × 4–6(–7) μm l/w (\( \bar{x} \) = 22 × 5.6 µm, n = 50), dark brown, subcylindrical to fusiform, or reniform, slightly curved, with acute to rounded apex, and rounded to truncate base, 3–7-septate, not constricted at the septa, thick-walled, smooth-walled, with guttules, having flattened, mucous caps at both ends.
Culture characteristics: Colonies on PDA reaching 35–38 mm diam. after 2 weeks at room temperature (20–30 °C). Colony medium dense, slightly irregular in shape, flattened to slightly raised, surface slightly rough with greyish-green hyphal tufts, edge undulate, with entire margin, floccose; from above, slightly radiating, white to cream at the margin, pale brown at the middle, separated from the margin with brown to dark green concentric ring near the edge, greyish green to dark green at the centre; from below, white to cream margin, brown to dark brown at the centre; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead hanging branches of Lonicera maackii, 20 April 2017, R. Phookamsak, KIB031 (KUN-HKAS 102233, holotype), ex-type living culture, KUMCC 18-0159 (KIB031A), KUMCC 18-0160 (KIB031B).
GenBank numbers: ITS = MK356380, LSU = MK356354, SSU = MK356368, TEF1-α = MK359071 (KUMCC 18-0159); ITS = MK356381, LSU = MK356355, SSU = MK356369, TEF1-α = MK359072, RPB2 = MK359078 (KUMCC 18-0160).
Notes: In the NCBI BLASTn search of ITS sequences, Wojnowiciella kunmingensis most closely matches W. dactylidis (Wijayaw. et al.) Hern.-Restr. & Crous, W. spartii (W.J. Li et al.) Hern.-Restr. & Crous and Wojnowicia italica Qing Tian et al. with 99% similarity. ITS and TEF1-α genes could not resolve the novelty of the Wojnowiciella species in this study. A comparison of ITS and TEF1-α nucleotide bases shows that Wojnowiciella kunmingensis is not significantly different from W. dactylidis and other Wojnowiciella species as well as Wojnowicia italica. However, a comparison of RPB2 sequence data shows that Wojnowiciella kunmingensis differs from W. dactylidis and Wojnowicia italica in 21/856 bp (2.4%) and 20/954 bp (2.1%), respectively.
Multigene phylogenetic analyses based on a combined LSU, SSU, TEF1-α and ITS sequence data show that Wojnowiciella kunmingensis forms a sister lineage with W. dactylidis and clusters with other Wojnowiciella species (Fig. 29). However, W. kunmingensis differs from W. dactylidis in having 3–7-septate conidia, while W. dactylidis has 7–11-septate conidia (Liu et al. 2015a). Wojnowiciella leptocarpi Crous et al. also has 3–7-septate conidia, with mucous caps at both ends. However, W. kunmingensis has longer conidia ((16–)18–24(–27.5) × 4–6(–7) μm versus 26–36 × 4–6 µm; Hernandez-Restrepo et al. 2016), that are brown to dark brown, and smooth-walled. The conidia of W. leptocarpi are orange brown, thick-walled, and verruculose (Hernandez-Restrepo et al. 2016). A comparison of RPB2 sequence data shows that W. kunmingensis differs from W. leptocarpi in 29/851 bp (3.4%). Wojnowiciella lonicerae (Wijayaw. et al.) Hern.-Restr. & Crous was also collected from Lonicera, but it has 8–11-septate conidia (Liu et al. 2015a).
Wojnowiciella rosicola (W.J. Li et al.) Wanas., Phookamsak & K.D. Hyde, comb. nov.
Index Fungorum number: IF556171; Facesoffungi number: FoF05705
Basionym: Wojnowicia rosicola W.J. Li, Camporesi & K.D. Hyde, in Wanasinghe et al., Fungal Divers.: https://doi.org/10.1007/s13225-018-0395-7, [144] (2018)
Holotype: ITALY, Arezzo [AR], Montemezzano, on dead aerial branch of Rosa sp. (Rosaceae), 25 August 2014, E. Camporesi, IT 2200 (MFLU 17-2785); ex-type living culture, MFLUCC 15-0128.
Morphological description: See Wanasinghe et al. (2018) (Fig. 93, pp. 144–147).
Notes: Wojnowiciella rosicola was introduced by Wanasinghe et al. (2018) as Wojnowicia rosicola W.J. Li et al. The species clustered with other Wojnowiciella species in Phaeosphaeriaceae that were previously treated in Wojnowicia and recently treated as a synonym of Wojnowiciella by Crous et al. (2015a, b) and Hernandez-Restrepo et al. (2016). Based on morphological characteristics and phylogenetic analyses, the species is congeneric with Wojnowiciella. We therefore, treat W. rosicola as a new combination.
Pseudoastrosphaeriellaceae Phookamsak & K.D. Hyde
Pseudoastrosphaeriellaceae was introduced by Phookamsak et al. (2015b) to accommodate a monotypic genus Pseudoastrosphaeriella. This family is characterized by hemispherical to lenticular ascostromata, globose to subglobose ascomata with a flattened or rounded base, immersed beneath host epidermis, erumpent through host surface by a papilla, with short to long necks, trabeculate pseudoparaphyses, short pedicellate, cylindric-clavate to clavate asci, and hyaline or brown, septate ascospores and coelomycetous asexual morphs (Phookamsak et al. 2015b). Based on close morphological characteristics and phylogenetic support, Hyde et al. (2017) accommodated Carinispora K.D. Hyde in Pseudoastrosphaeriellaceae and this was followed by Wijayawardene et al. (2018a). In this study, we introduce a new genus Pseudoastrosphaeriellopsis typified by P. kaveriana in Pseudoastrosphaeriellaceae based on molecular phylogeny coupled with morphological characteristics.
Pseudoastrosphaeriellopsis Devadatha, Wanas., Jeewon & V.V. Sarma, gen. nov.
Index Fungorum number: IF555790; Facesoffungi number: FoF05706
Etymology: Generic epithet in resemblance to Pseudoastrosphaeriella
Saprobic on decaying stems and twigs of Avicennia marina and Suaeda monoica, black spots, with short necks on host surface. Sexual morphAscomata light brown, solitary to gregarious, immersed to erumpent, globose to subglobose with a rounded base, uni-loculate, coriaceous, brown, short papillate, ostiolate. Ostioles central, cylindrical, straight to oblique, with ostiolar canal lined by hyaline periphyses. Peridium thin- to thick-walled, unequally thickened, composed of two layers, inner stratum with hyaline to pale brown compressed cells of textura angularis, outer stratum with compact brown polygonal cells of textura angularis, fused with the host tissue. Hamathecium composed of numerous, filamentous, septate, branched, hyaline pseudoparaphyses. Asci 8-spored, bitunicate, cylindric-clavate to clavate, with a short, thick pedicel, apically rounded and thickened with an ocular chamber. Ascospores overlapping 1–2-seriate, brown, fusiform to broadly fusiform, slightly curved with round to acute ends, slightly constricted at the 3rd septum and rarely at other septa, broader in the middle and tapering towards the ends, septate, with hyaline apical and terminal ends, smooth-walled, lacking guttules and mucilaginous sheath. Asexual morph Undetermined.
Type species: Pseudoastrosphaeriellopsis kaveriana Devadatha, Wanas., Jeewon & V.V. Sarma
Notes: The genus Pseudoastrosphaeriella Phookamsak et al. was introduced by Phookamsak et al. (2015b) to accommodate P. aequatoriensis (K.D. Hyde & J. Fröhl.) Phookamsak & K.D. Hyde, P. africana (D. Hawksw.) Phookamsak & K.D. Hyde, P. bambusae Phookamsak & K.D. Hyde, P. longicolla Phookamsak & K.D. Hyde, P. papillata (K.D. Hyde & J. Fröhl.) Phookamsak & K.D. Hyde and P. thailandensis Phookamsak et al. based on morphology and multigene phylogenetic analyses. Some of the species were previously treated in Astrosphaeriella Syd. & P. Syd. Maximum likelihood tree (Fig. 37) generated based on a combined LSU, SSU, ITS, RPB2 and TEF1-α dataset reveals that our new taxon clusters within Pseudoastrosphaeriellaceae sister to Pseudoastrosphaeriella with 63% ML and 0.95 BYPP statistical support. Tree topologies generated under ML and Bayesian criteria from combined datasets were congruent, whereas maximum parsimonious tree was varied, which showed that our taxon forms a clade between Testudinaceae Arx and Tetraplosphaeriaceae Kaz. Tanaka & K. Hiray. without any statistical support. Further comparison of LSU nucleotides between our taxon and Pseudoastrosphaeriella thailandensis (GenBank: KT955478) resulted in 62/871 (7.1%) base pair differences which confirms its placement as a new genus in Pseudoastrosphaeriellaceae.
Phylogram generated from maximum likelihood analysis based on LSU, SSU, ITS, RPB2 and TEF1-α sequence dataset of representative families in Pleosporales showing phylogenetic affinities of Pseudoastrosphaeriellopsis kaveriana. Gloniopsis calami (MFLUCC 15-0739) and Hysterium rhizophorae (NFCCI-4250) were selected as the outgroup taxa. Bootstrap support values for maximum likelihood (green), equal to or greater than 60% and Bayesian posterior probabilities (purple) equal to or greater than 0.95 are given above each branch, respectively. The new isolate is in blue. Type strains are in bold
Pseudoastrosphaeriellopsis shares morphological similarity with Pseudoastrosphaeriella in having immersed ascomata underneath the host epidermis, erumpent, uni-loculate, coriaceous, brown, and short papillate asci, with trabeculate pseudoparaphyses (Phookamsak et al. 2015b). However, Pseudoastrosphaeriellopsis can be distinguished from Pseudoastrosphaeriella in having globose to subglobose ascomata, immersed in host tissue, with fusiform to broadly fusiform ascospores.
Pseudoastrosphaeriellopsis kaveriana resembles Neotrematosphaeria biappendiculata (Kaz. Tanaka et al.) Thambug. et al., but differs in having immersed ascomata, with pore-like ostiole, and ascospores lacking appendages, whereas, the latter has semi-immersed ascomata, with crest-like ostiole, and ascospores with appendages at both ends (Thambugala et al. 2015). Biappendiculispora japonica Thambug. et al. differs from Pseudoastrosphaeriellopsis kaveriana in having slit-like ostioles, bulbous pedicels and 7–9 transverse septa ascospores with appendages (Thambugala et al. 2015). Pseudoastrosphaeriellopsis kaveriana also shares similarities with Trematosphaeria wegeliniana L. Holm & K. Holm, T. hydrophila Sacc. and T. crassisepta Kaz. Tanaka et al. in having 5-septate ascospores and overlapping ascospore dimensions, but is clearly distinct in having immersed ascomata beneath the host epidermis, short papilla and light brown, fusiform to broadly fusiform, slightly curved ascospores (Tanaka et al. 2015). Trematosphaeria wegeliniana and T. hydrophila lack a detailed description from type material and their taxonomic position remains unclear (Tanaka et al. 2015).
Thambugala et al. (2015) transferred Trematosphaeria terricola G.S. Gong to Alpestrisphaeria terricola (G.S. Gong) Thambug. & K.D. Hyde and Trematosphaeria biappendiculata (KTC 1124) to the new genus Neotrematosphaeria biappendiculata based on their morphological resemblance to Lophiostomataceae and multigene phylogeny. Our combined multiloci phylogenetic analysis also reveals that taxonomic position of some species in Trematosphaeria remains unclear and this needs further collection and revision.
Pseudoastrosphaeriellopsis kaveriana Devadatha, Wanas., Jeewon & V.V. Sarma, sp. nov.
Index Fungorum number: IF555791; Facesoffungi number: FoF05707, Fig. 38
Pseudoastrosphaeriellopsis kaveriana (AMH-9912, holotype). a Ascomata semi-immersed and released ascospores on the decaying wood of Avicennia marina.b, c Longitudinal sections of ascoma. d Ostiole. e Section of peridium comprising hyaline to pale brown cells of textura angularis. g Cellular pseudoparaphyses. h–k Immature and mature asci. f, l–o Ascospores. p Germ tubes developed from terminal ends of ascospore. Scale barsb = 100 μm, c, d = 50 μm, e–p = 10 μm
Etymology: Specific epithet in reference to the river Kaveri.
Holotype: AMH-9912
Saprobic on decaying stems and twigs of Avicennia marina and Suaeda monoica, black spots ascomata, with short necks on host surface. Sexual morphAscomata 225–335 µm high, 220–345 µm diam., immersed to erumpent, globose to subglobose, solitary to gregarious, coriaceous, brown, short papillate, ostiolate. Ostioles 110–165 µm long, 50–90 µm diam., central, cylindrical, straight to oblique, with ostiolar canal lined by hyaline periphyses. Peridium 15–25 µm wide, less developed at the base compared to the sides and top, composed of two layers, inner stratum with 3–5 layers of hyaline to pale brown compressed cells of textura angularis, outer stratum with compact brown polygonal cells of textura angularis, fused with the host cells and fungal tissue. Hamathecium composed of numerous, 1–3 µm wide, filamentous, septate, branched, hyaline pseudoparaphyses, embedded in a gelatinous matrix, anastomosing between and above the asci. Asci 85–145 × 15–18 µm (\( \bar{x} \) = 108 × 17 µm, n = 40), 8-spored, bitunicate, cylindric-clavate to clavate, with a short, thick pedicel, apically rounded and thickened, with an ocular chamber. Ascospores 25–40 × 5–10 µm (\( \bar{x} \) = 34 × 7 µm, n = 50), overlapping 1–2-seriate, brown, fusiform to broadly fusiform, slightly curved with round to acute ends, broader in the middle and tapering towards the ends, 5-septate, slightly constricted at the 3rd septum and rarely at other septa, smooth-walled, hyaline at both ends, from the central septum upper half is shorter and lower half is longer, lacking guttules and mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinated on SWA within 24 h, germ tubes arising from terminal ends of the ascospore. Colonies on MEA reaching 30–45 mm diam. after 25 days of incubation at room temperature, pale olive buff to olive buff, reverse brown, velvety, surface raised, irregular, with light yellow exudates.
Material examined: INDIA, Tamil Nadu, Tiruvarur, Muthupet mangroves (10.4°N 79.5°E), on decaying stems and twigs of Avicennia marina (Forssk.) Vierh. (Acanthaceae), 28 November 2015, B. Devadatha, AMH-9912 (holotype); isotype at Pondicherry University, Puducherry), ex-type living culture, NFCCI-4221; ibid. on stems and twigs of Suaeda monoica Forssk. ex J.F. Gmel), 28 November 2015, B. Devadatha, PUHD33.
GenBank numbers: ITS = MG947599, LSU = MG947595, SSU = MG947598, TEF1-α = MG968955, RPB2 = MG948547.
Roussoellaceae Jian K. Liu et al.
We follow the latest treatment and updated accounts of Roussoellaceae in Liu et al. (2014), Dai et al. (2017a), Tibpromma et al. (2017), Hyde et al. (2018b), Jayasiri et al. (2019) and Jiang et al. (2019). Phylogenetic affinities of the family were discussed by Jaklitsch and Voglmayr (2016) where Roussoellaceae was treated as a synonym of Thyridariaceae. However, Tibpromma et al. (2017) reinstated Roussoellaceae based on multigene phylogenetic analysis which showed Roussoellaceae is a well-resolved family in Pleosporales and this was concurred by Wanasinghe et al. (2018), Wijayawardene et al. (2018a), Jayasiri et al. (2019) and Jiang et al. (2019). Seven genera were accepted in this family based on molecular data and morphological characteristics (Wijayawardene et al. 2018a).
Taxonomic status of genera in Roussoellaceae is doubtful and needs to be clarified due to taxa in these genera have similar conidial characters and phylogeny has always shown that they are monophyletic (Liu et al. 2014; Dai et al. 2017a; Tibpromma et al. 2017; Hyde et al. 2018b; Jiang et al. 2019). However, Neoroussoella Phookamsak et al. and Roussoellopsis I. Hino & Katum. are distinct from Roussoella but these two genera usually cluster with Roussoella species (Liu et al. 2014; Phookamsak et al. 2014a; Dai et al. 2017a; Tibpromma et al. 2017; Hyde et al. 2018b; Jiang et al. 2019). Wanasinghe et al. (2018) introduced Pseudoneoconiothyrium (Phukhams. et al.) Phukhams. et al. and Pararoussoella to accommodate roussoella-like taxa in Thyridariaceae. We use increased taxon sampling in our phylogenetic analysis (Fig. 39) and show that the two genera erected by Wanasinghe et al. (2018), cluster with Roussoella species in Roussoellaceae.
Maximum likelihood phylogenetic tree based on a combined LSU, SSU, ITS, TEF1-α and RPB2 sequence dataset. The tree is rooted to Torula herbarum (CBS 111855). Bootstrap support values for ML (left) equal to or greater than 60% and the Bayesian posterior probabilities (right), equal to or greater than 0.95 BYPP are indicated above the nodes. Ex-type strains are in bold and the newly generated sequences are indicated in blue
Jayasiri et al. (2019) clarified Neoroussoella based on multigene phylogenetic analysis coupled with morphological characteristics, N. entadae Jayasiri et al. and N. leucaenae Jayasiri et al. were introduced. Roussoella solani Crous & M.J. Wingf. was transferred to Neoroussoella as N. solani (Crous & M.J. Wingf.) Jayasiri & K.D. Hyde. In addition, Roussoella mukdahanensis Phookamsak et al. was transferred to Pararoussoella as P. mukdahanensis (Phookamsak et al.) Jayasiri & K.D. Hyde. In this study, Neoroussoella heveae Senwanna et al. and Roussoella elaeicola Konta & K.D. Hyde are introduced as novel species based on morphological characteristics coupled with multigene phylogenetic analysis. In addition, Neoroussoella leucaenae is described on Hevea brasiliensis Müll.Arg. from Thailand for the first time.
Neoroussoella J.K. Liu et al.
We follow the latest treatment and updated accounts of Neoroussoella in Jayasiri et al. (2019).
Neoroussoella heveae Senwanna, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF555287; Facesoffungi number: FoF04825, Fig. 40
Neoroussoella heveae (MFLU 17-1983, holotype). a Conidiomata immersed in host substrate. b Section through the conidioma. c Conidioma wall. d–f Conidiogenous cells. g, h Colony on MEA (g = from above, h = from below). i Conidia. j Germinated conidium. Scale barsa = 200 µm, b = 100 µm, c = 20 µm, d–j = 5 µm
Etymology: Name refers to the host genus Hevea, from which the fungus was collected.
Holotype: MFLU 17-1983
Saprobic on Para rubber (Hevea brasiliensis). Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 90–130 μm high, 115–180 μm diam., pycnidial, immersed, visible as small, brown to black dots on the host surface, solitary, scattered, globose, uni-loculate, glabrous, ostiole not observed. Conidiomata walls 7–15 µm wide, thick-walled, composed of several cell layers of dark brown to black, pseudoparenchymatous cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–7 × 2–5 μm (\( \bar{x} \) = 5.2 × 3.4 μm, n = 15), lining the inner cavity, difficult to distinguish from pycnidial wall, enteroblastic, phialidic, integrated, simple, short, hyaline, ampulliform to doliiform, thin-walled, smooth. Conidia 2.5–5 × 2–4 µm (\( \bar{x} \) = 4 × 3, n = 50), globose or subglobose to ellipsoidal, initially hyaline, becoming brown to dark brown, aseptate, with one or two guttules, rough-walled, verruculose.
Culture characteristics: Colonies on MEA reaching 20–25 mm diam. after 2 weeks at 25–30 °C, colony from above, circular, medium dense, edge entire, velvety to woolly, white at the margin, white to yellowish in the middle, geenish grey to grey at the centre; from below, white at the margin, greenish grey at the centre, with concentric rings radiate.
Material examined: THAILAND, Phayao Province, Muang District, Mae Ka, on twig of Hevea brasiliensis (Euphorbiaceae), 5 December 2016, C. Senwanna, RBPY018 (MFLU 17-1983, holotype), ex-type living culture, MFLUCC 17-0338.
GenBank numbers: ITS = MH590693, LSU = MH590689, SSU = MH590691.
Notes: Neoroussoella heveae is introduced based on morphological and phylogenetic evidence. Our strain forms a separate lineage, clusters with Neoroussoella species (78% ML; Fig. 39). Neoroussoella heveae differs from the asexual morph of Neoroussoella species in having verruculose conidia, whereas, other Neoroussoella species have smooth-walled conidia.
Neoroussoella leucaenae Jayasiri, E.B.G. Jones & K.D. Hyde, Mycosphere 10(1): 1–186 (2019), Fig. 41
Holotype: THAILAND, Krabi Province, Mueang Krabi District, on decaying pod carpel of Leucaena sp. (Fabaceae), 31 August 2017, S.C. Jayasiri, C 356 (MFLU 18-2159; MFLU 18-2160, isotype), ex-type living culture MFLUCC 18-1544, KUMCC 18-0266.
Saprobic on dead twigs of Hevea brasiliensis. Sexual morphAscomata 130–195 µm high, 150–170 µm diam., visible as raised, aggregated, small, dark brown to black dots on the host surface, immersed, erumpent through host epidermis by minute papilla, scattered, solitary, sometimes gregarious, globose to subglobose, occasionally irregular in shape, uni-loculate, glabrous, ostiole central with minute papilla. Peridium (7–)10–18 µm, composed of several layers, of hyaline to dark brown pseudoparenchymatous cells, inner layer comprising flattened, hyaline to brown cells, arranged in a textura prismatica to textura angularis, outer layer comprising thick, dark brown to black cells, arranged in a textura angularis. Hamathecium composed of dense, 1–3 µm wide, hyaline, septate, filamentous, anastomosed pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci (35–)42–68(–75) × 4–5(–6) µm (\( \bar{x} \) = 53 × 5.5, n = 15), 8-spored, bitunicate, cylindrical, with a short pedicel, apically rounded, with an indistinct ocular chamber. Ascospores 7–10(– 15) × 3–4.5(– 6.5) µm (\( \bar{x} \) = 9.4 × 4.4, n = 40), overlapping 1-seriate, hyaline when young and medium to dark brown when mature, ellipsoidal to ovoid, with rounded ends, 1-septate, constricted at the septum, rough-walled, with two guttules, and longitudinal striations. Asexual morph See Jayasiri et al. (2019).
Culture characteristics: Colonies on PDA reaching 15–25 mm diam. after 3 weeks at 25–30 °C, circular, medium dense, edge entire, velvety; from above, light grey at the margin, white to light grey at the centre; from below, white at the margin, light to dark brown at the centre.
Material examined: THAILAND, Phayao Province, Muang District, Mae Ka, on dead twigs of Hevea brasiliensis, 3 January 2017, C. Senwanna, RBPY028 (MFLU 17-1985), living culture, MFLUCC 17-0346.
Known hosts and distribution: On decaying pod carpel of Leucaena sp. and Pterocarpus sp., Hevea brasiliensis (Thailand) (Jayasiri et al. 2019; this study).
GenBank numbers: ITS = MH590694, LSU = MH590690, SSU = MH590692, TEF1-α = MH590688.
Notes: Neoroussoella leucaenae (MFLU 17-1985) is found on Para rubber in Thailand for the first time. Phylogenetically our strain (MFLUCC 17-0346; Fig. 39) clusters with the type strain of N. leucaenae (MFLUCC 18-1544) and strain MFLUCC 17-0927 with moderate support (76% ML and 1.00 BYPP; Fig. 39). There is different only one and three nucleotide base positions in ITS and TEF1-α among N. leucaenae strains MFLUCC 18-1544, MFLUCC 17-0927 and MFLUCC 17-0346, which confirms that they are conspecific.
Roussoella Sacc.
We follow the latest treatment and updated accounts of Roussoella in Liu et al. (2014), Tibpromma et al. (2017), Hyde et al. (2018b) and Jiang et al. (2019).
Roussoella elaeicola Konta & K.D. Hyde, sp. nov.
Index Fungorum number: IF555291; Facesoffungi number: FoF04834, Fig. 42
Roussoella elaeicola (MFLU 15-0022, holotype). a, b Ascomata on host substrate. c Section of ascoma. d Peridium. e Pseudoparaphyses. f–h Asci. i–l Immature ascospores. m, n Mature ascospores. o, p Ascospores with poroid ornamentation. q Germinated ascospore. r Culture characteristic on MEA from above and below. Scale barsa, b = 1000 μm, c = 100 μm, d = 50 μm, e–h, q = 20 μm, i–p = 5 μm
Etymology: Name reflects the host genus Elaeis.
Holotype: MFLU 15-0022
Saprobic on dead petiole of Elaeis guineensis. Sexual morphAscomata 315–410 µm high, 325–350 μm diam., solitary, immersed in host tissue, erumpent through the host surface with minute papilla, globose to subglobose, uni-loculate, ostiole central with papilla, lacking periphyses. Peridium 25–70 µm wide, thin-walled, composed of several layers of small, compressed, hyaline to light brown pseudoparenchymatous cells of textura angularis, outer layers fusing with the host. Hamathecium composed of dense, 0.9–2.8 μm broad, filamentous, septate, trabeculate pseudoparaphyses, embedded in mucilage. Asci 70–140 × 6–9 μm (\( \bar{x} \) = 100 × 8 µm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, pedicellate, rounded apex with an indistinct ocular chamber. Ascospores 10–15 × 3–6 μm (\( \bar{x} \) = 12 × 4 µm, n = 30), slightly overlapping 1-seriate, hyaline to light brown when immature, ellipsoid with obtuse ends, becoming dark brown at maturity, 1-septate, with large guttules in each cell, constricted at the septum, rough-walled with poroid ornamentation, surrounded by mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinated on MEA within 24 h with germ tube was produced from both cells. Colony on MEA, at first whitish, felty, azonate, with fluffy margin, after incubation for 3–4 weeks appear circular, with fluffy, dense, pale brown mycelium in the middle and dense mycelium in the outer ring on the surface with smooth margin; from below, brown to dark brown in the middle and pale brown to yellow at the margin hyphae, septate, branched, and smooth.
Material examined: THAILAND, Chiang Rai Province, on dead petiole of Elaeis guineensis (Arecaceae), 25 November 2014, S. Konta, HR02d (MFLU 15-0022, holotype), ex-type living culture, MFLUCC 15-0276.
GenBank numbers: ITS = MH742329, LSU = MH742326 (MFLUCC 15-0276a); ITS = MH742330, LSU = MH742327 (MFLUCC 15-0276b); LSU = MH742328, SSU = MH742331 (MFLUCC 15-0276c).
Notes: Roussoella elaeicola (MFLU 15-0022) is introduced as a novel species and it was collected from oil palm (Elaeis guineensis) in Chiang Rai, Thailand for the first time. Multigene phylogenetic analyses show that R. elaeicola has a close relationship with R. euonymi Crous & Akulov and Pseudoneoconiothyrium rosae Phukhams et al. (Fig. 39) which were collected from Euonymus europaeus L. (Celastraceae) in Ukraine and on dead aerial spines of Rosa canina L. (Rosaceae) in Italy, respectively (Crous et al. 2018; Wanasinghe et al. 2018). However, we could not compare the morphological characteristics of our new species with these two species as Roussoella euonymi and Pseudoneoconiothyrium rosae are only known as asexual morph species, while our new taxon is represented by the sexual morph. One of the distinctive characters of Roussoella elaeicola is the distinctive trabeculate pseudoparaphyses (sensu Liew et al. 2000). A comparison of ITS nucleotide base pairs indicates that Roussoella elaeicola differs from R. euonymi and Pseudoneoconiothyrium rosae in 37/513 bp (7.2%) and 38/514 bp (7.4%). Roussoella elaeicola can be distinguished from other Roussoella species by its ascospores having poroid ornamentation, similar to R. scabrispora (Liu et al. 2014). However, R. elaeicola and R. scabrispora form a distinct lineage in Roussoellaceae.
Sulcatisporaceae Kaz. Tanaka & K. Hiray.
Sulcatisporaceae was introduced by Tanaka et al. (2015) to accommodate three genera: Magnicamarosporium Kaz. Tanaka & K. Hiray., Neobambusicola Crous & M.J. Wingf. and Sulcatispora Kaz. Tanaka & K. Hiray. and is typified by Sulcatispora. The family is characterized by globose to subglobose, ostiolate, papillate ascomata, a thick-walled peridium with a poorly developed base, and trabeculate, anastomosed pseudoparaphyses, bitunicate, clavate asci, and hyaline, broadly fusiform, 1-septate ascospores with an entire sheath. The asexual morphs are coelomycetous, with ellipsoid to subglobose, hyaline to dark brown, muriform or phragmosporous conidia (Crous et al. 2014b; Tanaka et al. 2015; Phukhamsakda et al. 2017). Five species from three genera are accommodated in this family. We introduce a new species, Neobambusicola brunnea (MFLU 18-1393) based on a phylogenetic analysis of a combined LSU and ITS sequence dataset (Fig. 43).
Phylogram generated from RAxML analysis based on a combined LSU and ITS sequence dataset. Tree is rooted with Camarosporium aloes (CPC 21572) and C. quaternatum (CBS 483.95). Bootstrap values ≥ 50% are indicated at the nodes. The new species is indicated in blue. Ex-type strains are indicated in bold
Neobambusicola Crous & M.J. Wingf.
Neobambusicola was introduced as a monotypic genus by Crous et al. (2014b) and is typified by N. strelitziae Crous & M.J. Wingf., isolated from leaves of Strelitzia nicolai in South Africa. The genus was represented by its asexual morph and is characterized by erumpent, globose, dark brown, ostiolate conidiomata, a thin-walled of peridium, subcylindrical to ampulliform, phialidic conidiogenous cells and hyaline to olivaceous, fusoid-ellipsoid, smooth-walled, 1-septate conidia with hyaline, smooth, guttulate to granular, aseptate, subglobose to subcylindrical microconidia (Crous et al. 2014b). Crous et al. (2014b) treated the genus in Bambusicolaceae. Tanaka et al. (2015) introduced a new family Sulcatisporaceae to accommodate Neobambusicola, Magnicamarosporium and Sulcatispora. Only Neobambusicola strelitziae was accommodated in Neobambusicola, but we introduce a second species N. brunnea which is represented by the sexual morph.
Neobambusicola brunnea Y. Chen & Norphanphoun, sp. nov.
Index Fungorum number: IF555293; Facesoffungi number: FoF05708, Fig. 44
Etymology: The specific epithet “brunnea” refers to the brownish ascospores.
Holotype: MFLU 18-1393
Saprobic on dead stem of herbage. Sexual morphAscomata 80–205 μm high, 90–260 μm diam., semi-immersed, blackish, irregular in shape, scattered on surface of stem, uni-loculate, glabrous, ostiolate, apapillate. Ostioles 35.5–68 μm diam., dark, circular and sunken at the apex of ascoma. Peridium 30–40 μm thick, two layered, thick-walled, outer layer irregular, comprising dark brown cells of textura angularis and inner layer slightly, irregular of light brown cells. Hamathecium comprising 1–2 μm wide, filamentous, branched or simple, septate, anastomosed, cellular pseudoparaphyses, embedded in a hyaline gelatinous matrix. Asci 70–90 × 8–10 μm (\( \bar{x} \) = 83.6 × 9 μm, n = 10), 4- or 8-spored, bitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with an ocular chamber. Ascospores 11–18 × 5–6 μm (\( \bar{x} \) = 12.9 × 5.8 μm, n = 20), 1-seriate, brown to dark brown, oblong to ellipsoidal, with rounded ends, 1-septate, slightly constricted at the septum, smooth-walled, with small guttules. Asexual morph Undetermined.
Material examined: CHINA, Guizhou Province, Qiandongnan Miao and Dong Autonomous Prefecture, Huangping District, on dead stem of herbage, 10 September 2017, Y. Chen, QDN001 (MFLU 18-1393, holotype).
GenBank numbers: ITS = MH644792, LSU = MH644791.
Notes: DNA was extracted directly from the ascomata, and a phylogenetic analysis of combined ITS and LSU sequence dataset shows that Neobambusicola brunnea is closely related to N. strelitziae with moderate support (87% ML; Fig. 43). A comparison of ITS and LSU pair wise shows that N. brunnea differs from N. strelitziae in 31 and 40 base positions, respectively. Therefore, N. brunnea is introduced as a novel species and this is the first report of the sexual morph of Neobambusicola.
Thyridariaceae Q. Tian & K.D. Hyde
Thyridariaceae was introduced by Hyde et al. (2013) to accommodate the genus Thyridaria Sacc. and is typified by T. broussonetiae (Sacc.) Traverso. The familial concept was solitary or gregarious, immersed to erumpent, globose, coriaceous ascomata, in valsoid configurations, with stromatic, pigmented, prosenchymatous tissues and ostioles with a disc-like ostiolar canal. Asci are fissitunicate, cylindrical to subclavate, pedicellate, with trabeculate pseudoparaphyses and ascospores are pigmented, ellipsoidal to fusiform and verruculose, with transverse eusepta or distosepta, and form coelomycetous Cyclothyrium asexual morphs (Hyde et al. 2013; Jaklitsch and Voglmayr 2016). Jaklitsch and Voglmayr (2016) excluded Cyclothyrium from Thyridariaceae and the genus was tentative placed in Pleosporales, genera incertae sedis (Wijayawardene et al. 2018a). Furthermore, they also synonymized Roussoellaceae under Thyridariaceae and accepted Neoroussoella, Thyridaria, Roussoella, Roussoellopsis and Parathyridaria Jaklitsch & Voglmayr. However, Tibpromma et al. (2017) reinstated Roussoellaceae and this was followed by subsequent authors (Hyde et al. 2018b; Wanasinghe et al. 2018; Wijayawardene et al. 2018a; Jayasiri et al. 2019; Jiang et al. 2019). Recently, Wanasinghe et al. (2018) introduced three new genera in Thyridariaceae viz. Cycasicola Wanas et al., Pseudoneoconiothyrium and Pararoussoella Wanas et al. However, in the present study Pseudoneoconiothyrium and Pararoussoella cluster with other Roussoella species in Roussoellaceae. Devadatha et al. (2018) also introduced a new genus Thyridariella Devadatha et al. in Thyridariaceae. Thyridariella clustered with Cycasicola in our study (Fig. 39). However, these two genera are represented by different morphs and we therefore, treat them as different genera until a link between these two genera is proven. In this study, we introduce a novel genus Liua to accommodate a novel species Liua muriformis in Thyridariaceae based on morphological distinctiveness and phylogenetic support.
Liua Phookamsak & K.D. Hyde, gen. nov.
Index Fungorum number: IF556175; Facesoffungi number: FoF05709
Etymology: In honour of Jian-Kui Liu, for his excellent work on taxonomic revision of Dothideomycetes.
Saprobic on Lonicera maackii. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata pycnidial, scattered, solitary, immersed, slightly raised, visible as small black dot on host surface, uni-loculate, globose to subglobose, glabrous, dark brown to black, ostioles central, apapillate, with pore-like opening. Conidiomata walls thin-walled, of equal thickness, composed of 3–5 cell layers, of flattened, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells holoblastic, phialidic, discrete, determinate, ampulliform to cylindrical, unbranched, aseptate, occasionally 1–2-septate, hyaline, smooth, arising from the inner cavity of the conidioma wall. Conidia phragmosporous to muriform, dark brown, oblong to ellipsoidal, or obovoid, (2–)3-transversely septate, with (0–)3 longitudinal septa, slightly constricted at the septa, smooth-walled.
Type species: Liua muriformis Phookamsak, H.B. Jiang & K.D. Hyde
Notes: In the NCBI BLASTn search of ITS sequences, Liua muriformis most closely matches with leaf litter ascomycete (strain its072) with 95% similarity and Cycasicola goaensis Wanas. et al. (MFLU 17-0581) with 93% similarity. Multigene phylogenetic analyses based on a combined LSU, SSU, ITS, TEF1-α and RPB2 sequence dataset show that L. muriformis forms a sister clade with C. goaensis and C. leucaenae Jayasiri et al. with high support (100% ML and 1.00 BYPP) and clusters with other two Thyridariella species in Thyridariaceae. Liua muriformis differs from C. goaensis and C. leucaenae in having dark brown, muriform or phragmosporous conidia, whereas, C. goaensis and C. leucaenae have pale yellowish, aseptate conidia (Wanasinghe et al. 2018; Jayasiri et al. 2019). Therefore, we introduce Liua as a new genus to accommodate L. muriformis based on its morphological distinct with Cycasicola.
Liua muriformis Phookamsak, H.B. Jiang & K.D. Hyde, sp. nov.
Index Fungorum number: IF556176; Facesoffungi number: FoF05710, Fig. 45
Liua muriformis (KUN-HKAS 102241, holotype). a Appearance of conidiomata on host. b Section through conidioma. c Section through conidioma wall. d–g Conidiogenous cells. h–l Conidia. m Germinated conidium. n, o Culture characteristics on PDA (n = from above, o = from below). Scale barsa = 200 μm, b = 50 μm, c = 20 μm, l, m = 10 μm, d–k = 5 μm
Etymology: The specific epithet “muriformis” refers to the holotype having muriform ascospores
Holotype: KUN-HKAS 102241
Saprobic on Lonicera maackii. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 80–150 μm high, 110–190 μm diam., pycnidial, scattered, solitary, immersed, slightly raised, visible as small black dot on host surface, uni-loculate, globose to subglobose, glabrous, dark brown to black, ostioles central, apapillate, with pore-like opening. Conidiomata walls 7–17 μm wide, thin-walled, of equal thickness, composed of 3–5 cell layers, of flattened, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis to textura prismatica. Conidiophores reduced to conidiogenous cells. Conidiogenous cells (3–)5–12.5(–17.5) × (2–)5–10 μm l/w (\( \bar{x} \) = 8.2 × 5.4 µm, n = 30), holoblastic, phialidic, discrete, determinate, ampulliform to cylindrical, unbranched, aseptate, occasionally 1–2-septate, hyaline, smooth, arising from the inner cavity of the conidioma wall. Conidia (12.5–)13–15(–17) × 7–9(–10) μm l/w (\( \bar{x} \) = 14.4 × 8.2 µm, n = 50), phragmosporous to muriform, dark brown, oblong to ellipsoidal, or obovoid, (2–)3-transversely septate, with (0–)3 longitudinal septa, slightly constricted at the septa, smooth-walled.
Culture characteristics: Colonies on PDA, reaching 30–32 mm diam. after 3 weeks at room temperature (20–30 °C). Colony dense, circular, flattened to slightly raised, surface rough, radially furrowed at the centre, smooth at the margin, with edge entire, velvety; from above, greyish green at the margin, pale yellowish to yellowish green at the centre; from below, dark green to greenish grey; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead hanging branches of Lonicera maackii, 20 April 2017, R. Phookamsak, KIB032 (KUN-HKAS 102241, holotype), ex-type living culture, KUMCC 18-0177.
GenBank numbers: ITS = MK433599, LSU = MK433598, SSU = MK433595, TEF1-α = MK426798, RPB2 = MK426799 (KUMCC 18-0177); ITS = MK433600, LSU = MK433592 (KUN-HKAS 102241, KIB0032F).
Dothideomycetes, orders incertae sedis
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Asterinaceae Hansf.
Asterinaceae was introduced by Hansford (1946) to accommodate obligate biotrophic, or epiphytic fungi which formed web-like, black colonies on the upper and lower surfaces of leaves, or stems, with or without appressoria of external mycelium, superficial, dimidiate ascomata with radiating star-like openings, fissitunicate asci, cylindrical, 2–6-celled, yellowish to brown ascospores and forming both coelomycetous and hyphomycetous asexual morphs (Hyde et al. 2013; Hongsanan et al. 2014, 2016; Guatimosim et al. 2015). Twenty-one genera are accepted in the family (Wijayawardene et al. 2018a). Updated molecular phylogeny and their evolutionary relationships based on molecular clock evidence were provided by Hongsanan et al. (2016) and Liu et al. (2017a).
Lembosia Lév.
The genus Lembosia was described by Léveillé (1845), based on the type species, L. tenella Lév. The genus is characterized by having lirelliform or V–Y-shaped ascomata, opening by a longitudinal fissure, absence of hypostroma, adhering to the host by superficial hyphae with lateral appressoria (hyphopodia), bitunicate asci disposed as an upright palisade layer, and 2-celled brownish ascospores (Hongsanan et al. 2014). More than 260 epithets are listed under Lembosia in Index Fungorum (2019). However, few species have molecular data. In this manuscript we introduce the new species Lembosia brigadeirensis (Fig. 46).
Phylogenetic tree was obtained by Bayesian inference methods using the sequences of the LSU region. The posterior probability values are indicated at the nodes. Strain numbers are indicated after species names. New sequence is in blue bold. The analyses included 30 strains including representative genera of Asterinales sensu stricto and Asterinales sensu lato. The tree is rooted with Venturia populina (CBS 256.38) and V. inaequalis (CBS 815.69)
Lembosia brigadeirensis Firmino, A.R. Machado & O.L. Pereira, sp. nov.
MycoBank number: MB822355; Facesoffungi number: FoF04108, Fig. 47
Lembosia brigadeirensis (VIC 44208, holotype). a Colony with open hysterothecia and surface mycelium. b Cross section of the ascomata. c Globose to lobate unicelular appressoria. d Parallel bitunicate asci. e Immature hyaline ascospores. f Brown and smooth ascospores. Scale barsa = 200 µm, b–f = 10 µm
Etymology: Name derived from the mountain range where the fungus was collected, Serra do Brigadeiro.
Holotype: VIC 44208
Epiphyllous on Epidendrum sp. Sexual morphColonies 4–6 mm diam., amphigenous, circular to irregular, single to confluent, dark brown, black. Hyphae 4–5 μm diam., straight to flexuous, irregularly branched, brown, septate, hyphal cells cylindrical, smooth. Appressoria 5.5–7.5 × 6–8 μm, few, entire to lobate, sessile, lateral, alternate to unilateral, never opposed, globose, unicellular, straight, brown, penetration peg central on the appressorial cell. Ascomata 530–1180 × 140–230 μm, superficial, hysterothecia, lirelliform, V–Y-shaped, mostly linear, on the top of mycelial mat, single to confluent, fringed at margins, massed in the centre of the colony, opening by longitudinal fissures, dark brown to black, wall of textura radiate to irregulata, cells isodiametric to cylindrical. Pseudoparaphyses up to 2.5 μm wide, cylindrical, filiform, septate, unbranched, hyaline. Asci 30–47.5 × 15–22 μm, 8-spored, bitunicate, fissitunicate, subclavate to cylindrical, disposed as an upright palisade layer. Ascospores 17.5–19.5 × 5.5–8 μm, cylindrical to oblong-clavate, ends rounded, straight or slightly arched, 1-septate, constricted at the median septum, hyaline, becoming brown at maturity, smooth-walled. Asexual morph Undetermined.
Material examined: BRAZIL, Minas Gerais, Araponga, Parque Estadual da Serra do Brigadeiro, on leaves of Epidendrum sp. (Orchidaceae), 10 September 2014, A.L. Firmino (VIC 44208, holotype).
GenBank numbers: ITS = MF667946, LSU = MF664531.
Notes: Lembosia brigadeirensis is a distinct species when compared with many other Lembosia species reported on Orchidaceae (Léveillé 1845; Horne 1905; Sydow 1939; Silva and Pereira 2008; Hosagoudar et al. 2009; Firmino and Pereira 2014). Lembosia brigadeirensis is most similar to L. sertiferae Syd., which has epiphyllous colonies, lobate appressoria, ellipsoid to oblong asci, and fusiform to oblong ascospores (Firmino and Pereira 2014). Lembosia bezerrae Firmino & O.L. Pereira has epiphyllous colonies, smaller appressoria, saccate to ovoid asci, and fusiform and smaller ascospores than L. brigadeirensis (Firmino and Pereira 2014). Lembosia epidendri Meir. Silva & O.L. Pereira has narrow hyphae, wider appressoria, smaller hysterothecia, saccate to ovoid asci, branched pseudoparaphyses, and larger and fusiform ascospores (Silva and Pereira 2008; Firmino and Pereira 2014). Lembosia dendrochili Lév. differs from L. brigadeirensis in having smaller hysterothecia, and larger asci (Léveillé 1845; Firmino and Pereira 2014). Lembosia rolfsii W.T. Horne differs from L. brigadeirensis in the subcuticular mycelium and conidia on superficial mycelium, and probably belonging to Maheshwaramyces (Hosagoudar et al. 2009; Firmino and Pereira 2014).
Based on LSU sequence data, Lembosia brigadeirensis is 97% similarity to Prillieuxina baccharidincola (Rehm) Petr. (GenBank no. KP143735), 95% similarity to Asterina melastomatis Lév. (GenBank no. KP143739) and 94% similarity to Alysidiella suttonii Cheew. & Crous (GenBank no. HM628777). Based on ITS sequence data, L. brigadeirensis is 88% similarity to Blastacervulus eucalypti H.J. Swart (GenBank no. GQ303271), Alysidiella suttonii (GenBank no. HM628774), and Heteroconium kleinzeense Crous & Z.A. Pretorius (GenBank no. EF110616). Phylogenetic analysis of LSU sequence dataset (Fig. 46) shows that Lembosia brigadeirensis forms a sister lineage with Prillieuxina baccharidincola (VIC42817) with high support (100% BYPP). Lembosia differs from Prillieuxina G. Arnaud and Asterina Lév. in having elliptical to cylindrical ascomata with longitudinal fissure. Alysidiella Crous and Blastacervulus H.J. Swart are asexual morph genera belonging to the same family as Lembosia (Asterinaceae), but with no known connection to a sexual morph. Heteroconium Petr. is also an asexual morph, but belonging to Herpotrichiellaceae (Chaetothyriales).
Botryosphaeriales Schoch et al.
Botryosphaeriaceae Theiss. & P. Syd.
Botryosphaeriaceae can be found as endophytes, saprobes and plant pathogens on various substrates worldwide (Liu et al. 2012b; Slippers et al. 2013; Dissanayake et al. 2016; Phillips et al. 2018; Jayawardena et al. 2019). The family comprises 28 genera and more than 190 species (Phillips et al. 2018; Tibpromma et al. 2018; Wijayawardene et al. 2018a). We follow the latest treatments and updated accounts in Dissanayake et al. (2016), Hyde et al. (2016), Yang et al. (2017), Phillips et al. (2018), Wanasinghe et al. (2018) and Jayawardena et al. (2019). Phylogenetic analyses based on a combined ITS, LSU, TEF1-α and TUB2 sequence dataset are provided for the genera Dothiorella (Fig. 48) and Sphaeropsis (Fig. 51).
Phylogenetic tree generated from maximum likelihood (RAxML) based on a combined ITS and TEF1-α. Maximum likelihood bootstrap value ≥ 70% and Bayesian posterior probabilities ≥ 0.95 BYPP are given at the nodes. The ex-type strains are in bold. The newly generated sequences are in blue. The tree is rooted with Neofusicoccum parvum (CMW9081)
Dothiorella Sacc.
We follow the latest treatment and updated accounts of Dothiorella in Yang et al. (2017). Updated phylogenetic analysis was retrieved from Dissanayake et al. (2017) and Wanasinghe et al. (2018).
Dothiorella acericola Phookamsak, Tennakoon & K.D. Hyde, sp. nov.
Index Fungorum number: IF556178; Facesoffungi number: FoF05711, Fig. 49
Dothiorella acericola (KUN-HKAS 102213, holotype). a Appearance of conidiomata on host. b, c Section through conidiomata. d Section through conidioma wall. e–g Conidiogenous cells (g = stained with congo red). h–j Conidia. k Germinated conidium. l Culture characteristics on PDA from above and below. Scale barsb, c = 200 μm, d = 50 μm, h = 20 μm, e–g, i–k = 10 μm
Etymology: The specific epithet “acericola” refers to the host genus Acer, on which the holotype was collected.
Holotype: KUN-HKAS 102213
Saprobic on dried twigs of Acer palmatum. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 220–360 µm high, 190–310 µm diam., pycnidial, aggregated, clustered, semi-immersed to erumpent, dark brown to black, globose to subglobose, uni- to bi-loculate, ostiole central, with minute papilla. Conidiomata walls 15–40 µm wide, composed of several layers of broad to flattened, dark brown to black, pseudoparenchymatous cells of textura angularis to textura prismatica, with flattened, hyaline cells towards the inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells (7–)9–15(–24) × 3–6(–7) μm l/w (\( \bar{x} \) = 13.2 × 4.5 µm, n = 30), holoblastic, phialidic, discrete, determinate, ampulliform to cylindrical, unbranched, aseptate, hyaline, smooth, arising from the inner cavity of the conidioma wall. Conidia 17–22(–23) × 7–10(–13) μm l/w (\( \bar{x} \) = 20.8 × 9.2 µm, n = 50), dark brown, oblong to ellipsoidal, 1-septate, slightly constricted at the septum, smooth-walled.
Culture characteristics: Colonies on PDA reaching 70–73 mm diam. after 1 week at 20–30 °C; initially medium sparse to dense, circular, or slightly irregular in shape, surface smooth, with edge entire to lobate; from above, initially white, becoming white-grey to grey; from below, grey to pale yellowish; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kunming, Panlong, Ciba, on dead hanging twigs of Acer palmatum Thunb. (Sapindaceae), 28 November 2015, R. Phookamsak, COE009 (KUN-HKAS 102213, holotype), ex-type living culture KUMCC 18-0137.
GenBank numbers: ITS = MK359449, TEF1-α = MK361182.
Notes: In the NCBI BLASTn search of ITS and TEF1-α sequences, Dothiorella acericola is most similar to D. viticola A.J.L. Phillips & J. Luque, with 99% and 98% similarities, respectively. Phylogenetic analyses of a combined ITS and TEF1-α sequence dataset show that D. acericola is sister to Spencermartinsia alpina Y. Zhang ter & Ming Zhang and distinct from D. viticola (Fig. 48). Dothiorella acericola, Spencermartinsia alpina and S. yunnana Zhang ter & Ming Zhang were collected from Yunnan, China, but they are phylogenetically distant (Zhang et al. 2016). Dothiorella acericola has the same size range of conidia as Spencermartinsia alpina and S. yunnana (Zhang et al. 2016). A comparison of TEF1-α nucleotide bases shows that D. acericola differs from Spencermartinsia alpina and S. yunnana in 11/225 bp (4.9%) and 13/225 bp (5.8%), respectively. Therefore, we introduce a new species, D. acericola in this study based on the guidelines of Jeewon and Hyde (2016). Yang et al. (2017) treated Spencermartinsia as a synonym of Dothiorella. Spencermartinsia alpina and S. yunnana should perhaps be transferred to the genus Dothiorella.
Dothiorella sarmentorum (Fr.) A.J.L. Phillips, A. Alves & J. Luque, Mycologia 97(2): 522 (2005)
Facesoffungi number: FoF04836, Fig. 50
Saprobic on a wide range of hosts. Sexual morph Undetermined. Asexual morphConidiomata 300–440 μm high, 215–300 μm diam., stromatic, solitary or scattered in small groups, immersed, uni-loculate, individual or aggregated, black, with globose to subglobose, ostiole. Conidiomata walls comprising several layers; outer layers thick-walled, dark brown cells of textura angularis; inner layers of thin-walled, lightly pigmented or hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells lining inner the conidioma cavity, holoblastic, hyaline, subcylindrical, proliferating at the same level giving rise to periclinal thickenings. Conidia 18–23 × 8–10 μm (\( \bar{x} \) = 21 × 9.4 μm, n = 30), ovoid, with a broadly rounded apex and truncate base, initially hyaline to lightly pigmented and aseptate, becoming dark brown, 1-septate, slightly constricted at the septum, smooth-walled.
Material examined: RUSSIA, Rostov region, Shakhty City, Alexandrovsky Park, on dead twigs of Platycladus orientalis (L.) Franco (Cupressaceae), 14 March 2016, T.S. Bulgakov (MFLU 16-1627).
Known hosts: Acer platanoides L., Aesculus hippocastanum L., Armeniaca vulgaris Lam., Cedrus atlantica (Endl.) Manetti ex Carrière, Chamaecyparis lawsoniana (A. Murray) Parl., Cornus sanguinea L., Coronilla emerus L., Crataegus sp., Cryptomeria japonica (L.f.) D.Don, Cupressus lusitanica Mill., Eriobotrya japonica (Thunb.) Lindl., Euonymus europaeus L., Forsythia europaea Degen & Bald., Malus pumila Miller, Menispermum canadense L., Paliurus spina-christi Mill., Persica vulgaris Mill., Pistacia spp., Populus nigra L., Prunus spp., Pyrus communis L., Quercus spp., Salix sp., Thuja spp., Ulmus spp., Vitis spp. (Farr and Rossman 2018).
Known distribution: Iran, Italy, Netherlands, New Zealand, Norway, Poland, Portugal, Serbia, Spain, Sweden, UK (Great Britain), Ukraine, the USA (California, Florida, Oregon, Washington) (Farr and Rossman 2018).
GenBank numbers: ITS = MH571673, TEF1-α = MH628155.
Notes: Dothiorella sarmentorum was introduced by Phillips et al. (2005). This species is a cosmopolitan distribution including many economical important trees (Phillips et al. 2005, 2013; Dissanayake et al. 2017). We isolated D. sarmentorum from Platycladus orientalis (Cupressaceae) for the first time (Farr and Rossman 2018). The morphological characters such as conidia shape, size and colour are similar to the type as described. However, we could not obtain a living culture from the isolated spores. Therefore, the morphology of the species is based only on characters on the host. Phylogenetic analyses of a combined ITS and TEF1-α sequence dataset (Fig. 48) show that our isolate (MFLU 16-1627) clusters with the type strain of D. sarmentorum (IMI63581b) and strain CBS 115038.
Sphaeropsis Sacc.
Sphaeropsis was introduced by Saccardo (1880b) to accommodate diplodia-like taxa and is typified by S. visci (Alb. & Schwein.) Sacc. with Phaeobotryosphaeria sexual morph (Phillips et al. 2008, 2013; Dissanayake et al. 2016; Wijayawardene et al. 2017a). Sphaeropsis has a cosmopolitan distribution on various hosts (Farr and Rossman 2018). The genus is characterized by pseudothecial, brown to black, uni-loculate ascomata, thick endotunica, bitunicate asci, with cellular pseudoparaphyses, brown, aseptate ascomata and asexual morph forms with stromatic conidiomata, with paraphyses and oval, oblong or clavate, aseptate conidia (Phillips et al. 2013). More than 600 species are listed under Sphaeropsis in Index Fungorum (2019). However, Phillips et al. (2013) re-circumscribed the genus and only five species were accepted based on morphological characteristics of the sexual and asexual morph connections and phylogenetic evidence (Phillips et al. 2008, 2013; Doilom et al. 2015, 2017; Dissanayake et al. 2016; Wijayawardene et al. 2017a). In this study, we report a new host record of S. eucalypticola from Bauhinia purpurea (L.) Benth. in Thailand.
Sphaeropsis eucalypticola A.J.L. Phillips, in Phillips et al., Stud Mycol 76: 158 (2013)
Facesoffungi number: FoF00169, Fig. 52
Holotype: THAILAND, Chiang Rai Province, Muang District, on dead twig of Eucalyptus sp., 8 August 2011, M. Doilom, MFLU 12-0753.
Saprobic on dead twigs. Sexual morphAscostromata 250–350 μm high, 170–250 μm diam. (ascostromata with papilla, not including subiculum or hypostroma), black, convex on host tissue, appearing through cracks in bark, scattered or clustered in small to large groups on a subiculum or hypostroma, 185–260 μm high at the base, aggregated, initially immersed, becoming erumpent, when cut horizontally locules visible as white contents and dark ascospore dots, uni-loculate or multi-loculate, globose to subglobose or flask-shaped. Papilla 60–95 μm long, 65–85 μm diam., ostiole with periphyses. Peridium 35–80 μm wide, thick-walled, composed of several layers of dark brown to black, coriaceous cells of textura angularis. Hamathecium comprising 2.5–4 μm wide, hyphae-like, hyaline, numerous, septate pseudoparaphyses, constricted at the septa. Asci 102–175 × 22–32 μm (\( \bar{x} \) = 130 × 27 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindric-clavate or clavate, with a short or long pedicel, apically rounded with an ocular chamber. Ascospores 27–33 × 11–14 μm (\( \bar{x} \) = 30 × 13 μm, n = 20), overlapping 2-seriate, hyaline when young, becoming pale brown or reddish brown when mature, ellipsoidal to ovoid, aseptate, sometime 2-septate, broader in the centre, with an apiculus at both ends, thick-walled, echinulate. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on PDA after 5–10 h. Germ tubes produced from germ pore of ascospores. Colonies on PDA, reaching the edge of the Petri dish after 5 days, flat or effuse, undulate, initially white, after 3 days becoming brownish grey to olive.
Material examined: THAILAND, Chiang Rai Province, Muang District, Tha Sud Subdistrict, Mae Fah Luang University campus grounds, on dead twigs of Bauhinia purpurea L. (Leguminosae), 14 March 2012, M. Doilom, MKBB031 (MFLU 18-1857), living culture, MFLUCC 12-0171.
Known hosts and distribution: Eucalyptus sp., Bauhinia purpurea, Tectona grandis L.f. (Thailand) (Liu et al. 2012b; Phillips et al. 2013; Doilom et al. 2017).
GenBank numbers: ITS = MK108956, TEF1-α = MK108958, TUB2 = MK108957.
Notes: A new isolate of Sphaeropsis eucalypticola (MFLUCC 12–0171) was collected from dead twigs of Bauhinia purpurea in Thailand. This new isolate shares a close phylogenetic affinity to the type of S. eucalypticola (100% ML, 100% MP and 1.00 BYPP) in our combined phylogeny of ITS, TEF1-α and TUB2 sequence data (Fig. 51). Sphaeropsis eucalypticola has been reported from Eucalyptus sp. and Tectona grandis in Thailand (Liu et al. 2012b; Doilom et al. 2017), but it has not been previously reported from Bauhinia purpurea (Fig. 52).
Phylogram generated from parsimonious tree based on combined ITS, TEF1-α and TUB2 sequence dataset. The tree is rooted to Barriopsis tectonae (MFLUCC 12-0381) and B. fusca (CBS 174.26). Maximum parsimony and maximum likelihood bootstrap values ≥ 70% and Bayesian posterior probabilities ≥ 0.95 are given at the nodes. The ex-type strains are in bold. The newly generated sequence is in blue bold
Sphaeropsis eucalypticola (MFLU 18-1857). a Ascostromata on dead twig of Bauhinia purpurea. b Ascostromata cut through horizontally showing the white contents with dark spots corresponding to asci with ascospores. c–e Vertical section of ascostromata. f Immature and mature asci with immature and mature ascospores. g–i Immature asci. j, k Asci with ascospores. l, m Immature ascospores. n, o Ascospores. p Germinated ascospore. q, r Colony on PDA after 2 months (q = above view, r = below view). Notesh, i, l stained in lactophenol cotton blue. Scale barsc, e, f = 100 µm. d = 50 µm. g, l–p = 10 µm. h–k = 20 µm
Microthyriales G. Arnaud
Microthyriales, genera incertae sedis
Parazalerion Madrid, Gené & Cano, gen. nov.
MycoBank number: MB824747; Facesoffungi number: FoF04480
Etymology: The name reflects the superficial morphological similarity between this genus and species of Zalerion sensu lato
Saprobic in soil. Sexual morph Undetermined. Asexual morph Hyphomycetous. Vegetative hyphae septate, branched, subhyaline to light olivaceous. Conidiophores reduced to conidiogenous cells. Conidiogenous cells subcylindrical to narrowly clavate, smooth-walled, light olivaceous. Conidia produced terminally or laterally on undifferentiated hyphae, or on short conidiophores. Conidial filament curved, sinuous or irregularly coiled, multi-septate, light olivaceous brown to mid brown, slightly to strongly constricted at the septa; groups of conidia often appear compactly intertwined, forming irregularly shaped masses of cells.
Type species: Parazalerion indica Madrid, Gené & Cano
Notes: Parazalerion is introduced as a novel monotypic conidial genus of Ascomycota. Morphologically, it resembles the marine genus Zalerion R.T. Moore & Meyers (Sordariomycetes, genera incertae sedis) in the production of irregularly coiled, dematiaceous, multi-septate conidia which often form knots of cells (Ellis 1976; Goos 1985; Campbell et al. 2005). The new genus, however, was found in a terrestrial habitat and is phylogenetically related to Spirosphaera minuta Hennebert (Fig. 53), which belongs in Dothideomycetes, relatively close to Microthyriaceae (Voglmayr et al. 2011). Furthermore, knots of cells originate from a single conidial filament in Zalerion, whereas in Parazalerion they originate from the intertwining of groups of conidia (Fig. 54). This particular development of knots of cells also distinguish Parazalerion from other similar genera, including Cirrenalia Meyers & R.T. Moore, Cumulospora I. Schmidt, Glomerulispora Abdel-Wahab & Nagah., Halazoon Abdel-Aziz et al., Hiogispora Abdel-Wahab & Nagah., Hydea K.L. Pang & E.B.G. Jones, Matsusporium E.B.G. Jones & K.L. Pang, Moheitospora Abdel-Wahab et al., Moleospora Abdel-Wahab et al. and Moromyces Abdel-Wahab et al. (Ellis 1971; Abdel-Wahab et al. 2010). The LSU-based phylogenetic tree demonstrates that Parazalerion is phylogenetically clearly distinct from those hyphomycete genera (Fig. 53).
Maximum likelihood tree based on partial LSU region, showing the phylogenetic relationships of Parazalerion and other ascomycetes including morphologically similar hyphomycetous genera. Bootstrap support values > 70% are shown near the internodes. The tree is rooted to Fibulochlamys chilensis (Agaricales). The original isolate numbers and GenBank accession numbers of LSU sequences are noted after the species names. Ex-type strains are indicated in bold
The closest match in the BLASTn search with ITS + LSU sequences of Parazalerion indica strain CBS 125443 is Spirosphaera minuta strain CBS 476.66 (GenBank no. HQ696659, 92% similarity, 92% query coverage). In our study, Spirosphaera minuta and Parazalerion indica appeared as sister taxa in the LSU-based phylogenetic tree (Fig. 53) and this relationship received 97% bootstrap support. Since the type species of Spirosphaera, S. floriformis Beverw., is a phylogenetically distant species belonging to Helotiales (Voglmayr et al. 2011), Spirosphaera minuta apparently needs to be reallocated to a different genus. Its definitive phylogenetic position deserves further study, but the fungus is also clearly different from Parazalerion. It occurs on submerged plant material and produces more or less globose conidia formed by a complexly coiled hyaline, branching filament (Hennebert 1968). Spirosphaera is a polyphyletic genus that needs to be revised. Another member of this genus, S. cupreorufescens Voglmayr is a member of Pleosporales (Voglmayr et al. 2011) and also needs to be segregated from Spirosphaera sensu stricto.
Parazalerion indica Madrid, Gené & Cano, sp. nov.
MycoBank number: MB824748; Facesoffungi number: FoF04481, Fig. 54
Etymology: Refers to the country where this fungus was collected, India.
Holotype: IMI 397928
Saprobic in soil. Sexual morph Undetermined. Asexual morph Hyphomycetous. Vegetative hyphae 1–1.5 µm wide, septate, branched, subhyaline to light olivaceous, smooth- and thin-walled. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2.5–5 × 1.5–2 µm, subcylindrical to narrowly clavate, smooth-walled, light olivaceous. Conidia produced terminally or laterally on undifferentiated hyphae, or on short conidiophores. Conidial filament curved, sinuous or irregularly coiled, with 1–3(–6) usually thick and dark septa, light olivaceous brown to mid brown, thick-walled, smooth to verruculose, slightly to strongly constricted at the septa, 8–19(–26) µm long, 4–5.5 µm wide at the widest part, with an obtuse apex; groups of conidia often appearing tightly intertwined, forming compact, irregularly shaped, light olivaceous brown to dark brown, 8–23 µm wide masses of cells.
Culture characteristics: Colonies reaching 21 mm. diam. on OA and 25 mm. diam. on PCA and PDA after 14 days at 25 °C. On OA light grey and floccose at the centre, light brown to dark brown and almost glabrous towards the periphery, with a fimbriate margin; reverse dark brown at the centre, light brown towards the periphery. On PCA white to cream, funiculose at the centre, almost glabrous towards the periphery, with a fimbriate margin; reverse concolorous with obverse. On PDA velvety to floccose, cream to dark brown, with a fimbriate margin; reverse concolorous with obverse. No diffusible pigments observed in any of the tested media.
Material examined: INDIA, Delhi, isolated from soil, 2 February 1997, H.C. Gugnani (IMI 397928, holotype), ex-type living cultures CBS 125443, FMR 9690.
GenBank numbers: ITS + LSU = MH100803.
Muyocopronales Mapook et al.
Muyocopronaceae K.D. Hyde
Muyocopronaceae was invalidly introduced by Luttrell (1951) [as ‘Myiocopronaceae’], and is typified by Muyocopron Speg. with M. corrientinum Speg. as the type species. The genus was introduced to accommodate epiphytic fungi, characterized by black, superficial, dimidiate-scutate, subcarbonaceous, ostiolate ascomata, without mycelium and bitunicate, ovoid to obclavate asci, containing subglobose to ellipsoidal, hyaline ascospores (Mapook et al. 2016; Tibpromma et al. 2016). Muyocopron has long historical discussion of its taxonomic placement (Saccardo 1883a; von Arx and Müller 1954, 1975; Eriksson and Hawksworth 1993; Lumbsch and Huhndorf 2007; 2010), until Hyde et al. (2013) re-defined the family Muyocopronaceae to accommodate Muyocopron. Mapook et al. (2016) introduced the order Muyocopronales to accommodate this family based on molecular data coupled with morphological characteristics. Only Muyocopron is accommodated in the family with more than 60 epithets listed (Hyde et al. 2013; Mapook et al. 2016; Tibpromma et al. 2016; Wijayawardene et al. 2018a; Index Fungorum 2019). In this study, a new host and geographical records of Muyocopron lithocarpi Mapook et al. is reported (Figs. 55, 56).
RAxML tree based on a combined LSU and SSU sequence dataset. Bootstrap support values for ML equal to or greater than 60% and Bayesian posterior probabilities equal to or greater than 0.95 BYPP are defined as ML/BYPP above the nodes. The tree is rooted to Lichenothelia convexa (L1607). Newly generated sequence is in blue and ex-type strains are in bold
Muyocopron Speg.
We follow the latest treatment and updated accounts of Muyocopron in Mapook et al. (2016) and Tibpromma et al. (2016).
Muyocopron lithocarpi Mapook, Boonmee & K.D. Hyde, in Mapook et al., Phytotaxa 265(3): 235 (2016), Fig. 56
Holotype: THAILAND, Chiang Rai Province, on fallen leaves of Lithocarpus lucidus (Fagaceae), 30 September 2014, A. Mapook (MFLU 15-1133), ex-type living culture MFLUCC 14-1106.
Saprobic on dead stems of herbaceous plant. Sexual morphAscostromata dry, black, circular, raised to umbonate on the host surface, without a subiculum, easily removed from the host, clustered, gregarious, or in groups of 2–3 locules, ostiolate. Ascomata 55–110 µm high, 175–380 µm diam., clustered, gregarious or in groups, superficial, black, with a central irregular ostiole. Peridium 10–27 µm wide, slightly thick-walled of unequal thickness, poorly developed at the base, slightly thick at the sides towards the apex, comprising two types of cell layers; outer layer composed of black carbonaceous, brittle cells, inner layer composed of hyaline to brown, pseudoparenchymatous cells of textura angularis to textura prismatica. Hamathecium comprising numerous, 2–3 µm wide, filiform, septate, anastomosed pseudoparaphyses. Asci (41–)47–78(–85) × (18–)21–28(–29) µm (\( \bar{x} \) = 59.6 × 24.5 µm, n = 45), 8-spored, bitunicate, ovoid to obclavate, or ampulliform, short pedicellate, apically rounded, apex thick with small ocular chamber. Ascospores (12–)14–19(–20) × (7–)8–10(–12) µm (\( \bar{x} \) = 17.2 × 9.8 µm, n = 60), overlapping 1–3-seriate, hyaline, subglobose to obovoid, with obtuse ends, 1-celled, rough-walled with small granules, and 1–3 large guttules. Asexual morph Undetermined.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Jinghong, Nabanhe, on dead stems of herbaceous plant, 21 November 2015, R. Phookamsak, XB016 (KUN-HKAS 102243).
Known hosts and distribution: Lithocarpus lucidus, Peltophorum sp. (Thailand); Cercis chinensis (Guizhou, China) (Mapook et al. 2016; Jayasiri et al. 2019).
GenBank numbers: LSU = MK447738, SSU = MK447740.
Notes: In molecular phylogenetic analysis our isolate clusters with Muyocopron lithocarpi Mapook et al. (Fig. 55). The morphology of our isolate is similar to M. lithocarpi described by Mapook et al. (2016), although our isolate has larger asci than in the original description (41–85 × 18–29 µm versus 45–65 × 15–28 µm) (Mapook et al. 2016). The species was collected from herbaceous plant in Yunnan, China for the first time.
Tubeufiales Boonmee & K.D. Hyde
Tubeufiaceae M.E. Barr
We follow the latest treatment and updated accounts of Tubeufiaceae in Brahmanage et al. (2017), Chaiwan et al. (2017), Liu et al. (2018), Lu et al. (2018a, b), Phookamsak et al. (2018), Tibpromma et al. (2018) and Jayasiri et al. (2019). There are 27 genera accommodated in this family based on molecular data coupled with morphological characteristics (Lu et al. 2018b; Tibpromma et al. 2018; Wijayawardene et al. 2018a; Jayasiri et al. 2019). In this study, a new species Pseudohelicomyces menglunicus is introduced from a rotten seed coat in Yunnan, China (Fig. 57).
Phylogram generated from the best scoring of the RAxML tree based on a combined ITS, LSU, TEF1-α and RPB2 sequence dataset of Pseudohelicomyces and related genus Helicomyces in Tubeufiaceae. Tubeufia tectonae (MFLUCC 17-1985) was selected as the outgroup taxon. Bootstrap support values for maximum likelihood (left) equal to or greater than 60% and the Bayesian posterior probabilities (right) equal or higher than 0.90 BYPP are indicated above the nodes. Ex-type and ex-epitype strains are in bold. Newly generated sequences are indicated in blue
Pseudohelicomyces Y.Z. Lu et al.
We follow the latest treatment and updated accounts of Pseudohelicomyces in Lu et al. (2018b) and Jayasiri et al. (2019).
Pseudohelicomyces menglunicus J.F. Li, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF555770; Facesoffungi number: FoF05763, Fig. 58
Pseudohelicomyces menglunicus (KUN-HKAS 85793, holotype). a, b Colonies on rotten seed coat. c Conidiophores with conidiogenous cells. d, e Conidiophores. f Conidiophores bearing conidia. g Conidiogenous cells. h–r Conidia. s Germinated conidium. Scale barsa = 0.5 cm, b, d, e = 100 µm, f, s = 50 µm, c, g–r = 20 µm
Etymology: The specific epithet “menglunicus” refers to the Menglun Town, Xishuangbanna, Yunnan, China, where the holotype was collected.
Holotype: KUN-HKAS 85795
Saprobic on seed coat. Sexual morph Undetermined. Asexual morphMycelium immersed on the substrate, composed of septate, branched, smooth, thin-walled, subhyaline to dark brown hyphae. Conidiophores (106–)130–220 × 9–13(–13.5) µm, macronematous or micronematous, paler towards the apex, hyaline to light brown, thin-walled, smooth, septate, branched, straight or flexuous, cylindrical, tapering towards the apex. Conidiogenous cells (20–) 23.5–32(–36) × (4–)4.5–5.5(–6) µm (\( \bar{x} \) = 4.5 × 5.3 µm, n = 10), holoblastic, integrated, intercalary and sometimes terminal, determinate or sympodial, occasionally small and discrete, denticulate, denticles cylindrical, often narrow, hyaline, smooth. Conidia (18–)20–33(–34) × (21.5–)25–30(–40) µm (\( \bar{x} \) = 22.5 × 27.2 µm, n = 20), pleurogenous or acropleurogenous, solitary, simple, subhyaline to paler yellowish brown, septate, slightly constricted at the septa, planate to cochleate, smooth, thin-walled, hygroscopic.
Culture characteristics: Conidia germinating on PDA within 14 h and germ tubes produced from all cells. Colonies growing on PDA, hairy, brown to dark brown, reaching 5 mm in 15 days at 23 °C, mycelium partly superficial, partly immersed, slightly effuse, radially striate, with irregular edge, subhyaline to dark brown; conidia sporulating within 15 days on PDA.
Material examined: CHINA, Yunnan Province, Xishuangbanna, on unidentified seed, 15 August 2014, J.F. Li, H-26 (KUN-HKAS 85795, holotype), ex-type living culture, MFLUCC14-0689.
GenBank numbers: ITS = MK335914, SSU = MK335915, TEF1-α = MK335916.
Notes: Pseudohelicomyces menglunicus resembles species of Helicosporium Nees and Neohelicosporium Y.Z. Lu et al. in morphological characters, but is obviously unique in conidiophores and conidia. Pseudohelicomyces menglunicus differs from Helicosporium and Neohelicosporium species in having flexuose, branched and hyphae-like conidiophores, and smooth-walled conidia. In the phylogenetic analysis (Fig. 57), P. menglunicus forms a separated lineage, sister to P. aquaticus Y.Z. Lu et al. with moderate support (76% ML and 0.97 BYPP). Jayasiri et al. (2019) introduced a new sexual morph species, P. quercus Jayasiri et al. on fruit of Quercus sp. from Thailand. The species also formed a clade with P. aquaticus and P. menglunicus (pre-analysis, data not shown). A comparison of ITS and TEF1-α nucleotide bases shows that P. menglunicus differs from P. quercus in 37/569 bp (6.5%) of ITS and 28/912 bp (3.1%) of TEF1-α. Hence, P. menglunicus is introduced as a new asexual morph species.
Class Eurotiomycetes O.E. Erikss. & Winka
We follow the latest treatment and updated account of Eurotiomycetes in Gueidan et al. (2014) and Geiser et al. (2015). The outline and notes of the genera in Eurotiomycetes was provided by Wijayawardene et al. (2017a, 2018a).
Subclass Chaetothyriomycetidae Doweld
Chaetothyriales M.E. Barr
Cyphellophoraceae Réblová & Unter.
Cyphellophoraceae was introduced by Réblová et al. (2013) to accommodate a monotypic genus Cyphellophora G.A. de Vries and is typified by C. laciniata G.A. de Vries which was isolated from human skin in Switzerland. Taxa have been reported as pathogens or endophytes on plants, or as soil borne, as well as infection on humans and animals (i.e., nails and skin) (Réblová et al. 2013; Feng et al. 2014; Gao et al. 2015; Yang et al. 2018a). Twenty-six epithets are listed in Index Fungorum (2019) with 23 possible species are accepted in this genus.
Cyphellophora G.A. de Vries
Most Cyphellophora species have been reported in their asexual morph. However, Yang et al. (2018a) reported the sexual morph of Cyphellophora on living leaves of Alnus nepalensis D. Don (Betulaceae) from China for the first time. We introduce a new sexual species, C. filicis, which was collected from dead fronds of a fern in Thailand. The new species is introduced based on its morphological distinctiveness and phylogenetic support (Fig. 59).
Cyphellophora filicis Hongsanan, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF556179; Facesoffungi number: FoF05712, Fig. 60
Cyphellophora filicis (KUN-KHAS 102220, holotype). a, b Appearance of ascomata on host surface. c Section through ascoma. d Section through peridium. e, f Asci embedded in a hyaline gelatinous matrix. g–l Ascospores. m Ascospore germination. n, o Culture characteristics on PDA (n = from above, o = from below). Scale barsa = 500 µm, b = 100 µm, c, d = 20 µm, e, f = 10 µm, g–l = 5 µm
Etymology: The specific epithet “filicis” (Latin: fern) refers to the host, from which the holotype was collected.
Holotype: KUN-KHAS 102220
Saprobic on dead fronds of a fern, without dark superficial hyphae, appearing as black dots on host surface. Sexual morphAscomata 40–60 µm high, 55–75 µm diam., immersed in host tissue, becoming erumpent, solitary, subglobose to globose, dark brown, uni-loculate, glabrous, ostiolate, with minute papilla. Peridium 3–10 µm wide, thin-walled, composed of 2–5 layers of flattened, brown pseudoparenchymatous cells of textura angularis. Asci 30–50 × (10–)12–15(–19) µm (\( \bar{x} \) = 41.5 × 13.6 µm, n = 30), 8-spored, bitunicate, ovoid to ampulliform, short pedicellate, with an ocular chamber. Ascospores (15–)17–20(–22) × (3–)4–5(–6) µm (\( \bar{x} \) = 18.6 × 4.7 µm, n = 30), overlapping 2–3-seriate, hyaline, ellipsoidal to fusiform, inconspicuously 3-septate, not constricted at the septa, narrowly round at the ends, sometimes curved at the middle, with a guttule in each cell. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 34–36 mm diam. after 3 weeks at 20–25 °C, colonies circular, dense, flat, slightly raised, surface dull, with edge entire, woolly to velvety; from above, dark grey at the margin, white-grey at the centre, separating from the margin by convex, concentric ring; from below dark greenish at the margin, with green yellowish at the centre; not producing pigmentation in agar.
Material examined: THAILAND, Chiang Rai Province, Doi Pui, on dead fronds of a fern, 2 February 2017, R. Phookamsak, DP002 (KUN-KHAS 102220, holotype), ex-type living culture, KUMCC 18-0144 (DP002A), KUMCC18-0145 (DP002B).
GenBank numbers: ITS = MK404056, LSU = MK404052, SSU = MK404054 (KUMCC 18-0144); ITS = MK404057, LSU = MK404053, SSU = MK404055 (KUMCC18-0145).
Notes: The phylogenetic tree (Fig. 59) shows that Cyphellophora filicis is closely related to a fungus in Chaetothyriales (T222) that is associated with ants nest and their runway galleries (Nepel et al. 2014). It is also related to C. fusarioides (B. Sutton & C.K. Campb.) Decock, C. laciniata G.A. de Vries, C. suttonii (Ajello et al.) Decock and C. vermispora A. Walz & de Hoog, but as a distinct new species in Cyphellophora (Fig. 59). We could not compare morphological characters of our new species and those Cyphellophora species due to the fact that C. filicis is sexual morph species, while those other species in Cyphellophora were found as asexual morph. The first record of the sexual characters in Cyphellophora (C. jingdongensis) was provided by Yang et al. (2018a). However, C. jingdongensis differs from C. filicis in growing on honey dew excretions from insects, with dark superficial mycelium, scattered, superficial ascomata, without short necks, ellipsoidal to cylindrical asci and 1–3-septate fusoid ascospores. Thus, we introduce C. filicis as a new species based on the sexual morph characters and phylogenetic evidence (Figs. 59, 60).
Herpotrichiellaceae Munk
The family Herpotrichiellaceae was introduced by Munk (1953) and placed in the order Chaetothyriales (Barr 1976; Réblová et al. 2013; Gueidan et al. 2014; Wijayawardene et al. 2018a). The family is characterized by small, superficial, inconspicuous, setose ascomata, fissitunicate asci and greenish grey to brown, phragmosporous or dictyosporous ascospores (Munk 1953; Hyde et al. 2016). Species of this family can be saprobes on decaying wood, bark and leaves and are also found as pathogens on humans and living plants, as well as parasites on fungi or lichens worldwide (Crous et al. 2007b; Untereiner et al. 2011; Wijayawardene et al. 2017a). Fifteen genera are accepted in this family (Wijayawardene et al. 2018a).
Capronia Sacc.
Capronia is a poorly understood ascomycete genus characterized by very small, setose ascomata, lacking paraphyses, fissitunicate asci, and septate, or muriform, hyaline or pigmented ascospores (Munk 1957a, b; Müller et al. 1987; Untereiner et al. 2011; Friebes 2012). Species of this genus are associated with a wide range of hosts as saprobes on rotting wood or bark and decaying stems and leaves of herbaceous plants, or pathogenic on plants as well as parasites on other fungi, or lichens (Untereiner 2000; Untereiner et al. 2011; Wijayawardene et al. 2017a). We introduce a new species Capronia camelliae-yunnanensis, collected on Camellia yunnanensis in China (Figs. 61, 62).
Phylogenetic tree generated from maximum likelihood (RAxML) based on a combined ITS, LSU and SSU sequence dataset of Herpotrichiellaceae. Maximum likelihood bootstrap support values greater than 50% and Bayesian posterior probabilities greater than 0.75 BYPP are indicated on the notes. The new isolate is in blue. The tree is rooted with Vonarxia vagans (CBS 123533)
Capronia camelliae-yunnanensis (HMAS 255435, holotype). a Blackish ascomata on decorticated bark of Camellia yunnanensis. b Vertical section of ascoma. c Peridial structure. d Paraphyses. e–h Asci. i Asci stained with Melzer’s reagent. j Apical ring stained with Melzer’s reagent. k, l Immature ascospores. m, n Mature ascospores. o Germinated ascospore. p, q Culture characteristics on PDA (p = from above, q = from below). Scale barsb = 50 μm, c, d = 20 μm, e–i = 10 μm, o = 5, j–n = 2 μm
Capronia camelliae-yunnanensis M. Raza, Z.F. Zhang & L. Cai, sp.nov.
Index Fungorum number: IF555356; Facesoffungi number: FoF04884, Fig. 62
Etymology: Named after the epithet of Camellia yunnanensis, the host of which the holotype was collected.
Holotype: HMAS 255435
Saprobic on Camellia yunnanensis. Sexual morphAscomata 175–200 μm high, 215–220 μm diam., scattered or clustered, solitary, superficial on decorticated bark of host, with papilla, globose to subglobose, lodged on a basal subiculum, which form loose hyphae penetrating the underlying cells, setose around the surface of the wall, setae up to 30 μm long. Peridium 20–30 μm wide, thick-walled, of equal thickness, composed of several layers of small, pseudoparenchymatous cells, inner layers comprising hyaline cells, arranged in a textura angularis, outer layers comprising brown to dark brown becoming blackened cells of textura prismatica. Hamathecium composed of dense, 3–5 μm wide, broad, filamentous paraphyses with indistinct septate, not constricted at the septa, embedded in a gelatinous matrix. Asci (51–)57–78(–80) × (5–)6–10(–12) μm (\( \bar{x} \) = 67.8 × 8.7 μm, n = 40), 8-spored, bitunicate, fissitunicate, broadly cylindrical, short pedicellate with knob-like pedicel, apically broad rounded, with a blunt ocular chamber. Ascospores (10–)13–17(–19) × (2–)3–5 μm (\( \bar{x} \) = 15.1 × 4.5 μm, n = 40), overlapping 1–3-seriate, hyaline and aseptate when young, becoming light brown to yellowish, ellipsoidal to fusiform, muriform, with 3–7 transverse septa and 1–3 longitudinal septa, slightly constricted at the septa, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 15–20 mm diam. after 5 weeks at 25 ± 2 °C, colonies circular, umbonate, smooth to woolly with entire edge, velvety, smooth at the margin; from above, light green at the fruiting zone, whitish green at the productive zone and ageing zone; from below, light green at the fruiting zone, dark green to blackish at the productive zone, grey at the ageing zone; not producing pigmentation in PDA.
Material examined: CHINA, Yunnan Province, Baoshan City, Longling County, on decorticated bark of Camellia yunnanensis Cohen Stuart (Theaceae), October 2015, M. Raza, HMAS 255435 (holotype), ex-type living culture, CGMCC3.19061.
GenBank numbers: ITS = MH807377, LSU = MH807378, SSU = MH807379.
Notes: Capronia camelliae-yunnanensis forms a well-supported clade, sister to C. pilosella (P. Karst.) E. Müll. et al. (100% ML and 1.00 BYPP; Fig. 61). Capronia camelliae-yunnanensis differs from C. pilosella in the shape and size of the ascospores (C. camelliae-yunnanensis, (10–)13–17(–19) × (2–)3–5 μm versus 12–14 × 4–4.5 μm, C. pilosella; Karsten 1873). Capronia pilosella has phragmosporous, fusoid to clavate-fusoid, 3-septate ascospores (Karsten 1873; Müller et al. 1987), while C. camelliae-yunnanensis has muriform, ellipsoidal to fusiform, ascospores with 3–7 transverse septa and 1–3 longitudinal septa.
Subclass Eurotiomycetidae Geiser & Lutzoni
Eurotiales G.W. Martin ex Benny & Kimbr.
Trichocomaceae E. Fisch.
The family Trichocomaceae was introduced by Fischer (1897). This is a large saprobic family in nature with the most well-known genera including Aspergillus P. Micheli ex Haller, Penicillium, and Paecilomyces Bainier. Species belonging to this family have the ability to produce secondary metabolites (mycotoxins or extrolites), and enzymes (Pitt and Hocking 2009; Samson et al. 2010; Houbraken et al. 2011).
Penicillium Link
The genus Penicillium was first described by Link in 1809. Species of Penicillium are well known and found abundantly in the soil, air, indoor environments and in contaminated foods (Frisvad and Samson 2004; Samson et al. 2010). According to Houbraken and Samson (2011), the genus Penicillium was divided into four subgenera: Aspergilloides Dierckx, Furcatum Pitt., Penicillium, and Biverticillium Dierckx, and 25 sections. Among the sections of Penicillium, section Citrina was introduced by Houbraken and Samson (2011) based on phylogenies derived from RPB1, RPB2, Tsr1, and Cct8 sequence data. Currently, section Citrina contains 40 species (Houbraken et al. 2011; Visagie et al. 2014a, b). Members of section Citrina are found in soil, leaf litter, indoor environments, and food (Pitt 1979; Pitt and Hocking 2009; Samson et al. 2010). The species of section Citrina are characterized by the production of relatively small conidia, symmetrically biverticillate conidiophores and ampulliform phialides. They are also known for their ability to produce a variety of extrolites, including mycotoxins, citrinin, and citreoviridin (Houbraken et al. 2011). While, evaluating fungal diversity in soil samples in Korea, a new species was isolated and is described here based on morphological characteristics and phylogenetic analyses (Fig. 63).
Phylogenetic tree based on maximum likelihood analysis of a combined beta tubulin (TUB2) and calmodulin (CMD) dataset for Penicillium dokdoense and related species within the sect. Citrina. Sequence of Penicillium corylophilum was used as outgroup taxon. Numbers at the nodes indicate the bootstrap values (≥ 50%) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are in blue and ex-type strains in bold
Penicillium dokdoense Hyang B. Lee & T.T.T. Nguyen, sp. nov.
Index Fungorum number: IF554459; Facesoffungi number: FoF013606, Fig. 64
Penicillium dokdoense (CNUFC-DDS11-1, holotype). a, e Colonies in yeast extract sucrose agar (YES). b, f Colonies in malt extract agar (MEA). c, g Colonies in Czapek yeast autolysate agar (CYA). d, h Colonies in creatine sucrose agar (CREA). (a–d: obverse view, e–h: reverse view). i–m Verticillate conidiophores and conidia on phialides. n Conidia. Scale barsi = 20 μm, j–l = 10 μm, m = 5 μm, n = 3 μm
Etymology: Named after the place where it was collected, Dokdo Island
Holotype: CNUFC-DDS11-1
Sexual morph Undetermined. Asexual morph Hyphomycetous. Colonies grow slowly on CYA, reaching 23.5–26 mm diam. at 25 °C in 7 days, grey-green, floccose in centre, soluble pigment absent, margin entire; reverse yellowish white with yellow-brown in centre. Conidiophores mostly biverticillate, sometimes monoverticillate, or divaricate, stipes smooth, vary greatly in length, septate, 2–3.5 μm wide. Metulae cylindrical, 2–5 per stipe, 10.5–17.5 × 2.5–4.2 μm. Phialides ampulliform, 3–9 per metula, 6.7–11.5 × 2–3.5 μm. Conidia roughened, globose to subglobose, or ellipsoidal, and dark blue-green, 2–3.5 × 2–3 μm.
Culture characteristics: The isolate grew over a wide range of temperatures with varying growth rates on MEA, CYA, YES and CREA. The average growth rates of CNUFC-DDS11-1 on MEA, CYA, YES, and CREA medium at 25 °C were 34.5, 24.5, 22, and 14.5 mm per 7 days, respectively. Optimal growth was observed around 25 °C, slow growth was observed below 10 °C, and no growth at 37 °C.
Material examined: REPUBLIC OF KOREA, from soil in Dokdo Island in the East Sea of Korea, June 2014, CNUFC-DDS11-1 (holotype); isotype in Korean Collection for Type Cultures (KCTC, Daejeon, Korea), ex-type living culture, JMRC:SF:013606.
GenBank numbers: ITS = MG906868, TUB2 = MH243037, CMD = MH243031 (CNUFC-DDS11-1); ITS = MG906869, TUB2 = MH243038, CMD = MH243032 (CNUFC-DDS11-2).
Notes: The data from combined sequence analyses of the two loci (Fig. 63) reveal that Penicillium dokdoense, P. terrigenum Houbraken et al., P. cf. terrigenum and P. copticola Houbraken et al. are closely related. Penicillium dokdoense shares several similarities with P. terrigenum as its growth on CREA is poor with no acid production, but this species differs from P. terrigenum in terms of reverse colour on CYA and YES, and colony features on CYA. Furthermore, P. dokdoense produces globose to subglobose, or ellipsoid conidia and biverticillate, monoverticillate, or divaricate conidiophores, in contrast to the mostly ellipsoid conidia and biverticillate sporangiophores of P. terrigenum. Penicillium terrigenum and P. copticola grew at a similar range of temperatures from 25 °C to 30 °C, whereas P. dokdoense grew slowly. Penicillium dokdoense grows and sporulates at 35 °C, while, P. cf. terrigenum does not grow above 30 °C. At 5 °C, P. dokdoense and P. copticola were both capable of growth, whereas P. terrigenum was not. When grown on CYA, colonies of P. dokdoense are weakly wrinkled, while colonies of P. terrigenum are strongly wrinkled. The results of morphological and comparative sequence analyses of P. dokdoense indicate that it is a distinct species from P. terrigenum, P. cf. terrigenum, and P. copticola. Thus, P. dokdoense is proposed.
Class Lecanoromycetes O.E. Erikss. & Winka
We follow the latest treatment and updated accounts of Lecanoromycetes in Miadlikowska et al. (2014) and Kraichak et al. (2018). The updated outline and notes of the genera in Lecanoromycetes was provided by Wijayawardene et al. (2017a, 2018a).
Lecanoromycetes, families incertae sedis
Micropeltidaceae Clem. & Shear
The family Micropeltidaceae was introduced by Clements and Shear (1931) as ‘Micropeltaceae’ and is typified by Micropeltis Mont. with M. applanata Mont. as the type species. The family comprises foliar, biotrophic epiphytes, which are mostly found on the lower leaf surface as small black dots. Micropeltidaceae species are characterized by superficial, flattened, black-blue or greenish to black thyriothecia, easily removed from the host surface, poorly developed at the base, the wall comprising interwoven hyphae, with a central ostiole and ascospores are septate and hyaline (Clements and Shear 1931; Wu et al. 2011; Hyde et al. 2013; Hongsanan et al. 2015; Hongsanan and Hyde 2017). Micropeltis phetchaburiensis sp. nov. is introduced based on its morphological characteristics coupled with phylogenetic analyses of a combined LSU and SSU sequence dataset (Fig. 65). The new species was collected from living leaves in Thailand.
Phylogram generated from maximum likelihood (RAxML) analysis based on combined LSU and SSU sequence dataset of representative families in Lecanoromycetes. Tree is rooted with Icmadophila ericetorum (AFTOL-ID 4846) and Siphula ceratites (P110). Maximum likelihood bootstrap (black) and maximum parsimony bootstrap (blue) values > 65% are given above the nodes. The scale bar indicates 0.05 changes. New isolate is in blue. Ex-type strains are indicated in bold
Micropeltis Mont.
We follow the latest treatment and updated account of Micropeltis in Hongsanan and Hyde (2017).
Micropeltis phetchaburiensis Dayarathne, Hongsanan & K.D. Hyde, sp. nov.
Index Fungorum number: IF555294; Facesoffungi number: FoF04841, Fig. 66
Etymology: Name reflects Phetchaburi Province in Thailand, from where the species was collected.
Holotype: MFLU 18-1408
Epiphytic appearing as small black dots, superficial, on the upper surface of living leaves, superficial hyphae absent. Sexual morphThyriothecia 75–90 × 140–160 μm diam. (\( \bar{x} \) = 80 × 150 μm, n = 5), solitary, superficial on the surface of hosts, circular, membranous, black, easy to detached, base poorly developed, with a central, irregular ostiole. Upper walls comprising an irregular, meandering arrangement of hyphae, from the central ostiole to the outside. Peridium 35–50 μm wide, composed of two strata, the outer stratum having bluish to black, occluded walls, inner stratum of greenish to hyaline, flattened cells. Hamathecium with evanescent pseudoparaphyses. Asci 50–68 × 11–16 μm (\( \bar{x} \) = 60 × 14 μm, n = 10), 8-spored, bitunicate, broadly cylindrical to fusiform, with a short pedicel, apically rounded with ocular chamber. Ascospores 16–20 × 2–4 μm (\( \bar{x} \) = 18 × 3 μm, n = 20), overlapping 2–3-seriate, hyaline, clavate, 3-septate, constricted at the septa, narrowly rounded at both ends, smooth-walled, surrounded by a mucilaginous sheath. Asexual morph Undetermined.
Material examined: THAILAND, Phetchaburi Province, Prachuap Khiri Khan, 77230 Bang Saphan, Ron Thong, on living leaves of an unidentified plant, 14 December 2015, M. Dayarathne, KLAP011 (MFLU 18-1408, holotype; HKAS102010, isotype).
GenBank numbers: LSU = MH656405, SSU = MH656406.
Notes: Micropeltis phetchaburiensis resembles M. dendrophthoes Hongsanan & K.D. Hyde and M. zingiberacicola H.X. Wu & K.D. Hyde in forming broadly cylindrical to fusiform asci and hyaline, clavate, septate ascospores (Wu et al. 2011; Hongsanan et al. 2015). Micropeltis phetchaburiensis can be distinguished from M. dendrophthoes and M. zingiberacicola in having 3-septate ascospores with the 4 cells being equal in length and width, while ascospores of the other two species are 4–5-septate and enlarged at the first cell and relatively longer lower end cells. In our phylogenetic analyses of a combined LSU and SSU sequence dataset, M. phetchaburiensis forms a basal lineage to M. dendrophthoes and M. zingiberacicola with moderate bootstrap support (97% ML and 93% MP; Fig. 65).
Class Leotiomycetes O.E. Erikss. & Winka
We follow the latest treatment and updated accounts of Leotiomycetes in Zhang and Wang (2015), Jaklitsch et al. (2016a) and Wijayawardene et al. (2018a) for the taxonomic outline of this class.
Helotiales Nannf. ex Korf & Lizoň
Lachnaceae Raitv.
Raitviir (2004) raised Nannfeldt’s tribe Lachneae (fam. Hyaloscyphaceae; Nannfeldt 1932) to the family level and designated Lachnum Retz. as the type genus. Han et al. (2014) showed that Lachneae formed a monophyletic lineage within Hyaloscyphaceae sensu lato, justifying the existence of Lachnaceae. Jaklitsch et al. (2016a) and Wijayawardene et al. (2018a) recognize 16 genera in this family. Phylogenetic relationships of selected lachnoid taxa were studied previously by Cantrell and Hanlin (1997) and Hosoya et al. (2010). We introduce a new monospecific genus Velebitea in the family Lachnaceae based on microscopic and macroscopic features.
Velebitea I. Kušan, Matočec & Jadan, gen. nov.
MycoBank number: MB827753; Facesoffungi number: FoF05713
Etymology: Named after the mountain, Velebit, on which it was collected.
Sexual morphAscomata apothecial, comparatively robust and medium sized, superficial, stipitate, solitary or gregarious, deeply cupulate when young, becoming shallowly cupulate to ± plate-shaped. Hymenium creamy whitish, yellowish with age, margin upright, covered with long whitish hairs, as well as whole excipulum and stipe throughout the base, excipulum paler than hymenium, stipe centrally attached, tapering towards the base, base brownish. Subhymenium equally thick or thicker than medullary or ectal excipulum, composed of hyaline textura epidermoidea-intricata, in living state clearly discerned from the medulla. Marginal texture of hyaline textura porrecta-prismatica, often with abundant yellow resinous inclusions, beset with ± tidily organized cylindrical marginal hairs which are always clearly separated from excipular ones by a small hairless area just at the point of hymenial base level, individual hairs ± straight, multi-celled, markedly shorter than excipular flank hairs, apical cell cylindric-obtuse to subclavate, walls thin and hyaline, cells without refractive content when in living state, only basal 1–2 cells with firmly attached KOH resistant granules, not stainable in cotton blue, with additional abundant loosely attached KOH soluble pale yellowish resinous granules, no crystals. Medullary excipulum composed of hyaline densely woven textura intricata, producing small hyaline crystalloid particles with age, sometimes having abundant yellow resinous inclusions, intercellular spaces overall slightly gelified. Ectal excipulum composed of single layer of textura prismatica with cells running ± parallel to the surface, walls somewhat thickened, especially in some outermost cells, basal areas regularly with highly refractive golden yellow resinous accumulations; surface beset with very untidily intricately organized flexuous excipular hairs, multi-celled, longer than marginal hairs, walls thin, with apical localized thickenings in some hairs, only basal 1–2 cells with firmly attached KOH resistant granules, regularly supplemented by KOH soluble loosely attached pale yellowish granules, surface irregularly embedded in abundant gel plaques clearly stained lilac in brilliant cresyl blue, in cotton blue walls not cyanophilic, yellow resinous accumulations dissolved. Overall texture and hairs in Lugol’s solution without any amyloid reactions, all yellow resinous accumulations in texture are rapidly soluble in KOH. Paraphyses cylindrical, apically obtuse, straight, not branching in the upper part, apical and often subapical cells contain hyaline non-refractive vacuoles. Asci 8-spored, elongated cylindrical-deltoid, apex conical obtuse, protruding above paraphyses at full maturity, arising from simple septate ascogenous cells, in Lugol’s solution apical apparatus moderately euamyloid of Calycina-type. Ascospores elongated fusoid, ± straight to bent, bilaterally symmetrical, always 1-celled in full maturity, smooth, hyaline, poles tapered to sub-obtuse, eguttulate or with several minute guttules, uninucleate, the remaining sporoplasm regularly occupied by several conspicuous non-refractive vacuoles, when freshly ejected without sheath; in Lugol’s solution cytoplasm without glycogen accumulations. Asexual morph Undetermined.
Type species: Velebitea chrysotexta I. Kušan, Matočec & Jadan
Notes: A data matrix for alignment was constructed to determine the phylogenetic position of Velebitea chrysotexta within Lachnaceae and also to test phylogenetic proximity to Hyaloscyphaceae (Amicodisca virella (P. Karst.) Huhtinen), and Tetracladium spp. Phylogenetic analysis (Fig. 67) included the ITS and LSU sequences generated from the holotype of Velebitea chrysotexta and other related sequence data, retrieved from GenBank as well as a newly sequenced collection of Neodasyscypha cerina (Pers.) Spooner (CNF 2/10442; ITS = MH886408, LSU = MH886412) which was collected from fallen decorticated branch of Fagus sylvatica L. in Croatia. Sequences of Hymenoscyphus fructigenus (Bull.) Gray were used as an outgroup taxon. Maximum likelihood analysis of the concatenated ITS and LSU alignment was performed by MEGA7 (Kumar et al. 2016), including 1361 total characters in the final dataset. The phylogeny based on concatenated analysis of ITS and LSU nests the genus Velebitea in the family Lachnaceae as a separate lineage having comparatively basal position not belonging to any of tested genera (Fig. 67).
Maximum likelihood phylogenetic tree based on a concatenated ITS and LSU sequence dataset. Sequences recovered during this study are shown in blue. The tree is rooted to Hymenoscyphus fructigenus. Maximum likelihood bootstrap support values greater than 50% and Bayesian posterior probabilities greater than 0.95 BYPP are shown at the nodes. The bar length indicates the number of nucleotide substitutions per site
Megablast search of NCBIs GenBank nucleotide database using the ITS sequence of Velebitea chrysotexta (CNF 2/10072, GenBank no. MH886407) shows that the closest hits belong to Lachnellula spp. with the similarities between 89% and 91% similarities. Lachnellula P. Karst. is a genus in Lachnaceae, strictly confined to coniferous hosts (Dharne 1965). On the other hand, the closest hits using the LSU sequence (GenBank no. MH886411) are Tetracladium furcatum Descals (GenBank no. EU883428; similarity = 907/1009 (90%), gaps = 45/1009 (4%)) and T. maxilliforme (Rostr.) Ingold (GenBank no. EU883429 and EU883430; both with similarity = 903/1008 (90%), gaps = 43/1008 (4%)). Tetracladium De Wild. is an aquatic hyphomycete genus (Letourneau et al. 2010) classified as Helotiales, incertae sedis (Wijayawardene et al. 2017a, 2018a), but phylogenetic affinity is close to Helotiaceae, and Hyaloscyphaceae (Wang et al. 2015). Certain similarity exists between Velebitea and Tetracladium in their ecological preference to a colder climate; the sexual morph of Velebitea lives in subalpine forests with beech (Fagus) while some members of the genus Tetracladium were recorded in cold snow-covered soil in a glacier zone (Kuhnert et al. 2012).
Together with the genus Dasyscyphella Tranzschel, Velebitea is different from all other genera in the family Lachnaceae by partially granulated hyaline hairs. Dasyscyphella is clearly polyphyletic (Hosoya et al. 2010; this study) and therefore in need of taxonomic rearrangement. Since the type species of the genus Dasyscyphella, D. cassandrae Tranzschel is not present in public DNA sequence databases, we included all other available species from this genus in our phylogenetic analysis because species currently ascribed to Dasyscyphella display highest similarity to our material based on non-molecular data. The polyphyly of the genus Dasyscyphella could be in line with wide diversity of certain non-molecular features that may play important role in future species distinguishing at generic level, such as existence of hair crystals, resinous exudates and its microchemical properties, hair wall granulation degree, hamathecial features, details in ecology, apothecial development and anatomy, despite of high similarity among many of species in terms of “standard” microscopical characters (e.g. spore and ascus shape/measurements). Many of these characters already efficiently delimitate some monophyletic lachnacean genera, viz. Capitotricha (Raitv.) Baral, Brunnipila Baral and Lachnellula. Dasyscyphella cassandrae, the type species of the genus, differs strikingly from Velebitea chrysotexta by very elongate flexuous-cylindric, septate ascospores, overall absence of KOH soluble resinous lumps and, together with all other known species currently ascribed to the genus Dasyscyphella in having only the apical (rarely also subapical) smooth hair cell. Whereas, hairs in V. chrysotexta are smooth except in basal 1–2 cells. Hairs in D. cassandrae (as well as in many other species) are longer at the margin than on excipular flanks, quite opposite in V. chrysotexta. A number of species currently ascribed to a genus Dasyscyphella have hairs bearing conspicuous calcium oxalate crystals and/or crystal druses (such as D. nivea positioned well aside from V. chrysotexta in our phylogenetic analysis) that are completely lacking in V. chrysotexta. Nearly all species currently ascribed in the genus Dasycyphella have either lanceolate or cylindrical-pointed paraphyses (as in D. cassandrae). There are however some species of the genus Dasyscyphella that according to Raitviir (2002), also Dennis (1949), produce resinous pigments comparable to Velebitea chrysotexta: Dasyscyphella claviculata (Velen.) Baral & Svrček, D. crystallina (Fuckel) Raitv., D. rubi Raitv., D. conicola (Rehm) Raitv. & Arendh., D. nivea (R. Hedw.) Raitv., D. tamajonica (Raitv. & R. Galán) Raitv. (all producing calcium oxalate crystals, mostly having different ecology), D. mughonicola (Svrček) Raitv. & Arendh. (whose asci arising from croziers, coniferous substrate), D. patuloides Raitv. & R. Galán (producing hyaline resin, asci arising from croziers, having phyllophilous ecology) and D. sulphuricolor (Peck) J.H. Haines (producing brownish resin that reacts vinaceous with KOH). Much work is still to be done to uncover true phylogenetic affinities of a number of species currently accommodated in Dasyscyphella which might belong to severally phylogenetic lineages representing separate genera.
Velebitea chrysotexta I. Kušan, Matočec & Jadan, sp. nov.
MycoBank number: MB827754; Facesoffungi number: FoF05714, Figs. 68, 69, 70
Velebitea chrysotexta. a Fructification growth in situ. b Apothecia, side view, resinous plaques visible. c Ascomata top view. d Living mature asci protruding above paraphyses tips. e Paraphyses. f Simple septate ascogenous cells. g Freshly ejected living mature ascospores. h Overmature ascospores. i Freshly ejected living mature ascospores. j Germinated ascospores on PDA after 24 h. k Amyloid reaction in asci. l Marginal hairs tips. m Marginal apothecial area largely beset by resinous matter. d–i, m in water mount, k, l in Lugol’s solution, j on PDA. c–h, l CNF 2/10072 (holotype), i, j, m CNF 2/10661, a, b, k CNF 2/10736. Photo by N. Matočec and I. Kušan. Scale barsa, c = 2 mm, b = 1 mm, d–i, k, l = 10 µm, j, m = 50 µm
Velebitea chrysotexta. a Apothecial section beset with resinous matter. b Excipular flank with hairs. c Excipular hair base. d Marginal texture with hairless area. e Marginal hairs. f Marginal hair tips in bright field (left) and phase contrast (right). g Excipular texture with thick subhymenial layer. h Medulla with crystalloid particles. i Large resinous accumulations in ectal excipulum. j Excipular hairs, apical wall thickenings present in some hairs, phase contrast (left), bright field (right). k Excipular hairs in dead state. All in water mount. b, d, f, j, k CNF 2/10072 (holotype), a, c, g, i CNF 2/10661, e, h CNF 2/10736. Photo by N. Matočec and I. Kušan. Scale barsa = 50 µm, b, d, e, g–k = 20 µm, c, f = 10 µm
Velebitea chrysotexta. a Marginal hairs with resinous lumps. b Stipe vestiture, side view. c Stipe texture and vestiture in section. d Stipe hairs. e Living mature ascospore. f Marginal hairs with rapidly floculated surface matter. g Marginal hairs after longer exposure. h Excipular hairs in living and dead state. i Excipular tissue displaying gelified areas (lilac and rosy). j Living excipular hairs. k Medullary excipulum. l Apothecial section and vestiture, dark field. m Apothecial vestiture, phase contrast. n Marginal hairs. o Hymenial elements. p Excipular tissue, resinous lumps dissolved. q Excipular hairs. a–d in water mount, e–k in brilliant cresyl blue, l–o in cotton blue, p, q in KOH. b–q CNF 2/10072 (holotype), a CNF 2/10661. Photo by N. Matočec and I. Kušan. Scale barsb, c, l, m = 50 µm, a, d, f–k, n, p, q = 20 µm, o = 10 µm, e = 5 µm
Etymology: Refers to its yellowish pigment inclusions in the apothecial texture
Holotype: CNF 2/10072
Sexual morphAscomata apothecial, superficial, stipitate, solitary or gregarious, deeply cupulate when young, becoming shallowly cupulate to ± plate shaped, 2.5–3.2 mm high, 1.1–3 mm diam. Hymenium 80–105 µm thick, creamy whitish in primordial stage, becoming creamy to pale ochre yellow, yellowing with age, margin upright, covered with whitish hairs, as well as whole excipulum and stipe throughout the base, excipulum paler than hymenium, completely covered with whitish hairs, stipe 1.5–2.2 × 0.6–1 mm, centrally attached, tapering towards the base, hairy throughout, base brownish. Subhymenium 32–47 µm thick, composed of hyaline textura epidermoidea-intricata, cells 3.4–5.1 µm wide, while in living state clearly discerned from the medulla. Marginal texture forming very thin layer, composed of hyaline textura porrecta-prismatica, often containing abundant yellow resinous accumulations, beset with ± tidily organized marginal hairs which are always clearly separated from excipular ones by small hairless area just at the point of hymenial base level, individual hairs ± straight but bending slightly towards the hymenial rim, projecting towards the marginal surface perpendicularly, 5–7-celled, 66–109 µm long, 3.7–5.6 µm wide at the base, 2.2–3.8 µm in the middle and 2.3–3.7 µm in the apical part, apical cell cylindric-obtuse to subclavate, individual cells prismatic, containing low refractive globules, walls hyaline and refractive, 0.2–0.5 µm thick, may be slightly thickened in dead state, cells without refractive content when still alive, wall of the basal 1–2 cells firmly granulate, additionally beset with loosely attached pale yellowish granules, no crystals; in brilliant cresyl blue numerous non-refractive hyaline vacuoles stained deep lilac-violet to violet after longer exposure, cytoplasm in dead apical cells turquoise blue, basal wall granules grey cyan to violet grey, walls unstained; after adding 2.5% KOH hair granules persistent, subhyaline golden yellow accumulations instantly dissolved, for few moments giving localized yellow solution area; in cotton blue hair walls not cyanophilic, apical cells cytoplasm pale blue, wall granules pale cyan, yellow resinous accumulations dissolved. Medullary excipulum 30–44 µm thick, composed of hyaline densely woven textura intricata intertwining with ± vertical cells, cells thin-walled, 2.2–3.7 µm wide, running predominantly parallel to the excipulum surface, producing hyaline crystalloid particles with age, sometimes containing abundant yellow resinous accumulations; in brilliant cresyl blue intercellular space slightly rosy-lilac (gelified); in cotton blue walls not cyanophilic. Ectal excipulum 38–52 µm thick, composed of textura prismatica, cells 13.6–46.7 × 4.5–10.9 µm, running ± parallel to the surface, walls somewhat thickened, especially in some outermost cells, basal area with highly refractive golden yellow resinous accumulations; surface ornamented with flexuous excipular hairs which are very untidily intricately organized, 4–11-celled, 92–156 µm long, 2.5–4.1 µm wide at the base, 2.2–3.1 µm in the middle part and 2.4–3.7 µm at the apex, individual cells prismatic, walls highly refractive, sometimes with localized apical thickenings, in dead state more pronounced, 0.7–0.8 µm thick, basal 1–2 cells firmly granulate, with additional loosely attached pale yellowish granules, no crystals; in brilliant cresyl blue textural cells’ cytoplasm lilac, thickest walls greenish cyan, slightly gelified, surface with revealed abundant, lilac irregular gel plaques; in cotton blue walls not cyanophilic, cytoplasm bluish, yellow resinous accumulations dissolved. Stipe excipulum of hyaline celled, wavy textura prismatica, cells 17.4–34.2 × 5.6–9.3 µm, walls thickened in the outermost cells, giving rise to hyaline hairs as those on ectal excipulum, 90–136 µm long, 3.8–4.4 µm wide at the base, 2.7–3.5 µm in the middle and 1.8–2.8 µm at the apical part, containing rich golden-yellow partly crystalloid accumulations. Overall texture and hairs in Lugol’s solution without any amyloid reactions, while resinous matter is rapidly soluble by KOH. Paraphyses cylindrical, apically obtuse, straight, not branching in the upper part, apical cell 19.3–35.7 × 2.1–3.2 µm, apical and often subapical cells contain hyaline non-refractive vacuoles in a living state (not vacuolar bodies), dead cells with highly refractive lemon yellow content; in Lugol’s solution, brilliant cresyl blue and cotton blue unstained. Asci 72.6–95.1 × 5.5–7.2 µm, elongated cylindrical deltoid, apex conical obtuse, protruding above paraphyses tips up to 12 µm at full maturity, pars sporifera 23.5–38.6 µm, 8-spored, arising from simple septate ascogenous cells, sometimes with lateral protuberance; in Lugol’s solution apical apparatus moderately euamyloid, of Calycina-type. Ascospores (8.2–)8.4–10.8–14.2(–15.2) × (2.1–)2.2–2.6–2.8 µm, Q = (3.2–)3.3–5.4–5.7(–6) (n = 125), elongated fusoid, ± straight to slightly bent or sickle shaped, rarely subsigmoid, bilaterally symmetrical, poles tapered to sub-obtuse, 1-celled, smooth, hyaline, overmatured 1-septate, eguttulate or with several minute guttules, 0.2–0.6 µm diam., uninucleate, nucleus centrally positioned, 1.3–1.4 µm in diam., the remaining sporoplasm regularly occupied by several conspicuous non-refractive vacuoles; 3–4 ascospores at uppermost positions in living mature asci markedly shorter, mostly 2-seriate, when freshly ejected without sheath; in Lugol’s solution cytoplasm partly yellowish, without glycogen accumulations, in brilliant cresyl blue wall unstained, no sheath revealed, after longer exposure internal small metachromatic corpuscles regularly formed. Ascospores in polysporic test cultures under axenic conditions obtained by shooting asci on PDA readily germinated nearly equally at both poles during 24 h at 24 °C. Asexual morph Undetermined.
Habitat and phenology: Saprobic on very rotten decorticated fragments and large branches of Fagus sylvatica (Fagaceae) hidden in the litter, in subalpine forests, fruit bodies appear in May and June.
Known distribution: The species is known so far only from Mt. Velebit, Croatia.
Material examined: CROATIA, Lika-Senj County, Sjeverni Velebit National Park, northern part of Mt. Velebit, Jurekovac area, 1050 m E-SE from Jurekovački kuk peak (1525 m), 44°45′16″N, 15°00′57″E, 1340 m a.s.l., forest of Abies alba Mill., Fagus sylvatica L. and Picea abies (L.) H. Karst., on decorticated rotten Fagus sylvatica stump base together with Chlorociboria aeruginosa (Oeder) Seaver ex C.S. Ramamurthi et al. and Hyaloscypha vitreola (P. Karst.) Boud., 27 May 2017, I. Kušan and N. Matočec (CNF 2/10072, holotype); ibid. 2 June 2018 (CNF 2/10661); Lika-Senj County, Paklenica National Park, southern part of Mt. Velebit, Velika Ruja in Javornik area, 1160 m NE-E-NE from Badanj peak (1638 m), 44°22′60″N, 15°27′22″E, 1365 m a.s.l., forest of Fagus sylvatica, on fallen decorticated branch of Fagus sylvatica, 25 June 2018, N. Matočec and I. Kušan (CNF 2/10736).
GenBank numbers: ITS = MH886407, LSU = MH886411 (CNF 2/10072, holotype).
Class Pezizomycetes O.E. Erikss. & Winka
We follow the latest treatment and updated accounts of Pezizomycetes in Jaklitsch et al. (2016a), Ekanayaka et al. (2018) and Wijayawardene et al. (2018a).
Pezizales J. Schröt.
Pezizaceae Dumort.
The family Pezizaceae was introduced by Dumortier (1829) with Peziza Fr. as the type genus. A systematic overview of the family was recently given by Jaklitsch et al. (2016a) who recognized 32 genera in this family. Ekanayaka et al. (2018) reviewed the families in the class Pezizomycetes and outlined 45 genera names belonging to Pezizaceae, including also some taxa synonymized by other authors.
Sarcopeziza Loizides et al.
A new genus in the Pezizaceae, Sarcopeziza Loizides et al. was recently erected (Agnello et al. 2018) to accommodate a single species, Peziza sicula Inzenga (Inzenga 1869). This species was previously typified and treated by Agnello et al. (2013) and Agnello et al. (2015). Since the original description of Inzenga (1869) and the modern presentations by Agnello et al. (2013) and Agnello et al. (2018) are not fully adequate and accurate for further advancement in taxonomy of the group, a more detailed re-description of microscopical features of Sarcopeziza sicula is provided here, based on careful cyto- and histochemical analyses.
Sarcopeziza sicula (Inzenga) Agnello, Loizides & P. Alvarado, Ascomycete.org 10(4): 179 (2018)
MycoBank number: MB827574; Facesoffungi number: FoF05715, Figs. 71, 72
Sarcopeziza sicula. a Ascomata and part of hymenium after Inzenga (1869), scanned from Venturella (2005). b Dried ascomata. c Ascus amyloidity. d Opercular fine structure. e Ascus bases with ascogenous cells. f Ascospore ornamentation. g Ascus bases with ascogenous cells. h Ascospores. i Mature ascospores in multi-guttulate state displaying single nucleus. j Mature ascospores during coalescence of lipid bodies. k Completely coalesced lipid body matter. l Ascospores with de Bary bubble present in some spores. c in Lugol’s solution, e, g, i–k in water mount, d, f, h, l in cotton blue. All from MCVE 25877 (epitype). Photo by N. Matočec and I. Kušan. Scale barsb = 1 cm, c–l = 10 µm
Sarcopeziza sicula. a Excipular structure (bright field). b Excipular structure (dark field). c Excipular structure, upper half with hymenium (dark field). d Excipular structure, lower half (dark field). e Subhymenium with minute crystalloid particles. f Upper medulla with minute crystalloid particles. g Upper medulla with lipid like globules. h Ectal excipulum with minute crystalloid particles. i Cortical cells with yellow cytoplasm and hyaline hyphoid outgrowths. j Subhymenium and upper medulla. k Upper medulla with largest cells. l Lower medulla. m Lower medulla with fasciculate texture. n Ectal excipulum. a, b, e, f, h in water mount, c, d, g, i–n in cotton blue. All from MCVE 25877 (epitype). Photo by N. Matočec and I. Kušan. Scale barsa–d = 200 µm, e–i = 20 µm, j–n = 50 µm
Basionym: Peziza sicula Inzenga, Funghi Siciliani, Centuria Seconda: 29 (1869)
= Sarcosphaera sicula (Inzenga) Pat., Bull. Soc. Hist. nat. Autun 17: 154 (1904)
Sexual morphAscomata apothecial, hypogeous to semi-hypogeous at first, becoming epigeous during development, firstly globose with pre-defined narrowly circular apical opening, 2–5 cm in diam., expanding when ripe, cracked into lobes, becoming star-shaped, solitary or gregarious, reaching 4.5–10 cm in diam., base substipitate to stipitate deeply buried in the substrate, hymenial surface vinaceous-purplish to livid vinaceous, matte, margin tapered in section, entire in youth, smooth, excipulum concolorous or somewhat darker than hymenium, gibbous, ± smooth to finely pruinose; flesh of homogenous soft waxy consistence, on cut not exuding milk, 2.5–4.2 mm thick, of the same colour as external surface with more pinkish tinge, odourless and flavourless. Hymenium 360–400 µm thick, arranged as a regular palisade. Subhymenium reduced and faintly discerned from the upper medulla only by scattered ascogenous hyphae, other cells indistinct from those in upper medulla though slightly smaller, continuing upwards as moniliform lower paraphysal cells. Upper medulla 950–1200 µm thick, composed of textura angularis richly intertwined with elements of short-celled textura intricata, nearly ± isodiametric cells gradually more frequent and larger towards lower medulla, 13.5–55 µm diam., elongated cells 8.5–88 × 5.3–16.5 µm, walls hyaline, thin, many cells contain minute refractive crystalloid particles in water mount, but without globular content, highly refractive globules though present in cotton blue, 0.8–5.2 µm diam., but crystalloid matter absent; lower zone with many cells having thickened yellowish and highly refractive septal rings. Lower medulla 500–900 µm thick, generally as central textura intricata, but cell bundles in lower half mostly oriented ± vertically as textura fasciculata, while on the border with upper medulla or in central areas hyphae may be oriented predominantly ± horizontally, hyphae in upper 1/3 of the whole layer often without strict orientation, cells cylindrical-hyphoid, 6.2–14.8 µm wide, without any type of refractive contents, walls subhyaline, some ± cylindrical cells densely set with rich accumulations of golden-yellow and highly refractive minute granules that may consolidate to form irregular cytoplasmic patches of isabelline, extracellular crystalloid content very sparse. Ectal excipulum compact area 220–375 µm thick, composed of textura angularis to textura prismatica, cells 11.4–28.5 × 6.2–24.5 µm, some cells somewhat elongated, more elongated cells dominant in upper zone and then of ± vertical orientation, walls thickened, subhyaline to yellowish, 0.7–0.9 µm thick in cortical cells, inner cells with thin, hyaline walls, containing minute to medium sized refractive crystalloid particles in water mount, but without globular content, highly refractive globules though present in cotton blue, 0.8–6.5 µm diam., but crystalloid matter absent; surface with occasional low pustules, ornamented chiefly by individualized, mostly scattered, (1–)2–6-celled terminal outgrowths, 21.4–125 × 9.5–14.7 µm, individual cells sub-cuboid to cylindrical, 9.2–40 × 9.1–14.7 µm, walls subhyaline, thin-walled; cortical cells beset with yellowish to isabelline-ochre highly refractive minute to larger crystalloid matter, cytoplasm of some cortical cells pale isabelline. In KOH refractive cellular content in upper medulla coalesced, lower medullar/ectoexcipular cells without refractive content, crystals not dissolved. Overall texture in Melzer’s reagent without dextrinoid reactions, refractive cellular granules coalesced to highly refractive large guttules; in congo red all cell walls ± stained; in cotton blue cell walls cyanophobic in entire excipulum, but some ectal excipular cells with bluish cytoplasm, medullar cell walls not cyanophilic. Paraphyses cylindrical, inconspicuous, apically obtuse to clavate, straight to apically bent, not branching in the upper part, apical cell 2.8–4.8 µm diam., middle and lower cells cylindric, moniliform cells exist near the margin, wall hyaline, thin; in Lugol’s solution unstained. Asci 355–446 × 14–17.3 µm, cylindrical, apex subtruncate with comparatively weakly pronounced functional operculum, 8-spored, base pleurorhynchous, with two ± tightly set septa, arising from non-repetitive croziers at widely variable levels, operculum 5.2–5.8 µm in diam., 0.7–0.9 µm thick, strictly apical, flat-lenticular with only slight indentation ring best visible under oil immersion in cotton blue, periascal mucus very thin without thick apical accumulation, film-like, evenly thick throughout, lateral wall 3-layered, subhyaline to yellowish, 0.7–0.9 µm thick; in Lugol’s solution periascal mucus in the broad upper area diffusely and moderately euamyloid, reaction gradually decreasing in intensity downwards, basally inamyloid, same in Melzer’s reagent; in congo red periascal mucus weakly pronounced, very thin, outermost wall layer strongly grey-red, median layer less stained, innermost layer unstained, operculum differentially stained red while thin operculum ring area remained unstained. Ascospores (13.2–)13.4–15.1–16.1(–16.7) × (8.1–)8.3–8.9–10, Q = 1.48–1.57–1.76(–1.77) (n = 50, in cotton blue), hyaline, ellipsoid, sometimes broadly oblong, radially symmetrical, with rounded poles, 1-celled, seemingly smooth under immersion lens when in water mount, many spores (especially when outside of the asci) slightly roughened in cotton blue but markings are not cyanophilic, measuring below 0.2 µm high and up to 0.3 µm diam., mature spores initially with multiguttulate pattern, lipid bodies, when still not coalesced 0.3–1.5 µm diam., lipid bodies in dead spores coalesced in various degree up to maximal, uni-guttulate stage (visible only in water mount), uninucleate (visible in spores having lipids in multi-guttulate pattern), wall 3-layered, 0.7–0.8 µm thick; in Lugol’s solution without glycogene accumulations; in KOH wall stable (not loosening); in cotton blue perispore weakly cyanophilic, wall not loosened, de Bary bubbles present in some mature spores, lipid bodies completely masked, sporoplasm unstained; in acetocarmine nucleus not differentially stained, only submature spores with weakly stained nucleus, perispore not loosened. Asexual morph Undetermined.
Material examined: ITALY, Province of Brindisi, San Pancrazio Salentino, San Antonio alla Macchia, 40°25′00″N, 17°50′00″E, 59 m a.s.l., on bare and mossy soil in a sunny Pinus halepensis Mill. forest with Eryngium campestre L. and Poaceae, 7 February 2008, A. Delle Donne and C. Agnello (MCVE 25877, epitype).
Habitat and phenology: On sandy or calcareous soils in frame of dry Mediterranean thickets (mostly dominated by Cistus spp.) and grasslands and parks, sometimes in vicinity of thermophilic Pinus spp., Pyrus spp. and Olea europea in thermo-Mediterranean bioclimatic belt, fruit bodies appear between January and April.
Known distribution: Cyprus, Greece, Israel, Italy, Spain and Tunisia.
GenBank numbers ITS = MH886405, LSU = MH886409 (MCVE 25877, epitype).
Notes: A data matrix for alignment was constructed to show a phylogenetic position of Sarcopeziza sicula within Pezizaceae, with a special emphasis on the genus Peziza. Phylogenetic analysis (Fig. 73) included the ITS and LSU sequences generated from the epitype collection of Sarcopeziza sicula and other related sequences retrieved from GenBank as well as a newly sequenced collection of Adelphella babingtonii (Berk. & Broome) Pfister, Matočec & I. Kušan (CNF 2/9430; ITS = MH886406, LSU = MH886410). Ascobolus crenulatus P. Karst. was used as an outgroup taxon. The maximum likelihood analysis of a combined ITS and LSU sequence dataset was conducted in MEGA 7 (Kumar et al. 2016) with a total of 1462 positions in the final dataset.
Maximum likelihood phylogenetic tree based on a concatenated ITS and LSU sequence dataset. Sequences recovered during this study are shown in blue and ex-type strains are in bold. The tree is rooted to Ascobolus crenulatus. Bootstrap values greater than 50% are indicated at the nodes. The bar length indicates the number of nucleotide substitutions per site
The phylogeny based on concatenate analysis of ITS and LSU nested the genus Sarcopeziza in the family Pezizaceae, in a species group along with Eremiomyces Trappe & Kagan-Zur, Hapsidomyces venezuelensis J.C. Krug & Jeng and Peziza phyllogena Cooke, next to the Terfezia-Tirmania, P. depressa, P. saniosa and Peziza ostracoderma clades (Fig. 73). Eremiomyces echinulatus (Trappe & Marasas) Trappe & Kagan-Zur and E. magnisporus G. Moreno et al. cluster with high support with Sarcopeziza sicula showing a high level of genetic similarity. Sarcopeziza sicula demonstrates a distant relationship to Peziza vesiculosa Bull. and Sarcosphaera coronaria (Jacq.) J. Schröt. which are type species of the genera where it was previously combined (Inzenga 1869; Patouillard 1904), suggesting taxonomic affinity outside of those genera.
Besides molecular evidence, Sarcopeziza sicula is different from Peziza sensu stricto [a core species group gathered around the type species, P. vesiculosa, cf. Hansen et al. (2001, 2002, 2005)], primarily by faintly visible, film-like periascal mucus that is revealed by moderately diffusely amyloid reaction in iodine mounts, gradually decreasing in strength downwards. Whereas, all species from Peziza sensu stricto have opercular (apical) thick accumulation of heavily amyloid mucus giving strong ring-like amyloid reaction confined to the ascal tops. Agnello et al. (2018) define asci as diffusely amyloid along the entire length of the asci and strongly amyloid at the apex (type I, cf. Hansen et al. 2001). Judging to both the authors’ microphotograph and own observations, the type of amyloidity belongs to type III instead of type I. The fine structure of the ascus wall lacks strong apical indentation ring and lentiform operculum in Sarcopeziza sicula, characters that are constantly present in all studied species in Peziza core species group as well as in ascobolacean representatives and species of Peziza succosa and P. succosella clade (pers. data, cf. Samuelson 1978).
Moreover, Sarcopeziza sicula is also different by ontogenetically and cytochemically comparatively stable multi-guttulate lipid configuration in fully developed ascospores and violet-purplish in flesh. Contrary to Agnello et al. (2013, 2018), the spores in this study are recognized as multi-guttulate when fully mature and in living state whose lipids coalesce in dead state towards uni-guttulate or more rarely bi-guttulate pattern. The process of lipid body coalescence in dead spores is regularly observed throughout Ascomycota (Baral 1992). The microphotograph of ripe asci in Agnello et al. (2018, Fig. 2f), is clearly displaying multi-guttulate pattern of living and fully mature ascospores, while Fig. 2c, d in Agnello et al. (2013) resemble dead ascospores containing subsequently coalesced lipid bodies.
Furthermore, the authors presumed that ascospore ornamentation is not constant character in this species. It is quite possible that ornamentation in this species is normally formed after spore ejection and extraascal dormancy. Both available ascomata from the epitype produced large number of dormant ascospores whose fine ornamentation is clearly visible in lactic acid cotton blue under oil-immersion.
From the genus Sarcosphaera Auersw. (its type and the only species S. coronaria), Sarcopeziza sicula is different by the absence of paraphysal internal content, different original lipid body configuration in mature ascospores (multi-guttulate vs. bi-guttulate), intensively pigmented flesh and the excipular surface, substipitate to stipitate apothecia, and lack of discernable odour (S. coronaria has somewhat chlorine scent).
Agnello et al. (2018) describe the subhymenium as 150–180 µm thick layer but we detected it as very reduced and unclearly discerned tissue from the upper medulla using high contrasting techniques on a number of entire, thin sections (ranging from hymenium up to ectal excipular layer) from the epitype material.
The closest pezizacean relatives of Sarcopeziza sicula revealed in our phylogenetic analysis are species of the genus Eremiomyces, Hapsidomyces venezuelensis and Peziza phyllogena (a member of the genus Peziza sensu lato). All so far produced DNA based phylogenies (along with the present one), however, clearly showed polyphyly in the genus Peziza, where numerous species attributed to the other genera (especially of hypogeous representatives) build up monophyletic clades along with certain members ascribed to a genus Peziza. Therefore, all species putatively ascribed to a genus Peziza should be carefully restudied using integrative taxonomic approach. Sarcopeziza sicula is readily recognizable from all other currently known genera in the family Pezizaceae by specific combination of macroscopical features (semi-hypogeous sarcosphaeroid apothecia with unique pigmentation), ecology (subpsammophilic and/or xeric habitats in thermo-Mediterranean zone) and microscopy, principally cytochemistry and fine structure of the asci and multi-guttulate lipid body configuration of the ascospores, characters that were proved to be informative for differentiation in Pezizaceae in a previous study (Pfister et al. 2009).
There is a number of similarities in excipular structures of semi-hypogeous species Sarcopeziza sicula and peridial/glebal microscopic structures of the closest relative, hypogeous species Eremiomyces magnisporus (cf. Alvarado et al. 2011): (1) the same texture in peridia of E. magnisporus and cortical layer of ectal excipulum in S. sicula; (2) cytoplasmic pigments in peridial cells of E. magnisporus and cortical layer cells of S. sicula; (3) same texture in glebal sterile veins in E. magnisporus and medullary excipulum in S. sicula; and (4) lipid-like globules in glebal sterile vein cells in E. magnisporus and medullary excipular cells in S. sicula.
The other hypogeous species Eremiomyces echinulatus which is a type species of the genus Eremiomyces, and E. innocentii Ant. Rodr. & Bordallo as well as phylogenetically close Kalaharituber pfeilii (Hennings) Trappe & Kagan-Zur (cf. Ferdman et al. 2005 and Crous et al. 2017) also possess very similar structures in peridial and glebal cells and texture. Very similar excipular texture exists in Peziza phyllogena too (pers. data) and in wider apothecial marginal area of Hapsidomyces venezuelensis (cf. Krug and Jeng 1984), two epigeous phylogenetically closest relatives. Nearly the same fine opercular structure in cotton blue mount is found in both Sarcopeziza sicula and Peziza phyllogena (pers. data). On the other hand, genera Eremiomyces and Kalaharituber Trappe & Kagan-Zur differ from Sarcopeziza sicula by their entirely hypogeous living strategy developing permanently closed stereothecia with solid gleba and producing perfectly globose coarsely aculeate ascospores.
The closest apothecial representatives have different living strategy also viz. fimicolous in Hapsidomyces venezuelensis and phyllophilous in Peziza phyllogena versus terricolous-subpsammophilous in Sarcopeziza sicula. Ascal amyloidity is also different: thin but wider and moderately strong apical ring-like reactive zone exist in Peziza phyllogena extending downwards with abruptly weakened intensity (pers. data) while Hapsidomyces venezuelensis has weakly amyloid reaction over entire ascus length (Hansen et al. 2001). Hapsidomyces venezuelensis has ascospores very much resembling those of Eremiomyces (though spinulose-reticulate). While, Peziza phyllogena has ellipsoid ascospores (as in Sarcopeziza sicula), though rather coarsely sculptured by cyanophilic verrucae and higher tuberculae around the polar areas (pers. data).
Class Sordariomycetes O.E. Erikss. & Winka
The classification of the families in Sordariomycetes follow Maharachchikumbura et al. (2016), Hongsanan et al. (2017) and Wijayawardene et al. (2017a, 2018a).
Subclass Diaporthomycetidae Senan. et al.
Atractosporales H. Zhang et al.
Conlariaceae H. Zhang et al.
Conlariaceae was introduced by Zhang et al. (2017a) in the order Atractosporales to accommodate the freshwater genus Conlarium, with two species, C. aquaticum W. Dong et al. and C. duplumascosporum F. Liu & L. Cai. We follow the latest treatment in Zhang et al. (2017a) and the new species, C. thailandense is introduced based on morphological characteristics coupled with phylogenetic analysis of combined LSU, SSU and ITS sequence data (Fig. 74).
Maximum likelihood (ML) majority rule consensus tree for the combined LSU, SSU and ITS sequence alignment for Atractosporales. The RAxML bootstrap support values (ML) greater than 70% and Bayesian posterior probabilities (BYPP) greater than 0.95 BYPP are given at the nodes (ML/BYPP). The ex-type strains are in bold and the new isolates are in blue. The tree is rooted to Lentomitella cirrhosa (ICMP 15131)
Conlarium F. Liu & L. Cai
Conlarium was introduced by Liu et al. (2012a) from a freshwater habitat in China to accommodate C. duplumascosporum with sexual and asexual morphs (Liu et al. 2012a). Recently, Zhang et al. (2017a) introduced the second freshwater taxon C. aquaticum from Thailand.
Conlarium thailandense X.D. Yu, H. Zhang & K.D. Hyde, sp.nov.
Index Fungorum number: IF555288; Facesoffungi number: FoF04830, Fig. 75
Etymology: Named after the country where it was collected, Thailand
Holotype: MFLU 17-1711
Saprobic on dead wood in terrestrial habitat. Sexual morph Undetermined. Asexual morph Hyphomycetous. Colonies sporodochial, broadly punctiform, gregarious, raised, dark brown to black. Mycelium mostly immersed on natural substratum, comprising branched, pale brown to hyaline, smooth and thin-walled hyphae. Conidiophores absent or reduced to conidiogenous cells. Conidiogenous cells monoblastic, holoblastic, integrated, determinate, cylindrical, hyaline, smooth, up to 5.5 μm long. Conidia 25–45 × 17–33 μm (\( \bar{x} \) = 35.9 × 26.9 μm, n = 20), acrogenous, solitary, dry, mostly irregular, subglobose to ellipsoidal, brown to dark brown, clathrate, muriform, 4–8-transversely septate, 4–6-longitudinally septate, slightly constricted at the septa, smooth and thin-walled, with a small, sub-rounded, dark brown ornamentation on the surface of each cell. Conidial secession schizolytic.
Culture characteristics: Colonies on PDA, irregular in shape, reaching 10–20 mm in 6 weeks at room temperature, black from above and below, umbonate, rough, dense, edge undulate.
Material examined: THAILAND, Chiang Rai Province, Mae Fah Luang University, on dead wood, 17 July 2017, S. Boonmee, DP6 (MFLU 17-1711, holotype), ex-type living culture, MFLUCC 17-2349.
GenBank numbers: ITS = MH624129, LSU = MH624127, SSU = MH624128.
Notes: Conlarium thailandense is similar to the asexual morph of C. duplumascosporum and C. aquaticum. They all have monoblastic, holoblastic conidiogenous cells and mostly irregular, brown, clathrate, muriform conidia (Liu et al. 2012a). However, they can be easily distinguished by the number of septa (0–2-transversely septate, 0–1-longitudinally septate in C. duplumascosporum, 6–12-transversely septate, 4–10-longitudinally septate in C. aquaticum and 4–8-transversely septate, 4–6-longitudinally septate in C. thailandense) and conidial size (15.5–35 × 11–26.5 μm in C. duplumascosporum, 45–70 × 20–57 μm in C. aquaticum and 25–45 × 17–33 μm in C. thailandense) (Liu et al. 2012a; Zhang et al. 2017a). Apart from number of septa and conidial size, a number of air-bubbles were also observed on mature conidia of C. thailandense, which have not been reported in C. aquaticum and C. duplumascosporum. Phylogenetic analyses of a combined LSU, SSU and ITS sequence alignment based on maximum likelihood and Bayesian inference show that C. thailandense is sister to C. aquaticum. A comparison of ITS pairwise indicates that C. thailandense differs from C. aquaticum in 13 base positions and is therefore novel following the guidelines of Jeewon and Hyde (2016).
Diaporthales Nannf.
Cytosporaceae Fr.
The family Cytosporaceae was introduced by Fries (1825) and recognized a family in Diaporthales, which comprises phytopathogens and saprobes (Wehmeyer 1975; Barr 1978; Eriksson 2001; Castlebury et al. 2002). Maharachchikumbura et al. (2015, 2016) listed 13 genera under Cytosporaceae. Senanayake et al. (2017) accepted five genera, which belong to Cytosporaceae based on morphological characteristics viz. Cytospora Ehrenb., Pachytrype Berl. ex M.E. Barr et al., Paravalsa Ananthap., Xenotypa Petr. and Waydora B. Sutton. Wijayawardene et al. (2018a) listed only genera Cytospora and Waydora in Cytosporaceae.
Cytospora Ehrenb.
Cytospora was introduced by Ehrenberg (1818) as type genus of the family Cytosporaceae in Diaporthales (Wehmeyer 1975; Barr 1978; Eriksson 2001; Castlebury et al. 2002; Norphanphoun et al. 2017). The genus was introduced as important pathogens, which cause cankers and dieback disease on branches of a wide range of hosts worldwide (Adams et al. 2005, 2006; Hyde et al. 2017, 2018b; Norphanphoun et al. 2017, 2018). There are 630 epithets for Cytospora (Index Fungorum 2019) with an estimated 110 species in Kirk et al. (2008). Norphanphoun et al. (2017) provided a comprehensive account of the morphology and molecular background of the genus. Several new taxa have recently been introduced (Hyde et al. 2016, 2017, 2018b; Norphanphoun et al. 2017, 2018; Senanayake et al. 2017; Tibpromma et al. 2017; Zhu et al. 2018). In this study, a novel species of Cytospora is introduced from Ulmus pumila L. based on molecular data coupled with morphological characteristics (Fig. 76).
Phylogram generated from maximum likelihood analysis based on a combined ITS, LSU, and ACT sequence dataset. The tree is rooted to Diaporthe eres (AFTOL-ID 935). Maximum likelihood bootstrap values ≥ 50% are given at the nodes. Newly generated sequence is indicated in blue. Ex-type strains are indicated in bold
Cytospora ulmicola Norphanphoun, Bulgakov, T.C. Wen & K.D. Hyde, sp. nov.
Index Fungorum number: IF555487; Facesoffungi number: FoF05184, Fig. 77
Cytospora ulmicola (MFLU 17-2080, holotype). a Herbarium label and specimens. b, d, e Appearance of conidiostromata on branches of Ulmus pumila. c, f Colonies on PDA (c = from above, f = from below). g Transverse sections through conidiostroma to show an arrangement of the locules. h Longitudinal section through conidiostroma. i Ostiolar neck. j Conidiostroma wall. k Conidiogenous cells. l Conidia. Scale barsb = 1000 µm, e = 100 µm, g, h = 200 µm, i = 50 µm, j = 20 µm, k, l = 10 µm
Etymology: The specific epithet “ulmicola” refers to the host plant genus Ulmus, on which the fungus was first collected.
Holotype: MFLU 17-2080
Associated with twigs and branches of Ulmus pumila L. (Ulmaceae). Sexual morph Undetermined. Asexual morphConidiostromata 1000–1700 × 500–800 µm diam., semi-immersed in host tissue, solitary, erumpent, scattered, discoid, circular to ovoid, with multi-loculate, pycnidial, embedded in stromatic tissue, with ostiole. Ostioles 200–500 µm long, with an ostiolar neck. Conidiomata walls comprising a few layers of pseudoparenchymatous cells of textura angularis, with inner layer thin, pale brown, outer layer brown to dark brown. Conidiophores unbranched or occasionally branched at the base, formed from the inner layer of the pycnidial wall. Conidiogenous cells (6–)9–14 × 1.3–1.8(–2.8) μm (\( \bar{x} \) = 11 × 2 μm, n = 15), blastic, enteroblastic, flask-shaped, phialidic, hyaline, and smooth-walled. Conidia (7–)8–9.5 × 1.5–1.7(–1.9) µm (\( \bar{x} \) = 8.5 × 1.6 µm, n = 30), unicellular, hyaline, oblong to allantoid, smooth-walled.
Culture characteristics: Conidia germinating on PDA within 12 h. Germ tubes produced from all sides. Colonies on PDA reaching 22–27 mm diam. after 2 weeks at room temperature, colonies irregular in shape, medium dense, flat to slightly raised, surface slightly rough, with small granular, to velvety, edge undulate with well-defined margin, from above pale orange to light brown at the margin, amber near the margin, with, zonate, pale yellowish to dandelion and dark amber at the centre; from below pale yellowish at the margin, with dark amber at the centre; not producing pigmentation in agar.
Material examined: RUSSIA, Rostov region, Shakhty City, street trees, on dead branch of Ulmus pumila L. (Ulmaceae), 27 May 2017, T.S. Bulgakov, T-1778 (MFLU 17-2080, holotype; PDD, isotype), ex-type living culture, MFLUCC 18-1227.
GenBank numbers: ACT = MH940216, ITS = MH940220, LSU = MH940218, TUB2 = MH986792.
Notes: Cytospora ulmicola (MFLU 17-2080) was found on a dead branch (with signs of necrosis) of Ulmus pumila in European Russia. Cytospora ulmicola resembles Cytospora species in having an ostiolar neck with multi-loculate conidiostromata and unicellular, oblong to allantoid conidia. Phylogenetic analysis based on a combined ITS, LSU and ACT sequence dataset indicates that C. ulmicola clusters with C. cotini Norph. et al. (MFLUCC 14-1050), C. ampulliformis Norph. et al. (MFLUCC 16-0583), C. gelida Norph. et al. (MFLUCC 16-0634), and C. ceratosperma (Tode) G.C. Adams & Rossman (MFLUCC 16-0625) (Fig. 76). However, C. ulmicola can be distinguished from related species based on molecular data. A comparison of nucleotide polymorphisms of ITS and ACT shows that C. ulmicola differs from C. cotini with one polymorphism of ITS; from C. ampulliformis with one polymorphism of ITS and ten polymorphisms of ACT; from C. gelida with four polymorphisms of ITS and 13 polymorphisms of ACT; and from C. ceratosperma with five polymorphisms of ITS and eight polymorphisms of ACT. Therefore, following the guidelines of Jeewon and Hyde (2016) we introduce it as a new species.
Melanconiellaceae Senan. et al.
Melanconiellaceae was introduced by Senanayake et al. (2017) including phytopathogenic and saprobic species to accommodate Greeneria Scribn. & Viala, Melanconiella Sacc. and Microascospora Senan. & K.D. Hyde in the order Diaporthales (Senanayake et al. 2018; Braun et al. 2018). We introduce a new monotypic genus Septomelanconiella to the family Melanconiellaceae to accommodate a single species collected on rose apple in Thailand.
Septomelanconiella Samarak. & K.D. Hyde, gen. nov.
Index Fungorum number: IF555301; Facesoffungi number: FoF04849
Etymology: The generic epithet “Septomelanconiella” reflects the septation of the conidia and parallel morphology to Melanconiella.
Saprobic on Syzygium samarangense in terrestrial habitats. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata pycnidial, immersed in the host, partially erumpent at maturity, mostly solitary or confluent, subglobose to irregular, to flattened and collabent, light brown. Conidiomata walls comprising 2–3 layers of hyaline cells, of textura angularis at the base, with light brown, thin outer layer. Conidiophores mostly reduced to conidiogenous cells, few with conidiophore and cylindrical conidiogenous cells. Conidiogenous cells enteroblastic, phialidic, integrated to discrete, cylindrical, determinate, hyaline, and finely roughened. Conidia cylindrical to clavate, one guttulate, becoming two guttules, hyaline, 1-septate when immature; mature conidia cylindrical to clavate, straight or slightly curved, brown, 1-euseptate, more often with 6 unequal lumina, guttulate, dark brown at the base.
Type species: Septomelanconiella thailandica Samarak. & K.D. Hyde
Notes: Septomelanconiella thailandica constitutes an independent lineage basal to Melanconiella syzygii Crous & M.J. Wingf. Despite average phylogenetic support, Septomelanconiella is distinguished from other species as it is characterised by 1-euseptate and luminate conidia. Septomelanconiella is similar to Melanconiella in having finely verrucose, brown, mature conidia.
Septomelanconiella thailandica can be phylogenetically distingushed from the other genera in Melanconiellaceae as circumscribed by Senanayake et al. (2017). Melanconiella syzygii was isolated from a prominent leaf spot of Syzygium and is characterized by 2–3 layers of peridium, 1–2-septate conidiophores and hyaline to light brown aseptate conidia (Crous et al. 2016a). Multigene phylogenetic analyses also reveal that Septomelanconiella thailandica is separated from Melanconiella syzygii. Wijayawardene et al. (2016) described Gloeocoryneum and Stegonosperiopsis and referred them to Ascomycota, genera incertae sedis. They have similar brown, septate conidia as in Septomelanconiella thailandica. However, Gloeocoryneum differs in having 2–5 conidial septa, while Stegonosperiopsis is characterized by cylindrical conidiophores. Based on morphology and phylogeny in this study, Septomelanconiella thailandica is introduced as a new genus and species (Figs. 78, 79).
Phylogram generated from maximum likelihood (RAxML) based on ITS, LSU and RPB2 partial sequence data analyses of 23 taxa and Pseudoplagiostoma oldii (CBS 124808) as an outgroup taxon. Bootstrap values for maximum parsimony (green) and maximum likelihood (blue) ≥ 50% and Bayesian posterior probabilities (black) ≥ 0.90 are given at the nodes. The newly generated sequence is in blue. The ex-type strains are in bold. The scale bar represents the expected number of nucleotide substitutions per site
Septomelanconiella thailandica (MFLU 18-0793, holotype). a Host. b Conidiomata on the substrate. c Vertical section of conidioma. d–h Conidiophores, conidiogenous cells and developing conidia. i–l Conidia. m Germinated conidium. n Culture on PDA from above after 16 days. o Culture on PDA from below after 16 days. Scale barsb = 1000 µm, c = 100 µm, m = 50 µm, d–l = 20 µm
Septomelanconiella thailandica Samarak. & K.D. Hyde, sp. nov.
Index Fungorum number: IF555302; Facesoffungi number: FoF04850, Fig. 79
Etymology: Name based on the country from which this species was collected, Thailand.
Holotype: MFLU 18-0793
Saprobic on recently dead twigs of Syzygium samarangense. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 360–500 μm diam., 130–200 μm high, pycnidial, immersed, partially erumpent at maturity, solitary or confluent, subglobose to irregular, to flattened and collabent, light brown. Conidiomata walls 28–37.5 μm wide, comprising 2–3 layers of hyaline cells, of textura angularis at the base, with light brown, thin outer layer. Conidiophores mostly reduced to conidiogenous cells, few with conidiophore and cylindrical conidiogenous cells. Conidiogenous cells 10.3–15.9 × 4.4–7.3 μm (\( \bar{x} \) = 13.2 × 5.9 μm, n = 20), enteroblastic, phialidic, integrated or discrete, cylindrical, determinate, hyaline, finely roughened. Conidia when immature, cylindrical to clavate, one guttulate, becoming two guttules, hyaline, one septate; mature conidia 37–52 × 15–23 μm (\( \bar{x} \) = 43.8 × 19.1 μm, n = 40), cylindrical to clavate, straight or slightly curved, brown, 1-euseptate, with 6 unequal lumina, guttulate, dark brown at base with opening 1.8–3.6 μm diam. (\( \bar{x} \) = 2.9 μm, n = 30).
Culture characteristics: Conidia germinating on PDA within 24 h, germ tubes produced from central part, often three hyphae. Colonies on PDA reaching 37 mm diam. in 2 weeks at 25 °C, hairy, white, superficial, rough surface, irregular edge, reverse yellowish brown.
Material examined: THAILAND, Chiang Rai Province, Muang District, Nang Lae, on dead twigs of Syzygium samarangense (Blume) Merr. & L.M. Perry (Myrtaceae), 25 January 2018, M.C. Samarakoon, SAMC091 (MFLU 18-0793, holotype; KUN-HKAS 102320, isotype), ex-type living culture, MFLUCC 18-0518, ICMP.
GenBank numbers: ITS = MH727706, LSU = MH727705, RPB2 = MH752072.
Pseudoplagiostomataceae Cheew. et al.
The monotypic family Pseudoplagiostomataceae was introduced by Cheewangkoon et al. (2010) to accommodate a cryptosporiopsis-like fungus isolated from Eucalyptus. Pseudoplagiostomataceae resembles Gnomoniaceae G. Winter based on morphological characters of its sexual morph, such as solitary, immersed, lacking stromatic ascomata with lateral beaks, asci with a distinct apical ring and 1-septate ascospores (Sogonov et al. 2008; Senanayake et al. 2017). Cheewangkoon et al. (2010) showed phylogenetically that Pseudoplagiostomataceae is closer to families with well-developed stromatic tissue such as Diaporthaceae Höhn. ex Wehm. and Pseudovalsaceae M.E. Barr, or families with stromatic and non-stromatic tissues such as Valsaceae Tul. & C. Tul. and Sydowiellaceae Lar.N. Vassiljeva. However, in our phylogenetic analyses it forms a fully-supported lineage, sister to Apoharknessiaceae Senan. et al. (Fig. 80).
Phylogenetic tree generated by maximum likelihood analysis of a combined ITS, LSU, TUB2 and TEF1-α sequence dataset of Pseudoplagiostomataceae and related families. Related sequences were obtained from GenBank. Thirty one strains are included in the analyses and the tree is rooted with Phaeoacremonium novae-zealandiae (CBS 110156) and P. hungaricum (CBS 123036). Tree topology of the ML analysis was similar to the MP and BI. The best scoring RAxML tree with a final likelihood value of -15510.220382 is presented. The matrix had 1138 distinct alignment patterns, with 50.26% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.226739, C = 0.283205, G = 0.257579, T = 0.232477; substitution rates AC = 1.368247, AG = 2.869863, AT = 1.349096, CG = 0.967468, CT = 5.138587, GT = 1.000000; gamma distribution shape parameter α = 0.391573. RAxML (black) and maximum parsimony (blue) bootstrap support values ≥ 60% are shown respectively above the nodes. Bayesian posterior probabilities ≥ 0.95 BYPP indicated in green. The scale bar indicates 0.06 changes. The ex-type strains are in bold and new isolates in blue bold
Pseudoplagiostoma Cheew. et al.
We follow the latest treatment and updated accounts of Pseudoplagiostoma in Cheewangkoon et al. (2010), Suwannarach et al. (2016) and Du et al. (2017). Based on phylogenetic analyses of a combined ITS, LSU, TUB2 and TEF1-α dataset coupled with morphological characteristics, a novel species, P. mangiferae is introduced.
Pseudoplagiostoma mangiferae Dayarathne, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF555434; Facesoffungi number: FoF05111, Fig. 81
Pseudoplagiostoma mangiferae (KUN-HKAS 102244, holotype). a Leaf blight symptom on living leaf of Mangifera sp. b Conidiomata on host surface. c, d Section through conidiomata. e, f Conidiomata walls at the side and the base respectively. g Paraphyses. h–k Conidiogenous cells. l–n Conidia. Scale barsc–e = 50 μm, f, g, l = 20 μm, h–k, m, n = 10 μm
Etymology: The specific epithet “mangiferae” refers to the host genus Mangifera, of which the species was first collected.
Holotype: KUN-HKAS 102244
Associated with the leaf blight symptom on Mangifera sp. having amphigenous, subcircular to irregular, medium brown with blackish brown, reverse medium brown, surrounded by a purple-brown margin, which is dark brown in reverse. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 70–140 µm high, 90–150 μm diam. (\( \bar{x} \) = 109 × 125 μm, n = 10), medium to dark brown, pycnidial with pale yellow drops of exuding conidia; subglobose, subcuticular to epidermal, with central rupture, breaking through plant tissue. Conidiomata walls 5–10 µm wide, thin-walled, composed of 2–4 layers of yellowish brown, pseudoparenchymatous cells, of textura angularis, intermixed with the host cells at the base and side. Paraphyses 0.8–2 μm wide, branched, septate, hyaline. Conidiophores absent. Conidiogenous cells 5–11 × 3.2–12.6 μm (\( \bar{x} \) = 9 × 6 μm, n = 30), proliferating enteroblastic, appearing as phialides with periclinal thickening and collarette, or with percurrent proliferation in the apical part; discrete, arising from the inner cell layer, hyaline, smooth, cylindrical to ampulliform, wider at the base, straight. Conidia 18–24 × 11–14 μm (\( \bar{x} \) = 22 × 13 μm, n = 50), hyaline, ellipsoidal, guttulate, smooth, thick-walled, aseptate, straight or slightly curved, frequently slightly narrow at the middle, with obtuse apex; base tapering to flat protruding scar.
Culture characteristics: Colonies on PDA reaching 80–85 mm diam. after 4 weeks at 20–25 °C, sparse to medium sparse, circular, flat, surface slightly rough with tufts of hyphae, edge entire, woolly to cottony, radiating with sparse mycelia in the middle part; from above, pink-white to cream, from below, pale yellowish; not producing pigmentation in agar.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Jinghong, Nabanhe, associated with leaf blight symtom on living leaf of Mangifera sp. (Anacardiaceae), 21 November 2015, R. Phookamsak, XB010 (KUN-HKAS 102244, holotype), ex-type living culture, KUMCC 18-0179.
GenBank numbers: ITS = MK084824, LSU = MK084825, TEF1-α = MK084822, TUB2 = MK084823.
Notes: Morphological characteristics clearly distinguish Pseudoplagiostoma mangiferae from other Pseudoplagiostoma species. The conidiogenous cells of P. mangiferae (5–11 × 3.2–12.6 μm) are wider than those of P. eucalypti (6–15 × 2–6 μm) (Cheewangkoon et al. 2010). Pseudoplagiostoma variabile is distinguished from P. mangiferae by its subglobose to bean-shaped, variable conidia shape (Cheewangkoon et al. 2010). The conidia of P. corymbiae (14–19 × 7–10 μm), P. eucalypti (14–22 × 6–11 μm), P. oldie (11–20 × 6–11 μm) and P. variabile (6.5–19 × 6.5–10.5 μm) are shorter than those of P. mangiferae (18.8–24 × 11.3–14.2 μm) while P. dipterocarpi (14–36 × 7–11 μm) has longer conidia (Cheewangkoon et al. 2010; Crous et al. 2012; Suwannarach et al. 2016). Furthermore, P. oldie is distinguished from P. dipterocarpi by its conidia turning brown when mature (Cheewangkoon et al. 2010). According to our multigene analyses, P. mangiferae shows a close phylogenetic relationship with P. dipterocarpi (Fig. 80). However, they form well-separated lineages with high support (94% ML, 98% MP and 0.97 BYPP). A comparison of ITS nucleotide bases shows that P. mangiferae differs from P. dipterocarpi in 67/512 bp (13.1%). We therefore, introduce P. mangiferae as a new species following the guidelines of Jeewon and Hyde (2016).
Schizoparmaceae Rossman
Schizoparmaceae was introduced by Rossman et al. (2007) and is typified by Schizoparme Shear. The family also includes Pilidiella Petr. & Syd., the asexual morph of Schizoparme and the closely related Coniella Höhn (Rossman et al. 2007). In a recent revision, these three genera were synonymized under the old name, Coniella (Alvarez et al. 2016).
Coniella Höhn.
The genus Coniella was introduced by von Höhnel (1918) and is typified by Coniella pulchella Höhn. (= C. fragariae (Oudem.) B. Sutton). This genus comprises endophytes, saprobes and plant pathogens (Van Niekerk et al. 2004; Alvarez et al. 2016; Chethana et al. 2017). A new host record collected on Prunus armeniaca L. from Russia is reported for C. vitis, which has been previously reported as a plant pathogen from Vitis vinifera L. (Vitaceae; Chethana et al. 2017) (Fig. 82).
Phylogenetic tree generated by maximum parsimony analysis of combined ITS, LSU, HIS3 and TEF1-α sequence dataset of Coniella species. Related sequences were obtained from GenBank. Forty four strains are included in the analyses, which comprise 2881 characters including gaps. Single gene analyses were carried out and compared with each species, to compare the topology of the tree and clade stability. The tree is rooted with Melanconiella sp. (CBS 110385). The maximum parsimonious dataset consisted of 2109 constant, 558 parsimony-informative and 214 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 2551 steps (CI = 0.509, RI = 0.708, RC = 0.360, HI = 0.491) in the first tree. Maximum parsimony bootstrap support values ≥ 70% are shown near the nodes. Bayesian posterior probabilities ≥ 0.95 BYPP indicated as thickened black branches. The scale bar indicates 10 changes. The ex-type strains are in bold
Coniella vitis Chethana, J.Y. Yan, X.H. Li & K.D. Hyde, Pl. Dis. 101: 2129 (2017).
Facesoffungi number: FoF02722, Fig. 83
Coniella vitis (MFLUCC 18-0093). a Host surface from which the saprobe was isolated. b Immature white pycnidia on PDA. c Mature black pycnidia with hyaline spore mass on PDA. d Conidiogenous cells. e Periclinal thickening at the apex of the conidiogenous cells. f–i Different shapes of conidia. j Upper-view (right) and the reverse view (left) of the colony on PDA. Scale barsa = 2 mm, c = 500 μm, d, e = 10 μm, f–i = 5 μm
Holotype: CHINA, Yanqing, Beijing, on white rot-infected berries of Vitis vinifera (Vitaceae), 13 May 2015, X–H Li, JZB3700001 (MFLU 16-2677), ex-type living culture, MFLUCC 16-1399.
Saprobic or pathogenic on branches and twigs of Prunus armeniaca L. (Rosaceae). Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 200–370 μm diam. (\( \bar{x} \) = 304.2 μm, n = 20), pycnidial, solitary, submerged in PDA, globose to slightly depressed globose, with verruculose wall, initially hyaline becoming dark brown to black at maturity, with a central ostiole. Conidiophores 5–9 × 2–4 μm (\( \bar{x} \) = 7.8 × 3.3 μm, n = 10), formed on a dense, cushion-like aggregation of hyaline cells, subcylindrical, hyaline, smooth, simple or branched below, mostly reduced to conidiogenous cells. Conidiogenous cells 9.5–16 × 2.5–4 μm (\( \bar{x} \) = 13.2 × 3.3 μm, n = 10), phialidic, percurrently proliferating, hyaline, simple, slender, smooth with a prominent periclinal thickening. Conidia 8.5–12 × 3.5–6.5 μm (\( \bar{x} \) = 10.2 × 4.9 μm, n = 40), l:w ratio 2, inequilateral, hyaline when immature becoming pale brown, aseptate, straight to slightly curved, narrowly ellipsoidal, often somewhat flattened on one side, both sides gradually tapering towards the subobtusely rounded apex, subtruncate base, smooth-walled, and multi-guttulate with one or two prominent guttules.
Culture characteristics: Colonies on PDA, reaching 8 cm diam. after 5 days at 28 °C, effuse, flat, mostly immersed mycelium, aerial mycelium mostly sparse, crenulated edges with concentric rings, white on surface and buff in reverse. Conidia in mass, hyaline.
Material examined: RUSSIA, Rostov Region, Shakhty City, on dead twigs (with signs of necrosis) of Prunus armeniaca L. (Rosaceae), 1 March 2016, T.S. Bulgakov, T1243 (MFLU 16-1537), living culture, MFLUCC 18-0093.
GenBank numbers: ITS = MH569466, LSU = MH569461, HIS3 = MH645901, TEF1-α = MH645902.
Known hosts and distribution: Vitis vinifera (China) and Prunus armeniaca (Russia) (Chethana et al. 2017; Farr and Rossman 2018).
Notes: Coniella vitis has been reported from Vitis vinifera as causing grape white rot in China (Chethana et al. 2017; Jayawardena et al. 2018). Based on our phylogenetic analysis of a combined ITS, LSU, HIS3 and TEF1-α sequence dataset of Coniella species (Fig. 82), our strain MFLUCC 18-0093 clusters together with the ex-type strain of Coniella vitis (MFLUCC 16-1399) with high bootstrap and Bayesian probabilities (100% MP and 1.00 BYPP). When comparing our strain with the type specimen of C. vitis (MFLUCC 16-1399), they are similar in morphology. However, our strain has slightly larger conidiomata, and larger conidiogenous cells compared to the type strain (Chethana et al. 2017).
Diaporthomycetidae, families incertae sedis
Distoseptisporaceae K.D. Hyde & McKenzie
Distoseptisporaceae was introduced by Su et al. (2016) and classified in Diaporthomycetidae, families incertae sedis by Wijayawardene et al. (2018a). The family consists of one hyphomycetous genus, Distoseptispora which is typified by D. fluminicola McKenzie et al. Seventeen Distoseptispora species have been introduced through previous studies, out of which eleven species are from freshwater, and five species are from terrestrial habitats (Hyde et al. 2016; Su et al. 2016; Xia et al. 2017; Luo et al. 2018; Tibpromma et al. 2018; Yang et al. 2018c) (Fig. 84).
Phylogenetic tree generated by maximum likelihood analysis of a combined ITS, LSU and TEF1-α sequence dataset of Distoseptisporaceae and other related families in Diaporthomycetidae. Related sequences were obtained from GenBank. Fifty one strains are included in the analyses, which comprised 2409 characters including gaps. Single gene analyses were carried out and compared with each species, to compare the topology of the tree and clade stability. The tree is rooted with Sordaria fimicola (SMH 4106, FGSC 2918). Tree topology of the ML analysis was similar to the MP and BI. The best scoring RAxML tree with a final likelihood value of -17019.410141 is presented. The matrix had 1186 distinct alignment patterns, with 38.62% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.237054, C = 0.262859, G = 0.290202, T = 0.209884; substitution rates AC = 1.172995, AG = 2.180726, AT = 1.594744, CG = 1.095103, CT = 6.416481, GT = 1.000000; gamma distribution shape parameter α = 0.352856. The maximum parsimonious dataset consisted of 1183 constant characters, 751 parsimony-informative and 475 parsimony-uninformative characters. The parsimony analysis of the data matrix resulted in the maximum of two equally most parsimonious trees with a length of 2985 steps (CI = 0.590, RI = 0.726, RC = 0.428, HI = 0.410) in the first tree. RAxML (black) and maximum parsimony (blue) bootstrap support values ≥ 60% are shown respectively above the nodes. Bayesian posterior probabilities ≥ 0.95 BYPP indicated in green. The scale bar indicates 0.1 changes. The ex-type strains are in bold and a new isolate is in blue
Distoseptispora thysanolaenae Goonas., Dayarathne, Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF555408; Facesoffungi number: FoF05011, Fig. 85
Distoseptispora thysanolaenae (KUN-HKAS 102247, holotype). On host substrate: a Appearance of colonies on host surface. b, d, e Conidiophores. c, f Conidiophores with attached conidia. g–i Variable shapes of conidia. In vitro: j Sporulation of conidia on PDA after 4 weeks. k–m Conidiophores with attached conidia. n–s Variable shapes of conidia. Scale barsa = 500 μm, b–i, k–r = 20 μm, s = 50 μm
Etymology: Refers to the host from which the species was isolated
Holotype: KUN-HKAS 102247
Saprobic on dead culms of Thysanolaena maxima. Sexual morph Undetermined. Asexual morph Hyphomycetous. Colonies effuse, brown to dark brown, hairy or fluffy, arising from subiculum, with tufts. Mycelium partly superficial, composed of branched, septate, smooth, brown hyphae. Conidiophores 30–80 × 3.5–5.5 μm (\( \bar{x} \) = 52 × 4.5 μm, n = 30), macronematous, mononematous, light to dark brown, 2–8-septate, smooth, usually flexuous or sometimes straight, unbranched, cylindrical, rounded at the apex. Conidiogenous cells monoblastic, integrated, terminal, determinate, hyaline to light brown, cylindrical. Conidia 21.5–80 × 6.5–12.8 μm (\( \bar{x} \) = 53 × 9.5 μm, n = 30), acrogenous, solitary, narrow and elongated obclavate, slightly curved, 8–14-distoseptate, thick-walled, light to dark brown, paler at the apex, tapering towards a rounded, flat apex, truncate with flat base, with conspicuous spore attachment loci, guttulate, smooth-walled.
Culture characteristics: Colonies on PDA reaching 43–45 mm diam. after 4 weeks at 20–25 °C, colonies dense, circular, flat, surface slightly rough, rugose with edge entire, floccose to velvety; colony from above, greenish grey at the margin, brown-grey at the centre, from below dark grey at the margin, black at the centre; not producing pigmentation in PDA. Sporulation on PDA after two months. Conidiophores 10–30(–47) × 3–5 μm (\( \bar{x} \) = 21.4 × 4.6 μm, n = 25), macronematous, micronematous, mononematous, light to dark brown, 1–3-septate, smooth, usually flexuous or sometimes straight, unbranched, cylindrical, rounded at the apex. Conidiogenous cells monoblastic, integrated, terminal, determinate, hyaline to light brown, cylindrical. Conidia (20–)30–70(–243) × 5–8(–11) μm (\( \bar{x} \) = 53 × 8.4 μm, n = 30), acrogenous, varied in shape, elongate cylindrical to obclavate, lanceolate, rostrate, 6–22(–50)-distoseptate, light brown to dark brown, tapering towards a rounded, sometimes bulbous apex, truncate at the base, with conspicuous spore attachment loci, smooth-walled.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Mengla County, Xishuangbanna Tropical Botanical Garden (XTBG), on dead culms of Thysanolaena maxima (Roxb.) Kuntze (Poaceae), 22 April 2017, R. Phookamsak, IS004 (KUN-HKAS 102247, holotype), ex-type living culture, KUMCC 18-0182 (IS004A), KUMCC 18-0183 (IS004B).
GenBank numbers: ITS = MK045851, LSU = MK064091, TEF1-α = MK086031.
Notes: Distoseptispora thysanolaenae is the sixth species in this genus to be introduced from a terrestrial habitat. The previously introduced species are D. martini (J.L. Crane & Dumont) J.W. Xia & X.G. Zhang, D. tectonae Doilom & K.D. Hyde, D. tectonigena Doilom & K.D. Hyde, D. thailandica Tibpromma & K.D. Hyde and D. xishuangbannaensis Tibpromma & K.D. Hyde (Hyde et al. 2016; Xia et al. 2017; Luo et al. 2018; Tibpromma et al. 2018). Distoseptispora thysanolaenae can be distinguished from these species by its 2–8-septate conidiophore that are 30–80 × 3.5–5.5 μm and narrow elongated, obclavate, light to dark brown conidia that are 21.5–80 × 6.5–12.8 μm, 8–14-distoseptate and smooth-walled. Distoseptispora martini has longer conidiophores (50–110 × 3.5–4.5 μm), shorter and wider conidia (15–20 × 11–16 μm) that are ellipsoid, oblate or subglobose (Xia et al. 2017).
Distoseptispora tectonae and D. tectonigena differ in their conidiophore dimensions (up to 40 × 4–6 μm, and up to 110 × 5–11 μm, respectively) and conidial dimensions ((90–)130–140(–170) × 13–14 μm, and 148–225(–360) × 11–12 μm, respectively) and number of septa. Distoseptispora thailandica and D. xishuangbannaensis have larger conidia (130–230 × 13.5–17 μm and 160–305 × 8–15 μm, respectively) and more septa (35–52-distoseptate, and up to 40-distoseptate, respectively) (Tibpromma et al. 2018).
Phylogenetic analyses based on a combined ITS, LSU and TEF1-α sequence dataset show that our taxon clusters with other Distoseptispora species (Fig. 84). The species is sister to D. guttulata J. Yang & K.D. Hyde (MFLUCC 16-0183). A comparison of ITS and TEF1-α nucleotide bases shows that D. thysanolaenae differs from D. guttulata with 59 nucleotide bases of ITS and 64 nucleotide bases of TEF1-α. Distoseptispora thysanolaenae shares similar morphological features with D. guttulata but can be distinguished from the latter in having shorter conidia (21.5–80 μm versus 75–130 μm; Yang et al. 2018c). Therefore, following the guidelines of Jeewon and Hyde (2016) we introduce it as a new species.
Diaporthomycetidae, genera incertae sedis
Proliferophorum G.N. Wang, H. Zhang, K.D. Hyde & Senan., gen. nov.
Index Fungorum number: IF555401; Facesoffungi number: FoF04847
Etymology: The generic epithet “Proliferophorum” refers to the proliferation of conidiophores.
Saprobic on decaying, submerged wood in freshwater habitat. Sexual morph Undetermined. Asexual morph Hyphomycetous. Colonies irregular, hairy, black, gregarious. Mycelium mostly immersed in substratum, consisting of branched, septate, subhyaline to pale brown, smooth hyphae. Conidiophores macronematous, mononematous, caespitose, cylindrical, unbranched, erect, straight and curved at the upper part, dark brown, light brown at the apex, 8–10-septate, not constricted at the septa, smooth, sometimes percurrently proliferating 1–2 times at broken ends, with few upper cells, guttulate. Conidiogenous cells holoblastic, polyblastic, terminal, sympodial, pale brown or subhyaline, with minute, truncate conidiogenous loci. Conidia fusiform to cylindrical, aseptate when young, 2–3-septate when mature, rarely up to 4-septate, slightly constricted at the septa, dark brown at central cells, pale brown at end cells, guttulate at some stage, dry, smooth.
Type species: Proliferophorum thailandicum G.N. Wang, H. Zhang, K.D. Hyde & Senan.
Notes: Proliferophorum is introduced as a monotypic genus in the subclass Diaporthomycetidae to accommodate a hyphomycetous species forming mononematous, caespitose conidiophores, sometimes percurrently proliferating 1–2 times at broken ends of conidiogenous cells and fusiform to cylindrical conidia. The genus was found on wood, similar to the genus Minimelanolocus R.F. Castañeda & Heredia, which has conidiophores that are generally conspicuous, mononematous, solitary or fasciculate, septate, erect, straight or flexuous, smooth or verrucose, and brown to dark brown with a melanised base (Castañeda-Ruiz et al. 2003). The conidia of Minimelanolocus are generally, cylindrical, naviculate, clavate, obclavate and pale brown to dark brown (Liu et al. 2015b). However, Proliferophorum differs from Minimelanolocus in its conidiophores that have a few upper guttulate cells and conidia having obvious droplets. In addition, LSU sequence data analysis shows the genus does not group with species in Chaetothyriales. Phylogenetic analyses (Fig. 86) of taxa within Diaporthomycetidae indicate that Proliferophorum forms a single lineage, between Phomatosporales Senan. et al. and Amplistromatales M.J. D’souza et al. The sporothrix-like asexual morph of Phomatosporales was reported from culture by Rappaz (1992).
Phylogram inferred from maximum likelihood analysis of a combined LSU, SSU and ITS sequence dataset using a GTRGAMMA model of evolution. Maximum likelihood bootstrap support greater than 80% and Bayesian posterior probability greater than 0.80 BYPP are indicated at the nodes. Newly introduced strain is in blue bold and type strains are in bold. The tree is rooted to Leotia lubrica (AFTOL-ID 1 = OSC 100001). The combined LSU, SSU and ITS sequence dataset comprised 67 strains (including the new strain and outgroup taxon) and manually adjusted dataset totally comprised 2228 characters including gaps. The best scoring RAxML tree was selected to represent the relationships among taxa, in which a final likelihood value of − 22073.200898
Amplistromatales comprises the families Amplistromataceae Huhndorf et al. and Catabotrydaceae Petr. ex M.E. Barr and their asexual morphs have been reported as acrodontium-like for Amplistroma Huhndorf et al. (Maharachchikumbura et al. 2015). Proliferophorum and acrodontium-like asexual morphs are distinct.
Proliferophorum thailandicum G.N. Wang, H. Zhang, K.D. Hyde & Senan., sp. nov.
Index Fungorum number: IF555400; Facesoffungi number: FoF04848, Fig. 87
Etymology: In reference to Thailand, where the species was collected.
Holotype: MFLU 17-1054
Saprobic on decaying, submerged wood in freshwater habitat. Sexual morph Undetermined. Asexual morphColonies irregular, hairy, black, gregarious. Mycelium mostly immersed in substratum, consisting of branched, septate, subhyaline to pale brown, smooth hyphae. Conidiophores macronematous, mononematous, caespitose, cylindrical, unbranched, erect, straight and curved at the upper part, dark brown, light brown at the apex, 8–10-septate, not constricted at the septa, 50–100 × 4–7 μm (\( \bar{x} \) = 82 × 5.5 μm, n = 10), smooth, sometimes percurrently proliferating 1–2 times at broken ends, with few upper cells guttulate. Conidiogenous cells holoblastic, polyblastic, terminal, sympodial, pale brown or subhyaline, up to 20–30 μm long, with minute, truncate conidiogenous loci. Conidia fusiform to cylindrical, aseptate when young, 2–3-septate when mature, rarely up to 4-septate, slightly constricted at the septa, dark brown at central cells, pale brown at end cells, guttulate at some stage, dry, smooth, 15–25 × 3–7 μm (\( \bar{x} \) = 23 × 5 μm, n = 20).
Culture characteristics: Colonies on PDA reaching 10 mm diam. within 15 days at 25 °C, colony circular, medium dense slightly raised to umbonate, surface slightly rough with edge entire, floccose to velvety, colony from above and below black; not producing pigmentation in agar.
Material examined: THAILAND, Chiang Rai Province, Longkhot Subdistrict, saprobic on decaying submerged wood in a stream, 1 September 2017, G.N Wang, 4.14.1 (MFLU 17-1054, holotype), ex-type living culture, MFLUCC 17-0293.
GenBank numbers: ITS = MK028344, LSU = MK028343, SSU = MK028345.
Subclass Hypocreomycetidae O.E. Erikss. & Winka
Glomerellales Chadef. ex Réblová et al.
Plectosphaerellaceae W. Gams et al.
Plectosphaerellaceae was introduced by Zare et al. (2007) to accommodate the genera Acrostalagmus Corda, Gibellulopsis Bat. & Maia, Musicillium Zare & W. Gams, Plectosphaerella and Verticillium Nees, with Plectosphaerella as the type genus and P. cucumeris Kleb. being the type species. Eleven genera are accepted in this family viz. Acrostalagmus, Brunneomyces A. Giraldo et al., Chordomyces Bilanenko et al., Gibellulopsis, Lectera P.F. Cannon, Longitudinalis Tibpromma & K.D. Hyde, Musicillium, Plectosphaerella, Sodiomyces A.A. Grum-Grzhim. et al., Stachylidium Link and Verticillium (Wijayawardene et al. 2018a).
Plectosphaerella Kleb.
We follow the latest treatment and updated accounts of Plectosphaerella in Zare et al. (2007), Carlucci et al. (2012), Hyde et al. (2017) and Su et al. (2017), with the updated phylogenetic analyses based on a combined ITS, LSU and TEF1-α which were retrieved from Hyde et al. (2017) and Su et al. (2017). A novel species, Plectosphaerella kunmingensis is introduced based on its holomorphic characteristics and phylogenetic affinities in Plectosphaerellaceae (Fig. 88)
Phylogenetic tree generated from maximum likelihood analysis based on a combined LSU, ITS and TEF1-α sequence dataset of the genera in Plectosphaerellaceae. Bootstrap support value of maximum likelihood (left) equal to or greater than 70% and Bayesian posterior probability equal to or greater than 0.95 BYPP are indicated above or below the nodes. Type strains are in black bold. The new species are in blue bold. The tree is rooted with Reticulascus clavatus (CBS 125296)
Plectosphaerella kunmingensis Phookamsak, J.F. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF556180; Facesoffungi number: FoF05716, Fig. 89
Plectosphaerella kunmingensis (KUN-HKAS 102246, holotype). a, b Culture characteristics on PDA (a = from above, b = from below). c Ascomata forming on PDA after 2 months. d Squash mount of the ascoma. e Section through ascoma. e, f Section through peridium. g–j Ascospores. k Ascospores stained in Melzer’s reagent. l Asci. m–p Conidiophores attached with conidia. q Conidia. Scale barsd, e = 50 μm, l = 20 μm, f, m–p, q = 10 μm, g–k = 5 µm
Etymology: The specific epithet “kunmingensis” refers to Kunming City, Yunnan, China where the species was first collected.
Holotype: KUN-HKAS 102246
Colonies forming on PDA. Mycelium composed of 1–3 µm wide, septate, branched, smooth and thin-walled, hyaline hyphae, partly immersed on PDA. Sexual morph forming black, obpyriform ascomata after 8 weeks at 20–25 °C. Ascomata 100–185 μm high, 80–110 μm diam., perithecial, superficial or immersed in culture colonies, scattered, solitary to gregarious, subglobose to ovoid, or obpyriform, uni-loculate, glabrous, ostiole at centre, with a minute papilla. Peridium 7–18 μm wide, thin-walled, of equal thickness, composed of 1–3 layers, of dark brown pseudoparenchymatous cells, arranged in a textura angularis. Asci (42–)45–55(–62) × 10–13 μm (\( \bar{x} \) = 51.9 × 11.6 μm, n = 15), 8-spored, unitunicate, cylindric-obclavate to obpyriform, subsessile, thick-walled at apex, with J-, subapical ring. Ascospores (8–)10–13(–15) × 3–5 μm (\( \bar{x} \) = 11.9 × 4.3 μm, n = 50), overlapping 1–2-seriate, hyaline, ellipsoidal to subfusoid, with round ends, 1-septate, smooth-walled with small guttules, becoming finely echinulate when stained by Melzer’s reagent. Asexual morph Hyphomycetous, Conidiophores 15–50(–58) × (1.5–)3–5 µm (\( \bar{x} \) = 38.5 × 4.2 µm, n = 20), macronematous or micronematous, produced from prostrate hyphae, lacking hyphal coils, sometimes branched, hyaline, yellowish brown at the base, thick-walled, smooth, septate, branched, straight or flexuous. Conidiogenous cells phialidic, determinate, integrated to discrete, hyaline, subcylindrical to ampulliform, smooth-walled, with periclinal wall thickening, with minute collarette. Conidia 7–10 × 4–7 µm (\( \bar{x} \) = 8.7 × 5.4 µm, n = 50), pleurogenous or acropleurogenous, formed in slimy heads at the apex of the phialides, hyaline, subglobose to ellipsoidal, aseptate, smooth, thin-walled, with granular contents. Chlamydospores not produced.
Culture characteristics: Colonies on PDA reaching 40–48 mm diam. after 3 weeks at 20–25 °C, dense, irregular in shape, flat to slightly raised, slightly rough at surface, with mycelia radiating outwards, sometimes forming sectors of different folds, fimbriate at edge, mucoid to floccose; from above, white yellowish to cream; from below, pale yellowish; not producing pigmentation in agar medium.
Material examined: CHINA, Yunnan Province, Kunming City, Kunming Institute of Botany, colonies forming on WA as a contaminated fungus, 5 July 2017, R. Phookamsak, KIB042 (dried culture herbarium: KUN-HKAS 102246, holotype), ex-type living culture, KUMCC 18-0181.
GenBank numbers: ITS = MH254296, LSU = MH254298, RPB2 = MH254297, TEF1-α = MH254295.
Notes: Phylogenetic analyses of a combined ITS, LSU and TEF1-α sequence dataset (Fig. 88) show the new species, Plectosphaerella kunmingensis forming a distinct lineage at the basal clade of Plectosphaerella in Plectosphaerellaceae with moderate support (76% ML and 0.96 BYPP). Plectosphaerella kunmingensis can be distinguished from other Plectosphaerella species in the lack of hyphal coils, as well as having partly coloured conidiophores. The sexual morph of P. kunmingensis differs from P. cucumerina in having smaller asci (P. kunmingensis, (42–)45–55(–62) × 10–13 μm versus 50–80 × 6–9 μm, P. cucumerina; Carlucci et al. 2012) and wider ascospores (P. kunmingensis, (8–)10–13(–15) × 3–5 μm versus (9–)10.5–14(–15) × 2.5–3(–4) μm, P. cucumerina; Carlucci et al. 2012) (Fig. 90).
Phylogram generated from maximum parsimony based on a combined ITS and LSU sequence dataset of the genus Leptobacillium and related genera in Cordycipitaceae. Bootstrap support value of maximum likelihood (left) and maximum parsimony (right) equal to or greater than 70% are indicated above the nodes. Type strains are in bold. The new strain is in blue bold. The tree is rooted with Gibellulopsis piscis (CBS 892.70)
Another species, Plectosphaerella sinensis was also found from China isolated from a healthy stem of Cucumis melo L. (Cucurbitaceae) (Su et al. 2017). Whereas, our isolate was found as a contaminated fungus on WA. Plectosphaerella kunmingensis has subglobose to ellipsoidal, aseptate conidia and lacks chlamydospores, while P. sinensis has ellipsoidal, 0–1-septate conidia with a slightly apiculate base, and forming intercalary or terminal, irregular, thick-walled chlamydospores (Su et al. 2017). In the BLASTn search on NCBI GenBank, the closest matches of ITS sequence of P. kunmingensis are P. nepalense (CBS 971.72) and P. cucumerina (XSD-75) with 95% similarities for both strains. In addition, a comparison of ITS nucleotide bases between P. kunmingensis and P. niemeijerarum (CBS 143233) shows that they differ in 42 base positions (8.08%/520 bp). Based on a molecular analyses coupled with morphological characteristics, we therefore, propose P. kunmingensis as a new species.
Hypocreales Lindau
Cordycipitaceae Kreisel ex G.H. Sung et al.
Cordycipitaceae was validated by Sung et al. (2007) to accommodate the type of Cordyceps Fr., C. militaris (L.) Fr. and most of Cordyceps species forming brightly coloured, fleshy stromata. Species of Cordycipitaceae have been reported as obligate saprotrophs, parasites and symbionts with insects and fungi or grasses, rushes or sedges (Sung et al. 2007; Schardl et al. 2013; Kepler et al. 2013, 2017; Li et al. 2016; Tibpromma et al. 2017; Huang et al. 2018). Eighteen genera are listed in this family (Wijayawardene et al. 2018a). We follow the latest treatment and updated accounts of Cordycipitaceae in Kepler et al. (2017).
Leptobacillium Zare & W. Gams
The genus Leptobacillium was introduced by Zare and Gams (2016) to accommodate saprotrophic and fungicolous phialidic hyphomycetes and Leptobacillium leptobactrum (W. Gams) Zare & W. Gams is the type species. We follow the latest treatment in Zare and Gams (2016). Leptobacillium leptobactrum var. calidius is reported for the first time in India.
Leptobacillium leptobactrumvar.calidius Zare & W. Gams, Mycol Progr 15: 1003 (2016)
Facesoffungi number: FoF04832, Fig. 91
Leptobacillium leptobactrumvar.calidius (NFCCI 4235). a Colony morphology on PDA (front view). b Colony morphology on PDA (reverse view). c Conidia produced in long chains from phialides. d Conidiophores bearing phialides producing conidia in chain. e Enlarged view of conidia. f Conidia produced in gloeosporic mass at the tip of phialides. Scale bars 10 µm
Holotype: GHANA, Atewa. CBS H-22406, a dried culture of CBS 748.73 (= IHEM 3708), from living lepidopteran larva collected by H.C. Evans.
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978)
Saprobic on phylloplane. Sexual morph Undetermined. Asexual morphConidiophores 36–173.5 × 1.2–2.8 μm (\( \bar{x} \) = 97 × 1.5 μm, n = 30), cylindrical, mainly solitary or in groups (2–3), simple to branched, smooth-walled, hyaline. Conidiogenous cells 3.5–66.5 × 0.8–2 μm (\( \bar{x} \) = 29.5 × 1.4 μm, n = 30), phialidic, cylindrical, septate, slightly tapering towards apex, 1–3 arising from single node of condiophores. Conidia 2.5–6.2 × 0.8–1.8 μm (\( \bar{x} \) = 4 × 1.2 μm, n = 30), produced in long chains (about 20 in a chain) and in gloeosporic masses, cylindrical, bacilliform, fusiform, smooth-walled, aseptate, hyaline. Chlamydospores absent.
Culture characteristics: Colonies on PDA reaching 15–28 mm diam. in 10 days, at 25 °C, white (4A1), margin smooth, entire, flat to slightly raised, puffy, sometimes produces exudates. Reverse yellowish white (4A2) to crème (4A3), wrinkled.
Known hosts and distribution: Hemileia vastatrix Berk. & Broome on Coffee (Brazil), technical equipment (France, Netherlands), living lepidopteran larva (Ghana), decaying wood (Poland), cyst of Heterodera glycines Ichinohe (USA) (Zare and Gams 2016).
Material examined: INDIA, Maharashtra, Pune, on phylloplane of Colocasia esculenta (L.) Schott (Araceae), 10 November 2017, S.K. Singh, living culture, NFCCI 4235, voucher specimen, AMH 10000.
GenBank numbers: ITS = MG786580, LSU = MG786581.
Notes: Phylogenetic analysis based on a combined ITS and LSU sequence dataset of 24 taxa shows that the Indian taxon (NFCCI 4235) clusters with type of Leptobacillium leptobactrum var. calidius Zare & W. Gams (CBS 748.73) (Fig. 90). Mega blast search of ITS sequence shows 99% similarity (505/508) with L. leptobactrum var. calidius (CBS 748.73) and same similarity (99% similarity, 592/594) for LSU analysis. In this isolate conidia are produced in long chains and in gloeosporic masses. Though slight variations in conidial length and width were observed, our isolate is similar to L. leptobactrum var. calidius (CBS 748.73) in overall morphological characteristics. In addition, our isolate optimally grows at 25–26 °C, the same as reported for L. leptobactrum var. calidius (CBS 748.73) with negligible growth at 30 °C. Optimum temperature for growth of L. leptobactrum var. leptobactrum is 18–21 °C (Zare and Gams 2016). Leptobacillium leptobactrum var. calidius and L. leptobactrum var. leptobactrum were treated as a synonym of L. leptobactrum (Index Fungorum 2019). However, L. leptobactrum var. calidius (CBS 748.73) forms a distinct lineage with the type strain of L. leptobactrum (CBS 771.69). Hence, based on similarity in morphological characteristics, temperature requirements, and phylogenetic analysis this Indian isolate is identified as L. leptobactrum var. calidius (Zare and Gams 2016). To our knowledge, this is the first report of this genus, species and variety isolated from phylloplane of a different host, Colocasia esculenta from India.
Hypocreaceae De Not.
Hypocreaceae was introduced by De Notaris (1844) and is typified by Hypocrea Fr. Species of Hypocreaceae are characterized by their brightly coloured, fleshy perithecial stromata (Rogerson 1970). The family comprises 18 genera and more than 450 species (Kirk et al. 2008; Wijayawardene et al. 2018a).
Trichoderma Pers.
Trichoderma is frequently isolated from various habitats including soil, decaying wood, and vegetable matter (Samuels 2006) and is known to play an important ecological role as biocontrol agents of plant diseases, producers of bioactive compounds, and pathogens of animals and mushrooms (Schuster and Schmoll 2010). Based on molecular data, more than 250 species are currently accepted in this genus (Bissett et al. 2015). Three novel species are introduced based upon their phylogenetic affinities clarified by maximum likelihood and Bayesian inference analyses of a combined TEF1-α and RPB2 sequence dataset (Fig. 92).
Phylogenetic tree for Trichoderma from a maximum likelihood analysis based on the combined TEF1-α and RPB2 alignments. Branch support values are given as ML bootstrap values (≥ 70% ML) and Bayesian posterior probabilities (≥ 0.95 BYPP). The scale bar indicates the number of nucleotide substitutions per site. New species names are in blue. The letter “T” indicates ex-type strains
Trichoderma koreanum S-Y. Oh, M.S. Park & Y.W. Lim, sp. nov.
MycoBank number: MB824661; Facesoffungi number: FoF04459, Fig. 93
Etymology: The specific epithet “koreanum” refers to the type locality.
Holotype: SFC20131005-S066.
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1963).
Sexual morph Undetermined. Asexual morph Hyphomycetous. Conidiophores branched at an acute or right angle to the main axis, mostly unpaired. Phialides 8.6–13.4 × 2.4–3.7 μm, l/w 2.5–4.9, 1.7–2.6 μm wide at the base (n = 30), typically formed in whorls of 3–5, subulate or lageniform. Conidia smooth, subglobose to ellipsoid, 3.1–4.4 × 2.6–3.3 μm, l/w 1.1–1.4(−1.5) (n = 30).
Culture characteristics: On CMD after 72 h colony radius 17–22 mm at 15 °C, 29–35 mm at 20 °C, 44–49 mm at 25 °C, 20–29 mm at 30 °C; colonies on CMD fill a 90 mm diam. Petri dish within 5 days at 25 °C. Colony hyaline, radial, indistinctly zonate, aerial hyphae spreading uniformly throughout the colony. Conidiation starting after 4 days, light green (M. 28A5) or deep green (M. 27E8) conidia forming in pustules, appearing around the margin of the colony. No chlamydospores observed. No distinct odour. Agar not pigmented. On PDA after 72 h colony radius 12–17 mm at 15 °C, 21–30 mm at 20 °C, 35–44 mm at 25 °C, 21–24 mm at 30 °C; colonies on PDA fill a 90 mm diam. Petri dish within 5–6 days at 25 °C. Colony radial, mycelium dense, not finely zonate, aerial hyphae abundant, more abundant in distance from the inoculum. Conidiation starting after 3 days, greyish yellow (M. 2C5) or greyish green (M. 29C5) conidia formed abundant on aerial hyphae. On SNA after 72 h colony radius 11–14 mm at 15 °C, 17–24 mm at 20 °C, 33–36 mm at 25 °C, 14–22 mm at 30 °C; colonies on SNA fill a 90 mm diam. Petri dish within 6 days at 25 °C. Colony hyaline, radial, mycelium loose, indistinctly zonate, aerial hyphae more abundant in distance from the inoculum. Conidiation starting after 3 days, greyish yellow (M. 2B4) or greyish green (M. 29C5) conidia formed abundant on aerial hyphae.
Material examined: REPUBLIC OF KOREA, Gyeongsangbuk Province, Uljin, Jinjosan Mountain, elev. 170 m, isolated from root of Pinus densiflora Siebold & Zucc. (Pinaceae) under a fairy ring of Tricholoma matsutake (S. Ito & S. Imai) Singer, October 2013, S.Y. Oh, PF066 (SFC20131005-S066, holotype), ex-type living culture KACC 48487; ibid., Gangwon Province, Hongcheon, Gongjaksan Mountain, elev. 300 m, isolated from root of P. densiflora under a fairy ring of T. matsutake, September 2013, S-Y. Oh, SFC20130926-S004.
GenBank numbers: ITS = MH050352, RPB2 = MH025988, TEF1-α = MH025979.
Notes: Morphologically and phylogenetically (Figs. 92, 93), Trichoderma koreanum is closely related to T. tomentosum Bissett, T. ceraceum P. Chaverri & Samuels, and T. linzhiense K. Chen & W.Y. Zhuang. However, T. tomentosum has shorter phialides (4.5–5 × 3–3.2 μm) and narrower conidia (3.2–3.5 × 2.2–2.5 μm). Trichoderma linzhiense grows faster at 25 °C (51–60 mm on CMD, 62–63 mm on PDA, and 50–51 mm on SNA after 3 days). Trichoderma ceraceum grows slower at 20 °C (15–17 mm on PDA and 6–14 mm on SNA) and has shorter phialides (6.5–7.7 × 3–3.5 μm).
Trichoderma pinicola S-Y. Oh, M.S. Park & Y.W. Lim, sp. nov.
MycoBank number: MB824662; Facesoffungi number: FoF04460, Fig. 94
Etymology: The specific epithet “pinicola” refers to the genus name of Pinus densiflora, the source of type strain
Holotype: SFC20130926-S233.
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1963).
Sexual morph Undetermined. Asexual morph Hyphomycetous. Conidiophores branched at an acute to the main axis, mostly paired. Phialides 7.8–13.3 × (2.5 −)2.6–4.1(−4.5) μm, l/w 1.9–4.4(−4.5), 1.8–3.3 μm wide at the base (n = 30), typically formed in whorls of 3 or rarely solitary, subulate or lageniform. Conidia 3.5–4.9(−5) × 2.8–3.5(−3.6) μm, l/w 1.1–1.6 (n = 30), smooth, subglobose to ellipsoid. Chlamydospores rare, globose, 5.2–10(−10.1) × 5.2–10 μm, l/w 1–1.02 (n = 10). No distinct odour. Agar not pigmented.
Culture characteristics: On CMD after 72 h colony radius 14–18 mm at 15 °C, 23–34 mm at 20 °C, 39–50 mm at 25 °C, 34–45 mm at 30 °C; colonies on CMD fill a 90 mm diam. Petri dish at 4 days at 25 °C. Colony hyaline, radial, not zonate, mycelium common, aerial hyphae inconspicuous. Conidiation starting after 4 days, greyish green (M. 28B5) or deep green (M. 28E8) conidia formed scarcely on aerial hyphae around the margin of the colony. On PDA after 72 h colony radius 12–15 mm at 15 °C, 18–28 mm at 20 °C, 30–38 mm at 25 °C, 21–36 mm at 30 °C; colonies on PDA fill a 90 mm diam. Petri dish within 6 days at 25 °C. Colony hyaline, radial, with wavy margin, mycelium common, aerial hyphae more abundant in colony centre. Conidiation starting after 9 days, greyish green (M. 27C6) or pastel green (M. 29A4) conidia formed on aerial hyphae, more abundant around the margin of the colony. On SNA after 72 h colony radius 9–12 mm at 15 °C, 19–24 mm at 20 °C, 30–43 mm at 25 °C, 19–33 mm at 30 °C; colonies on SNA fill a 90 mm diam. Petri dish within 5–6 days at 25 °C. Colony hyaline, radial, mycelium loose, spreading uniformly throughout the colony. Conidiation starting after 3–4 days, greyish green (M. 29C5) or deep green (M. 27E8) conidia formed abundant on aerial hyphae.
Material examined: REPUBLIC OF KOREA, Gangwon Province, Hongcheon, Gongjaksan Mountain, elev. 300 m, isolated from root of Pinus densiflora under a fairy ring of Tricholoma matsutake, September 2013, S-Y. Oh, PF233 (SFC20130926-S233, holotype), ex-type living culture KACC 48486; ibid., Hongcheon, Gongjaksan Mountain, elev. 300 m, isolated from root of P. densiflora under a fairy ring of T. matsutake, September 2013, S-Y. Oh, SFC20130926-S111.
GenBank numbers: ITS = MH050354, RPB2 = MH025993, TEF1-α = MH025981.
Notes: Trichoderma pinicola is morphologically similar to T. hirsutum K. Chen & W.Y. Zhuang, the latter has longer phialides (11.4–18.3 × 2.5–3.1 μm) and lacks chlamydospores in CMD. Phylogenetically (Fig. 92), T. pinicola is closely related to T. simplex K. Chen & W.Y. Zhuang. However, T. simplex has more abundant aerial hyphae and conidia formation, and faster growth at 25 °C (49–56 mm on CMD and 52–56 mm on PDA after 3 days). In addition, T. pinicola showed wavy margin on PDA.
Trichoderma rugulosum M.S. Park, S-Y. Oh & Y.W. Lim, sp. nov.
MycoBank number: MB824663; Facesoffungi number: FoF04461, Fig. 95
Etymology: The specific epithet refers to the wrinkle in colony reverse on PDA.
Holotype: SFC20180301-001
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1963).
Sexual morph Undetermined. Asexual morph Hyphomycetous. Conidiophores symmetry, branches mostly paired. Phialides typically formed in whorls of 3–4, ampulliform to lageniform, 5.9–10.3(−10.5) × 2.1–3.6(−3.9) μm, l/w 1.9–3.7(−3.8), (1.2 −)1.3–1.9 μm wide at the base (n = 30). Conidia green, smooth, globose, subglobose or broadly ellipsoidal, (2.5–)2.6–3.2 × (2.3–)2.3–2.9 μm, l/w 1–1.2(−1.3) (n = 30).
Culture characteristics: On CMD after 72 h colony radius 10–14 mm at 15 °C, 24–29 mm at 20 °C, 62–69 mm at 25 °C, 69–75 mm at 30 °C; colonies on CMD fill a 90 mm diam. Petri dish within 4 days at 25 °C. Colony hyaline, radial, mycelium loose, indistinctly zonate, aerial hyphae more abundant in distance from the inoculum. Conidiation starting after 3 days, deep green (M. 27E8) or greyish green (M. 27c4) conidia forming in pustules. No chlamydospores observed. No distinct odour. Agar not pigmented. On PDA after 72 h colony radius 9–11 mm at 15 °C, 13–21 mm at 20 °C, 38–43 mm at 25 °C, 22–42 mm at 30 °C, colonies on PDA fill a 90 mm diam. Petri dish within 6 days at 25 °C. Colony radial, mycelium dense, zonate, aerial hyphae abundant, spreading uniformly throughout the colony, forming wrinkle in distance from the inoculum in reverse colony, at 30 °C colony radial, mycelium dense, with wavy margin. Conidiation starting after 3 days, effuse in aerial hyphae or in densely disposed granules, more abundant in 4–5 concentric rings, yellowish green (M. 30B8). No chlamydospores observed. No distinct odour. Olive yellow (M. 3C8) pigment diffusing into the agar. On SNA after 72 h colony radius 3–6 mm at 15 °C, 7–11 mm at 20 °C, 14–29 mm at 25 °C, 10–21 mm at 30 °C; colonies on SNA a 90 mm diam. the Petri dish within 8–9 days at 25 °C. Colony hyaline, radial, mycelium loose, not finely zonate, aerial hyphae common. Conidiation starting after 3 days, deep green (M. 27E8) or yellowish green (M. 30B8) conidia forming in pustules, pustules forming in 2–3 concentric rings.
Material examined: REPUBLIC OF KOREA, Jeju Province, Chuja-do Island, isolated from Chondria crassicaulis Harvey, September 2017. M.S. Park, F181 (SFC20180301-001, holotype), ex-type living culture KACC 48485; ibid., Chuja-do, isolated from Sargassum thunbergii (Mertens ex Roth) Kuntze, September 2017, M.S. Park, SFC20180301-002.
GenBank numbers: ITS = MH050353, RPB2 = MH025986, TEF1-α = MH025984.
Notes: Trichoderma rugulosum is isolated from Chondria crassicaulis and Sargassum thunbergii (seaweeds) in South Korea. Morphologically and phylogenetically, T. rugulosum is closely related to species in the T. harzianum complex. However, these species have faster growth at 30 °C on SNA (32–70 mm on SNA after 3 days). T. rugulosum shows a wrinkle in colony reverse on PDA at 25 °C and wavy margin on PDA at 30 °C, features not seen in members of the T. harzianum complex.
Hypocreales, genera incertae sedis
Emericellopsis Beyma
Emericellopsis Beyma (1940) was introduced with E. terricola Beyma as the type species. Emericellopsis species have been mostly isolated as saprobes from marine environments, soda lakes, and terrestrial habitats (Grum-Grzhimaylo et al. 2013). We describe a novel species from Korea based on phylogenetic analysis of a combined ITS and TUB2 sequence dataset (Fig. 96).
Phylogenetic tree of Emericellopsis koreana (CNUFC-MOG1-1 and CNUFC-MOG1-2) and related species based on maximum likelihood analysis of a combined ITS and TUB2 sequence dataset. Sequences of Bionectria spp. were used as outgroup taxa. Bootstrap values (≥ 50%) from 1000 replications are indicated at the nodes. New taxa are shown in blue and ex-type strains in bold
Emericellopsis koreana Hyang B. Lee, S.J. Jeon & T.T.T. Nguyen, sp. nov.
Index Fungorum number: IF554458; Facesoffungi number: FoF05732, Fig. 97
Emericellopsis koreana (CNUFC-MOG1-1, holotype). a, b Colonies on malt extract agar (MEA) (a: obverse view, b: reverse view). c Conidiophores with branched form in the basal region (LM). d, g Unbranched conidiophores, conidial heads (LM). e, f Conidia on slimy head and single conidia (LM). g–i Curved or straight conidiophore and ellipsoid or oblong-ellipsoid conidia (SEM). Scale barsc–g = 20 µm, h = 4 µm, i = 5 µm
Etymology: Named after the country where it was collected, Korea.
Holotype: CNUFC-MOG1-1
Sexual morph Undetermined. Asexual morph Hyphomycetous observed from MEA, acremonium-like. Mycelia consisting of hyaline, smooth-walled, septate hyphae, single or in bundles. Conidiophores mostly simple and orthotropic. Conidiogenous cells (15.5–)31.5–40(–59) µm long, tapering from 2(–2.5) µm at the base to 0.5 µm at the apex. Conidia ellipsoid or oblong-ellipsoid, smooth-surfaced, 3–4(–5) × 1.5–2(–2.5) µm (n = 50), hyaline, adhering in slimy heads. No chlamydospores observed.
Culture characteristics: The isolate grows over a wide range of temperatures with varying growth rates. The average growth rates of CNUFC-MOG1-1 on MEA, CYA, and PDA medium at 25 °C were 27.5, 17, and 15.5 mm after 7 days, respectively. Optimal growth was observed around 25 °C, slow growth was observed below 10 °C, and no growth at 40 °C.
Material examined: REPUBLIC OF KOREA, Jeonnam Province, garden of the Chonnam National University located in Gwangju (35°10’20.2”N 126°53′57.2″E), from gut of a mosquito larva, 15 September 2016, H.B. Lee, CNUFC-MOG1-1 (holotype), ex-type living culture, JMRC:SF:013604.
GenBank numbers: ITS = MH173304, TUB2 = MH243035 (CNUFC-MOG1-1); ITS = MH173305, TUB2 = MH243036 (CNUFC-MOG1-2).
Notes: Emericellopsis koreana, which forms a subclade with E. donezkii Beliakova, E. humicola (Cain) Cain ex Grosklags & Swift, E. persica Papizadeh et al. and Emericellopsis sp., differs by having smaller conidia. In the phylogenetic tree based on a combined ITS and TUB2 sequence dataset (Fig. 96), the strains CNUFC-MOG1-1 and CNUFC-MOG1-2 form a separate branch from other related species of Emericellopsis and is considered to be a new species.
Subclass Savoryellomycetidae Hongsanan et al.
Savoryellales Boonyuen et al.
Savoryellaceae Jaklitsch & Réblová
Savoryellaceae was introduced by Jaklitsch (2015) and is typified by Savoryella E.B.G. Jones & R.A. Eaton. The family is characterized by immersed to superficial, globose to pyriform, coriaceous, papillate ascomata, clavate to cylindrical, unitunicate asci, with an inamyloid apical ring, paraphyses, ellipsoidal to fusiform, versicolorous, septate ascospores, with or without polar mucilaginous pads or appendages and dematiaceous, hyphomycetous asexual morphs (Jaklitsch 2015). Most species of Savoryellaceae are aquatic occurring on submerged wood in freshwater, marine and brackish habitats worldwide (Jaklitsch 2015; Xia et al. 2017). The generic type, Savoryella was re-circumscribed by Maharachchikumbura et al. (2016) and the higher level classification of the families in Savoryellomycetidae was revised by Hongsanan et al. (2017) based on phylogenetic and molecular clock evidence. Six genera are accepted in this family (Wijayawardene et al. 2017a, 2018a).
Canalisporium Nawawi & Kuthub.
The genus Canalisporium was introduced by Nawawi and Kuthubutheen (1989) to accommodate muriform asexual morph species, with conidia that are flattened dorsiventrally, comprising a single layer of regularly arranged cells, which are supported by a small, thin-walled, cuneiform, pale basal cell. There are 12 species in this genus (Zhao et al. 2013). Five species lack molecular data, viz. C. kenyense Goh et al., C. microsporum G.Z. Zhao, C. nigrum G.Z. Zhao, C. panamense A. Ferrer & Shearer and C. variabile Goh & K.D. Hyde (Goh et al. 1998; Sri-Indrasutdhi et al. 2010; Zhao et al. 2013). In this study, Canalisporium kenyense was collected from submerged decaying wood in a freshwater stream in Thailand. The taxon is re-circumscribed and illustrated as the reference specimen (Figs. 98, 99).
Phylogram generated from maximum likelihood analysis of a combined LSU and ITS sequence dataset of species in Canalisporium Nawawi & Kuthub. and Savoryella E.B.G. Jones & R.A. Eaton (Savoryellaceae). Twenty-three strains are included in the combined sequence dataset, consisting of 1768 total characters with gaps (655 characters for ITS and 1113 for LSU). Pleurotheciella rivularia Réblová et al. (CBS 125238) is the outgroup taxon. The best scoring RAxML tree with a final likelihood value of -7016.458694 is presented. RAxML bootstrap support values equal to or greater than 75% and Bayesian posterior probabilities equal to or higher than 0.95 BYPP are given above the nodes (ML/BYPP). Hyphen (“–”) indicates a value lower than 75% for RAxML and a posterior probabilities lower than 0.95 for Bayesian inference analysis. Newly generated sequences are in blue. Ex-type strains are in bold
Canalisporium kenyense Goh, W.H. Ho & K.D. Hyde, Can J Bot 76: 148 (1998)
Facesoffungi number: FoF04845, Fig. 99
Holotype: KENYA, Mt. Kenya, Castle Forest, on rotten wood, 25 Jan. 1984, P.M. Kirk, 1593a, IMI 285428a.
Saprobic on decaying wood submerged in a freshwater stream. Sexual morph Undetermined. Asexual morph Hyphomycetous, dictyosporous. Conidiophores macronematous, mononematous, unbranched, septate, up to 25 × 3–6 µm, hyaline to pale brown, smooth-walled. Conidiogenous cells holoblastic, monoblastic, integrated, terminal, determinate, hyaline to pale brown, smooth-walled. Conidia acrogenous, solitary, subglobose, flattened, muriform, smooth, pale brown to dark brown, 40–50 × 25–30 × 13–20 μm with 2 straight columns of vertical septa and 4–6 rows of transverse septa, slightly constricted at the septa, apical rows of cells darker than the basal rows, dark and thickly banded at the septa, canals in the septa obscured by dark pigmentation, apex comprising a single cell, the number of cells per conidium varies from 13 to 20, base comprising a single cell, cuneiform, sometimes swollen, thin-walled, pale brown, or comprising three thin-walled, pale brown, small cells in a row.
Material examined: THAILAND, Chiang Rai Province, Muang District, Nang Lae Nai Village, on submerged decaying wood in a freshwater stream, 31 December 2016, Y.Z. Lu, CR01 (MFLU 17-1086, reference specimen designated here); ibid., KUN-HKAS 97477.
GenBank numbers: ITS = MH701998, LSU = MH701999, TEF1-α = MH708885.
Known hosts and distribution: On decaying wood (Kenya), submerged wood (Hong Kong), decorticated branches of dead tree (China), decaying branches of unidentified plant (China) (Goh et al. 1998; Zhuang 2001; Zhao et al. 2013; Farr and Rossman 2018).
Notes: Our new collection resembles Canalisporium kenyense in morphological characters of the conidiophores, conidiogenous cells and conidia, and their measurements align with those of the holotype (IMI 285428a) (Goh et al. 1998) and other specimens of C. kenyense (Zhao et al. 2013). Therefore, we identify our new collection as C. kenyense which is the first report from Thailand. Phylogenetically, C. kenyense forms a distinct lineage within the genus Canalisporium with strong support (96% ML and 1.00 BYPP; Fig. 98). Based on morphological characters and phylogenetic support, we designate our collection MFLU 17-1086 as a reference specimen.
Subclass Sordariomycetidae O.E. Erikss. & Winka
Chaetosphaeriales Huhndorf et al.
Chaetosphaeriaceae Réblová et al.
Chaetosphaeriaceae is a widespread, species-rich family, which was invalidly introduced by Locquin (1984) to accommodate Chaetosphaeria Tul. & C. Tul., Loramyces W. Weston, Niesslia Auersw., Rhagadostoma Körb. and Zignoëlla Sacc., (Réblová et al. 1999; Hyde et al. 2017). Réblová et al. (1999) re-described and validated Chaetosphaeriaceae and accepted Ascocodinaea Samuels et al., Chaetosphaeria, Melanochaeta E. Müll. et al., Melanopsammella Höhn., Porosphaerella E. Müll. & Samuels, Porosphaerellopsis Samuels & E. Müll. and Striatosphaeria Samuels & E. Müll. Currently, 38 genera are accepted in Chaetosphaeriaceae (Hyde et al. 2017; Tibpromma et al. 2018; Wijayawardene et al. 2018a). We introduce Thozetella lithocarpi sp. nov. from Lithocarpus sp. and record Macaranga tanarius as a new host record for Chaetosphaeria panamensis based on both morphological and phylogenetic analyses (Fig. 100).
Phylogram generated from maximum likelihood analysis of a combined LSU, ITS and TUB2 sequence dataset of Chaetosphaeriaceae. Maximum likelihood bootstrap support values greater than 70% and Bayesian posterior probabilities (BYPP) above 0.95 are shown above the nodes. The new isolates are in blue and ex-type strains in bold. The tree is rooted with Gelasinospora tetrasperma (AFTOL-ID 1287) and Sordaria fimicola (CBS 508.50)
Chaetosphaeria Tul. & C. Tul.
The saprobic genus, Chaetosphaeria was introduced by Tulasne and Tulasne (1863) based on the type species, C. innumera Berk. & Broome ex Tul. & C. Tul., and currently comprise 165 epithets (Index Fungorum 2019). Chaetosphaeria is placed in Chaetosphaeriaceae (Chaetosphaeriales) based on molecular data (Réblová et al. 1999; Huhndorf et al. 2004; Maharachchikumbura et al. 2015), although it was previously placed in Lasiosphaeriaceae by Barr (1990). Chaetosphaeria species have a diverse distribution having been recorded from both temperate and tropical countries (i.e. Canada, China, Europe, Hong Kong, New Zealand, Thailand) (Hyde et al. 1999, 2017; Réblová 2000; Perera et al. 2016; Wijayawardene et al. 2017a). Host-specificity of the taxa in this group has not yet been proven given that they have been recorded from various plant families (i.e. Arecaceae, Euphorbiaceae, Pinaceae) (Hyde et al. 1999; Perera et al. 2016; Farr and Rossman 2018).
Chaetosphaeria panamensis Huhndorf & F.A. Fernández, Fungal divers 19:33 (2005)
Facesoffungi number: FoF02657, Fig. 101
Chaetosphaeria panamensis (MFLU 18-0087). a Appearance of ascomata on host. b Close-up of the ascoma. c, d Section of ascoma. e Section through ostiole. f Section of peridium. g Paraphyses. h–j Asci. k–n Ascospores. o Colony from above. p Colony from below. Scale barsc, d = 100 µm, e = 50 µm, f = 30 µm, g–j = 60 µm, k–n = 20 µm
Holotype: PANAMA, Barro Colorado Island National Monument, Shannon trail, 50 to 150 m, [9.1667, -79.8333], on decorticated wood, 23 August 1997, SMH, FAF, SMH3596 (in F).
Saprobic on decaying wood of Macaranga tanarius. Sexual morphAscomata 200–250 μm diam., 180–230 μm high, black, scattered, solitary, sparse, semi-immersed in the host at the base of ascomata, becoming superficial, globose, coriaceous, setose, ostiolate, papillate. Setae scattered over entire ascomata, dark brown, stiff, pointed, 50–60 μm long. Papilla central, short, brown, with periphyses. Peridium 25–35 μm thick, composed of two cell layers, outer layer comprising 4–5 layers of thick, dark brown cells, arranged in textura angularis to textura globosa, inner layer comprising several layers of flattened, brown pseudoparenchymatous cells, arranged in textura prismatica. Paraphyses 3–4 μm wide at the base, tapering towards the apex, numerous, septate, arising from the basal cavity, embedded in a hyaline gelatinous matrix. Asci 125–150 × 9–12 μm (\( \bar{x} \) = 137.5 × 11.2 μm, n = 10), 8-spored, unitunicate, arising from the basal hymenium, cylindrical, rounded at the apex, with a J-, apical ring. Ascospores 50–60 × 3–4 μm (\( \bar{x} \) = 56.4 × 3.5 μm, n = 20), fasciculate, hyaline, filiform, straight to gently curved, with rounded ends, slightly tapering to base, 7-septate, smooth-walled, lacking a gelatinous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 8 mm diam. after 2 weeks at 25–30 °C, colonies medium dense, circular, surface slightly rough with edge fimbriate, effuse, velvety, margin, slightly irregular; from above, light brown to yellowish at the margin, light brown to grey at the centre, from below white to yellowish at the middle, light brown at the margin, grey to whitish at the centre, white to light purple at the middle; mycelium cream to whitish with tufting; not producing pigmentation in PDA.
Material examined: TAIWAN, Chiayi, Shihnong Forest Area, decaying wood of Macaranga tanarius (L.) Müll.Arg (Euphorbiaceae), 25 June 2017, D.S. Tennakoon, DTW027 (MFLU18-0087), living culture, FU30910.
Known hosts and distribution: Decorticated wood (Panama), Pinus sp. (Thailand), Macaranga tanarius (Taiwan) (Huhndorf and Fernández 2005; Perera et al. 2016).
GenBank numbers: ITS = MH974685, LSU = MH974686.
Notes: Chaetosphaeria panamensis was introduced by Huhndorf and Fernández (2005), and was collected from decorticated wood in Panama. In this study, C. panamensis is reported on another host, Macaranga tanarius from Taiwan. Phylogenetic analyses based on a combined LSU, ITS and TUB2 sequence dataset show that our strain (FU30910) clusters with the ex-type strain of C. panamensis (SMH 3596) and another representative strain (MFLUCC 15-1011) with high support (100% ML and 1.00 BYPP; Fig. 100). Based on morphological characters (superficial, globose ascomata with setae, cylindrical asci and filiform, slightly curved, 7-septate, hyaline ascospores) and molecular data of ITS region, our isolate is identical to the type (SMH 3596; Huhndorf and Fernández 2005) and hence, the new isolate is identified as C. panamensis. This is the first record of C. panamensis on decaying wood of Macaranga tanarius from Taiwan.
Thozetella Kuntze
We follow the latest treatment and updated accounts of Thozetella in Tibpromma et al. (2018).
Thozetella lithocarpi R.H. Perera & K.D. Hyde, sp. nov.
Index Fungorum number: IF555300; Facesoffungi number: FoF04923, Fig. 102
Etymology: Referring to the host genus, Lithocarpus.
Holotype: MFLU 16-1068
Saprobic on Lithocarpus fruits. Sexual morph Undetermined. Asexual morph Hyphomycetous. Colonies effuse, superficial, sessile sporodochial, greenish white. Sporodochia subulate, scattered, sessile, of greenish white mass, with a white spore mass at the apex. Microawns 24–54 µm long, 3–3.5 µm wide, visible as small hairs on the sporodochial mass, aseptate, variously-shaped, sigmoid or sickle-shaped, apex straight, hyaline, smooth-walled, thick-walled. Conidiophores 21–30 × 1.5–3.5 µm, macronematous, packed in a bundle, usually ramose. Conidia 20–35 × 2–3 µm (\( \bar{x} \) = 26 × 2.7 µm, n = 25), falcate, inequilateral, truncate at both ends, hyaline, smooth-walled, with a single filiform setula at each end, 6.9–8.7 µm long.
Culture characteristics: Colonies on MEA reaching 35 mm diam. after 3 weeks, margins effuse, grey to dark brown, flat, lacking aerial mycelium, reverse dark brown to black.
Material examined: THAILAND, Chiang Mai Province, on a dried seed of Lithocarpus sp. (Fagaceae; 19 species of Lithocarpus have been reported from Chiang Mai Province, according to BGO plant database), 22 December 2015, R.H. Perera, S-7 (MFLU 16-1068, holotype), ex-type living culture, MFLUCC 16-0194.
GenBank numbers: ITS = MH810433, LSU = MH810432.
Notes: Thozetella lithocarpi (MFLUCC 16-0194) shows a close relationship with T. pandanicola Tibpromma & K.D. Hyde, T. pinicola S.Y. Yeung et al. and T. nivea (Berk.) Kuntze (Fig. 100). However, T. lithocarpi can be distinguished from T. pandanicola and T. pinicola in having larger conidia and presence of microawns (Jeewon et al. 2009; Tibpromma et al. 2018). Thozetella lithocarpi produces microawns with a straight apex, while they are undulating to geniculate in T. nivea (Barbosa et al. 2011). A comparison of nucleotides in ITS gene region shows that T. lithocarpi differs from T. pandanicola and T. pinicola by 9 nucleotides (1.9%) and T. nivea by 18 nucleotides (3.9%). Our new species is also compared with Thozetella species that lack molecular data. Thozetella lithocarpi produces comparatively larger conidia (20–35 × 2–3 μm versus 11–17 × 2–2.5 μm) and smaller microawns (24–54 µm long, 3–3.5 µm wide versus 40–100 μm long, 2.5–4 μm wide) than T. cubensis R.F. Castañeda & G.R.W. Arnold (Castañeda-Ruiz and Arnold 1985). Thozetella canadensis Nag Raj produces verrucose microawns, while T. lithocarpi produces smooth-walled microawns (Nag Raj 1976). Thozetella aculeata Prisc. Silva & Grandi, T. buxifolia Allegr. et al., T. effusa B. Sutton & G.T. Cole, T. radicata (E.F. Morris) Piroz. & Hodges, T. serrata Whitton et al., T. submersa F.R. Barbosa & Gusmão and T. tocklaiensis (Agnihothr.) Piroz. & Hodges also produces smaller conidia than those of T. lithocarpi. Thozetella ypsiloidea J.S. Monteiro et al. differs from T. lithocarpi by its Y-shaped microawns. Microawns of T. serrata have a serrated edge, while microawns have a straight apex in T. lithocarpi (Monteiro et al. 2016).
Coniochaetales Huhndorf et al.
Coniochaetaceae Malloch & Cain
Coniochaetaceae was established to accommodate two genera Coniochaeta (Sacc.) Cooke and Coniochaetidium Malloch & Cain by Malloch and Cain (1971). Species of Coniochaetaceae can be distinguished from other families in having ascospores with elongated germ slits. Cultures are frequently pink or orange and have a yeast-like appearance. They usually grow better at low temperatures. Conidia are produced in abundance as phialospores or rarely as aleuriospores (Malloch and Cain 1971; Huhndorf et al. 2004; Wanasinghe et al. 2018). We followed the latest phylogenetic analyses and the updated accounts of Coniochaeta in Nasr et al. (2018), Samarakoon et al. (2018) and Wanasinghe et al. (2018). A new species is introduced based on its morphological distinctiveness coupled with strong phylogenetic support (Fig. 103).
Phylogram generated from maximum likelihood analysis for Coniochaeta simbalensis using a combined LSU and ITS sequence dataset based on the Tamura–Nei model (Tamura and Nei 1993). Phylogenetics analyses were conducted in MEGA7 (Kumar et al. 2016). Chaetosphaeria innumera and C. pygmaea were used as outgroup taxa. Type strains are indicated in bold. Newly generated sequence is indicated in blue
Coniochaeta (Sacc.) Cooke
We follow the latest treatment and updated accounts of Coniochaeta in Samarakoon et al. (2018) and Wanasinghe et al. (2018).
Coniochaeta simbalensis S. Rana & S.K. Singh, sp. nov.
MycoBank number: MB824288; Facesoffungi number: FoF04831, Fig. 104
Coniochaeta simbalensis (AMH 9941, holotype). a Colony morphology on PDA (front view). b Colony morphology on SDA (front view). c Colony morphology on PCA (front view). d Hyphal wall septate, thickened, guttulate, and showing anastomoses. e Terminal to intercalary chlamydospores. f Phialides with gleosporic mass of conidia. g Adelophialide with gleosporic mass of conidia (magnified view). h Discrete phialides and adelophialides. i Ventricose phialides in group with conidia. j Discrete phialides and dispersed conidia. k Mass of conidia. Scale bars = 10 µm
Etymology: The specific epithet “simbalensis” refers to the place of collection.
Holotype: AMH-9941
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Saprobic on mushroom rhizospheric soil. Sexual morph Undetermined. Asexual morph Hyphomycetous. Hyphae thin- to thick-walled, simple to branched, smooth-walled, constricted near the septa, wall thickened, guttulate, anastomosis observed. Conidiophores 1.2–6.3 µm wide, starting in 2–3 days from slender, thin hyaline to thick, smooth-walled hyphae, mostly reduced to conidiogenous cells. Phialides 1.8–60 × 1–3.7 µm (\( \bar{x} \) = 14.3 × 2.2 µm, n = 30), variable, produced laterally from superficial hyphae, solitary or in groups (1–3), ventricose, base narrow, and middle swollen with elongated narrow tip (collar) tapered collula, variable in length, all curved, reduced in size to dentate structure like adelophialides to elongated branched. Conidia 2.2–8.8 × 1.3–3.3 µm (\( \bar{x} \) = 4.5 × 2 µm, n = 30), 1-celled, oval to cylindrical to subglobose, produced sarcinately in gleosporic mass, rarely found in zipper-like arrangement, yeast-like cells observed frequently, monopolar budding seen in conidia, sporulation abundant, variable in size. Chlamydospores 5–8.6 × 3.4–6.7 µm (\( \bar{x} \) = 6.8 × 4.9 µm, n = 30), light to dark olivaceous brown, observed frequently, terminal to intercalary, solitary or in branched chains, wall thickened and darkened, constricted near the septa, variable in shape, globose, cylindrical to pyriform, sometimes produced laterally from short and long stalk of ~ 16–28 µm. Stalks septate, simple to branched, light to olivaceous brown, smooth-walled.
Culture characteristics: Colonies on PDA reaching 39–45 mm diam. after 2 weeks at 25 °C; from above brownish grey (9D2) with margins fading to dull red (9B3), flat, sulcate, entire with smooth margin; from below, brownish grey (9E2) with outer margins fading to pale orange (6A3), sulcate. Colonies on MEA reaching to 15–20 mm diam. after 2 weeks at 25 °C; from above brownish grey (6F2), slightly raised, sulcate, and irregular; from below grey (6E1). Colonies on PCA reaching 29–34 mm diam. after 2 weeks at 25 °C; from above smoke brown (4F3); from below, silver grey (4E2) with a margin of nearly 5 mm yellowish white (4A2) in flat, glazy, margin entire and irregular.
Material examined: INDIA, Himachal Pradesh, Kangra Dist, Simbal (31.9754 N” 76.6507 E”), Mushroom rhizospheric soil, 8 July 2017, S. Rana, (AMH 9941, holotype), ex-type living culture, NFCCI 4236.
GenBank numbers: ITS = MG825743, LSU = MG917738.
Notes: Coniochaeta simbalensis differs from other Coniochaeta species based on the sequence analysis. On megablast analysis, ITS sequence of C. simbalensis shows 94.92% (468/493) similarity and 25 gaps (5%) with C. cateniformis (Perdomo et al.) Gené & Guarro (UTHSC 01-1644, type), 92.23% (487/528) similarity and 29 gaps (5%) with C. canina (Deanna A. Sutton et al.) Deanna A. Sutton et al. (UTHSC 11-2460, type), 93.37% (366/392) similarity and 5 gaps (1%) with C. hoffmannii (J.F.H. Beyma) Z.U. Khan et al. (CBS 245.38, type) and 87.36% (491/562) similarity and 42 gaps (7%) with C. acaciae Samarakoon et al. (MFLUCC 17-2298). Interestingly, the sources of isolation of these species are quite distinct. Coniochaeta cateniformis (UTHSC 01-1644) was isolated from canine bone marrow, C. canina (UTHSC 11-2460) from bone aspirate, canine breed German Shepard, C. hoffmannii (CBS 245.38) from butter (Khan et al. 2013) and C. acaciae (MFLUCC 17-2298) from dead trunk and branches of Acacia sp. (Samarakoon et al. 2018), whereas, C. simbalensis was isolated from rhizospheric soil of unidentified mushroom growing in soil.
Coniochaeta simbalensis produces brownish grey (9D2) colonies with margins fading to dull red (9B3) and sulcate on PDA, whereas C. cateniformis and C. canina produces orange white and non-sulcate colonies. Similarly, C. simbalensis produces brownish grey (6F2), colonies on MEA as against orange red to yellowish red colonies produced by C. acaciae. Phialides of highly variable length were frequently observed in vitro by C. simbalensis. Conidia are highly variable in shape and size in C. simbalensis, ranging from oval to cylindrical to subglobose, non-truncate, and straight; whereas conidia are ovoidal to ellipsoidal, truncate and smaller in C. cateniformis, and ellipsoidal, straight to slightly curved in C. canina (Perdomo et al. 2013; Troy et al. 2013) and ellipsoidal in C. acaciae (Samarakoon et al. 2018). Coniochaeta simbalensis also readily produces chlamydospores in culture, a feature absent in C. acaciae and C. prunicola Damm & Crous (Damm et al. 2010). Therefore, based on phylogenetic inference (Fig. 103), morphological and cultural distinctness C. simbalensis is proposed as a new species.
Phyllachorales M.E. Barr
Phyllachoraceae Theiss. & H. Syd.
We follow the latest treatment and updated accounts of Phyllachoraceae in Dayarathne et al. (2017) and Mardones et al. (2017, 2018). Updated phylogenetic analysis (Fig. 105) was retrieved from Dayarathne et al. (2017).
Phylogram generated from maximum likelihood analysis based on a combined LSU, SSU and ITS sequence dataset of taxa in Phyllachorales and related orders. Bootstrap support values for maximum likelihood (left) equal to or greater than 70% and the values of Bayesian posterior probabilities (right) equal to or greater than 0.90 are given above the nodes. The new isolate is in blue. The tree is rooted with Xylaria polymorpha (MUCL49884) and X. hypoxylon (STMA07069). Ex-type strains are indicated in bold
Tamsiniella S.W. Wong et al.
Tamsiniella was introduced as a monotypic genus by Wong et al. (1998) to accommodate the freshwater fungus, T. labiosa S.W. Wong et al., which was collected from submerged wood in a small stream in Australia and Hong Kong (Wong et al. 1998). The genus is characterized by dark brown, immersed to semi-immersed, subglobose ascomata, with periphyses, papillate, thin-walled, pale brown peridium, paraphyses, 8-spored, unitunicate, cylindrical asci, with an unusual J-, lip-like, refractive apical ring, and hyaline, ellipsoidal-fusiform, aseptate ascospores, with narrow, roughed mucilaginous sheath (Wong et al. 1998). The asexual morph of this genus is unknown (Wijayawardene et al. 2017b). Based on the general morphology of the ascus apical ring observed with light microscopy and scanning, transmission electron microscopy, a new genus was established (Wong et al. 1998). Wong et al. (1998) suggested to place the genus in its own family due to a unique character of its apical ring. Wijayawardene et al. (2018a) treated the genus in Sordariomycetes, genera incertae sedis. We collected a specimen from a small river in Yunnan, China. Based on morphological comparison, our collection is typical of the type of Tamsiniella. We therefore, designate our collection as an reference specimen based on morphological characteristics and geographical distribution (Fig. 105).
Tamsiniella labiosa S.W. Wong, K.D. Hyde, W.H. Ho & S.J. Stanley, Can J Bot 76(2): 334 (1998)
Facesoffungi number: FoF05052, Fig. 106
Tamsiniella labiosa (MFLU 18-1191, reference specimen). a–c Appearance of black ascomata superficial on host. d Vertical section of ascoma. e Structure of peridium. f Paraphyses. g–j Unitunicate asci. k–m Ascospores. n Germinated ascospore o Colony on PDA (from above). p Colony on PDA (from below). Scale barsd = 50 μm, e, j–n = 10 μm, f–i, o = 20 μm
Holotype: AUSTRALIA, North Queensland, Mount Lewis, on submerged wood in a small stream, July 1993, T.M. and K.D. Hyde, ML9 (HKU (M) 2276; IFRD199-014).
Saprobic on decaying wood submerged in freshwater. Sexual morphAscomata 100–120 μm high, 130–150 μm diam. [type: 130–225 μm high, 180–250 μm diam.], black, gregarious or scattered, superficial, subglobose to ellipsoidal, uni-loculate, thin-walled, laterally ostiolate, with a mass of spores oozing when old. Peridium 6–9 μm wide [type: 5–11 μm wide], comprising 3–4 layers of dark brown, thick-walled, compressed cells of textura angularis. Hamathecium comprising numerous, ca 6 μm wide at the base, 2 μm diam. at the apex [type: 4–5 μm wide], cylindrical, unbranched, hyaline, septate, paraphyses, slightly constricted at the septa, tapering towards the apex. Asci 80–110 × 7.5–9 μm (\( \bar{x} \) = 93 × 8.5 μm, n = 15) [type: 80–102 × 8–10.5 μm], 8-spored, unitunicate, cylindrical to cylindric-clavate with a short, twisted pedicel, apically obtuse and inwardly concave, slightly wider than subapical apparatus, with J-, a refractive, lip-like apical ring, 1.9–2 × 2.9–3.1 μm. Ascospores 17–20 × 3.5–5 μm (\( \bar{x} \) = 19 × 4.5 μm, n = 15) [type: (12–)15–21 × 3.8–4.5(–5) μm], overlapping 1-seriate, hyaline, aseptate, fusiform to ellipsoidal, straight or curved, guttulate, thin-walled, with a thin, hyaline, mucilaginous sheath, 1–2 μm wide. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 20 mm in 12 days at 25 °C, circular, white to yellow–brown from above, white to light yellow from below, surface smooth with sparse mycelium, dry, edge entire.
Material examined: CHINA, Yunnan Province, Pingbian, on submerged wood in a small river, 20 September 2017, W. Dong, WF-33A (MFLU 18-1191, a reference specimen is designated here; ibid., KUN-HKAS 101711), living culture, MFLUCC 18-1018 = KUMCC 18-0060.
Known hosts and distribution: On submerged woods or twigs in Australia, Brazil, China, Hong Kong (Wong et al. 1998; Barbosa et al. 2013; this study).
GenBank numbers: ITS = MK034865, LSU = MK034866, SSU = MK034867.
Notes: Our isolate shares the size range of asci, ascospores, peridium and paraphyses with the type of Tamsiniella labiosa (see description). However, it has a slightly smaller ascomata compared to the type. Our isolate was collected from submerged wood in Yunnan, China, whereas the type specimen was collected from submerged wood in North Queensland, Australia. Based on morphological comparison and geographical distribution, we hence, designate our collection as a reference specimen of T. labiosa.
In phylogenetic tree, Tamsiniella labiosa forms a distinct lineage in the order Phyllachorales M.E. Barr with moderate support (77% ML and 0.99 BYPP, Fig. 105). Phyllachorales species are distinctive as they are biotrophic on various hosts (Pearce and Hyde 1994; Dayarathne et al. 2017; Mardones et al. 2017, 2018). Phyllachorales species are characterized by deep black stromata of various shapes; pseudostroma inside the host tissue and usually beneath an epidermal clypeus; perithecia usually strongly melanized; cylindrical to clavate asci with an inconspicuous apical ring; and globose to filiform, mostly hyaline, 1-celled, rarely brown or septate ascospores (Parbery 1967; Cannon 1991; Dayarathne et al. 2017; Mardones et al. 2017, 2018). However, our collection was isolated from decaying wood submerged in freshwater, which was the same habitat as another freshwater ascomycete genus Ascovaginospora Fallah et al. in Phyllachoraceae (Wijayawardene et al. 2018a). Therefore, Tamsiniella is accommodated in Phyllachoraceae based on phylogenetic analyses.
Sordariales Chadef. ex D. Hawksw. & O.E. Erikss
Lasiosphaeriaceae Nannf.
Lasiosphaeriaceae was established by Nannfeldt (1932), circumscribed by species with black ascomata and cylindrical asci, brown to hyaline ascospores, and typified by Lasiosphaeria Ces. & De Not. The family was re-circumscribed by Maharachchikumbura et al. (2015, 2016) and 35 genera were accepted in the family. Based on phylogeny, Lasiosphaeriaceae is sister to Chaetomiaceae with high support and this family has been shown to be paraphyletic, with many genera polyphyletic (Chang et al. 2010; Kruys et al. 2015; Maharachchikumbura et al. 2015, 2016). Wijayawardene et al. (2018a) listed 32 genera in Lasiosphaeriaceae.
Zopfiella G. Winter
Zopfiella was established by Winter (1884) to introduce Z. tabulata (Zopf) G. Winter and Z. curvata (Fuckel) G. Winter. Phylogenetic studies reported that the genera Zopfiella, Triangularia Boedijn, Cercophora Fuckel and Podospora Ces. are polyphyletic (Miller and Huhndorf 2005; Cai et al. 2005, 2006a, b; Chang et al. 2010; Kruys et al. 2015; Maharachchikumbura et al. 2015, 2016). A new species Z. indica, is introduced within Lasiosphaeriaceae based on evidence from morphology and DNA sequence data (Fig. 107).
Phylogram generated from maximum parsimony analysis based on a combined LSU, TUB2, ITS and RPB2 gene regions of Zopfiella and related taxa in Lasiosphaeriaceae. Bootstrap support values for maximum likelihood (green), maximum parsimony (blue) equal to or greater than 75% and the values of Bayesian posterior probabilities (purple) equal to or greater than 0.95 BYPP are given above each branch respectively. The new isolate is in blue. Ex-type strains are indicated in bold. The tree is rooted with Coniochaeta discoidea (Udagawa & Furuya) Dania García et al. (SANK12878)
Zopfiella indica Devadatha, Jeewon & V.V. Sarma, sp. nov.
Index Fungorum number: IF554286; Facesoffungi number: FoF04269, Fig. 108
Zopfiella indica (AMH-9907, holotype). a, b Ascomata superficial on the bark of intertidal mangrove wood. c Squash mount of ascoma. d Section through peridium. e Hyaline filiform paraphyses. f–i Immature and mature asci. j–o Ascospores with apical and basal cauda. Scale barsc = 100 μm, h–i = 50 μm. d–g, j–o = 10 μm
Etymology: Refers to country of its origin
Holotype: AMH-9907
Saprobic on the bark of intertidal mangrove wood. Sexual morphAscomata 320–555 µm high, 220–405 µm diam. (\( \bar{x} \) = 455.4 × 317 µm, n = 10), perithecial, lacking stromatic tissues, superficial, pyriform, solitary to gregarious, coriaceous, dark brown to black, enclosed with copious, flexuous, septate, light brown to dark brown, short to long hyphae, prevalent at the base, about 75–200 µm long, 1–2.5 µm thick, ostiolate. Ostiole 50–130 µm long, 40–125 µm diam. (\( \bar{x} \) = 105 × 104 µm, n = 10), black, short papillate, straight to curved, darker than remaining part of the perithecium, filled with brown cells. Periphyses 1–2 µm wide (\( \bar{x} \) = 1.7 µm, n = 20). Peridium 5–25 µm wide (\( \bar{x} \) = 13.2 µm, n = 10), membranous to coriaceous, pale brown to dark brown, comprising two layers, inner stratum flattened with several layers of hyaline to pale brown cells of textura prismatica, outer stratum comprising 2–3 layers of textura angularis. Paraphyses 1–2.5 µm wide (\( \bar{x} \) = 1.9 µm, n = 20), hyaline, filiform. Asci 115–200 × 15–45 µm (\( \bar{x} \) = 147.1 × 26.5 µm, n = 40), 8-spored, unitunicate, cylindrical to clavate, evanescent, short pedicellate, 20–75 µm × 2.5–7.5 µm (\( \bar{x} \) = 46 × 5 µm, n = 40), apical ring indistinct. Ascospores 23–35 × 10–20 µm (\( \bar{x} \) = 29.9 × 15.7 µm, n = 50), partially overlapping 1–2-seriate, hyaline at first turning golden yellow and olivaceous brown to dark brown at maturity, apical cell ovoid to ellipsoidal, rugose, apical cauda, single, lash-like, attached to the apical part of the terminal cell, 8–55 × 3–8 µm (\( \bar{x} \) = 20 × 5 µm, n = 20), smooth-walled, collapsing, primary appendage attached to the base of the pedicel and similar to apical cauda, 15–25 × 3–8 µm (\( \bar{x} \) = 20 × 5 µm, n = 20), mostly collapsing. Asexual morph Undetermined.
Culture characteristics: Ascospores germinated on SWA within 24 h, germ tubes arise from ends of the ascospore. Colonies on MEA reaching 70–85 mm diam. after 25 days of incubation at room temperature, initially deep olive grey, becoming dark olive grey at maturity, surface umbonate, margin entire, velvety, circular, reverse dark olive grey.
Material examined: INDIA, Tamil Nadu, Tiruvarur District, Muthupet mangroves (10.4°N 79.5°E), on the bark of intertidal mangrove wood, 28 November 2015, B. Devadatha, AMH-9907 (holotype), ex-type living culture, NFCCI-4217.
GenBank numbers: ITS = KY863506, LSU = KY863507, SSU = MF168941, RPB2 = MF182396, TEF1-α = MF182400, TUB2 = MF406208.
Notes: Multigene phylogenetic analyses indicate that Zopfiella indica shares a sister relation with Z. karachiensis (S.I. Ahmed & Asad) Guarro and Triangularia tanzaniensis R.S. Khan & J.C. Krug in a strongly supported monophyletic clade (Fig. 107). Zopfiella indica is clearly distinguished from Z. karachiensis and Triangularia tanzaniensis based on its ascospores having ovoid to ellipsoidal, rugose head cell, apical cauda attached to apical part of the terminal cell and primary appendage attached to the basal pedicel.
Species belonging to Podospora are predominantly coprophilous whereas those belonging to Zopfiella predominantly thrive in soil or on plant substrata and less on dung. Given the existing taxonomic confusion vis-a-vis the genera Zopfiella and Podospora, we prefer to place Z. indica in the genus Zopfiella based on its occurrence on decaying wood, and presence of a septum in the dark cell.
Recent phylogenetic analysis showed that Zopfiella is polyphyletic, interspersed with species belonging to other genera and hence is in need of revision along with other closely related genera such as Podospora and Triangularia, both at the morphological and molecular level, by including a larger representation of the species belonging to these genera. Phylogenetic analyses carried out by Cai et al. (2006b) also indicated that Zopfiella does not constitute a monophyletic group. They felt that the characters of non-ostiolate ascomata, and the absence of gelatinous appendages in ascospores that are considered important in delineating Zopfiella from Podospora (Guarro et al. 1991) are not reliable in understanding phylogenetic relationships (Cai et al. 2006b). This is because they found non-ostiolate Zopfiella species interspersed in different clades in the trees suggesting multiple origins of this morphological character. Also they found that the presence or absence of gelatinous appendages are phylogenetically less informative as different Zopfiella species grouped in different clades that also include many species possessing elaborate gelatinous appendages. Cai et al. (2006b) suggested that Zopfiella should be restricted to species with a septum in the dark cell.
There are two strains under the name Zopfiella karachiensis (IFO32902 and CBS 657.74) in GenBank, which were included in our phylogenetic analysis. However, these two strains formed a separated lineage in our phylogenetic tree (Fig. 107). Zopfiella karachiensis (IFO32902) is sister to Z. lundqvistii (NBRC30585); whereas, Z. karachiensis (CBS 657.74) clustered with Triangularia tanzaniensis and Z. indica (our present strain). Taxonomic revision of genera in this family is needed based on multigene phylogenetic analyses.
Subclass Xylariomycetidae O.E. Erikss. & Winka
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Amphisphaeriaceae G. Winter
The family Amphisphaeriaceae was introduced by Winter (1887) to accommodate Amphisphaeria and allied taxa. Amphisphaeriaceae is mainly characterized by perithecial, semi-immersed to erumpent ascomata, dark peridium, unitunicate, cylindrical asci, with J + or J-, apical rings, pale to dark brown, septate ascospores and forming coelomycetous asexual morphs (Senanayake et al. 2015; Maharachchikumbura et al. 2016). Three genera are accepted in this family, Amphisphaeria Ces & De Not., Griphosphaerioma Höhn., and Lepteutypa Petr. (Wijayawardene et al. 2018a). An updated evolutionary relationship of the family in Xylariomycetidae was presented by Samarakoon et al. (2016) and Hongsanan et al. (2017).
Amphisphaeria Ces. & De Not.
Amphisphaeria was established by Cesati and De Notaris (1863) without assigning a generic type. Petrak (1923) proposed A. umbrina (Fr.) De Not. as the lectotype of Amphisphaeria. Hyde et al. (1996) epitypified and described A. umbrina. Senanayake et al. (2015) re-circumscribed Amphisphaeria and introduced A. sorbi Senan. & K.D. Hyde with the link between the sexual and asexual morphs. We follow the latest treatment and updated accounts of Amphisphaeriaceae in Senanayake et al. (2015) and Maharachchikumbura et al. (2016). Based on phylogenetic analyses of a combined LSU, SSU and ITS sequence dataset (Fig. 109) coupled with morphological characteristics, we therefore, introduce a new species from intertidal branches and twigs of Suaeda monoica Lam. (Amaranthaceae) in India.
Phylogram generated from maximum likelihood analysis based on a combined LSU, SSU and ITS gene regions of Amphisphaeriaceae and other related taxa. Bootstrap support values for maximum likelihood (green), maximum parsimony (blue) equal to or greater than 75% and the values of Bayesian posterior probabilities (purple) equal to or greater than 0.95 BYPP are given above each branch respectively. The new isolate is in blue. Ex-type strains are in bold. The tree is rooted with Xylaria hypoxylon (CBS 122620)
Amphisphaeria mangrovei Devadatha & V.V. Sarma, sp. nov.
Index Fungorum number: IF554279; Facesoffungi number: FoF04273, Fig. 110
Etymology: Named after the fungal habitat from marine environment, where the fungus found.
Holotype: AMH-9948
Saprobic on intertidal branches and twigs of Suaeda monoica.Sexual morphAscomata 150–280 μm high, 140–250 µm diam. (\( \bar{x} \) = 192 × 211 µm, n = 10), immersed to erumpent, globose to subglobose, gregarious to solitary, coriaceous, brown, short papillate, ostiolate. Ostioles 45–60 µm long, 35–45 µm diam. (\( \bar{x} \) = 50 × 40 µm, n = 10), short, periphysate, brown, 0.5–2 µm (\( \bar{x} \) = 1.5 µm, n = 20). Peridium equal in thickness, 10–20 µm wide (\( \bar{x} \) = 13 µm, n = 10), both at the base and sides, comprising two layers, inner stratum with 3–4 layers of hyaline to light brown cells of textura angularis and outer stratum with 2–3 layers of light brown to brown cells of textura angularis, fusing with the host tissue. Paraphyses 1–2 µm wide (\( \bar{x} \) = 1.7 µm, n = 20), filamentous, septate, longer than asci, embedded in a gelatinous matrix. Asci 80–130 × 5–10 µm (\( \bar{x} \) = 100 × 8 µm, n = 30), 8-spored, unitunicate, cylindrical to obclavate, apically rounded with a J-, apical ring, short pedicellate. Ascospores 12–15 × 4–6 µm (\( \bar{x} \) = 13 × 5 µm, n = 50), partly overlapping 1-seriate, light brown, one median septate, ellipsoidal, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on SWA within 24 h, germ tubes arising from both ends of the ascospore. Colonies on MEA reaching 80–90 mm diam. after 7 days of incubation at room temperature, cream to white from above, yellow and white at margin from below, granular and cottony, undulate, umbonate, irregular.
Material examined: INDIA, Tamil Nadu, Tiruvarur, Muthupet mangroves (10.4°N 79.5°E), on intertidal branches and twigs of Suaeda monoica, 29 October 2016, B. Devadatha, AMH-9948 (holotype), ex-type living culture, NFCCI-4247.
GenBank numbers: ITS = MG844283, LSU = MG767311, SSU = MG844279.
Notes: Amphisphaeria mangrovei differs from other Amphisphaeria species in having smaller ascomata, asci and ascospore dimensions and the marine habitat. Amphisphaeria mangrovei has similar morphological characters to A. sorbi such as clypeate ascomata, asci with J-, apical ring and single, median septate, ellipsoidal ascospores (Senanayake et al. 2015). However, A. sorbi has larger ascomata, asci and ascospores, and slightly constricted ascospores with a thick mucilaginous sheath. Amphisphaeria umbrina and A. vibratilis (Fuckel) E. Müll. have clypeate ascomata, asci with J + , discoid subapical rings and larger ascospores. Phylogenetic analyses of a combined LSU, SSU and ITS regions reveal that A. mangrovei is sister to A. sorbi and A. umbrina with significant support (96% ML, 90% MP and 1.00 BYPP; Fig. 109). Based on morphological characters and molecular phylogenetic analyses, a new species A. mangrovei is introduced.
Sporocadaceae Corda.
Jaklitsch et al. (2016b) proposed the family Sporocadaceae based on morphological observations and phylogenetic analyses of a concatenated ITS-LSU sequence dataset with asexual morph genera that are acervular coelomycetes having hyaline, pale or dark brown, septate conidia. The type genus is Seimatosporium Corda (Jaklitsch et al. 2016b). The family Sporocadaceae hitherto includes 22 genera (Jaklitsch et al. 2016b; Wijayawardene et al. 2017a, 2018a). We follow the latest treatment and updated accounts of Sporocadaceae in Jaklitsch et al. (2016b), Maharachchikumbura et al. (2017) and Wanasinghe et al. (2018). The updated phylogenetic analyses are derived from Maharachchikumbura et al. (2017) and Wanasinghe et al. (2018).
Bartalinia Tassi
We follow the latest treatment and updated accounts of Bartalinia in Jaklitsch et al. (2016b) and Wanasinghe et al. (2018). In this study, Bartalinia kunmingensis is introduced from Zea mays (Poaceae) in Yunnan, China based on morphological characteristic and phylogenetic analyses of ITS and LSU sequence data (Fig. 111).
Phylogram generated from maximum likelihood analysis based on ITS and LSU sequence dataset of the representative species in Sporocadaceae. The updated sequence dataset was derived from Wanasinghe et al. (2018). Sixty strains are included in the sequence analyses. Phlogicylindrium eucalyptorum (CBS 111689) and Phlogicylindrium uniforme (CBS 131312) are used as outgroup taxa. Bootstrap support values for ML equal to or greater than 50% are given above the nodes. Newly generated sequences are in blue. Ex-type strains are indicated in bold
Bartalinia kunmingensis Thiyag., Wanas., Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF556209; Facesoffungi number: FoF05717, Fig. 112
Etymology: The specific epithet “kunmingensis” refers to Kunming City, Yunnan, China, where the holotype was collected.
Holotype: KUN-HKAS 102242
Saprobic on dead leaves of Zea mays. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 80–110 μm high, 110–140 μm diam. (\( \bar{x} \) = 126.2 × 99 μm, n = 10), pycnidial, dark brown to black, immersed, slightly raised, solitary to gregarious, uni-loculate, globose to subglobose, glabrous, ostiolate, with a minute papilla. Conidiomata walls 8–20 µm wide, slightly thick-walled, of equal thickness, comprising several cell layers of brown, pseudoparenchymatous cells of textura angularis, paler towards the inner layers. Conidiophores arising from the inner cavity, reduced to conidiogenous cells. Conidiogenous cells (3.6–)4–7.5 × 2–5 μm (\( \bar{x} \) = 5.4 × 2.8 μm, n = 30), holoblastic, phialidic, rarely with 1–2 percurrent proliferations, discrete, hyaline, ampulliform to subcylindrical, or obclavate, aseptate, smooth-walled. Conidia (17.5–)20–25 × 3–4 μm (\( \bar{x} \) = 22.1 × 3.9 μm, n = 30), cylindrical to subcylindrical, straight to slightly curved, 4-septate, not constricted at the septa, with longest cell at the second from base, bearing appendages; basal cell 2.5–4 μm long (\( \bar{x} \) = 3.2 µm), obconic, truncate at base, hyaline, thin and smooth-walled, second cell from the base 6.5–8 μm long (\( \bar{x} \) = 7.4 µm), pale yellowish, third cell 4–5.5 μm long (\( \bar{x} \) = 4.8 μm), pale yellowish, fourth cell 4–5.5(–6) μm long (\( \bar{x} \) = 5 μm), pale yellowish, apical cell 2–3(–3.7) μm long (\( \bar{x} \) = 2.9 μm), conical, hyaline and smooth-walled, forming three-branched tubular, flexuous, 10–20 µm long apical appendages; basal appendages 5–6 µm long, single, absent at immature state, tubular, unbranched, centric.
Culture characteristics: Colonies on PDA reaching 38–40 mm diam. after one week at room temperature. Colony dense, irregular in shape, flat, slightly raised, surface smooth, with edge undulate, floccose to fluffy, entire margin, forming black stromatic after 2 months; from above, white yellowish to cream at the margin, grey yellowish to dark yellowish, slightly radiated outwards colony, from below, black; not producing pigmentation on agar medium.
Material examined: CHINA, Yunnan Province, Kumming, Kunming Institute of Botany, on dead leaves of Zea mays L. (Poaceae), 5 November 2015, D.S. Tennakoon, COE002 (KUN-HKAS 102242, holotype), ex-type living culture, KUMCC 18-0178.
GenBank numbers: ITS = MK353083, LSU = MK353085, SSU = MK353148, RPB2 = MK492668, TEF1-α = MK492656.
Notes: Phylogenetic analyses of a combined ITS and LSU sequence dataset (Fig. 111) show that Bartalinia kunmingensis (KUMCC 18-0178) clusters with Bartalinia species and is sister to B. robillardoides Tassi (CBS 122705, ex-epitype strain). A comparison of ITS region shows that B. kunmingensis is not significant different from B. robillardoides (only two differentiated nucleotide bases); however, B. kunmingensis is different from B. robillardoides in 207/890 bp (23.2%) in RPB2 region. We therefore, identify our isolate as a new species which was found from corn (Zea mays) in China. Bartalinia kunmingensis differs from B. robillardoides (CBS H-21728) in having smaller conidiomata and paler yellowish conidia. (Crous et al. 2014a).
Robillarda Sacc.
The genus Robillarda was introduced by Saccardo (1880a) and is typified by R. sessile Sacc. This genus contains about 38 species (Crous et al. 2015a). The asexual morph has been reported with its unique characteristics such as solitary or gregarious, separate, subglobose, unicellular, immersed, ostiolate, glabrous pycnidia, holoblastic, ampulliform, hyaline, conidiogenous cells, originating on the inner wall of the pycnidium and ellipsoidal, 1-septate, smooth-walled, hyaline conidia with single branched, apical appendage (Crous et al. 2015a; Borse et al. 2016; Wijayawardene et al. 2016). In this study, the new species, Robillarda mangiferae is introduced from leaf blight on mango in Yunnan, China.
Robillarda mangiferae Thiyag., Wanas., Phookamsak & K.D. Hyde, sp. nov.
Index Fungorum number: IF556210; Facesoffungi number: FoF05718, Fig. 113
Etymology: The specific epithet “mangiferae” is based on the host genus Mangifera, from which the taxon was isolated.
Holotype: KUN-HKAS 102245
Associated with a leaf blight symptom on Mangifera. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 250–310 μm high, 300–340 μm diam., black, pycnidial, semi-immersed to erumpent, solitary, scattered, irregular in shape, uni-loculate, glabrous, minutely ostiolate, with beak-like papilla. Conidiomata walls 8–27 μm wide, thin-walled, of unequal thickness, slightly thick at the sides, composed of two types of cell layers, inner layers comprising hyaline, flattened, pseudoparenchymatous cells of textura angularis to textura prismatica; outer layers comprising dark brown to black, thick-walled, coriaceous cells, of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 5–10 × 3–7 μm (\( \bar{x} \) = 6.9 × 4.8 μm, n = 30), holoblastic, proliferating percurrently 1–3 times, discrete, subcylindrical to ampulliform, hyaline, aseptate, smooth-walled, arising from the inner cavity. Conidia (7.5–)10–11(–12) × (2.5–)3–4(–4.5) μm (\( \bar{x} \) = 10.9 × 3.5 μm, n = 50), hyaline, oblong to ellipsoidal, or subfusoid, narrower towards the basal cell, straight, (0–)1-septate, thin and smooth-walled, apical cell developed into a branched appendage; appendages 25–35 × 1–2.5 μm (\( \bar{x} \) = 29.3 × 1.7 μm, n = 50), dividing into 2–3 branches, straight, non-flexuous, broadly tubular, narrower towards apex, inconspicuously septate at the apex.
Culture characteristics: Colonies on PDA reaching 80–85 mm diam. after 1 week at 20–25 °C, sparse to medium sparse, circular, flat, surface slightly rough with white tufts hyphae, or small granular, edge entire, floccose, forming small, black pycnidia on colony and embedded in media agar after 3 weeks; from above, white-grey to greenish grey, from below, white to cream at the margin, radiated with pale brown to black concentric ring at the middle, white-grey at the centre; not producing pigmentation in agar.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Jinghong, Nabanhe, associated with leaf blight symptom on living leaf of Mangifera sp., 21 November 2015, R. Phookamsak, XB011 (KUN-HKAS 102245, holotype), ex-type living culture, KUMCC 18-0180.
GenBank numbers: ITS = MK353084, LSU = MK353086, SSU = MK353149.
Notes: Robillarda mangiferae resembles Robillarda species in having oblong to subfusoid, septate conidia, with an apical cell modified into a branched appendage. However, R. mangiferae can be distinguished from other Robillarda species by its appendage being straight, non-flexuous, broad tubular and narrower towards the apex, with inconspicuous septa at the apex, as well as its conidiogenous cells being holoblastic and proliferating percurrently. Robillarda species have flexuous, narrow tubular, aseptate appendages and holoblastic conidiogenous cells, proliferating sympodially or percurrently near the apex (Crous et al. 2015a; Wijayawardene et al. 2016). Phylogenetic analyses of a combined ITS and LSU sequence dataset show that R. mangiferae clusters with other Robillarda species and Ellurema indica Nag Raj & W.B. Kendr. [current name = Hyalotiopsis Punith., proposed by Réblová et al. (2016), and Wijayawardene et al. (2016)] in Sporocadaceae, but the species forms a distinct lineage at the base of this clade with moderate support (92% ML and 1.00 BYPP; Fig. 111). Robillarda mangiferae differs from Ellurema indica in having oblong to ellipsoidal, or subfusoid, (0–)1-septate conidia, with straight, non-flexuous, broadly tubular apical appendages. The asexual morph of Ellurema indica (= Hyalotiopsis) has cylindrical to fusiform or obclavate conidia, with more than 1-septate and 2–3 apical bi- or tri-furcate, filiform, flexuous appendages (Wijayawardene et al. 2016, 2017a). Based on morphological characteristics and phylogenetic analyses of a combined ITS and LSU sequence dataset (Fig. 111), we hence, introduce a new species, R. mangiferae in this study.
Xylariales Nannf.
Diatrypaceae Nitschke
We follow the latest treatment and updated accounts of Diatrypaceae in de Almeida et al. (2016), Shang et al. (2017, 2018) and Senwanna et al. (2017). A higher level classification with divergence time estimates for Diatrypaceae was provided by Hongsanan et al. (2017) and placed the family in order Xylariales (Xylariomycetidae, Sordariomycetes) and a similar scheme is followed in Wijayawardene et al. (2018a). A novel species, Peroneutypa mangrovei is introduced in Diatrypaceae based on analysis of a combined ITS and TUB2 sequence dataset (Fig. 114), coupled with morphological characteristics. In addition, the new genus Neoeutypella is introduced as a monotypic genus to accommodate N. baoshanensis. The new genus was collected from dead wood in Baoshan, China. The sexual and asexual morphs are described and illustrated.
Phylogram generated from maximum likelihood, maximum parsimony and Bayesian inference analyses based on a combined ITS and TUB2 sequence dataset of diatrypaceous species. Bootstrap support values for maximum likelihood (black) and maximum parsimony (green) equal to or greater than 60% and Bayesian posterior probabilities (blue) equal to or greater than 0.95 BYPP are shown above the nodes. The new isolates are in blue and ex-type strains are in bold. The tree is rooted to Lopadostoma turgidum (LT2 and CBS 133207)
Neoeutypella M. Raza, Q.J. Shang, Phookamsak & L. Cai, gen. nov.
Index Fungorum number: IF555373; Facesoffungi number: FoF04927
Etymology: The generic epithet “Neoeutypella” refers to the taxon resembling Eutypella.
Saprobic on dead wood of Pinus armandii. Sexual morphAscostromata entostromatic, carbonaceous, visible as black, solitary to gregarious, globose to long irregular in shape on host surface, erumpent through host epidermis, producing yellow pigments surrounding ascostroma. Ascomata perithecial, black, immersed to semi-immersed in stromatic tissues, aggregated, globose or subglobose, ostiolate, papillate, slightly conspicuous, with periphyses. Peridium thickened unequally, two-layered, outer layer comprising 5–7 layers of thick-walled, hyaline to dark brown cells of textura angularis, inner layer comprising 3–5 layers of thin-walled, hyaline to brown cells of textura prismatica. Hamathecium comprising aseptate, filamentous paraphyses, tapering toward the apex, embedded in hyaline gelatinous matrix. Asci 8-spored, unitunicate, spindle-shaped, long pedicellate, apically rounded with refractive cytoplasmic strands, amyloid, with a J + , subapical ring. Ascospores overlapping 1–3-seriate, allantoid, slightly or moderately curved, initially hyaline, becoming pale brown at maturity, aseptate, mostly with small 1–2 guttules. Asexual morphHyphae branched, smooth, hyaline, septate. Conidiophores long, branched, with phialides, mononematous, macronematous, hyaline. Conidiogenous cells smooth-walled, hyaline, holoblastic, discrete, phialidic, doliiform, ampulliform or irregular in shape. Conidia filiform, solitary, aseptate, smooth-walled, unbranched, hyaline to pale yellow.
Type species: Neoeutypella baoshanensis M. Raza, Q.J. Shang, Phookamsak & L. Cai
Notes: Neoeutypella resembles Eutypella (Nitschke) Sacc. in forming large entostroma, 8-spored, spindle-shaped asci and allantoid ascospores, with a libertella-like asexual morph. Phylogenetic analyses of maximum likelihood, maximum parsimony and Bayesian inference based on the combined ITS and TUB2 sequence dataset (Fig. 114) show that Neoeutypella baoshanensis groups with Eutypella caricae (De Not.) Berl. (strains EL51C and GL08362). Sequences of these two strains are available in GenBank, but no morphological description is available for comparative studies. The two Eutypella caricae strains and Neoeutypella form a distinct lineage from Eutypella sensu stricto. Acero et al. (2004) mentioned that these two strains might have been misidentified and a taxonomic revision of these species is needed. Eutypella caricae and Neoeutypella are phylogenetically closely related to Diatrypella banksiae Crous which produced an asexual morph (Crous et al. 2016a). Nevertheless, Neoeutypella can be differentiated from D. banksiae in shape and size of conidia. Neoeutypella baoshanensis (see below) has filiform conidia [(16.5–)25–37(–40) × 1.2–1.9 µm], whereas Diatrypella banksiae has spindle-shaped conidia [(25–)27–30(–35) × 1.5(–2) µm] (Crous et al. 2016a).
Based on morphological comparison of our new taxon and Eutypella caricae described by Saccardo (1882) and Berlese (1902), Neoeutypella baoshanensis (99% similarity in ITS, 90% similarity in TUB2) differs from Eutypella caricae in having larger asci (N. baoshanensis, (45–)52–110(–125) × (19–)26–37(–40) versus 35–45 × 6–7, E. caricae) and ascospores (N. baoshanensis, (30–)35–43(–50) × (8–)9–11(–12) versus 9–11 × 2.5–3, E. caricae) (Saccardo 1882; Berlese 1902). Neoeutypella baoshanensis has spindle-shaped asci and pale yellowish to pale brown ascospores, whereas E. caricae has clavate asci and hyaline ascospores (Saccardo 1882; Berlese 1902). Based on phylogenetic support coupled with morphological differences, we therefore introduce our isolate as a new species in the new genus Neoeutypella.
Neoeutypella baoshanensis M. Raza, Q.J. Shang, Phookamsak & L. Cai, sp. nov.
Index Fungorum number: IF555372; Facesoffungi number: FoF04928, Fig. 115
Neoeutypella baoshanensis (HMAS 255436, holotype). a Blackish ascostromata surrounded by yellow pigments on Pinus armandii. b Vertical section of ascostroma. c Ostiole. d, e Peridial structure. f Paraphyses stained with Indian ink. g Asci attached with paraphyses stained with Indian ink. h Immature ascus. i–k Mature asci. l J+, apical ring stained with Melzer’s reagent. m–q Ascospores. r Germinated ascospore. s, t Culture characteristics on PDA (s = from above, t = from below). u Conidiogenous cells with conidia. v Phialides with young developing conidia. w, x Conidiogenous cells. y Conidia. Scale barsb = 1000 μm, c, d = 50 μm, e–g = 20 μm, h–k, r, u, y = 10 μm, l–q = 5 μm
Etymology: The specific epithet “baoshanensis” refers to the locality Baoshan (Yunnan, China), where the holotype was collected.
Holotype: HMAS 255436
Saprobic on dead wood of Pinus armandii. Sexual morphAscostromata 650–1100 μm diam., entostromatic, carbonaceous, black, solitary to gregarious, globose to long irregular in shape on host surface, erumpent through host epidermis, producing yellow pigments surrounding ascostroma. Ascomata perithecial, 500–770 high, 450–530 diam., black, immersed to semi-immersed in stromatic tissues, aggregated, globose or subglobose, ostiolate, papillate, slightly conspicuous, with periphyses. Peridium 145–250 wide, thickened unequally, two-layered, outer layer comprising 5–7 layers of thick-walled, hyaline to dark brown cells of textura angularis, inner layer comprising 3–5 layers of thin-walled, hyaline to brown cells of textura prismatica. Hamathecium 3–7 μm wide (\( \bar{x} \) = 4.7 μm, n = 20), comprising aseptate, filamentous paraphyses, tapering towards the apex, embedded in hyaline gelatinous matrix. Asci (60–)75–85(–90) × (5.5–)6.5–7.5(–8) μm (\( \bar{x} \) = 77.5 × 8 μm, n = 25), 8-spored, unitunicate, spindle-shaped, long pedicellate, apically rounded with refractive cytoplasmic strands, amyloid, with a J + , subapical ring. Ascospores (8.5–)10–11.5(–13) × (2–)2.3–2.5(–3) μm (\( \bar{x} \) = 10.8 × 2.4 μm, n = 50), overlapping 1–3-seriate, initially hyaline, becoming pale brown at maturity, allantoid, slightly or moderately curved, aseptate, mostly with 1–2 small guttules. Asexual morphHyphae branched, smooth, hyaline, septate, 1.5–3.5 μm diam. Conidiophores long, branched, with phialides, mononematous, macronematous, hyaline. Conidiogenous cells (12–)14–35.5(–40) × 4–13(–15) μm (\( \bar{x} \) = 25 × 8.5 μm, n = 20), smooth-walled, hyaline, holoblastic, discrete, phialidic, doliiform, ampulliform or irregular in shape. Conidia (16.5–)25–37(–40) × 1.2–1.9 μm (\( \bar{x} \) = 29 × 1.5 μm, n = 50), filiform, solitary, aseptate, smooth-walled, unbranched, hyaline to pale yellow.
Culture characteristics: Colonies on PDA reaching 6.5–7 mm diam. after 1 week at 25 ± 2 °C, circular, flat, slightly raised, surface dull with edge undulate, filamentous at the margin; from above, white with cotton consistency; from below, pale yellow and not producing pigment in PDA medium. Asexual morph produced on PDA after 3 weeks and colony becomes black from below.
Material examined: CHINA, Yunnan Province, Baoshan City, Longling County, on dead wood of Pinus armandii Franch. (Pinaceae), October 2015, M. Raza, HMAS 255436 (holotype), ex-type living culture, LC 12111.
GenBank numbers: ITS = MH822887, TUB2 = MH822888.
Peroneutypa Berl.
We follow the latest treatment and updated accounts of Peroneutypa in Senwanna et al. (2017) and Shang et al. (2018).
Peroneutypa mangrovei Devadatha & V.V. Sarma, sp. nov.
Index Fungorum number: IF554285; Facesoffungi number: FoF04271, Fig. 116
Etymology: Specific epithet in reference to the habitat.
Holotype: AMH-9944
Saprobic on decaying wood of Avicennia marina.Sexual morphAscostromata absent or poorly developed between perithecial necks, perithecia solitary to gregarious, up to four in groups, dark brown to black, immersed becoming raised to erumpent through the host tissue with median necks. Ascomata 250–525 µm high, 100–330 µm diam. (\( \bar{x} \) = 375 × 202 µm, n = 10), erumpent to immersed, globose to subglobose, gregarious to solitary, ostiolate, with short beaks, periphysate, brown to black. Ostiolar canals 50–85 µm wide (\( \bar{x} \) = 67 µm, n = 5), with moderate neck length 100–350 µm (\( \bar{x} \) = 231 µm, n = 5), cylindrical, straight, dark brown to black. Periphyses filamentous, short, 0.5–2 µm wide (\( \bar{x} \) = 1.5 µm, n = 10). Peridium 15–35 µm wide (\( \bar{x} \) = 22 µm, n = 10), comprising two layers, inner stratum with many layers of hyaline cells of textura angularis and outer stratum with 2–3 layers of light brown to black cells of textura angularis. Hamathecium composed of numerous, 1–2 µm (\( \bar{x} \) = 1 µm, n = 20) wide, filamentous, septate paraphyses, longer than asci, embedded in a gelatinous matrix. Asci 14–20 × 3–4 µm (\( \bar{x} \) = 17 × 3.5 µm, n = 20), 8-spored, unitunicate, cylindrical to clavate, short pedicellate, apically rounded to truncate, with a J-, apical ring. Ascospores 3–5 × 1–1.5 µm (\( \bar{x} \) = 4 × 1 µm, n = 30), overlapping 1–3-seriate, hyaline to pale yellow, straight to allantoid, aseptate, smooth-walled, lacking guttules, light brown in mass. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 50–80 mm diam. after 15 days of incubation at room temperature, initially white, becoming light grey at maturity, reverse light yellow, cottony, punctiform, flat, circular, entire.
Material examined: INDIA, Puducherry, Thengaithittu mangroves (11.5°N 79.5°E), on decaying wood of Avicennia marina (Forssk.) Vierh. (Acanthaceae), 12 March 2016, B. Devadatha (AMH-9944, holotype), ex-type living culture, NFCCI-4246.
GenBank numbers: ITS = MG844286, LSU = MG844278, SSU = MG844282, TUB2 = MG844282.
Notes: Peroneutypa mangrovei shares similar morphological characters such as J-, apical ring asci, overlapping ascospores dimensions with P. diminutispora D.A.C. Almeida et al. (de Almeida et al. 2016). However, it differs in having perithecia single or in groups of up to four, shorter ascomata and ostiole, longer asci (14–20 × 3–4 µm) and its exclusive occurrence in marine habitat. Peroneutypa diminutispora has perithecia arranged in a single layer, occurring singly or in groups of up to seven, shorter asci (8–14 × 3.5–5 μm) that are urn-shaped (de Almeida et al. 2016). Peroneutypa cosmosa (Speg.) Carmarán & A.I. Romero has larger asci (18–25 × 5–7 μm) with J+, apical rings and longer ascospores (6–8 μm) (Carmarán et al. 2006). Peroneutypa longiasca Senwanna et al. and P. mackenziei Q.J. Shang et al. have longer ascospores (5–7 μm) (Senwanna et al. 2017; Shang et al. 2017). This is the first report of Peroneutypa species from a marine habitat (Jones et al. 2015). ITS and TUB2 gene phylogeny also supports P. mangrovei as distinct forming an independent lineage, sister to Eutypa microasca E. Grassi & Carmarán, and cluster with Peroneutypa diminutispora and P. cosmosa (Fig. 114). A comparison of ITS nucleotides between P. mangrovei and P. dimunitispora (GenBank no. KM396647) results in 7.4% (47 nucleotides) and 5.5% (26 nucleotides) along with P. cosmosa (GenBank no. KF964568).
Hypoxylaceae DC.
We follow the latest treatment and updated accounts of Hypoxylaceae in Daranagama et al. (2018) and Wendt et al. (2018).
Hypoxylon Bull.
Hypoxylon is the largest genus in Hypoxylaceae with over 140 accepted species classified on the basis of morphology, phylogeny and chemotaxonomy (Kuhnert et al. 2014; Wijayawardene et al. 2017a). More than 1000 epithets are listed in Index Fungorum (2019). We introduce a new species Hypoxylon teeravasati based on morphology and multigene analysis (Fig. 117).
Phylogram generated from maximum likelihood analysis based on ITS, LSU, RPB2 and TUB2 gene regions of Hypoxylaceae and related taxa in Xylariales. Bootstrap support values for maximum likelihood (green), maximum parsimony (blue) equal to or greater than 75% and the values of Bayesian posterior probabilities (purple) equal to or greater than 0.95 are given above each branch respectively. The new isolate is in blue. Ex-type strains are indicated in bold. The tree is rooted with Xylaria hypoxylon (CBS122620)
Hypoxylon teeravasati Devadatha, V.V Sarma & E.B.G Jones, sp. nov.
Index Fungorum number: IF554278; Facesoffungi number: FoF04272, Fig. 118
Hypoxylon teeravasati (AMH-9906, holotype). a Ascostromata on the decaying wood of Avicennia marina.b Cross section of ascostroma. c, d Longitudinal sections of ascostroma. e Peridium. f Filamentous paraphyses. g–k Immature and mature asci (g–i = Asci showing apical bluing in Lugol’s solution). l Immature ascospore. m–o, q Mature ascospores. p Mature ascospore with dehiscent perispore in KOH. r Germinating ascospore. s KOH extractable pigments. Scale barsc, d = 100 μm, e–r = 10 μm
Etymology: The specific epithet “teeravasati” in Sanskrit, refers to the coastal environment where the fungus thrives
Holotype: AMH-9906
Colour codes follow: A mycological colour chart (Rayner 1970).
Saprobic on decaying wood of mangrove trees. Sexual morphAscostromata 310–770 × 400–655 μm diam. (\( \bar{x} \) = 533 × 498 µm, n = 10), glomerate to hemispherical, effuse-pulvinate, connected to each other by thick stromatal tissue at the base, with very conspicuous perithecial mounds, surface burnt sienna (plate 7, D8), with KOH extractable pigments eye brown (plate 7, F6), the tissue below the perithecial layer inconspicuous. Perithecia 300–650 μm diam. (\( \bar{x} \) = 442 μm, n = 10), immersed, sphaerical. Ostioles papillate, without apparent disk formation. Peridium 45–100 μm (\( \bar{x} \) = 68 μm, n = 10), comprising several layers of textura angularis and outer layer of pseudoparenchyma. Hamathecium composed of 1–3 μm diam., hyaline, aseptate and filiform paraphyses, longer than asci. Asci 65–160 × 7–13 μm (\( \bar{x} \) = 105 × 8.2 μm, n = 20), 8-spored, unitunicate, cylindrical, pedicellate, with apical ring bluing in Lugol’s solution, discoid, 1–2.5 × 2–3.5 μm (\( \bar{x} \) = 1.9 × 3.1 μm, n = 20). Ascospores 9–15 × 4–7 μm (\( \bar{x} \) = 11.7 × 7.6 μm, n = 30), 1-seriate, unicellular, ellipsoid-inequilateral, with narrowly rounded ends, brown to dark brown, with a straight germ slit more or less running the entire spore-length, perispore dehiscent in 10% KOH, epispore smooth. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on SWA within 24 h, germ tubes arising from terminal ends of the ascospore. Colonies on MEA at room temperature reaching 45–60 mm diam. within 25 days, honey yellow at centre and olive buff at margin, reverse clove brown with yellow exudates and dawn grey at margin, honey yellow diffusible pigments, filiform, umbonate, circular and velvety.
Material examined: INDIA, Tamil Nadu, Tiruvarur, Muthupet mangroves (10.4°N 79.5°E), on decaying wood of Avicennia marina (Acanthaceae), 28 November 2015, B. Devadatha, AMH-9906 (holotype), ex-type living culture, NFCCI-4216; ibid. on decaying branches and twigs of Suaeda monoica Forssk. ex J.F. Gmel, PUFD4 (paratype at Pondicherry University, Puducherry).
GenBank numbers: ITS = KY863509, LSU = MF385274, SSU = MF385273, RPB2 = MG986895, TEF1-α = MF182401, TUB2 = MG986894.
Notes: Multigene analysis shows that Hypoxylon teeravasati shares a strongly supported sister relationship with H. jaklitschii Sir & Kuhnert and H. lenormandii Berk. & M.A. Curtis as a basal taxon. All three taxa constitute a single monophyletic clade with high support (Fig. 117), but their relationships with other Hypoxylon species are not well-resolved. We found distinct nucleotide base pair differences between H. teeravasati and H. jaklitschii across ITS and TUB2 genes [36 within ITS and 23 within TUB2] which is in agreement with guidelines by Jeewon and Hyde (2016) to establish new species. Hypoxylon teeravasati, H. lenormandii, H. jaklitschii and H. croceum J.H. Mill. share similar stromatal characters and overlapping asci dimensions. However, H. teeravasati can be easily distinguished from H. lenormandii, H. jaklitschii and H. croceum in having a burnt sienna surface, with KOH extractable pigments eye brown and larger perithecia. Hypoxylon lenormandii has surface greyish sepia, fuscous, or brown vinaceous, dull orange brown to dark brown granules beneath and between perithecia, with KOH extractable pigments hazel, fulvous, umber or ochreous (Kuhnert et al. 2015; Liu et al. 2015a). Hypoxylon jaklitschii has a sepia to dark brick surface, pruinose, with orange brown or dark brown granules immediately beneath the surface and between perithecia, with KOH extractable pigment umber or dark brick and smaller ascospores (9.5–12 × 4–5.5 versus 9–15 × 4–7) (Kuhnert et al. 2015). Hypoxylon croceum is distinguished from H. teeravasati in having an initially sepia or fuscous surface, becoming dark brown at maturity and abelline or hazel KOH extractable pigments (Miller 1933). Hypoxylon croceum is the only report of Hypoxylon species from a marine habitat (Jones et al. 2015). Hypoxylon teeravasati is saprobic on decaying branches and twigs of Avicennia marina and Suaeda monoica, exclusively from marine environments. Hypoxylon lenormandii and H. jaklitschii have been reported from terrestrial habitats.
Phylum Basidiomycota R.T. Moore
We follow the latest treatment of Basidiomycota in Zhao et al. (2017).
Class Agaricomycetes Doweld
The classifications of the families in Agaricomycetes herein follow Hibbett et al. (2014) and Zhao et al. (2017). The subclasses, orders and families of Agaricomycetes are listed in alphabetical order.
Subclass Agaricomycetidae Parmasto
Agaricales Underw.
Agaricaceae Chevall.
We follow the latest treatments and updated accounts of Agaricaceae in Zhao et al. (2016), Zhou et al. (2016) and Hyde et al. (2017). Three specimens were collected and preliminary BLAST using ITS sequence data indicated that two taxa belong to Agaricus sect. Xanthodermatei and the third to Coprinus. Based on distinctive morphological characteristics and phylogenetic support, two novel species of Agaricus sect. Xanthodermatei as well as Coprinus trigonosporus sp. nov. are introduced in this study. An updated phylogenetic tree based on maximum likelihood and Bayesian inference analyses also confirms their placement (Fig. 119).
Maximum likelihood tree of Agaricus sect. Xanthodermatei based on LSU, TEF1-α and ITS sequences with Agaricus campestris (LAPAG370) as the outgroup taxon. The bootstrap values and Bayesian posterior probabilities more than 50%/0.90 (BS/BYPP) are indicated at the nodes. The branches in bold mean the related PP > 0.95, “T” refers to sequences from type specimen
Agaricus L.: Fr.
We follow the latest treatment and updated accounts of Agaricus in Thongklang et al. (2014), Karunarathna et al. (2016), Zhao et al. (2016) and Zhou et al. (2016). Detailed taxonomic revision of taxa in Agaricus sect. Xanthodermatei was discussed by Chen et al. (2016), Kerrigan (2016), Mahdizadeh et al. (2016), Zhao et al. (2016) and Parra et al. (2018).
Agaricus memnonius M.Q. He & R.L. Zhao, sp. nov.
Fungal names: FN570535; Faceoffungi number: FoF03940, Fig. 120
Etymology: The Latin epithet “memnonius” meaning “brown-black” refers to the colour of the pileus surface.
Holotype: HMAS 0278359
Pileus 50 mm diam., plane or plano-concave, disc black or black-brown, slightly depressed, margin straight, exceeding lamellae; surface dry, with black-brown fibrillose scales against white background, scales triangular, appressed, extremely denser at disc, scattered towards the margin. Lamellae up to 3 mm broad, free, crowded, pink, edge even, intercalated with lamellulae. Annulus superous, double, membranous, white, pendant, upper side smooth, lower side cogwheel, white, edge light brown. Stipe 57 × 5 mm (8 mm at base), white, hollow, cylindrical with slightly bulbous base, surface dry, smooth, silky, with rhizomorphs. Context fleshy, white. Odour unknown. KOH reaction: positive yellow. Schäffer’s reaction: negative. Basidia 15–19.5 × 6.5–9.3 μm, clavate, hyaline, 4-spored, smooth. Basidiospores 4.5–5.3 × 3.3–4.1 μm, (\( \bar{x} \) = 5 ± 0.2 × 3.6 ± 0.2, Q = 1.2–1.5, Qm = 1.4 ± 0.1, n = 20), ellipsoid, smooth, thick-walled, brown. Pleurocystidia absent. Cheilocystidia not very conspicuous, can be single and multiseptate (generally no more than three elements), the terminal element clavate, cylindrical, 12.1–24.8 × 6.9–13.7 μm. Pileipellis a cutis composed of hyphae of 4.6–14.4 μm diam., smooth, cylindrical, slightly constricted at septa, pigment intracellular, light brown or brown.
Material examined: CHINA, Sichuan Province, Miyi County, 13 September 2015, ZRL20151118 (HMAS 0278359, holotype).
Host and habitat: Solitary on soil in forest with bamboo around.
Distribution: Sichuan Province (China).
GenBank numbers: ITS = MG763128, LSU = MG765263, TEF1-α = MG765265.
Notes: The phylogenetic trees generated by maximum likelihood and Bayesian inference analyses (Fig. 119) show that Agaricus memnonius forms a distinct lineage within Agaricus sect. Xanthodermatei in the clade Xan II and the tree topology is similar with previous studies (Zhao et al 2016; Zhou et al. 2016; Parra et al. 2018).
Agaricus memnonius has relatively small and slender basidiomes, which are similar to the European species A. laskibarii L.A. Parra & Arrillaga, A. xanthodermulus Callac & Guinb. and A. parvitigrinus Guinb. & Callac (Parra 2013). However, A. memnonius has black-brown and triangular scales on the pileus, while the other three species have greyish brown and not triangular scales. Some species described from China also have small and slender basidiomes, such as A. gregariomyces J.L. Zhou & R.L. Zhao and A. karstomyces R.L. Zhao. However, A. gregariomyces has larger and elongate basidiospores (5.6–6.3 × 3.5–4.0 μm, Q = 1.6–1.9; Zhou et al. 2016). Agaricus karstomyces has dot-like scales on the pileus, while A. memnonius has triangular scales. Based on phylogenetic and morphological studies, A. memnonius is introduced as a new species and is characterized by its distinct phylogenetic position in section Xanthodermatei, small and slender basidiome, black-brown and triangular scales on the pileus, and the single and multiseptate cheilocystidia.
Agaricus langensis M.Q. He & R.L. Zhao, sp. nov.
Fungal names: FN570534; Facesoffungi number: FoF03941, Fig. 121
Etymology: The Latin epithet “langensis’’ meaning “originating from Lang” refers to the Lang County where the holotype was collected.
Holotype: HMAS 0278317
Basidiomes flavescent when rubbed. Pileus 26–49 mm diam., parabolic when young, then convex, disc slightly subumbonate, margin straight, sometimes with appendiculate remains of veil; surface dry, with grayish brown fibrillose scales against white background, scales appressed, covering the whole pileus, denser at disc, scattered towards the margin. Lamellae up to 5 mm broad, free, crowded, pink, edge even, intercalated with lamellulae. Annulus up to 6 mm in diam., superous, double, membranous, white when fresh, yellowish when dry, pendant, upper side smooth, lower side cogwheel, white, edge light brown. Stipe 59–76 × 6–7 mm (7–10 mm at base), white, hollow, cylindrical, surface dry, smooth or slightly fibrillose, with rhizomorphs. Context fleshy, white. Odour unknown. KOH reaction: positive yellow. Schäffer’s reaction: negative. Basidia 18.5–25.3 × 6.7–8.9 μm, clavate, hyaline, 4-spored, smooth. Basidiospores 6.3–8.3(–8.5) × 3.7–5.1 μm, (\( \bar{x} \) = 7.2 ± 0.6 × 4.4 ± 0.3, Q = 1.4–1.9, Qm = 1.6 ± 0.1, n = 20), ellipsoid, elongate, smooth, thick-walled, brown. Pleurocystidia absent. Cheilocystidia absent. Pileipellis a cutis composed of hyphae of 4.8–12.1 μm diam., smooth, cylindrical, slightly constricted at septa, hyaline, light brown or brown.
Material examined: CHINA, Tibet, Lang County, Gongga Village, Alt. 3384 m, 29°16′ N, 93°11′ E, S-Y. Su, ZRL20152282 (HMAS 0278317, holotype).
Habit and habitat: Scattered on soil in forest.
Distribution: Tibet (China).
GenBank numbers: ITS = MG763129, LSU = MG765264, TEF1-α = MG765266.
Notes: Agaricus langensis (HMAS 0278317) belongs to a clade called Xan III (Thongklang et al. 2014; Parra et al. 2018) which is present (but not always indicated) in all previous multigene trees of Agaricus sect. Xanthodermatei (Zhao et al. 2016, Zhou et al. 2016, Parra et al. 2018) and which includes in our tree A. flavidodiscus L.A. Parra et al., A. langensis and 32 other species (Fig. 119). The new species forms a sister lineage with Agaricus sp. (ZRL2012629) with moderate support (75% ML and 1.00 BYPP) in our phylogenetic analyses (Fig. 119). Agaricus parvitigrinus Guinb. & Callac resembles A. langensis in the field, because both have small to medium sized basidiomes, a convex pileus covered by greyish fibrillose scales. However, A. langensis has larger basidiospores than those of A. parvitigrinus (5.8 × 3.7 μm; Parra 2013). Agaricus menieri Bon, A. xanthodermulus Callac & Guinb. and A. xanthodermus Genev. have similar sized basidiospores, but have distinct cheilocystidia (Parra 2013), a feature lacking in A. langensis. Agaricus tibetensis J.L. Zhou & R.L. Zhao also has small to medium sized basidiomes, same sized basidiospores as A. langensis, and no cheilocystidia (or rare in A. tibetensis), and both species originated from Tibet (Zhou et al. 2016). However, their ITS sequences differ at 33 base positions, and the molecular phylogeny also indicates that they are different species.
Based on the phylogenetic analyses and morphological characteristics, we introduce this new species, which is characterized by its distinct phylogenetic position in sect. Xanthodermatei, small to medium sized basidiomes, relatively large basidiospores and absence of cheilocystidia.
Coprinus Pers.
Traditionally, Coprinus comprised all coprinoid species (black spore print and mature lamellae, plicate-sulcate pileus) and it was the type genus of the family Coprinaceae Overeem & Weese. On the basis of earlier phylogenetic studies, Redhead et al. (2001) transferred most Coprinus species into four genera of the new family Psathyrellaceae Vilgalys et al. Coprinus comatus (O.F. Müll.) Pers., and a few related species remained in Coprinus, which was transferred to family Agaricaceae (Moncalvo et al. 2002; Vellinga 2004). Coprinus species have rather small to large basidiomes with squamulose pileus, ring-like partial veil and deliquescent lamellae. A phylogenetic tree is presented in Fig. 122.
Maximum likelihood phylogenetic tree of Coprinus trigonosporus and closely related species based on ITS sequence dataset and calculated with MEGA6.0 software (Tamura et al. 2013). The new species is shown in blue. Maximum likelihood bootstrap values greater than 50% are indicated at the nodes. The tree is rooted with Tulostoma kotlabae and T. niveum. The bar indicates the number of nucleotide substitutions per site
Coprinus trigonosporus Tkalčec & Mešić, sp. nov.
MycoBank number: MB826852; Facesoffungi number: FoF05719, Fig. 123
Etymology: The species is named after its basidiospores that are often rounded triangular in frontal view.
Holotype: CNF 1/6594
Pileus up to 24 mm broad and 32 mm high when still closed, 32–46 mm broad at maturity, ellipsoid to oblong at first, later obtusely conical to subapplanate with broad subumbonate centre, often radially splitting, when young entirely covered with a dense, white universal veil forming imbricate, rather large scales with mostly upturned lower edge, later veil splitting up into patches of different sizes (from one scale to group of scales) except at the centre, surface strongly plicate-sulcate, white at first, becoming pink brown to vinaceous brown and soon black, margin sometimes with few small, white, appendiculate remnants of partial veil at maturity. Lamellae free, crowded, broad, often sinuous when young, white at first, later turning pink to vinaceous brown, soon becoming black, weakly deliquescent. Stipe 34–56 × 5.5–10 mm, tapering upwards or subcylindrical, with subbulbous, obconical, buried base (up to 15 mm wide), white, central, hollow, dry, ± tomentose when young, (sub) glabrous at maturity, partial veil remaining as a white, narrow ring at the top of the base. Context white. Odour and taste fungoid. Spore print black. Basidia 30–62 × 12–24 µm, clavate, 4-spored, thin-walled or moderately thick-walled (up to 1 µm), first hyaline, at maturity partially (in the middle part) or entirely with pale to dark brown parietal pigment, surrounded by 6–12 hymenophysalides (pseudoparaphyses). Hymenophysalides 15–42 × 6–14 µm, clavate, thin-walled, sometimes moderately thick-walled (up to 0.8 µm), hyaline to pale brown. Basidiospores [500/5/1] (9.3–)10.7–14.6–18.6(–20.8) × (8.1–)9.6–11.8–14.3(–15.1) × (7.8–)8.5–10–11.6(–12.3) µm, averages of different basidiomes 13.9–15.3 × 11.7–12.2 × 9.8–10.3 µm, Qf = 0.9–1.2–1.7, Qs = 1.2–1.4–1.8, av. Qf = 1.1–1.3, av. Qs = 1.4–1.5, rounded triangular, cordiform, ovoid or subglobose in frontal view (rarely ellipsoid), with rounded to flat base and rounded apex, ovoid to ellipsoid in side view, flattened, smooth, moderately thick-walled (up to 1 µm), with 1.5–3.5 µm wide, slightly to strongly eccentric, rarely central germ-pore (sometimes ring-like protruding), medium to dark brown in H2O and KOH, semitransparent, non-amyloid and non-dextrinoid. Pleurocystidia absent. Cheilocystidia 16–58 × 11–28 µm, broadly to narrowly clavate, thin-walled, hyaline. Pileipellis a cutis, composed of repent, thin-walled, 5–15 µm wide, subhyaline to brown hyphae with intracellular pigment. Veil cells on the pileus 12–170 × 2–38 μm, cylindrical to inflated, in chains, often constricted at the septa, occasionally branched, sometimes with individual, simple, subglobose to finger-like excrescences, thin-walled, rarely moderately thick-walled (up to 0.8 µm), smooth, rarely finely encrusted, hyaline. Stipitipellis a cutis of parallel, repent, thin-walled, hyaline, 1.5–10 µm wide hyphae. Clamp connections absent; pseudoclamps present on some septa in veil, pileipellis and trama.
Material examined: SAUDI ARABIA, Jizan Province, village, 8 km S from Harub, 17°22′03″N, 42°52′10″E, 257 m a.s.l., on sandy soil in a courtyard, 22 September 2010, leg. M. Čerkez, CNF 1/6594 (holotype).
Habit and habitat: In group (approximately 20 basidiomes), on sandy soil with some sheep dung, among scattered herbaceous plants and bushes in a courtyard.
Distribution: So far known only from the type locality in the Kingdom of Saudi Arabia, Jizan Province.
GenBank numbers: ITS = MH422561, LSU = MH422563.
Notes: Based on a megablast search of NCBIs GenBank nucleotide database, the closest hit using ITS sequence for our new species is Coprinus vosoustii Pilát [GenBank no. JF907844, similarity = 627/668(94%), Gaps = 9/668(1%)]. Phylogenetic analysis based on the ITS sequence data of Coprinus sensu stricto shows that C. trigonosporus formed a distinct lineage and clustered with C. vosoustii and C. sterquilinus (Fr.) Fr.. Morphologically, C. trigonosporus is best characterised by a considerable number of cordiform and rounded triangular basidiospores. Other species in the genus have ellipsoid or ovoid basidiospores (van de Bogart 1976; Moreno and Heykoop 1998; Cacialli et al. 1999; Uljé 2005; Crous et al. 2016b). The rather peculiar microscopic characteristic of C. trigonosporus is the large number of hymenophysalides (6–12) surrounding the basidium.
Amanitaceae E.-J. Gilbert
Amanitaceae is defined as those agarics having bilateral, divergent lamellae trama and a longitudinally acrophysalidic stipe context. The family comprises five genera, Amanita Pers., Catatrama Franco-Mol., Limacella Earle, Limacellopsis Zhu L. Yang et al. and Myxoderma Fayod ex Kühner (Cui et al. 2018). In this family, Amanita is the most species-rich genus (Bas 2000; Tulloss 2005; Yang 2005; Cui et al. 2018). More than 1000 species have been described worldwide with ca. 600 accepted names; about 60 taxa have been reported from India (Yang 2000; Bhatt et al. 2003; Tulloss and Yang 2016).
Amanita Pers
Amanita was recently discussed by Ariyawansa et al. (2015a), Tulloss and Yang (2016), Tulloss et al. (2016) and Cui et al. (2018). We follow the latest treatment and updated accounts of Amanita in Tulloss and Yang (2016), Tulloss et al. (2016), Tibpromma et al. (2017) and Cui et al. (2018). Two novel species (belonging to Amanita sect. Amanita and A. sect. Vaginatae), collected from the north-western part of Indian Himalaya are introduced together with their morphology and phylogenetic placements (Figs. 128, 131). In addition, A. altipes and A. melleialba are reported from Thailand for the first time, which was established based on morphology and DNA sequence analyses of LSU and RPB2 regions (Fig. 124).
Phylogenetic tree of Amanita species inferred from a combine LSU and RPB2 sequence dataset using maximum likelihood. Bootstrap values (BS) ≥ 50% are shown above the branches. The first records of Amanita species from Thailand in this study are in blue font. Voucher collection identifiers are provided after each species name. Type specimens are indicated in bold
Amanita altipes Zhu L. Yang, M. Weiss & Oberw., Mycologia 96(3): 636 (2004)
Facesoffungi number: FoF04857, Fig. 125a
Holotype: China, Yunnan Province, Lijiang Country, Laojunshan, altitude 3800 m., on soil under Abies, Betula, Picea, Quercus and/or Salix, 14 August 2000, Zhu L. Yang 2915, KUN-HKAS 36609.
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes medium-sized. Pileus 70 mm in wide, parabolic when young, convex to plano-convex, depressed at centre, broadly umbonate, yellowish to yellow (3A4–7), pale yellow toward margin, often with dark yellow to brownish (5D6–8) over disk, viscid when moist; universal veil mostly over disc as felty, floccose patches, 2–5 mm wide, thick, yellowish to yellow to dirty yellow (3A2–5); margin tuberculate-striate (0.13–0.41R), non-appendiculate. Lamellae 2–4 mm broad, free to nearly free, crowded to plentiful, white to yellowish (2A1–3); lamellulae of 2–5 lengths, crowed, truncate; context white, hollow in centre. Stipe 150 × 16 mm. (length includes bulb), with tapering upward, yellowish (3A2–4), becoming whitish toward stipe base, often covered with yellow to yellowish (3A4–6) squamules above partial veil, white, with yellowish to whitish squamules or fibrils under partial veil, white (1A1). Bulb subglobose to ovate, 8–32 mm diam., white. Universal veil on stipe base as very short volval limb on bulb margin, floccose patches or warts near apex of bulb, yellow to yellowish (3A6–8). Partial veil subapical, membranous, 18 mm below apex of stipe, surface yellowish, with a yellow edge. Odour indistinct. Lamellar trama bilateral, divergent; mediostratum 50–60 μm wide; filamentous hyphae 1.8–8 μm wide, branching, hyaline, of abundant fusiform to subellipsoid, inflated cells (60–90 × 12–33 mm); vascular hyphae not observed. Subhymenium 30–50 μm thick; inflated cells dominating, in 2–3 layers, ovoid to subellipsoid, 10–25 × 8–18 μm, subtended by concatenated partially inflated hyphal segments. Basidia 32–70 × 10–14 μm, narrowly clavate to clavate, mostly 4-spored, occasionally 2-spored, with sterigmata up to 7 μm long; clamps absent. Basidiospores [60/1/1] (7.5–)8–10(–11) × (7–)7.5–9.5(–10) μm, (Q = 1–1.1(–1.2); Q’= 1.07 ± 0.06), smooth, hyaline, colourless, thin-walled, inamyloid, globose to subglobose, rarely broadly ellipsoid, apiculus rather variable, sublateral, very prominent to rather small, cylindric to truncate-conic; contents monoguttulate or occasionally granular; white in deposit. Lamellar edge sterile; filamentous hyphae 3–7 μm wide, hyaline, colourless or pale yellow, thin-walled; inflated cells dominating, mostly globose to subglobose and sometimes ovoid, 15–25 × 10–18(–24) μm, colourless, thin-walled. Pileipellis up to 155 μm thick; upper layer (70–130 μm thick) strongly gelatinized, composed of interwoven, thin-walled, colourless, filamentous hyphae 3–8 μm wide; lower layer (50–65 μm thick) composed of compactly arranged, filamentous hyphae 3–8 μm wide; vascular hyphae rare. Universal veil on pileus filamentous hyphae 2.5–6.2 μm wide, branching, with slightly inflated elements; inflated cells very abundant to nearly dominant, globose to subglobose to ovoid or ellipsoid to subfusiform (45–90 × 15–30 μm), in chains of 2–4, thin-walled, colourless vascular hyphae occasional. Universal veil on stipe base composed 5–6 irregularly arranged elements; filamentous hyphae very abundant 2.2–15 μm wide; inflated cells abundant to very abundant, fusiform to long ellipsoid, 40–80 × 10–35 μm, colourless or with intracellular pale brown pigment, thin- to slightly thick-walled, terminal or in chains of 2–3 and then terminal, becoming rare toward inner layer; filamentous hyphae abundant; vascular hyphae occasional. Stipe trama longitudinally acrophysalidic; filamentous hyphae 2.2–14 μm wide; acrophysalides subfusiform to clavate, 250–450 × 25–40 μm; vascular hyphae rare. Partial veil filamentous hyphae 2–5 μm wide, gelatinized, branching, hyaline, inflated cells terminal, thin-walled, ellipsoid to long ellipsoid, 15–70 × 10–24 μm, colourless, or with intracellular pale brown pigment, thin-walled; vascular hyphae rare. Clamp connections absent in all parts of basidiomes.
Material examined: THAILAND, Lampang Province, Along road number 1252, 18.935, 99.390833, elev.1450 m, 14 June 2013, B. Thongbai, BZ201342 (MFLU 14-0065).
Host and habitat: Solitary in forests dominated by Fagaceae.
Distribution: Known from China and Thailand.
GenBank numbers: LSU = MH716040, RPB2 = MH727686.
Notes: Amanita altipes, originally described from south-western China by Yang et al. (2004), resembles several species in the section Amanita, such as A. elata (Massee) Corner & Bas, A. gemmata (Fr.) Bertillon, A. orientigemmata Zhu L. Yang & Yoshim. Doi, A. russuloides (Peck) Sacc., and A. xylinivolva Tulloss et al. Amanita altipes shares some morphological similarities with these yellow to yellowish species which have been discovered in either Europe or Asia. Molecular analyses indicate that A. altipes is a distinct species closely related to A. melleialba. The combined gene phylogenetic analyses indicate that Thai A. altipes sequences clustered with A. altipes sequences from China with 100% ML support (Fig. 124).
Amanita flavoalba Mehmood & R.P. Bhatt, sp. nov.
MycoBank number: MB820829; Facesoffungi number: FoF04390, Figs. 126, 127
Etymology: Referring to the yellow centre and whitish margin of the pileus.
Holotype: CAL 1405.
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes small to medium-sized. Pileus 40–60 mm wide, initially hemispherical then convex to plane, slightly umbonate, yellow to vivid yellow or cadmium yellow (2A7–8) over centre, white to yellowish white (1A2–2A2) toward margin, viscid to sub-viscid when moist, shiny; context 3–4 mm thick, thinning slowly toward margin, white, unchanging when bruised or exposed; margin striate (0.1–0.2R), non-appendiculate. Universal veil on pileus as membranous to sub-membranous patches, 2–4 mm wide, white or snow white (1A1), distributed irregularly over the pileus surface. Lamellae free to narrowly adnate, leaving decurrent line on stipe, crowded (8–10 lamellae/10 mm at margin), white, unchanging, 3–8 mm broad. Lamellulae truncate to subtruncate, plentiful, in several lengths. Stipe 70–95 × 5–7 mm (length includes bulb), nearly cylindric or slightly tapering upward, slightly flaring at apex, white to yellowish white (1A2), finely fibrillose above and below annulus; context white, unchanging when cut or bruised, stuffed. Partial veil medium to subapical, membranous, white. Bulb 17–19 × 15–17 mm, subglobose to ovoid, remnants of universal veil on top of bulb white, sub-membranous to shaggy or as cottony patches. Odour indistinct. Taste not recorded. Spore print white. Macrochemical test no reaction to 5% KOH on pileal surface and context. Subhymenium wst-near = 25–40 µm; wst-far = 38–55 μm thick, with basidia arising from short inflated hyphal segments up to 8 × 10 µm. Hymenophoral trama bilateral, divergent; wcs = 30–45 µm, composed of long ellipsoid to subfusiform inflated cells (up to 65 × 30 µm), filamentous hyphae 3–7 µm wide, thin-walled, colourless, hyaline. Basidia (42–)45–55(–60) × (10–)10.5–11.5(–12) µm, thin-walled, colourless; sterigmata 3–4 µm long; clamp connections not observed at the base of basidia. Basidiospores [60/3/2](8–)8.5–10.5(–11) × (7–)7.5–9(–9.5) µm, (L = 9.5 µm; W = 8.3 µm; Q = (1.09–)1.11–1.17(–1.21); Q’ = 1.14), subglobose, sometimes broadly ellipsoid, thin-walled, hyaline, smooth, non-amyloid, apiculus sublateral, up to 1.5 µm high; contents monoguttulate. Lamellae edge sterile, with inflated, clavate or subglobose to pyriform cells, 14–35 × 8–13 µm, dominating, thin-walled, colourless. Pileipellis 110–130 µm thick, in two layers; upper layer 40–50 μm thick, slightly gelatinized, colourless, filamentous hyphae 2–4 µm wide, subradially arranged to interwoven; lower layer 70–80 thick, filamentous hyphae 2–5 µm wide, subradially to densely arranged with yellowish brown intracellular pigment, thin-walled. Universal veil on pileus filamentous hyphae 3–7 µm wide, dominating, hyaline; inflated cells globose to subglobose 20–50 × 26–47 µm, broadly ellipsoid to ellipsoid or fusiform, 37–64 × 12.5–20 µm, thin-walled, colourless, hyaline. Pileus context filamentous hyphae 5–8 µm wide, colourless, hyaline, thin-walled, inflated cells 30–90 × 10–30 µm, colourless, thin-walled; vascular hyphae not observed. Universal veil on stipe base filamentous; hyphae 4–9 µm wide, dominating, branching, hyaline, thin-walled; inflated cells subglobose to ovoid 18–36 × 21–37 µm, broadly ellipsoid to subclavate 39–74 × 12.5–20 µm, abundant to very abundant. Partial veil filamentous hyphae 2–4 μm wide, branching, thin-walled, colourless, hyaline, with inflated cells up to 25 × 13 µm. Stipe context longitudinally acrophysalidic; acrophysalides dominating 190–280 × 26–36 μm, filamentous hyphae (3–7 μm wide). Clamp connections absent in all tissues.
Material examined: INDIA, Uttarakhand, Bageshwar District, Dhakuri, 2570 m, N30°04.962′ E79°55.159′, 2 August 2016, T. Mehmood, TM 16-1249 (CAL 1405, holotype); ibid., Dhakuri, 3 August 2016, T. Mehmood, TM 16-1280.
Host and habitat: On ground under Abies pindrow (Royle ex D. Don) Royle in temperate coniferous forest
Distribution: Indian Himalaya.
GenBank numbers: LSU = KY861748 (CAL 1405), MF695813 (TM 16-1280).
Notes: A combination of macro- and micromorphological features like inamyloid basidiospores and bulbous stipe base place A. flavoalba in Amanita [subg. Amanita] sect. Amanita (Yang 1997).
Amanita flavoalba is distinct from other known species of Amanita sect. Amanita by a combination of characteristics: smaller basidiomes, yellow to vivid yellow pileus centre and whitish to yellowish white toward pileus margin, membranous to submembranous universal veil on pileus, subglobose basidiospores, universal veil on the pileus composed of abundant filamentous hyphae mixed with scattered inflated cells. In the field, A. flavoalba is easily recognized by its yellow pileus with yellowish white margin, easily detachable universal veil on pileus in the form of membranous to submembranous patches and its occurrence under Abies pindrow. The presence of subglobose basidiospores, very abundant filamentous hyphae mixed with scattered inflated cells in universal veil on the pileus are also quite striking.
Amanita flavoalba might be confused with A. melleialba Zhu L. Yang et al. (originally described from China), however, the latter possesses a honey pileus with longer marginal striation, subconical to granular universal veil remnants on pileus, ellipsoid to sometimes broadly ellipsoid basidiospores (7.5–9.5 × 6–7 μm, with Q = 1.41) and it occurs in subtropical forests dominated by plants of Fagaceae (Ariyawansa et al. 2015a).
Amanita flavoalba is similar to A. pseudosychnopyramis Yang Y. Cui et al., but the latter has yellowish brown to brownish pileus, universal veil remnants as conical to pyramid, grey to brownish grey warts and it occurs in subtropical forests of Fagaceae (Ariyawansa et al. 2015a).
Amanita parvipantherina Zhu L. Yang et al. and A. subparvipantherina Zhu L. Yang et al. are easily segregated from A. flavoalba by the universal veil remnants on pileus in the form of subconical to granular warts (Yang et al. 2004; Ariyawansa et al. 2015a). Amanita parvipantherina also has broadly ellipsoid to ellipsoid basidiospores (8.5–11.5 × 6.5–8.5 μm, Q = 1.38; Yang et al. 2004) and A. subparvipantherina has broadly ellipsoid to ellipsoid basidiospores (9–11.5 × 6.5–8 μm, with Q = 1.38; Ariyawansa et al. 2015a). Amanita flavoalba resembles A. altipes by its yellowish pileus but A. altipes pileus has a brownish tinge and its basidiospores are globose to subglobose (8–10 × 7.5–9.5 μm, with Q = 1.07; Yang et al. 2004). Amanita elata (originally described from Singapore) differs from A. flavoalba by its pale dingy to ochraceous buff pileus with very faint sulphur-yellow tinge, exannulate stipe, smaller basidiospores (7–8.5 × 6.8–7.7 μm) and its occurrence in tropical forests (Corner and Bas 1962).
Our molecular phylogenetic analysis shows closeness (though well separated in Fig. 128) with a few taxa (in Amanita sect. Amanita) like A. xylinivolva (originally described from Colombia; GenBank no. FJ890036), A. crenulata Peck (originally described from the USA; GenBank no. HQ539687) and A. breckonii Thiers & Ammirati (originally reported from the USA; GenBank no. KJ535440).
Maximum likelihood phylogram of Amanita showing the position of Amanita flavoalba. Phylogenetic analysis was conducted in MEGA 6.0 (Tamura et al. 2013) based on LSU sequence data. Bootstrap support values (> 50%) obtained from maximum likelihood analysis are shown above or below the branches at nodes. New taxon is highlighted in blue. Type specimens are indicated in bold. Amanita morrisii Peck, A. brunneolocularis Tulloss & Franco-Mol. and A. orsonii Ash. kumar & T.N Lakh. were used as the outgroup taxa
Amanita xylinivolva can be easily separated by its basidiomes without a pileal umbo, white to off-white to dingy grey volval remnants, whitish buff lamellae, marked to abrupt bulb and microscopically, globose to subglobose basidiospores with comparatively lower Q value (8–10.2 × 7.2–9.5 μm, Q′ = 1.08; Tulloss et al. 1992). Amanita crenulata has a brownish beige to greyish pileus, universal veil on pileus as flocculose to subpyramidal warts and subglobose to broadly ellipsoid basidiospores (Q′ = 1.08) (Peck 1900). Amanita breckonii has ellipsoid to elongate basidiospores (9.8–12.8 × 6.2–8.7 µm, Q′ = 1.65) and clamp connections at the base of basidia (Thiers and Ammirati 1982). Macro- and micromorphology coupled with the LSU-based phylogenetic analysis corroborate A. flavoalba as a novel species.
Amanita melleialba Zhu L. Yang, Qing Cai & Yang Y. Cui, in Ariyawansa et al., Fungal Divers: https://doi.org/10.1007/s13225-015-0346-5, [163] (2015), Fig. 125b
Holotype: CHINA, Yunnan Province, Puer City, Caiyanghe Nature Reserve, Fagaceae (elev. 1300 m), 11 July 2014, G. Wu 1339 (HKAS 83446).
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes small-sized. Pileus 30–48 mm wide, parabolic when young, planoconvex to plane at maturity, depressed at centre, yellowish (4A3–4) to yellow (3B5–6), becoming yellowish to whitish (3A2) toward margin, slightly viscid when moist; universal veil mostly over disc, scarce towards margin, as granular or small warts dirty white (5B2), cream to yellowish (4A2–3), honey; margin tuberculate-striate (0.4–0.6R), non-appendiculate. Lamellae 2–4 mm broad, free to nearly free, crowded, white (1A1); lamellulae of 3–4 lengths, truncate. Stipe 40–75 × 4–7 mm (length includes bulb), slightly tapering upward, white (1A1) to dull white ground (1A2), often covered with floccose squamules above partial veil, white (1A1), floccose squamules to granules under partial veil, white (1A1); context stuffed to nearly hollow in centre, thin, white (1A1). Bulb subglobose to napiform, 6–12 mm wide, white (1A1). Universal veil on stipe base as very short volval limb on bulb margin and floccose squamules to granules dirty white (1A2). Partial veil subapical, membranous, 15–30 mm below apex of stipe, white (1A1) to cream (1A2), with a yellow floccose edge. Odour indistinct. Lamellar trama bilateral, divergent; mediostratum 30–60 μm wide; filamentous hyphae 1.8–8 μm wide, branching, hyaline, with slightly inflated elements; vascular hyphae not observed. Subhymenium 30–60 μm thick; inflated cells dominating, in 3–4 layers, subglobose to ovoid, 10–19 × 8–17 μm, subtended by concatenated partially inflated hyphal segments. Basidia 32–50 × 10–14 μm, narrowly clavate to clavate, mostly 4-spored, occasionally 2-spored, with sterigmata up to 5 μm long; clamps absent. Basidiospores [60/1/2] (7.2–)7.3–9.4(–10) × (5.3–)6.5–7(–7.3) μm, Q = 1.2–1.28(–1. 56); Q’ = 1.4 ± 0.06, smooth, hyaline, colourless, thin-walled, inamyloid, ellipsoid, sometimes broadly ellipsoid; apiculus rather variable, sublateral, very prominent to rather small, cylindric to truncate-conic; contents monoguttulate or occasionally granular; white in deposit. Lamellar edge sterile; filamentous hyphae 3–7 μm wide, hyaline, colourless or pale yellow, thin-walled; inflated cells dominating, mostly globose to subglobose and sometimes ovoid, 9–30 × 9–18(–24) μm, colourless, thin-walled. Pileipellis up to 155 μm thick; upper layer (50–70 μm thick) strongly gelatinized, composed of interwoven, thin-walled, colourless, filamentous hyphae 3–8 μm wide; lower layer (50–85 μm thick) composed of compactly arranged, filamentous hyphae 3–8 μm wide; vascular hyphae rare. Universal veil on pileus filamentous hyphae, 2.5–6.2 μm wide, branching, with slightly inflated elements; inflated cells very abundant to nearly dominant, globose to subglobose to ovoid or ellipsoid to subfusiform (20–60 × 10–30 μm), in chains of 2–4, thin-walled, colourless; vascular hyphae occasional. Universal veil on stipe base composed of two layers; inflated cells abundant to very abundant, fusiform to ellipsoid, 20–40 × 10–30 μm, colourless or with intracellular pale brown pigment, thin- to slightly thick-walled, terminal or in chains of 2–3 and then terminal, becoming rare toward inner layer; filamentous hyphae abundant; vascular hyphae occasional. Stipe trama longitudinally acrophysalidic; filamentous hyphae 2.2–15 μm wide; acrophysalides subfusiform to clavate, 120–350 × 25–45 μm; vascular hyphae rare. Partial veil filamentous hyphae 2–5 μm wide, gelatinized, branching, hyaline, inflated cells terminal, thin-walled, ellipsoid to long ellipsoid, 15–65 × 10–28 μm, colourless, thin-walled; vascular hyphae rare. Clamp connections absent in all parts of basidiomes.
Material examined: THAILAND, Chiang Rai Province, Chiang-khong District, Huay-sor Subdistrict, Nensomburn Village, 5 August 2015, B. Thongbai, BZ201518 (MFLU 15-3316).
Habit and habitat: Gregarious in forests dominated by Fagaceae.
Distribution: Known from China and now Thailand.
GenBank numbers: LSU = MH716041, RPB2 = MH727687.
Notes: Amanita melleialba belongs to subg. Amanita sect. Amanita. It was originally described from southwestern and central China. In the field, the distinguishing morphological characteristics of A. melleialba include its small basidiomes, universal veil mostly over disc as granular or small warts as well as stipe base. Amanita melleialba is very similar to A. parvipantherina, also known from China. However, A. parvipantherina has larger basidiospores (8.5–11.5 × 6.5–8.5 μm) and is distributed in mixed forests with Pinus yunnanensis (Yang et al. 2004; Yang 2005, 2015, Ariyawansa et al. 2015a). Combined-gene phylogenetic analyses show that Thai A. melleialba cluster with A. melleialba strains from China with 100% ML support (Fig. 124).
Amanita subtropicana Mehmood & R.P. Bhatt, sp. nov.
MycoBank number: MB824531; Facesoffungi number: FoF04391, Figs. 129, 130
Etymology: Referring to the subtropical region of Uttarakhand, the type locality.
Holotype: CAL 1660
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes small to medium-sized. Pileus 30–65 mm wide, olive brown (5F4–5) yellowish brown (5F6–8) to brown (6E4) over centre, honey yellow to khaki (4D4–6) toward margin, initially hemispherical, then convex to plano-convex, slightly umbo, dry, shiny; context 8–12 mm thick, white, unchanging when cut or bruised; margin tuberculate-striate, up to 14 mm wide, non-appendiculate. Universal veil remnants on pileus as felted, subconical to subpyramidal warts. Lamellae free, crowded, (8–12 per 10 mm at margin), 5 mm broad, initially white (1A1), fading yellowish white (1B2) with age. Lamellulae truncate, unevenly distributed. Stipe 90–160 × 10–20 mm, nearly cylindrical, slightly tapering upward, with apex slightly expended, initially white (5A1), covered with white farinose squamules; context white, hollow, unchanging. Partial veil absent. Universal veil remnants on stipe base white (1B1) to yellowish white (1C1), felted warts, mostly in complete ring, sometimes irregularly distributed. Odour indistinct. Taste not recorded. Spore print white. Subhymenium wst-near = 25–40 µm; wst-far = 45–60 μm; basidia arising from subglobose to irregular shaped cells, 6–16 × 4–14 µm. Hymenophoral trama bilateral, divergent; wcs = 36–55 μm wide, with inflated (clavate to fusiform) cells, 40–60 × 10–14 μm; filamentous, undifferentiated hyphae 3–7 µm wide, thin-walled, colourless, hyaline. Basidia (32–)46–60(–68) × (12–)13–15(–16) µm, 2- to 4-spored, thin-walled, colourless; sterigmata 3–5 μm long; clamp connections not observed at the base of basidia. Basidiospores [80/4/2] (7–)8.5–11(–12) × (5.5–)6–8(–8.5) µm, (L = 10.5 µm; W = 7.5 µm; Q = (1.19–)1.33–1.47(–1.6); Q’ = 1.41), broadly ellipsoid to ellipsoid, non-amyloid, hyaline, thin-walled, smooth, apiculus sublateral, up to 1.7 µm high; contents monoguttulate. Lamellar edge cells sterile with inflated cells, subglobose to pyriform, 20–48 × 18–36 μm, thin-walled, colourless, hyaline or sometimes with yellowish brown pigments. Pileipellis 80–140 μm thick, in two layers; gelatinized suprapellis, 25–50 μm thick, composed of compactly arranged filamentous, undifferentiated hyphae 2–5 μm wide, thin-walled, colourless, hyaline; ungelatinized subpellis, 55–90 μm thick, composed of filamentous, undifferentiated hyphae 3–7 μm wide, radially arranged with yellowish brown intracellular pigments. Pileus trama filamentous, undifferentiated hyphae 3–8 μm wide; with broadly ellipsoid to ellipsoid cells, 30–65 × 10–20 μm. Universal veil on pileus with inflated cells globose to subglobose, 20–44 × 18–38 μm, broadly ellipsoid to ellipsoid, 16–62 × 12–38 μm; filamentous, undifferentiated hyphae 3–6 µm wide. Universal veil on stipe base is similar to that of pileus surface. Stipe context longitudinally acrophysalidic; acrophysalides 118–224 × 24–32 μm; filamentous, undifferentiated hyphae 2–10 μm wide, thin-walled hyaline. Clamp connections absent in all tissues.
Material examined: INDIA, Tehri Garwhal, Byasi, 510 m, N30°04.00′ E78°28.09′, 9 August 2015, T. Mehmood, TM 15-915 (CAL 1660, holotype); ibid., Byasi, 18 August 2017, T. Mehmood, TM 17-1574.
Habit and habitat: Solitary to scattered, on ground under Shorea robusta C. F. Gaertn. (Dipterocarpaceae) in subtropical mixed forest.
Distribution: Indian Himalaya.
GenBank numbers: LSU = MG913204 (TM 17-1574); MG923799 (TM 15-915).
Notes: In the field, Amanita subtropicana is distinct from other known species of Amanita sect. Vaginatae by a combination of macroscopic characteristics: olive brown to yellowish brown pileus over centre, honey yellow to khaki colour towards margin which is covered by felted, subconical, subpyramidal to warty universal veil remnants on the pileal surface and broadly ellipsoid to ellipsoid basidiospores and putative association with Shorea robusta in subtropical broadleaf forest. Amanita ceciliae (Berk. & Broome) Bas, A. liquii Zhu L. Yang et al., A. griseofolia Zhu L. Yang, and A. cinctipes Corner & Bas are species of sect. Vaginatae which are somewhat similar to A. subtropicana on the basis of friable universal veil at the stipe base.
Amanita ceciliae, a European species, can be easily segregated from A. subtropicana by its yellow brown to grey-brown or olive-brown pileus with greyish to brownish universal veil remnants (Phillips 1990; Breitenbach and Kränzlin 1995). Amanita liquii, originally described from southwestern China, differs from A. subtropicana by its dark brown to blackish pileus with dark universal veil remnant and globose to subglobose basidiospores. Furthermore, it is associated with Abies and Picea (Yang et al. 2004). Amanita griseofolia differs by its grey to brownish grey pileus and felted to verrucose universal veil and globose to subglobose basidiospores (10–13.5 × 9.5–13 μm; Yang 2004). Amanita cinctipes (originally described from Singapore) is distinct from A. subtropicana by its mouse grey to greyish brown pileus with abundant universal veil remnants on the base of the stipe forming 2–4 rings and globose to subglobose basidiospores (Corner and Bas 1962).
Three Indian species, Amanita cornelii Mehmood et al., A. emodotrygon Mehmood et al. and A. rajendrae Mehmood et al. (GenBank no. KX528072, KX539266 and MF170174, respectively, in Fig. 131) of sect. Vaginatae are easily segregated from A. subtropicana by their distinct saccate universal veil on stipe base (Das et al. 2017b; Tibpromma et al. 2017).
Maximum likelihood phylogram of Amanita showing the position of Amanita subtropicana. Phylogenetic analysis was conducted in MEGA 6.0 (Tamura et al. 2013) based on LSU sequence data. Bootstrap support values (> 50%) obtained from maximum likelihood analysis are shown above or below the branches at nodes. The new taxon is highlighted in blue and bold on the tree. Type specimens are in bold. Amanita caesareoides Lj.N. Vassiljeva and A. caesarea (Scop.) Pers. were used as the outgroup taxa
Initial BLASTn search results of LSU sequence from Indian collection (TM 17-1574) against the NCBI database exhibited 87% similarity with 100% query coverage of Amanita subtropicana to “Amanita sp. Aus09” (GenBank no. KY349232). Phylogenetically (Fig. 131), A. subtropicana might have some closeness with Amanita sp. (Aus09), A. madagascariensis L.P. Tang et al. and A. strobilaceovolvata (Beeli) E.-J. Gilbert. Unfortunately, no morphological description of Amanita sp. (Aus09) is available for comparison of important morphological characteristics. Amanita madagascariensis originally described from East Africa can easily be separated by its dirty white or dull white pileus covered with greyish to brownish grey universal veil remnants and occurrence under Eucalyptus (Tang et al. 2015). Amanita strobilaceovolvata, originally described from the Congo, has a yellow to pale yellow pileus, a strobiloid saccate universal veil and subglobose to broadly ellipsoid basidiospores (8–10 × 7–9 μm) (Gilbert 1940; Tang et al. 2015).
Hygrophoraceae Lotsy
The family Hygrophoraceae contains 25 genera and over 600 species (Lodge et al. 2014). Most species in the family prefer a humicolous habitat, except for a few that are lignicolous, ectomycorrhizal, or on mosses (Griffith et al. 2002).
Hygrocybe (Fr.) P. Kumm
Hygrocybe is cosmopolitan in distribution (Senthilarasu et al. 2010). Currently, 457 species are listed as legitimate in Index Fungorum and MycoBank (2019). The genus is characterized by the presence of variously coloured basidiomes with waxy lamellae, absence of veilar remnants on the pileus margin as well as stipe, and white, inamyloid, smooth basidiospores (Boertmann 1995; Babos et al. 2011; Lodge et al. 2014; Hosen et al. 2016). Most species predominantly grow on soil, except for a few found on tree trunks or on logs (Lodge et al. 2006).
Hygrocybe lucida K. Acharya & A.K. Dutta, sp. nov.
MycoBank number: MB826973; Facesoffungi number: FoF05720, Figs. 132, 133
Etymology: Refers to the bright colouration of the pileus.
Holotype: CUH AM123
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes small to medium-sized. Pileus 6–23 mm diam., convex to broadly convex when young, becoming somewhat infundibuliform at maturity; surface somewhat sticky when moist, greyish orange (5B5–6) to orange (6A6–7) or reddish orange (7A7) with a slightly darker (7A8, 7B8) disc when young, becoming light orange (6A5) to orange (6A6–7) with reddish orange (7A7, 7B7) to orange-red (8A7, 8B7–8) centre, no colour change on bruising or with age, smooth and glabrous, often slightly fibrillose; margin at first entire, turning wavy at maturity. Lamellae ca. 2–3 mm broad, adnexed to broadly adnate, thick, white (1A1), regular, often forked towards margin from half-way to the stipe, distant, with lamellulae of 1–2 lengths; edge concolorous with the sides, smooth. Stipe 4–11 × 1.5–3 mm, central, orange (5A6–7) to deep orange (5A8) or greyish orange (5B6), cylindrical, rarely slightly broader towards the apex, mostly glabrous, semi moist, often slightly fibrillose, hollow. Odour and taste indistinct. Lamellar trama regular, made up of 3.5–7.5 µm broad, cylindrical, IKI-, thin-walled, hyaline, hyphae, individual compartments 29–76 µm long, tubuliform elements absent. Basidia dimorphous; macrobasidia 50–53(–58) × 5–7(–8.5) µm, narrowly clavate to cylindric-clavate, thin-walled, with refractive oleaginous contents, 4-spored; microbasidia 32–35(–40) × 4–5(–7) µm, narrowly clavate, thin-walled, with refractive contents, 4-spored. Basidioles 32–39(–58) × 5–6(–7) µm, narrowly clavate, thin-walled, hyaline, with refractive contents. Basidiospores vaguely dimorphous; macrospores 9–10–10.3(–10.8) × (6–)6.5–7–7.2 µm, Q = 1.4–1.45–1.5, ellipsoid, hyaline, thin-walled, inamyloid, with refractive oleaginous contents when viewed in KOH, not constricted at the middle; microspores (6.4–)7–7.2–7.5(–7.9) × (3.9–)5–5.5–6.5(–6.8) µm, Q = 1.1–1.3–1.8 µm, subglobose to ellipsoid, hyaline, thin-walled, IKI-, with few refractive oleaginous contents in KOH, not constricted at the middle. Lamellar edge fertile. Cheilo- and pleurocystidia absent. Pileipellis an ixotrichodermium, made up of erect, mostly unbranched to often branched, cylindrical hyphae, (5–)7–10(–15) µm broad, hyaline, IKI-, thin-walled, hyphal end often broader. Pileus tramal hyphae 7–9 µm broad, hyaline, IKI-, thin-walled. Stipitipellis an ixocutis with thin-walled hyphae, 3.5–5.5 µm wide, hyaline. Stipe trama hyphae 7–8 µm broad, interwoven, hyaline, thin-walled, IKI-. Caulocystidia absent. Clamp connections present in all parts of the basidiome including the base of basidia.
Material examined: INDIA, West Bengal, North-24-Parganas, near Basirhat, 22°39′10.0″N, 88°52′00.3″E (alt. 9 m), 9 August 2015, K. Acharya & A.K. Dutta, CUH AM123 (holotype); ibid., North-24-Parganas, Basirhat, 22°38′36.1″N, 88°53′35.3″E (alt. 9 m), 12 August 2015, K. Acharya & A.K. Dutta, CUH AM126.
Habit and habitat: Uncommon, solitary or scattered, on humus-rich soil.
Distribution: India.
GenBank numbers: ITS = MH599084 (CUH AM123); ITS = MH599083 (CUH AM126).
Notes: The distinguishing features of Hygrocybe lucida include small basidiome size, a convex (when young) to somewhat infundibuliform (at maturity), orange to reddish orange pileus that does not change colour on bruising or with age; white, adnexed to broadly adnate lamellae; an orange or greyish orange stipe; dimorphous basidiospores and basidia; absence of cheilo- and pleurocystidia; an ixotrichodermium-type pileipellis; regular lamellae trama composed of short hyphal elements (29–76 µm long); an ixocutis-type stipitipellis; absence of caulocystidia; and presence of clamp connections in all parts of the basidiome. This combination of features place the present species in sect. Firmae of subgen. Pseudohygrocybe (Pegler and Fiard 1978; Pegler 1986; Cantrell and Lodge 2001; Lodge and Ovrebo 2008). The morphological placement is further supported by the molecular phylogenetic analysis based on ITS sequence data (Fig. 134).
Phylogram generated from maximum likelihood (RAxML) analysis using a GTR + I+G model of nucleotide evolution based on ITS sequence dataset. Ampulloclitocybe clavipes and Cantharocybe gruberi were used as the outgroup taxa following Lodge et al. (2014). Maximum likelihood bootstrap support values greater than 50% are indicated above or below the nodes. Sequences used in this study mostly have been sampled from a previous study of Lodge et al. (2014). The newly generated sequences are placed in blue font to highlight its phylogenetic position in the tree. GenBank accession numbers for all of the sequences are indicated in the tree
Within sect. Firmae, species with more or less similar size of the macrospores include Hygrocybe alwisii (Berk. & Broome) Pegler, H. diversicolor (Petch) Pegler, and H. earlei (Murrill) Pegler. Hygrocybe alwisii, previously reported from India, and differing by a larger pileus (45–55 mm), adnexed to almost free lamellae, a longer stipe (60–70 mm), and a repent epicutis type of pileipellis (Pegler 1986; Leelavathy et al. 2006). Hygrocybe diversicolor has a larger pileus (up to 50 mm) that is olivaceous brown to blackish brown with appressed squamulose surface, longer stipe (30–35 mm), purplish grey to purplish black, and epicutis type of pileipellis (Pegler 1986). Hygrocybe earlei, a species known only from Cuba and Trinidad, has a pileus that is initially campanulate and becomes expanded with a broad low umbo, and free to adnexed lamellae. Hygrocybe cf. earlei, previously known only from Panama, has an umbonate pileus, pale yellow lamellae with white edge, a pure white, much longer stipe (up to 40 mm), smaller macrobasidia (24–46 μm long) and microbasidia (22–30 μm long), and presence of an intermittent thin gelatinous coating in the pileipellis (Lodge and Ovrebo 2008).
Hygrocybe batistae Singer, described from Brazil and later found in Colombia and Puerto Rico, has similarly coloured basidiomes but differs by its rugulose to rugose pileus, caespitose basidiomes, and presence of coralloid hyphae in the pileipellis (Lodge and Pegler 1990; Cantrell and Lodge 2001; Lodge et al. 2014). Hygrocybe neofirma S.A. Cantrell & Lodge also has similarly coloured basidiomes but has a star-shaped perforation at the pileus centre, light yellow to brilliant yellow lamellae, and larger macrospores (12.8–17.6 × 8–10.4 μm; Cantrell and Lodge 2001).
Hygrocybe hypohaemacta (Corner) Pegler, previously placed within sect. Firmae and recently transferred to sect. Velosae based on phylogenetic analysis by Lodge et al. (2014), appears to be close to H. lucida in some morphological characteristics. However, H. hypohaemacta has a convex to plano-convex pileus that is covered by a thick (up to 1.5 mm) greyish gluten, discolorous lamellae with pale golden edge, longer stipe (up to 60 mm), somewhat differently sized macrospores (7–11 × 5–8 µm), presence of polymorphic cheilocystidia, and an epicutis-type pileipellis (Pegler and Fiard 1978).
Hygrocybe trinitensis (Dennis) Pegler and H. siparia (Berk.) Singer are comparable in having similar smaller pilei (Pegler and Fiard 1978). Hygrocybe trinitensis differs by the presence of coral red lamellae that are broadly adnate with a decurrent tooth, a longer stipe (up to 50 mm), smaller macrospores (7–9 × 4.5–5.5 µm, Q = 1.56), smaller macro- and micro-basidia, and an epicutis-type pileipellis that contains red vacuolar pigments. Hygrocybe siparia, described from Brazil, has an umbilicate pileus with squamulose surface, lamellae with a decurrent tooth, longer macrospores (10–13 µm long, Q = 1.53), and smaller macro-basidia (40–48 µm long).
Among phylogenetically related taxa (Fig. 134), Hygrocybe firma (Berk. & Broome) Singer differs by its yellow pileus, strongly decurrent lamellae, and longer macrospores (11–18 µm long) (Berkeley and Broome 1871; Young and Mills 2002). Hygrocybe andersonii Cibula & N.S. Weber, originally described from Horn Island of Mississippi, has densely cespitose growth habit, brownish orange to deep orange lamellae, longer stipe (up to 42 mm long) reddish brown, and large bacilliform basidiospores (14–20.7 × 3.8–5.6 µm; Cibula and Weber 1996).
Marasmiaceae Roze ex Kühner
Marasmiaceae is a family of pale-spored agarics. It has a worldwide distribution and comprises about 54 genera and 1590 species (Kirk et al. 2008). Several of these taxa were previously considered under Tricholomataceae sensu lato (Moncalvo et al. 2002; Matheny et al. 2006). Most species are saprobes that play a leading role in nutrient-recycling and form prominent components of forest ecosystems (Cannon and Kirk 2007). A novel species, Marasmius indojasminodorus is introduced within Marasmiaceae. An updated phylogenetic tree of ITS sequence data based on maximum likelihood and Bayesian inference analyses of the genus Marasmius is provided (Fig. 135). Detailed literature and updated accounts of Marasmius were provided by Wannathes et al. (2009) and Tibpromma et al. (2017).
Consensus phylogram (50% majority rule) obtained from MCMC analysis of one million generations from a Bayesian inference analysis based on ITS region for Marasmius taxa and two outgroup sequences (Crinipellis brunneipurpurea and C. malesiana). Maximum likelihood (RAxML) bootstrap support (left) ≥ 50% and Bayesian posterior probabilities (right) ≥ 0.50 are indicated above the nodes. Sequences used in this study mostly have been sampled from a previous study (Tan et al. 2009; Wannathes et al. 2009). The newly generated previously described type specimen sequence of Marasmius midnapurensis and the sequences of the newly described taxa for the present study are placed in blue font to highlight its phylogenetic position in the tree. Ex-type strains are in bold. GenBank accession numbers for all of the sequences are indicated in the tree
Marasmius Fr.
We follow the latest treatment and updated accounts of Marasmius in Komura et al. (2016) and Tibpromma et al. (2017).
Marasmius indojasminodorus A.K. Dutta, K. Acharya & K. Das, sp. nov.
MycoBank number: MB820566; Facesoffungi number: FoF03247, Figs. 136, 137
Etymology: Refers to an Indian look-a-like of the Thai taxon Marasmius jasminodorus
Holotype: CAL 1514
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Pileus 12–21 mm diam., conical to convex when young, becoming broadly convex at maturity, often with a small umbonate centre, smooth to minutely pruinose, moist to semi moist, hygrophanous; disc rugulose, olive-brown (4D5–8) or light brown (5D7) to yellowish brown (5D–E8, 5F7–8), turning translucent with KOH; margin smooth when very young, rugulose-striate in age, slightly paler, yellowish brown (5D4–6, 5E4–5), turning translucent with KOH. Lamellae 2–2.5 mm broad, adnexed, subdistant (L = 14–16, l = 2–3), white (1A1) to cream, non-marginate, concolorous, non-intervenose. Stipe 15–40 × 1–1.5 mm, central, cylindrical, hollow, glabrous to slightly velutinous, non-insititious, overall white when young, at maturity apex becoming white to cream, base light brown (6D6) to brown (6D8), strigose at base, yellowish white to brownish white. Context 1 mm broad at centre, gradually thinner towards margin, creamy white, turning translucent with KOH. Odour strong, fragrant, sweet, like jasmine. Taste slightly bitter. Lamellar trama composed of 6–7(–7.5) µm broad, interwoven, cylindrical, hyaline, dextrinoid, non-gelatinous, thin- to thick-walled hyphae. Basidia not observed. Basidioles 21–22.5(–26) × (4–)6–6.5(–7.5) µm, fusoid to clavate, hyaline, thin-walled. Basidiospores (6.5–)8–8.7–10(–10.5) × 3.5–3.7–4 µm, Q = 1.8–2.4–2.8, ellipsoid, often curved in profile, hyaline, inamyloid, thin-walled. Pleurocystidia absent. Cheilocystidia composed of Siccus-type broom cells; main body 10–12(–15) × (3.5–)6–7(–7.5) µm, clavate to subclavate, hyaline, thin- to moderately thick-walled; apical setulae (3–)6.5–7(–9.5) µm long, cylindrical, acute to obtuse, yellowish in KOH, thick-walled. Pileipellis hymeniform, consisting of Siccus-type broom cells; main body (10–)12.5–14(–16.5) × (5–)6.5–7(–8) µm, cylindrical to clavate or broadly clavate, hyaline, inamyloid, thin- to moderately thick-walled; apical setulae (3.5–)5–7(–10.5) µm long, cylindrical, obtuse, yellowish with KOH, thick-walled. Pileus trama hyphae 3.5–5.5 µm broad, interwoven, cylindrical, often branched, hyaline, dextrinoid, thin-walled. Stipitipellis hyphae 3.5–4.5 µm broad, parallel, yellowish, cylindrical, dextrinoid, thin-walled. Stipe trama hyphae 4–7(–9) µm broad, parallel, hyaline, dextrinoid, cylindrical, non-gelatinous, thin- to moderately thick-walled. Caulocystidia of two types: a) abundant non-setulose cells, (17–)28–32(–36) × 6–7(–11) µm, cylindrical to irregular in outline, often seldom branched, rarely bilobed at apex, thin- to moderately thick-walled; b) Siccus-type brooms cells with main body 20–21.5(–25) × 3.5–4.5(–5) µm, uncommon, scattered, cylindrical to irregular in outline, hyaline, thin-walled; apical setulae 10–12.5 µm, cylindrical to irregular in outline, thin- to moderately thick-walled. Clamp connections present in all the tissues.
Material examined: INDIA, West Bengal, Howrah District, Acharya Jagadish Chandra Bose Indian Botanic Garden, 22°33′35.3″N, 88°17′21.5″E (alt. 12.2 m), 23 September 2015, A.K. Dutta & S. Paloi, AKD 135/2015 (CAL 1514, holotype); ibid., 22°33′38.4″N, 88°17′22.6″E (alt. 15.1 m), 23 September 2015, A.K. Dutta & S. Paloi, AKD 139/2015 (CAL 1515).
Host and habitat: Uncommon, scattered to gregarious, on dead and decayed leaves and wood of dicotyledonous plants.
GenBank numbers: ITS = KY785172, LSU = KY785174 (CAL 1514); ITS = KY785171, LSU = KY785173 (CAL 1515).
Notes: The most distinctive combination of features of Marasmius indojasminodorus includes a small to medium sized (12–21 mm diam.), convex to broadly convex, rugulose-striate yellowish brown pileus with a rugulose, olive brown to light brown or yellowish brown disc; adnexed, subdistant (14–16), white to cream, lamellae with lamellulae of 2–3 lengths; a non-insititious, glabrous to slightly velutinous stipe coloured white to cream at apex and light brown to brown at base, with yellowish white to brownish white basal mycelium; strong, sweet, jasmine like odour; ellipsoid basidiospores with a mean size of 8.7 × 3.7 µm (Q = 1.8–2.8); Siccus-type cheilocystidia with main body 10–15 × 3.5–7.5 µm; absence of pleurocystidia; and two types of caulocystidia, Siccus-type in combination with numerous non-setulose cells, cylindrical to irregular in outline (17–36 × 6–11 µm). This combination of features undoubtedly place the present taxon within Marasmius ser. Atrorubentes of sect. Sicci (Wannathes et al. 2009).
Among taxa that possess jasmine-like odour, Marasmius jasminodorous Wannathes et al. (Wannathes et al. 2009), described from Northern Thailand, differs by its colouration of pileus (dark reddish brown towards disc with light brown to brownish orange margin), basal mycelium of the stipe (brownish orange), and larger basidiospores with a mean range of 10.1 × 3.6 µm (Qm = 2.8). Marasmius odoratus V.A. Farook & Manim., recently described from Kerala state of India, primarily differs by the presence of “Globulares-type” cells in the pileipellis and is categorized under sect. Globulares (Farook and Manimohan 2015).
Among other similar species belonging to sect. Sicci, and phylogenetically close taxa (Fig. 135), M. midnapurensis A.K. Dutta et al., described from India, primarily differs from M. indojasminodorus by the absence of jasmine-like odour, longer basidiospores (10.7–15 µm long, Qm = 3), comparatively broader main-body of cheilocystidia (up to 10 µm broad), and longer (up to 50 µm), and non-setulose caulocystidia (Dutta et al. 2014). The Indonesian taxon, M. araucariae var. siccipes Desjardin et al. differs by its non-rugulose pileus, orange brown all over or with darker disc, discolorous lamellae with orange-brown edge, glabrous stipe, absence of any fragrant odour, and distinctly longer (11–12 × 3–4 µm), subfusoid basidiospores (Desjardin et al. 2000). Marasmius ochroleucus Desjardin & E. Horak, described from New Caledonia and subsequently reported from Northern Thailand, primarily differs by its light yellow to cream pileus, much crowded (L = 20–24, l = 3–4), and intervenose lamellae, and presence of only one type of caulocystidia shaped cylindrical to fusoid-ventricose (Wannathes et al. 2009). The Korean taxon M. occultatiformis Antonín et al. has brownish orange to brownish red pileus and slightly paler towards margin, presence of more lamellae (L = 25) finely pubescent towards edge, whitish basal mycelium, somewhat differently sized basidiospores (mean of 7.8 × 4 µm, Q = 1.95), and absence of caulocystidia (Antonín et al. 2012). Marasmius araucariae Singer, described from Argentina, has castaneous-ferruginous or ferruginous pileus, more lamellae (16–24), comparatively longer stipe (up to 70 mm long) with fulvous-white basal mycelium, absence of any odour, larger basidiospores (9–12.5 × 2.7–4.5 µm), and only one type of caulocystidia, subcylindric, 50 × 5.5 µm (Singer 1976). Marasmius napoensis Singer differs by its larger pileus (up to 60 mm diam.) with deeply sulcate margin, distant lamellae, longer stipe (60–77 mm long), absence of any strong odour, and presence of only Siccus-type caulocystidia (Singer 1976).
Marasmius pellucidus Berk. & Broome, although phylogenetically related as revealed in Fig. 135, possess Globulares-type of cells in the pileipellis and is categorized under a sect. Globulares (Wannathes et al. 2009). The other distinguishing features of M. pellucidus from that of M. indojasminodorus includes a plano-campanulate to almost applanate pileus, ivory to pale orange towards disc with white to cream or pale yellowish white margin, and presence of comparatively more lamellae (up to 26; Wannathes et al. 2009).
Omphalotaceae Bresinsky
The family Omphalotaceae was proposed by Bresinsky in Kämmerer et al. (1985) to accommodate the genera Omphalotus Fayod and Lampteromyces Singer. The members of the family are characterized by fleshy, brightly coloured and often luminescent basidiomes with a glabrous or fibrillose pileus, decurrent lamellae, central or eccentric stipe devoid of any veil, hyaline, smooth, globose or ellipsoid and inamyloid basidiospores, clavate basidia often intergrading with cystidioles, the absence of cystidia and monomitic hyphal system with clamp connections (Cannon and Kirk 2007). Species of Omphalotaceae are saprobic or necrotrophic on wood or litter (Moncalvo et al. 2002). Moncalvo et al. (2002), in their broad systematic treatment of the euagarics using LSU sequence, observed that several genera that were traditionally classified in various families or tribes of Agaricales (Singer 1986) and Caripia montagnei, a reduced form that was generally placed in the Stereales (Hawksworth et al. 1995) were clustered together in Omphalotaceae clade together with lentinuloid and omphalotoid species. In their overview of phylogeny of major clades of Agaricales, Matheny et al. (2006) recovered Omphalotaceae as monophyletic family.
Marasmiellus Murrill
Marasmiellus is a predominantly tropical and subtropical genus that encompasses more than 400 species known worldwide (Wilson and Desjardin 2005; Blanco-Dios 2015). Traditionally, the genus had been placed in family Tricholomataceae (Singer 1986). While Kirk et al. (2008) treated it in the Marasmiaceae, DNA-based molecular phylogenetic studies of Moncalvo et al. (2002) and Wilson and Desjardin (2005) indicated that this genus belongs to the Omphalotaceae. Marasmiellus is characterized by collybioid, omphalioid or pleurotoid basidiomes, slightly decurrent, intervenose lamellae, smooth, white or off-white and inamyloid basidiospores, and pileipellis or stipipellis showing a Rameales-structure (Singer 1973; Pérez-De-Gregorio et al. 2011). Most of the species are saprobes occurring on dead and rotting plant material and some are host specific. A few species are found on living hosts. According to Wilson and Desjardin (2005), the genus is polyphyletic. In the course of our studies on the agarics of Kerala State, India, we came across a remarkable species of Marasmiellus. It is formally described here as a new species based on both morphology and molecular phylogeny (Fig. 138).
Phylogram derived from maximum likelihood (RAxML) analysis using ITS sequence dataset. Maximum likelihood (RAxML) bootstrap support (left) ≥ 50% and Bayesian posterior probabilities (right) ≥ 0.90 BYPP are indicated above the nodes. Marasmiellus bicoloripes is highlighted in blue. Marasmius rotula and M. capillaris are selected as the outgroup taxa
Marasmiellus bicoloripes K.P.D. Latha, K.N.A Raj & Manim., sp. nov.
MycoBank number: MB820689; Facesoffungi number: FoF03251, Figs. 139, 140
Etymology: Referring to the bicoloured stipe of the basidiomes.
Holotype: CAL 1524.
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978) and the Online Auction Color Chart (Anonymous 2004).
Basidiomes small, somewhat omphalinoid. Pileus 5–20 mm diam., initially convex, becoming plano-convex with a small umbo surrounded by a shallow depression at maturity; surface brown (6F4/OAC733) all over when young, becoming greyish brown (6D3/OAC730) at the centre and brownish beige (6E3/OAC723) towards the margin, not hygrophanous, faintly pellucid-striate, appressed-fibrillose all over; margin decurved to somewhat straight, initially entire, becoming somewhat wavy. Lamellae adnate or subdecurrent, somewhat thick and waxy, often furcate and anastomosing, close, brownish orange (6E2/OAC724), up to 2 mm wide, with lamellulae of 2–3 tiers; edge finely torn, concolorous with the sides. Stipe 12–14 × 1–2 mm, central, terete, equal or slightly tapering towards the base, solid; surface initially white all over, becoming dark grey (5E1, 5F1/OAC904) towards the base at maturity, appressed-fibrillose all over. Odour and taste not distinctive. Lamellar trama subregular, hyphae 3–9 µm wide, hyaline, thin-walled, inamyloid. Subhymenium poorly developed pseudoparenchymatous. Basidia 20–31 × 4–6 µm, clavate or occasionally pedicellate clavate, hyaline, thin-walled, 4- or 2-spored; sterigmata up to 5 µm long. Basidiospores 4–8 × 3–4(–5) (6.5 ± 1.5 × 3.5 ± 0.6) µm, Q = 1.3–2.7, Qm = 1.9, oblong-ellipsoid, pip-shaped or subfusiform, often with a stretched-out apicular end, with prominent guttules, hyaline, inamyloid, smooth, thin-walled. Lamellar edge heterogeneous. Pleurocystidia absent. Cheilocystidia 20–32 × 4–8 µm, scarce, scattered, versiform: clavate with short or long, apical diverticulate projections or with a mucronate apex, cylindrical with a mucronate apex, utriform with a rostrate apex or flexuous, thin-walled, hyaline. Pileus trama interwoven; hyphae 3–10 µm wide, hyaline, thin-walled, inamyloid. Pileipellis a differentiated cutis with occasional nodulose-diverticulate hyphae; hyphae 3–18 µm wide, with a yellowish brown plasmatic pigment and strong, yellowish brown spiral encrustions, thin- to slightly thick-walled. Stipitipellis an epicutis; hyphae 3–6 µm wide, hyaline or with a yellowish brown plasmatic pigment, thick-walled (up to 1 µm thick), often giving rise to short or long, sometimes diverticulate side branches, 17–108 × 3–5 µm, slightly thick-walled towards the base. Caulocystidia absent. Clamp connections observed on all hyphae.
Material examined: INDIA, Kerala State, Ernakulam District, Pooyamkutty, 22 November 2014, K.P.D. Latha, DKP331 (CAL 1524, holotype).
Host and habitat: In small group among on a decaying twig.
GenBank numbers: ITS = KY807129, LSU = KY817233.
Notes: Marasmiellus bicoloripes is characterised by omphalinoid basidiomes with a greyish brown, faintly striate and fibrillose pileus, brownish orange, anastomosing and somewhat thick and waxy lamellae, a white stipe turning dark grey towards the base, oblong-ellipsoid to subfusiform basidiospores, a hymenium devoid of pleurocystidia, a heterogeneous lamellar edge with scattered cheilocystidia, a cutis-type pileipellis with occasional nodulose-diverticulate hyphae, an epicutis-type stipitipellis with frequent nodulose-diverticulate hyphae and presence of clamp connections. Owing to the combination of characteristics such as the stipe tending to greyish at the base, the cutis-type pileipellis with occasional diverticulate hyphae and the short basidiospores (less than 10.4 µm), this species can be placed either in sect. Candidi (Bat.) Sing. or in sect. Dealbati Sing. (Singer 1986) of Marasmiellus.
Marasmiellus cibodasensis Retnowati, an Indonesian species described invalidly by Retnowati (2012), differs in having a flattened pileus with a wrinkled surface, subdistant, non-anastomosing lamellae, an apically tapered, granulose and bulbous stipe which is not darkening towards the base, ellipsoid basidiospores, a fertile lamellar edge devoid of cheilocystidia and a gregarious habit on wood. Marasmiellus rhizomorphigenus Antonín et al., known from Republic of Korea (Antonín et al. 2010), differs in having a greyish to whitish pileus with a pubescent to tomentose surface, whitish to pale yellowish and distant lamellae, a stipe with a pubescent to furfuraceous surface, presence of rhizomorph, larger basidiospores (13.5–17 × 4.5–6.5 µm), well-developed, larger hymenial cystidia (34–70 × 8–14 µm) and presence of pileo- and caulocystidia. Marasmiellus koreanus Antonín et al. also described from Republic of Korea (Antonín et al. 2010), has larger, robust basidiomes with a brownish orange, rugulose pileus, light yellow lamellae, whitish to light yellow stipe, versiform cheilocystidia and stipitipellis with copious caulocystidia. Marasmiellus candidus (Fr.) Singer, a widespread species in Europe (Noordeloos 1995), differs in having a pure white, pruinose to tomentose pileus with a grey-brown centre, a pruinose stipe with a bulbous base, oblong to cylindrical and larger basidiospores [(10.5–)11.5–15(–17.5) × (7.5–)8.0–12.5 µm], copious and larger cheilocystidia (45–90 × 6–12.5 µm), an irregular cutis-type pileipellis with a transition to a trichoderm and the stipitipellis with caulocystidia.
The phylogenetic trees inferred from the ML and BI analyses of the ITS sequence dataset show identical topology and the ML phylogram is presented in Fig. 138. The tree reveals two distinct clades, the Tetrapyrgos and the Marasmiellus clades, as shown by Wilson and Desjardin (2005) and subsequently confirmed by Antonín et al. (2010). Marasmiellus bicoloripes nests in the Tetrapyrgos clade with 82% ML and 1.00 BYPP support. Within this clade, M. bicoloripes together with collections of M. rhizomorphigenus, M. tenerrimus (Berk. & M.A. Curtis) Singer and M. candidus formed a cluster where M. bicoloripes was found to be a lineage distinct from other species with significant support (73% ML and 1.00 BYPP). It has to be emphasized here that the clade Tetrapyrgos does not exactly correspond to the genus Tetrapyrgos as the latter is primarily characterised by tetrahedral basidiospores and a stipe arising from a basal pad (Honan et al. 2015) while some species including M. bicoloripes and M. candidus that form part of the clade Tetrapyrgos do not show these features.
Psathyrellaceae Vilgalys et al.
Based on molecular studies, Redhead et al. (2001) introduced the family Psathyrellaceae to accommodate the genera Psathyrella (Fr.) Quél. and Lacrymaria Pat., together with related species in the polyphyletic genus Coprinus sensu lato, which were transferred to the genera Coprinellus P. Karst., Coprinopsis P. Karst. or Parasola Redhead et al. A few species related to Agaricus remained in Coprinus sensu stricto. Therefore, the concept of family Coprinaceae Overeem & Weese was abandoned and Coprinus sensu stricto was transferred to Agaricaceae Chevall. Afterwards, several smaller genera were included in Psathyrellaceae. Kirk et al. (2008) consider the family to contain 12 genera.
Coprinopsis P. Karst.
Coprinopsis is a large genus that contains around 200 species (Kirk et al. 2008). They are saprotrophic on soil, wood, vegetable refuse, dung or burnt ground, and produce agaricoid, rather fragile basidiomes which are often short lived and/or deliquescent, with black to blackish spore deposit (Vesterholt 2012). The phylogenetic tree is presented in Fig. 141.
Maximum likelihood phylogenetic tree of Coprinopsis kubickae and related species based on ITS sequence alignment, calculated with MEGA6 software (Tamura et al. 2013). The sequence obtained in this study is shown in blue. Ex-type strains are indicated in bold. Maximum likelihood bootstrap values greater than 50% are indicated at the nodes. The tree is rooted with Coprinellus xanthothrix and C. domesticus. The bar indicates the number of nucleotide substitutions per site
Coprinopsis kubickae (Pilát & Svrček) Redhead, Vilgalys & Moncalvo, in Redhead et al., Taxon 50(1): 229 (2001)
Facesoffungi number: FoF05721, Fig. 142
Basionym: Coprinus kubickae Pilát & Svrček, Česká Mykol. 21: 142 (1967).
Holotype: Czechoslovakia, on culm of Juncus.
Pileus up to 10 mm wide when expanded, sphaerical at first, then ellipsoid to ovoid, paraboloid, convex, finally applanate or plano-concave with revolute margin, plicate-sulcate except at the centre, whitish to pale brown when young, then pinkish, finally brownish grey except brownish central disc, non-deliquescent, covered with scarce, minute, brownish to brown flocculose veil. Lamellae free, distant, L = 22–34, l = 1–3, white at first, becoming pinkish, then reddish brown with whitish and flocculose edge, finally brown-black and deliquescent. Stipe 16–28 × 0.8–1.2 mm, cylindrical or gradually thickened towards the base, which is often (sub) bulbose or with basal disc (up to 2 mm wide), central, hollow, dry, minutely fibrillose-floccose, often tomentose at the base, white. Odour and taste not observed. Spore print brown-black. Basidia 14–31 × 9–12 μm, clavate, 4-spored, thin-walled, hyaline, surrounded by 5–8 hymenophysalides (pseudoparaphyses). Basidiospores [100/2/1] 8–9.6–11.1 × 6.9–8.2–9.3 µm, Q = (1–)1.05–1.17–1.30(–1.38), mostly subglobose, broadly ellipsoid or ovoid, but also globose and ellipsoid, not flattened, with rounded to slightly conical base and rounded apex, smooth, thin-walled to moderately thick-walled (up to 0.7 µm), with 1.2–1.8 μm wide (inner diameter), central to eccentric germ-pore, medium rusty brown in H2O and NH4OH, medium brown in KOH, but soon becoming medium brown grey, semi-transparent, non-amyloid and non-dextrinoid. Cheilocystidia 30–90 × 9–24 µm, narrowly utriform to utriform, oblong, subcylindrical, conical or narrowly clavate, thin-walled to moderately thick-walled (up to 0.7 µm), hyaline, scattered. Pleurocystidia 50–110 × 13–24 µm, similar to cheilocystidia, but somewhat more elongated, mostly narrowly utriform, narrowly conical or subcylindrical, scattered. Veil composed of 2–6 μm broad, branched, thin-walled, rather sparsely diverticulate, hyaline to light yellowish brown hyphae, mostly with minutely to coarsely encrusted, light to dark brown pigment. Pileipellis a cutis, composed of repent, hyaline, thin-walled, 1.5–5 µm wide hyphae. Stipitipellis a cutis of parallel, repent, thin-walled, hyaline, 1–8 µm wide hyphae, sparsely diverticulate, with ascending hyphal tufts in places. Clamp connections present and abundant in all tissues.
Material examined: CROATIA, Karlovac County, Fishpond Draganići, 10 km NE from Karlovac, 45°33′40″N, 15°38′21″E, 107 m a.s.l., 7 September 2013, leg. M. Čerkez, CNF 1/6614.
Host and habitat: Solitary or in small groups on dead Phragmites, Typha, Juncus, Carex, Glyceria and Acorus species in wet habitats, as well as on rich soil and rotten straw in greenhouses (Uljé 2005; Nagy 2007; Gierczyk et al. 2011). From Canada reported on wood submerged in an alkali lake (Anastasiou 1967; as Coprinus amphibius Anastasiou). Our collection was collected on wet remnants of Phragmites australis, on the marshy edge of a fishpond.
Distribution: Known from about 15 European countries and Canada, rare.
GenBank number: ITS = MH422562.
Notes: Coprinopsis kubickae is primarily characterized by its branched, diverticulate and thin-walled veil elements, presence of clamp connections, smooth, globose to broadly ellipsoid, not flattened spores, rather small basidiomes and by living on plant (almost always herbaceous) remnants in wet habitats. Morphologically (according to branched and diverticulate veil), it belongs to section Alachuani (Singer) D.J. Schafer. However, the morphological concept of section Alachuani is not supported by molecular data and several Alachuani species are mixed with species of sections Atramentarii (Fr.) D.J. Schafer and Lanatuli (Fr.) D.J. Schafer in our ITS based phylogram (Fig. 141). Still, Coprinopsis kubickae nests in the clade with the rest of Alachuani species. We report Coprinopsis kubickae as new to Croatian mycobiota.
Boletales E.-J. Gilbert
Boletaceae Chevall.
Boletaceae is an important Basidiomycete family with a global distribution, most of which form ectomycorrhizal associations with trees. Most species have been described from temperate regions, and little research had been carried out on tropical boletes before 2010. In the past decade, however, phylogenetic analyses based on multigene dataset and including tropical taxa, have greatly improved our understanding of the systematics of the group, with many new genera and species being published (e.g., Halling et al. 2012; Hosen et al. 2013; Wu et al. 2015, 2016; Henkel et al. 2016). This molecular revolution has left a number of Boletaceae species (especially in the large genus Boletus) to be re-examined and recombined in the light of modern classification of the family. A novel species, Baorangia major is introduced based on distinct morphological characteristics coupled with phylogenetic analysis of a combined ATP6, RPB2, and TEF1-α dataset (Fig. 143). Two new combinations, Baorangia rufomaculata and Lanmaoa pallidorosea are also designated in this study.
Maximum likelihood tree inferred from three-gene dataset (ATP6, RPB2 and TEF1-α). Retiboletus griseus, R. fuscus, and Rhodactina rostratispora were used as the outgroup taxa. Sequences retrieved from GenBank were originally published in Halling et al. (2012), Wu et al. (2014, 2015, 2016), Zhao et al. (2014a, b), Raspé et al. (2016), and Vadthanarat et al. (2018). Maximum likelihood bootstrap values greater than 70% are indicated at the nodes. The bar indicates the number of nucleotide substitutions per site. Type specimens are indicated in bold. Newly generated sequences are in blue
Baorangia G. Wu & Zhu L. Yang
Baorangia was first recognized by Wu et al. (2014, as ‘clade 51’), and later formally described by Wu et al. (2015). Typical characteristics are a thin hymenophore that is 3–5 times thinner than the pileus context, yellow tubes and pores that stain blue when bruised, a light yellow context that slowly stains pale blue when cut, and a trichodermium to interwoven trichodermium pileipellis (Wu et al. 2015). Three species are recorded in the genus, B. bicolor (Kuntze) G. Wu et al., B. emilei (Barbier) Vizzini et al. and B. pseudocalopus (Hongo) G. Wu & Zhu L. Yang, which is the generic type (Kirk et al., continuously updated).
Baorangia major Raspé & Vadthanarat, sp. nov.
MycoBank number: MB824250; Facesoffungi number: FoF05722, Figs. 144, 145
Etymology: The specific epithet “major” from Latin, refers to the size of mature basidiomes, the largest in the genus.
Holotype: MFLU 12-0040
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes large when mature. Pileus (7–)16–22(–23) cm diam., at first hemispherical to convex, becoming convex to plano-convex and sometimes slightly depressed at the centre, greyish red (11C–E5) to greyish ruby (12B–E5), more rarely greyish pink (around 10A3), paler in spots and in age; margin involute at first, later inflexed, slightly exceeding; concolorous or abruptly paler (yellowish white; 2A2); surface even, dry, dull, subtomentose; context 1.7–3.8 cm thick half-way to the margin, firm at first, soft in age, off-white to yellowish white (1A2), slightly marmorated above the hymenophore, blueing when bruised, sometimes only weakly, then slowly getting yellow (3A5–6) with time, as in worm wounds. Stipe central, terete, cylindrical to subclavate (4.8–)5.2–8.5(–15) × 1.5–3.3(–4.5) cm; surface finely and densely dotted, subceraceous, dull, with off-white to pale yellow basal tomentum; context solid, yellowish white (2A2) at the top, more intensely yellow towards the base, marmorated, often with dark red spots, especially in the lower half, quickly and intensely blueing when cut. Hymenophore tubulate, decurrent; tubes 2–5 mm long, not separable, yellow (2–3A7), sometimes stained red at places (old bruises) or when old, quickly and intensely blueing when bruised; pores 2–5 mm wide at mid-radius when mature, mostly angular, elongated and radially arranged near the stipe, yellow (2A8), quickly and intensely blueing when bruised, sometimes reddish when very old. Odour fungoid to slightly fruity. Taste mild, fungoid. Spore print olive-brown. Macrochemical reactions: KOH pale orange on pileus and stipe, paler on context, null or merely slightly orangish on hymenium; NH4OH null. Basidia 4-spored, (36–)36–42.2–55(–55) × (8.5–)8.5–9.3–11(–11) µm (n = 20), narrowly clavate, sometimes curved, hyaline, with sterigmata up to 5 µm long, without basal clamp connection. Basidiospores (6–)7.5–8.1–9(–10) × (4–)4–4.6–5(–5.5) µm Q = (1.44–)1.5–1.8–2.1(–2.4) [N = 5/5/265]. From the type (7.5–)8–8.6–9.5(–9.5) × (4–)4–4.5–5(–5) µm, Q = (1.6–)1.69–1.9–2.13(–2.13) [n = 55], ellipsoid to ovoid, thin-walled, smooth, yellowish to greenish hyaline in water, KOH or NH4OH, yellowish in Melzer’s reagent. Cheilocystidia thin-walled, hyaline, of two different shapes, the first clavate to broadly clavate, (21–)21–29.3–37(–37) × (11.5–)11.5–13.1–15(–15) µm, the second fusiform to broadly fusiform, (26–)26–38.9–57(–57) × (10–)10–13.3–15(–15) µm. Pleurocystidia infrequent, fusiform to narrowly fusiform with obtuse apex, (44–)44–54.4–70(–70) × (8–)8–9.5–12(–12) µm, thin-walled, hyaline. Hymenophoral trama divergent, 90–112 µm wide, composed of 4–10 µm wide, hyaline hyphae, with regular mediostratum 30–45 µm wide. Pileipellis a trichoderm to tangled trichoderm, 170–190 µm thick, made of moderately interwoven and somewhat anastomosing, thin-walled, hyaline hyphae; terminal cells cylindrical with rounded apex, 20–58 × 4–9 µm, mostly hyaline to slightly pale yellowish brown at places in KOH. Pileus context made of moderately interwoven, hyaline, thin-walled hyphae, 4–13 µm wide. Stipitipellis a hymeniderm, 62–88 µm thick, mostly composed of basidiole-like cells, 12–43 × 4–10 µm. Caulocystidia infrequent, of three shapes, the first clavate to broadly clavate, (13–)23.8(–31) × (10–)12.5(–15) µm, thin-walled, hyaline, the second fusiform to broadly fusiform, (21–)31.5(–45) × (9.5–)11.6(–15), thin-walled, hyaline, and the third cylindrical, slightly curved, mostly hyaline, at places slightly yellowish brown in KOH. Stipe context composed of parallel, 4–12 µm wide, hyaline and somewhat anastomosing hyphae. Clamp connections not seen in any tissue.
Material examined: THAILAND, Chiang Mai Province, Mae Taeng District, around Mushroom Research Center, N19°07.1′–E98°44.0′, elev. 900 m, 2 July 2010, P. Sysouphanthong, OR070 (MFLU!, BR!); ibid., N19°07.2′–E98°43.9′, elev.915 m, 17 June 2011, O. Raspé & S.C. Karunarathna, OR197 (MFLU!, BR!); N19°06.5′–E98°44.5′, elev.1075 m, 18 June 2011, O. Raspé & S.C. Karunarathna, OR209 (MFLU, holotype; BR, isotype); N19°06.6′–E98°44.5′, elev.1055 m, 7 June 2012, O. Raspé & K. Wisitrassameewong, OR404; N19°07.2′–E98°43.9′, elev. 910 m, 29 July 2013, O. Raspé & B. Thongbai, OR657 (MFLU!, BR!); CHINA, Yunnan Province, Cangyuan County, Banhongxiang, along the road, N23°18.6′–E99°05.3′, elev. 1010 m, 10 July 2012, O. Raspé & R.L. Zhao OR486 (HMAS).
Habit and habitat: Mostly gregarious, sometimes fasciculate or solitary, on soil in forests dominated by Lithocarpus spp., Castanopsis spp., sometimes mixed with Dipterocarpus spp.
Distribution: Known from Chiang Mai Province, Thailand and Yunnan Province, China.
GenBank numbers: ATP6 = MG897421, RPB2 = MG897441, TEF1-α = MG897431 (OR209, holotype); ATP6 = MG897422, RPB2 = MG897442, TEF1-α = MG897432 (OR404), ATP6 = MG897423, RPB2 = MG897443, TEF1-α = MG897433 (OR486).
Notes: Baorangia major can easily be recognised in the field by its large basidiomes when mature, decurrent hymenophore with large, angular, radially arranged (at least near the stipe), and 1–3 times compound pores, red stipe that immediately and intensely turns blue when injured or merely touched. In their diagnosis of Baorangia, Wu et al. (2015) mentioned “context which stains pale blue slowly when cut”. While this fits B. major pileus context, it does not fit its stipe context, which quickly and intensely stains blue when cut. Baorangia bicolor (Kuntze) G. Wu et al. and B. rufomaculata (see below) somewhat resembles B. major, but the latter can easily be distinguished by the large angular pores and intense blueing.
Baorangia rufomaculata (Both) Raspé & Vadthanarat, comb. nov.
MycoBank number: MB824251; Facesoffungi number: FoF05723, Fig. 146
Basionym: Boletus rufomaculatus Both, Bull. Buffalo Soc. Nat. Sci. 36: 221 (1998)
Holotype: USA, Western New York, under Fagus grandifolia Ehrh., Quercus rubra L., Tsuga canadensis (L.) Carrière and Acer saccharum Marshall, Both 2831 (BUF).
Basidia 4-spored, (21–)21–24.6–31(–31) × (8–)8–8.9–10(–10) µm, clavate, hyaline, sterigmata 3–5 µm long, without basal clamp connection. Basidiopores (9–)9–10.6–12(–12.5) × (3.5–)3.5–3.9–4.5(–4.5) µm Q = (2.1–)2.3–2.7–3.2(–3.6) [N = 50], subcylindrical with slight suprahilar depression, thin-walled, smooth, slightly greenish to yellowish hyaline in water, yellowish hyaline in 5% KOH or NH4OH, brownish yellow in Melzer’s reagent. Cheilocystidia (18–)18–31.4–45(–45) × (6–)6–7.9–10(–10) µm, frequent, fusiform to broadly fusiform, thin-walled, hyaline. Pleurocystidia (42–)42–59.5–72(–72) × (7–)7–8.2–9.5(–9.5) µm, infrequent, narrowly fusiform, thin-walled, hyaline. Hymenophoral trama regular to slightly divergent, 90–190 µm wide, composed of (3–)5–7(–12) µm wide hyaline hyphae. Pileipellis a subcutis to tangled trichoderm, 120–220 µm thick, made of loosely to moderately interwoven, thin-walled, hyaline hyphae; terminal cells cylindrical with rounded apex, 22–45 × 5–8 µm, mostly hyaline, at places yellowish hyaline in KOH. Pileus context made of moderately interwoven hyaline, thin-walled hyphae, 3–12 µm wide. Stipitipellis a hymeniderm, 85–120 µm thick; terminal cells clavate to narrowly clavate with rounded apex, thin-walled, 14–47 × 3–12 µm; below the hymeniderm parallel, hyaline hyphae anastomosing at places. Stipe context composed of parallel, branched, hyaline, thin-walled hyphae, 7–14 µm wide. Caulocystidia rare, 23–30 × 7–9 µm, fusiform or clavate, thin-walled, hyaline. Clamp connections not seen in any tissue.
Material examined: USA, New York, Erie Co., Town of Orchard Park, Chestnut Ridge Park, N42°43.1′–W78°45.3′, elev. 330 m, 6 August 1997, E. Both 4144 (CFMR).
Distribution: North America.
GenBank numbers: ATP6 = MG897415, RPB2= MG897435, TEF1-α = MG897425.
Notes: The specimen we studied (Both 4144) was already sequenced by Nuhn et al. (2013) for LSU, RPB1, and TEF1-α genes, but under the wrong number “4414”. Our TEF1-α sequence is 100% identical to the one published by Nuhn et al. (2013), except for the insertion of an “N” at position 215 in the latter, which is likely an artefact. Our measurements of microscopic characteristics slightly deviate from the original description of Both (1998). However, the specimen we studied was collected and identified by Both from the type locality. Therefore, we are confident that it belongs to the same species.
Lanmaoa G. Wu & Zhu L. Yang
The genus Lanmaoa was recognized by Wu et al. (2014, as ‘clade 49’), and later formally described by Wu et al. (2015). The typical characteristics of the genus are a thin hymenophore which is 3–5 times thinner than the pileus context and stains blue when bruised, a light yellow context that slowly stains pale blue when cut, and an interwoven trichodermium to subcutis pileipellis (Wu et al. 2015). Seven species are recorded in the genus, L. angustispora G. Wu & Zhu L. Yang, L. asiatica G. Wu & Zhu L. Yang (generic type), L. carminipes (A.H. Sm. & Thiers) G. Wu et al., L. flavorubra (Halling & M. Mata) G. Wu et al., L. fragrans (Vittad.) Vizzini et al., L. pseudosensibilis (A.H. Sm. & Thiers) G. Wu et al. and L. roseocrispans Bessette et al. (Kirk et al., continuously updated).
Lanmaoa pallidorosea (Both) Raspé & Vadthanarat, comb. nov.
MycoBank number: MB824252; Facesoffungi number: FoF05724, Fig. 147
Basionym: Boletus pallidoroseus Both, Bull. Buffalo Soc. nat. Sci. 36: 218 (1998).
Holotype: USA, New York, on ground under Quercus rubra and Fagus grandifolia, Both 3026 (BUF).
Basidiospores and basidia not observed on the immature specimen studied. Cheilocystidia (14–)14–19.4–25(–25) × (5–)5–6.5–8(–8) µm, frequent, fusiform, thin-walled, hyaline. Pleurocystidia (28–)28–33.9–39(–39) × (6.5–)6.5–7.9–9(–9) µm, infrequent, narrowly fusiform, thin-walled, hyaline. Hymenophoral trama divergent, 110–175 µm wide, composed of (3.5–)4–7(–12) µm wide hyaline hyphae. Pileipellis a trichoderm, 220–300 µm thick, composed of thin-walled, hyaline hyphae; terminal cells cylindrical with rounded apex, 28–70 × 4–8 µm, mostly hyaline, at places brownish to yellowish hyaline in KOH. Pileus context made of strongly interwoven, hyaline, thin-walled hyphae, 4–6 µm wide. Stipitipellis a hymeniderm, 55–80 µm thick, composed of pointing out, clavate, thin-walled terminal cells 13–28 × 4–9 µm, which are mixed with caulocystidia. Stipe context composed of interwoven, sub-irregular, thin-walled, hyaline hyphae, 4–9 µm wide. Caulocystidia of two types, the first one very frequent, fusiform to broadly fusiform, (19–)19–28.4–39(–39) × (7–)7–8.4–11(–11.5) µm, thin-walled, hyaline, the second one infrequent, broadly clavate, (11–)11–20.5–27(–27) × (7–)7–9.4–11(–11) µm, thin-walled, hyaline. Clamp connections not seen in any tissue.
Material examined: USA, New York, Erie Co., Orchard Park, Chestnut Ridge Park, N42°43.1′–W78°45.3′, elev. 330 m, 19 August 2000, E. Both 4432 (CFMR).
Distribution: North America.
GenBank numbers: ATP6 = MG897417, RPB2 = MG897437, TEF1-α = MG897427.
Notes: Although the specimen we examined is immature and we could not check basidiospore and basidia dimensions, we are confident that it belongs to the species described by Both (1998). Indeed, it was collected and identified by E. Both himself from the type locality.
Cantharellales Gäum.
Clavulinaceae Donk
Clavulinaceae was established by Donk (1933) as a tribe, but later Donk (1961) raised the tribe to the rank of family. The family Clavulinaceae comprises four genera, Burgella Diederich & Lawrey, Clavulina J. Schröt., Membranomyces Jülich and Multiclavula R.H. Petersen (Kirk et al. 2008). We follow the treatment and updated accounts of Clavulinaceae in He et al. (2016) and Tibpromma et al. (2017). A novel species, Clavulina thindii is introduced based on distinct morphology and maximum likelihood phylogeny of ITS sequence data (Fig. 148).
Maximum likelihood phylogeny of Clavulina showing the position of novel species Clavulina thindii (shown in blue and bold). The ITS sequence dataset was aligned with online version of MAFFT v. 7 (Katoh and Standley 2013) and no manual editing was done within the alignment. One-thousand bootstrap replicates were analyzed to obtain nodal support values. Analysis was conducted with MEGA 6.0 (Tamura et al. 2013). Hydnum rufescens and H. repandum were used as the outgroup taxa. Maximum likelihood bootstrap values greater than 50% are indicated at the nodes. The bar indicates the number of nucleotide substitutions per site. Ex-type strains are indicated in bold
Clavulina J. Schröt.
Clavulina is distributed worldwide and characterized by clavarioid to coralloid, occasionally resupinate and effused, simple or branched basidiomes, monomitic hyphal system, with clamp connections in most species; smooth, hyaline, subglobose to broadly ellipsoid basidiospores usually with one large guttule; basidia with two or up to six cornuted sterigmata and often develop a transversal septum (Corner 1950, 1970; Petersen 1988a; Henkel et al. 2005, 2011; Uehling et al. 2012a; He et al. 2016). Species of Clavulina occur in forests dominated by ectomycorrhizal (ECM) trees of the leguminous genera (Fabaceae) (Henkel et al. 2011). Approximately 82 species have been described in the genus Clavulina (Corner 1950, 1970; Thind 1961; Petersen 1967, 1983, 1985, 1988a, b; Thind and Sharda 1984; Roberts 1999; Thacker and Henkel 2004; Henkel et al. 2005, 2011; Douanla-Meli 2007; Duhem and Buyck 2007; Trappe and Castellano 2007; Olariaga and Salcedo 2012; Uehling et al. 2012a, b; Wartchow 2012; He et al. 2016).
Clavulina thindii U. Singh, sp. nov.
MycoBank number: MB824527; Facesoffungi number: FoF04878, Figs. 149, 150
Clavulina thindii (CAL 1661, holotype). a Fresh basidiomes. b Apices of basidiome. c Transverse section through hymenium. d Basidium with post-partial septa and basal clamp (indicated by white arrow). e Hymenophoral tramal hyphae showing clamps (indicated by white arrows). f Basidiospores. Scale barsa = 20 mm, c–f = 10 µm
Etymology: To commemorate Prof. K.S. Thind for his contribution to Indian mycobiota.
Holotype: CAL 1661
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Basidiomes 41–66 × 30–49 mm, coralloid, terrestrial, profusely branched, solitary to scattered; sterile stipe 12–28 × 7–12 mm, white to greyish white, glabrous; fertile branches 30–38 × 2.5–4.5 mm, forming 4–7 ranks in multiple planes, branching pattern polychotomous to dichotomous-ascending, initially violet white to pale violet (16A2–3), maturing to lilac grey to lilac (16B2–3), wax white to greyish yellow (2B3–4) with drying, smooth; hymenium ripening over distal two-thirds of basidiomes, amphigenous, interface with stipe clearly demarcated; apices rounded-acuminate to irregularly coronate or blunt, concolorous with branches when young, becoming brownish orange (6C8) at maturity; context subsolid to hollow, concolorous with branches. Taste and Odour pleasant. Basidiospore deposit not obtained. Basidia 22–48 × 6–10 µm, subcylindrical to subclavate or clavate, with numerous granular contents, post-partial septa observed on some basidia, basally clamped, 2-spored; sterigmata up to 7 µm long, cornuted; basidioles numerous, subcylindrical to clavate. Hymenophoral tramal hyphae sub-regular to irregular; noninflated tramal hyphae 5–8 µm, smooth, thin-walled, hyaline, lacking internal contents; inflated tramal hyphae 11–15 µm, smooth, thin-walled, hyaline, lacking internal contents. Cystidia absent. Basidiospores 6.5–7.5–8 × 6–6.4–7 µm (n = 30, Q = 1.07–1.15–1.25), subglobose to broadly ellipsoid, smooth, pale yellow in KOH, inamyloid, with one translucent guttule; apicules up to 1 µm long. Clamp connections present.
Material examined: INDIA, Uttarakhand, Pauri Garhwal District, Gangoti-Adwani forest, alt. 1948 m, N 30°05.799′ E 078°43.927′, 3 October 2016, U. Singh, US 1428 (CAL 1661, holotype); ibid., 31 August 2017, U. Singh, US 1585.
Host and habitat: Solitary to scattered, on ground, under trees of Acacia dealbata A. Cunn. in mixed temperate forest.
Distribution: Known only from India.
GenBank numbers: ITS = MG892054 (US 1428); ITS = MG892055 (US 1585).
Notes: Clavulina thindii is distinct from all other species of Clavulina by the ITS sequence data and combination of morphological features: violet to lilac profusely branched basidiomes (42–66 × 38–49 mm) with white to greyish white stipe and rounded-acuminate to irregularly coronate or blunt apices, subsolid to hollow context, subglobose to broadly ellipsoid basidiospores, cylindrical to subclavate basidia with two cornuted sterigmata, absence of cystidia, noninflated to inflated tramal hyphae and presence of clamp connections.
In field, Clavulina thindii may appear similar to C. livida Shu Z. Yan et al. with both species possessing large sized, branched basidiomes with rounded-acuminate apices which turn brown at maturity, smooth basidiospores with one large guttule, subcylindrical to clavate basidia with sometimes post-partial septa, absence of cystidia, noninflated to inflated tramal hyphae and presence of clamp connections. However, the latter has greyish basidiomes with olive stipe, larger (11.6–12.9 × 10.7–12.5 µm), globose to subglobose basidiospores and larger basidia (51.5–76.5 × 7–12.3 µm) (He et al. 2016).
Clavulina amethystina (Bull.) Donk shares similarities with C. thindii in having lilac to violet, branched basidiomes with obtuse to blunt apices, but the former has completely lilac to violet basidiomes (the stipe of Clavulina thindii is white to greyish white), becoming dark grey with age and ellipsoid (Lm/Wm ≈ 1.55–1.62) basidiospores (Corner 1950; Olariaga et al. 2009).
Clavulina cinerea (Bull.) J. Schröt, C. rugosa (Bull.) J. Schröt, C. coralloides (L.) Schröt. (= C. cristata), C. subrugosa (Cleland) Corner and C. samuelsi R.H. Petersen are also closely related to C. thindii. However, these five species show morphological differences from C. thindii. Clavulina cinerea differs from C. thindii by its white to dark grey basidiomes with acute to blunt apices, indistinct or not well-delimited stipe due to basitonic branching and ovoid (Lm/Wm = 1.1–1.3) basidiospores (Bulliard 1788; Olariaga et al. 2009). Clavulina rugosa is distinguished by its simple to sparsely branched, white basidiomes, noncristate and normally obtuse apices and relatively larger basidiospores (9–14 × 8–12 µm, Lm × Wm = 9.9–10.6 × 8.2–8.8) (Corner 1950; Olariaga et al. 2009). Clavulina coralloides differs from C. thindii by its white to grey basidiomes with not well-delimited stipe due to basitonic branching, cristate apices, comparatively larger and subglobose basidiospores (7–11 × 6.5–10 µm) (Holmskjold 1790; Corner 1950; Olariaga et al. 2009). Clavulina subrugosa can be separated from C. thindii by its simple or filiform to flattened branched, white to pale greyish brown basidiomes and rather large basidiospores (6.8–8.6 × 5.8–7.2 µm) (Corner 1950; Petersen 1988a). Clavulina samuelsii differs from C. thindii in having tilleul buff fruiting body, comparatively larger basidiospores (7.2–9.4 × 6.1–7.6 µm) and presence of cystidia (Petersen 1988a).
Clavulina mussooriensis Corner et al. (an Indian species) shares similarities with C. thindii in possessing profusely branched basidiomes and sometimes subglobose basidiospores but the former has cream-white to greyish brown basidiomes with cristate to acute apices and larger basidiospores (7–9 × 5.6–8 µm) (Corner et al. 1958). Clavulina limosa K.S. Thind & Sharda (also originally reported from India) differs from C. thindii by simple (unbranched), slimy, creamish white to pallid-cream basidiomes and globose to subglobose basidiospores (Thind and Sharda 1984).
Thind (1961) reported Clavulina amethystinoides (Peck) Corner from India, but the conspecificity has not yet been verified with help of sequence data from the Indian collections. Clavulina amethystinoides shares some similarities with C. thindii in possessing violet basidiomes and 2-spored basidia, but differs in having simple or sparsely branched basidiomes, comparatively larger and globose to subglobose basidiospores (9–12.3 × 8.8–10.5 µm). Moreover, C. amethystinoides (originally reported from USA) differs from C. thindii by having simple or irregular branches, slightly palmate branching, pale drab, pinkish tan, fawn or very pale lilac basidiomes, pale livid flesh, eventually blackening tips with distinctly different colour and larger basidiospores (7–10 × 6–8 µm) (Corner 1950).
Clavulina thindii is commonly collected and consumed by local villagers. ‘Angalchyun’ (fingers like mushroom) is the vernacular name used for this species in the local language (Garhwali). However, inhabitants refer this vernacular name (Angalchyun) for all clavarioid fungi. The ITS dataset consisting of 49 sequences (including our isolates: US 1428, represented by GenBank no. MG892054 and US 1585, represented by GenBank no. MG892055) of Clavulina and Hydnum L. was analyzed by maximum likelihood phylogenetic inference (Fig. 148). Two sequences isolated from Indian materials appear to be nested amongst other sequences of Clavulina and form a distinct clade being sister to Chinese collections of C. livida (represented by KU219603, KU219604, KU219605 and KU219606). The combination of morphological features and phylogenetic analysis indicates that Clavulina thindii is a new species, distinct from all the known taxa of Clavulina.
Polyporales Gäum.
Phanerochaetaceae Jülich
The family Phanerochaetaceae was described by Jülich (1981) with Phanerochaete as generic type, and also including the genera Meruliopsis Bondartsev, Phlebiopsis Jülich and Scopuloides (Massee) Höhn. & Litsch. This family was characterized by mostly resupinate basidiome, thick-walled hyphae, often without clamps, basidia narrowly clavate and spores hyaline, smooth and thin-walled (Larsson 2007; Ryvarden and Melo 2014; Yuan et al. 2017). Justo et al. (2017) revised the family on the basis of molecular and morphological data and, in addition to Phanerochaete, they include the following thirteen genera: Bjerkandera P. Karst., Donkia Pilát, Hapalopilus P. Karst., Hyphodermella J. Erikss. & Ryvarden, Oxychaete Miettinen, Phaeophlebiopsis Floudas & Hibbett, Phanerina Miettinen, Phlebiopsis Jülich, Pirex Hjortstam & Ryvarden, Porostereum Pilát, Rhizochaete Gresl. et al., Riopa D.A. Reid and Terana Adans. Most of these genera are corticioid and rarely polyporoid, with the hyphal system monomitic or sometimes dimitic, and most have hyphae without clamps; cystidia are often present and spores are thin-walled, hyaline and smooth. All species of this family produce a white-rot.
Phanerochaete P. Karst.
Phanerochaete is a genus of widespread corticioid fungi that causes white rot on all kinds of decayed wood and plays a key role in forest ecosystems. The genus contains 167 legitimate names (MycoBank 2019). Since 2008 eight new species and one subspecies have been described (Nakasone 2008; Ghobad-Nejhad et al. 2015; Floudas and Hibbett 2015; Liu and He 2016; Sádlíková and Kout 2017; Spirin et al. 2017), and six new combinations have been proposed (Nakasone 2008; Hjortstam et al. 2009; Melo et al. 2012; Floudas and Hibbett 2015; Volobuev et al. 2015; Miettinen et al. 2016).
Phanerochaete was described by Karsten (1889) to accommodate Thelephora alnea Fr. and T. odora Fr., and therefore based on two elements, without designation of a type. Cooke (1953) considered T. alnea as generic type and later Corticium decolorans (= Phanerochaete velutina) was chosen as lectotype by Eriksson et al. (1978a). Recently, Spirin et al. (2017) reinstated T. alnea as the generic type. Long neglected, Phanerochaete was reintroduced by Donk (1957) with the two initial species. Several years later, the taxonomy of the genus was discussed by Donk (1962), Parmasto (1968), Eriksson et al. (1978b) and Burdsall (1985). Donk (1962) amended Phanerochaete, recognizing that the generic limits were uncertain and suggesting that other taxa should be included. Parmasto (1968) accepted twenty species separated in two subgenera, Phanerochaete and Phanericium Parm.; cystidiate and acystidiate species, respectively. Eriksson et al. (1978b) recognized twelve species in Northern Europe divided into three groups, Phanerochaete velutina group (= subgen. Phanerochaete), P. tuberculata group (= subgen. Phanericium Parm.), and P. septocystidiata group, the last one with only one species characterized by cystidia regularly septate. Burdsall (1985) recognized 42 species in three subgenera, Phanerochaete, Phanericium, and Scopuloides (Massee) Burdsall, the latter with cystidia and subiculum usually distinct.
Phylogenetic studies carried out in Phanerochaete sensu Burdsall (1985) showed that the genus is polyphyletic (De Koker et al. 2003; Wu et al. 2010a) and its species are distributed in nine phylogenetic linages across the phlebioid clade within order Polyporales (Floudas and Hibbett 2015). According to Floudas and Hibbett (2015) one of these linages, Phanerochaete sensu stricto, includes species mostly found in the subgenus Phanerochaete sensu Burdsall (1985).
From a morphological point of view, the species included in the Phanerochaete sensu stricto clade in Floudas and Hibbett (2015) and in our LSU analysis (Figs. 151, 152) are characterized by their basidiomes being resupinate, membraceous, white, yellowish orange, or red to brown; the subiculum well-developed and the margin mostly fibrillose, with or without cordons. The hyphal system is monomitic; the subicular hyphae thin to thick-walled, loosely interwoven, simple septate or with scattered single to multiple clamps; the subhymenial hyphae thin-walled, without clamps. The cystidia are cylindrical to subulate, thin-walled to almost metuloid, without a basal clamp. Spores are thin-walled, inamyloid and non dextrinoid.
Phylogram generated from Bayesian inference analysis of LSU sequence data of species of Phanerochaete sensu stricto from Floudas and Hibbett (2015) and the new species is marked in grey square. Maximum parsimony and maximum likelihood bootstrap support ≥ 50% (10.000 replicates both), and Bayesian posterior probabilities ≥ 0.50 BYPP are shown above the nodes. The tree is rooted with Pirex concentricus and Hyphodermella rosae. Newly generated sequences are in blue, types are in bold
Phylogram generated from Bayesian inference analysis of ITS sequence data of species of Phanerochaete sanguinea complex from Floudas and Hibbett (2015) and the new species is marked in grey square. Maximum parsimony and maximum likelihood bootstrap support ≥ 50% (10,000 replicates both), Bayesian posterior probabilities ≥ 0.50 BYPP are shown above the nodes. The tree is rooted with three Phanerochaete species
We follow the treatment and updated accounts of Floudas and Hibbett (2015) and Miettinen et al. (2016); the new species, Phanerochaete australosanguinea is introduced.
Phanerochaete australosanguinea Telleria, M. Dueñas & M.P. Martín, sp. nov.
MycoBank number: MB825276; Facesoffungi: FoF05725, Figs. 153, 154
Phanerochaete australosanguinea (20102Tell., MA-Fungi 91309, holotype). a Basidiome dry specimen. b Strands, dry specimen. c Substrate stained red. d Basal hyphae thick-walled with clamp. e Probasidia and cystidium. f Basidium stained in congo red. g Cystidium with secondary septa stained in congo red. h Spores. Scale barsa–c = 1 cm, d–h = 10 µm
Etymology: From Latin “australis” which means southern and “sanguinea” refers to the morphological similarity of this species with Phanerochaete sanguinea (Fr.) Pouzar
Holotype: 20102Tell, MA-Fungi 91309
Colour codes follow: ISCC-NBS Centroid Colour Charts (Kelly and Judd 1976)
Basidiomes resupinate, orbicular to confluent, membranaceous, closely adnate; hymenophore smooth to slightly cracked in mature specimens; brownish orange (54. Br O–37. m r O) when young, in mature specimens orange yellow (70. l. OY–71. m. OY); the edge especially differentiated by dark reddish orange (38. d. r O). Margin fibrillose and white; with well-developed dark strands sometimes present. KOH 3% reaction (+) turning green that quickly change to brown. Subiculum byssoid, white to cream, lighter in colour than the hymenophore. Hyphal system monomitic. Subicular hyphae thick-walled, loosely interwoven, sparsely branched, growing parallel to the substrate, (5–)8–10 µm wide, sometimes with orange brown content, with single clamps. Subhymenial hyphae thin-walled and short-celled, branched, densely interwoven, growing perpendicular to the substrate, 3–5 µm wide. Cystidia thin-walled, cylindrical to tapering, sometimes with secondary septa, 44–66 × 4–5 µm. Basidia narrowly cylindrical to claviform, four sterigmata, 40–46 × 4–5 µm. Spores ellipsoid, 4.5–5.5 × 3–3.5 µm, thin-walled, sometimes with oil drops in the protoplasm, Q (L/W) = 1.29.
Material examined: CHILE, Los Lagos, Palena, Hornopirén, comuna Hualainhué, Huinay Biological Reserve, path to Cerro del Tambor, 42°22′54″S 72°24′53″W, 202 msl, on unidentified wood, 8 May 2013, M. Dueñas, M.P. Martín & M.T. Telleria, 20102Tell., (MA-Fungi 91309, holotype); ibid., 20098Tell. (MA-Fungi 91308); 20114Tell. (MA-Fungi 91310).
Host and habitat: Known from the Valdivian temperate rain forest, Chilean Patagonia, South America, growing on decayed wood and associated with white rot.
GenBank numbers: ITS = MH233925, LSU = MH233928 (20098Tell., MA-Fungi 91308); ITS = MH233926, LSU = MH233929, (20102Tell., MA-Fungi 91309, holotype); ITS = MH233927 (20114Tell., MA-Fungi 91310).
Notes: Based on LSU analyses (Fig. 151), Phanerochaete australosanguinea clusters into the Phanerochaete sensu stricto clade in the P. sanguinea complex, according to the maximum likelihood phylogeny of the concatenated ITS, LSU, RPB1 and RPB2 obtained by Floudas and Hibbett (2015). Most species of this complex cause red–orange staining of the substrate, such as P. australosanguinea (Fig. 153c). In the LSU analyses, P. australosanguinea is a sister species of P. sanguineocarnosa Floudas & Hibbett. Morphologically, this is corroborated by both species sharing well-developed dark strands, the KOH reaction turning quickly green changing to brown on the basidiome; the subicular hyphae having developed clamps, and cystidia in both species are cylindrical, tapering to the apex, with secondary septa and of similar size. However, the spores are ellipsoid to broadly ellipsoid in P. australosanguinea (Q = 1.29), while those of P. sanguineocarnosa, described by Floudas and Hibbett (2015), are sub-ellipsoid to ellipsoid (Q = 1.8).
In the ITS analyses (Fig. 152), Phanerochaete australosanguinea also clusters in the P. sanguinea complex. However, the newly generated sequences are very distinct from those of the P. sanguinea complex of Floudas and Hibbett (2015). These authors included mainly species and specimens from the Northern hemisphere (except one sequence from Tasmania). The three collections from Chile form a basal and highly supported group (99% ML, 97% MP and 1.00 PP) indicating that P. australosanguinea is distinct from other species of the P. sanguinea complex by its geographical distribution. So far, P. australosanguinea has been reported from Valdivian temperate rain forest in Chilean Patagonia, where it is probably frequent and could have been confused with P. sanguinea (Fr.) Pouzar reported from the Patagonian Andes forest of Southern Argentina by Greslebin and Rajchenberg (2003).
Russulales Kreisel ex P.M. Kirk et al.
Russulaceae Lotsy
Russulaceae is one of the most speciose, and morphologically diverse ectomycorrhizal mushroom families (Miller et al. 2006; Hyde et al. 2016). This family has a worldwide distribution of more than 1200 species (Kirk et al. 2008). Multigene molecular phylogenetic analyses consistently recovered this family as a monophyletic clade which is now comprised of four genera: Russula Pers., Lactarius Pers., Lactifluus (Pers.) Roussel and Multifurca Buyck & Hofstetter (Buyck et al. 2008, 2010). We follow the treatments and the updated accounts of taxa in Russulaceae in Li et al. (2016), Hyde et al. (2016, 2017), De Crop et al. (2017) and Tibpromma et al. (2017). Four novel species viz. Lactarius olivaceopallidus, Lactifluus midnapurensis, Russula choptae and R. uttarakhandia are introduced in Russulaceae based on morphological distinctiveness and phylogenetic support. An updated phylogenetic tree of Lactarius, Lactifluus, Russula subg. Russula and subg. Incrustatula are provided. Detailed discussion and reviewed literatures were provided in Li et al. (2016), Hyde et al. (2016, 2017), De Crop et al. (2017) and Tibpromma et al. (2017).
Lactarius Pers.
Lactarius is one of the most prevalent agarics which form obligate symbiotic associations with many wide spread genera of trees (Verbeken et al. 2001; Le et al. 2007; Das and Verbeken 2011; Verbeken and Nuytinck 2013; Das et al. 2015; Hyde et al. 2016; Uniyal et al. 2017). Multigene based phylogenetic studies resulted segregation of paraphyletic Lactarius sensu lato into Lactifluus Pers. (Roussel) and Lactarius (including all angiocarpic species). Lactarius sensunovo consists of three cosmopolitan subgenera: Lactarius subg. Lactarius, L. subg. Plinthogalus (Burl.) Hesler & A. H. Sm. and L. subg. Russularia (Fr. ex Burl.) Kauffman (Verbeken and Nuytinck 2013; Das et al. 2015). Recent studies indicate the high species richness within L. subg. Lactarius in the Indian Himalayan region (Sharma and Das 2003; Das and Sharma 2005; Das and Verbeken 2011; Das 2013; Das et al. 2015; Uniyal et al. 2017). A novel species within L. subg. Lactarius sect. Uvidi, collected from Himalayan temperate forest (India) is presented here with morphological details and ITS phylogeny (Fig. 155).
Phylogeny of Lactarius olivaceopallidus inferred from maximum likelihood analysis of ITS sequence dataset using RAxMLGUI 1.5 (Silvestro and Michalak 2012). Multiple sequence alignment was performed using MAFFT v.7 (Katoh and Standley 2013) with default settings. Alignment was manually edited with Bioedit v 7.2.5 (Hall 1999). To change the multiple alignment format, Alignment Transformation Environment (ALTER) was used (Glez-Peña et al. 2010). One-thousand bootstrap replicates were analyzed to obtain nodal support values. TreeGraph2 was used for visualization of the phylogram (Stöver and Müller 2010). The novel species is shown in blue and type strains are in bold. Species of sister genus Lactifluus were selected as the outgroup taxa
Lactarius olivaceopallidus Uniyal, sp. nov.
MycoBank number: MB820694; Facesoffungi number: FoF04839, Figs. 156, 157
Lactarius olivaceopallidus (CAL 1401, holotype). a, c Fresh basidiomes in field and basecamp. b Latex on gills. d Transverse section through thick pileipellis. e Hyphal arrangement in pileipellis. f, g Pleurocystidia. h Cheilocystidia and marginal cells. i Pseudocystidia. j, k SEM images of basidiospores. Scale barsa = 20 mm, d = 100 μm, e, g–i = 10 μm, f = 25 μm, j–k = 2 μm
Etymology: Due to pale olive colour of basidiomes.
Holotype: CAL 1401
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Pileus 21–88 mm diam., convex, centrally depressed, finally infundibuliform, olive yellow (3C6) at centre, greyish yellow (4C4–7) toward margin, paler at margin, surface slimy to gelatinous, viscid at centre, sticky when dry, azonate to faintly zonate near margin, pale yellow (3A3) then yellow (3A7) with 30% KOH; margin decurved to inrolled. Lamellae yellowish white (1A2), pale yellow (3A2) in age, often with greyish yellow (4B4) spots, greyish violet (15D5) on bruising, adnate to broadly adnate, close (10–17 L + l/cm), including lamellulae), some forked near stipe, lamellulae numerous. Stipe 21–45 × 10–23 mm, yellowish white (4A2) to pale yellow (4A3), spotted with light yellow (4A5) scrobiculi, stuffed to hollow. Context up to 14 mm thick in pileus, yellowish white (1A2), pale yellow (3A3) with KOH, pale yellow (3A2) in stipe, immediately turning violet (15C6) on exposure. Latex yellowish white (3A2), rather abundant, immediately turning dull lilac (15C3) on gills, context, white paper and after 2–3 minutes on glass slide. Odour pleasant. Taste acrid. Basidia 51–72 × 9.5–16 µm, subclavate, 2–4-spored, sterigmata 5–9 µm long. Basidiospores 7.5–8.7–9.5 × 7–7.7–8.5 µm, (n = 40, Q = 1.06–1.12–1.21), subglobose to broadly ellipsoid; ornamentations amyloid, 1.7–2 µm high, mostly composed of broad ridges with wavy to even margin, triangular narrow ridges with round apices and small isolated warts, forming a dense incomplete reticulum; plage distally amyloid. Pleuromacrocystidia 51–113 × 9–17.5 µm, abundant, subclavate to fusiform, mostly round to blunt and acute apices, sometimes mucronate, contents dense. Pleuropseudocystidia up to 9 µm thick, nonemergent, cylindrical with rounded nonforked apex. Lamellae edge sterile composed of cheilomacrocystidia and marginal cells. Cheilomacrocystidia 34–63 × 8.5–12 µm, broadly fusiform, round to blunt apex. Marginal cells 13–23 × 4–6 µm, cylindric to subfusiform with rounded apex. Hymenophoral trama mainly composed of sphaerocytes and abundant lactifers, lactifers up to 14 µm wide. Pileipellis an ixocutis, 300–630 µm thick, composed of densely packed septate hyphae, often shriveled, forming bundles in outer parts, 1–4 µm thick. Lactifers in pilear trama up to 11 µm wide. Stipitipellis an ixocutis, 65–102 µm thick, hyphae 1–4 µm wide, septate, not shriveled.
Material examined: INDIA, Uttarakhand, Pauri Garhwal, Chaubatta, alt 1904 m, N30° 09.681′ E78° 51.222′, 19 July 2016, P. Uniyal, PU-1110 (CAL 1401, holotype); ibid., Pauri Garhwal, Chaubatta, 16 July 2016, P. Uniyal, PU 15-1090.
Host and habitat: Gregarious, under Quercus leucotrichophora A. Camus in temperate mixed forest.
GenBank numbers: ITS = KY440363 (CAL1401), ITS = MF741705 (PU 15-1090).
Notes: Lactarius olivaceopallidus differs from other members of Lactarius sect. Uvidi due to presence of pale olive basidiomes, abundant yellowish white latex, very high (≤ 2 µm) ornamentations of basidiospores and very thick (≤ 630 µm) ixocutis type of pileipellis. The combination of characteristics like viscid to slimy, azonate to slightly zonate pileus, ixocutis pattern of pileipellis and presence of white latex turning dull lilac on exposure undoubtedly place the present taxon in Lactarius sect. Uvidi.
In the field, Lactarius olivaceopallidus can easily be recognized by its pale olive basidiome, slimy to gelatinous pilear surface, pale yellow lamellae turning lilac when damaged, scrobiculate stipe surface and yellowish white latex that immediately changes into lilac on gills. Microscopically, very thick pileipellis (≤ 630 µm) and basidiospores with very high prominences (up to 2 µm) in form of a dense incomplete reticulum are also distinct.
Studies have suggested that poor molecular resolution in Lactarius sect. Uvidi has been a problem, as species in this group are dissimilar but genetically very close (Das et al. 2015; Barge et al. 2016). Species of L. sect. Uvidi in present study are well-separated (Fig. 155) from species belonging to other sections of L. subg. Lactarius. Two collections of the present species L. olivaceopallidus (CAL1401 and PU 15-1090) are distinctly separated in a relatively long branch from its closely related species. Morphologically, L. olivaceopallidus can be confused with L. uvidus (Fr.) Fr. (GenBank no. KJ742416, KJ742417; Fig. 155), L. violascens (J. Otto) Fr. and L. aspideus (Fr.) Fr. But, L. uvidus (originally described from Europe) differs in having greyish pink to light vinaceous grey pileus, cream to pale pinkish buff lamellae, larger basidiospores (8.4–11.3 × 6.6–8.5 µm), with shorter ornamentations (up to 0.7 µm) in form of rounded warts and obtuse broad ridges forming an incomplete reticulum. Lactarius violascens (originally reported from Europe) shares similarity in occurrence under deciduous trees but quite different due to greyish buff to brownish violet, azonate or zonate pileus, extracellular pigmentation in pileipellis appearing as dark brown granules and larger basidiospores (7.6 –11.3 × 6.4–8.5 µm) (Heilmann-Clausen et al. 1998). Like L. olivaceopallidus, a pale basidiome is also present in L. aspideus (originally reported from Europe) which differs from L. olivaceopallidus due to smaller (10–70 mm in diam.) pileus, non-scrobiculate stipe, unchanging latex on isolation from flesh, shorter ornamentations (up to 0.5 µm) in basidiospores, zebroid pattern forming complete to nearly complete reticulum and very thin (40–70 µm) pileipellis (Heilmann-Clausen et al. 1998).
Lactarius salicis-reticulatae Kühner (GenBank no. KR090958, KR090959; Fig. 155) is another lilac staining species which is also phylogenetically close (94% similarity for 93–96% query coverage using BLAST) to the present novel species, however, it has subdistant to distant lamellae, mild tasting watery white latex, association with Salix and larger basidiospores (8.5–11.5 × 8–10 μm) (Barge and Cripps 2016).
Genetically, Lactarius repraesentaneus Britzelm. (GenBank no. KR090950, KR090954, KR090948, KR090953, JF908312 and KR090952; Fig. 155) is the closest relative (only 95–96% similarity for 93–96% query coverage using BLAST) to L. olivaceopallidus, but morphology of the two species is very different. Lactarius repraesentaneus (originally reported from Europe) has orange-yellow to honey pileus and conspicuously bearded pileus margin (hairs up to 5 mm long). Moreover, L. repraesentaneus is reported growing in ecological association with Picea, Betula and Salix in temperate to arctic-alpine areas of North America and Europe. Microscopically, it has longer basidiospores (8–10.5 µm) with shorter (≤ 1 µm high) ridges and ixotrichoderm type of pileipellis. Phylogenetic analyses have suggested 99.4% similarity of L. repraesentaneus with L. dryadophilus Kühner (GenBank no. KR090901), which is very similar to L. repraesentaneus (Barge et al. 2016). Lactarius dryadophilus (originally reported from Europe) is also genetically close to L. olivaceopallidus (95% similarity for 94% query coverage using BLAST) but quite different due to white to pale yellow pileus with bearded margin and larger basidiospores (9.3–11.8 × 7.2–9.2 μm) having very low (≤ 0.3 µm high) ornamentations (Heilmann-Clausen et al. 1998; Barge et al. 2016).
In Asia, Lactarius sect. Uvidi is represented by L. formosus H.T. Le & A.Verbeken and L. pyriodorus K. Das & Verbeken. Lactarius formosus (originally reported from Thailand) is distinct from the present taxon by possessing greyish yellow to yellowish buff, zonate, scaly pileus with bundles of glutinous hairs and cutis to trichoderm type of thick (120–140 µm) pileipellis (Le et al. 2007). Lactarius pyriodorus K. Das & Verbeken (originally reported from Indian Himalaya) cannot be mistaken in the field for L. olivaceopallidus as the former displays brown vinaceous pileus, watery white latex, distinctive pear-like fruity odour and association with Abies. Microscopically, L. pyriodorus has larger basidiospores (8–8.9–10.5 × 6.9–7.5–8.8 μm), with lower (≤ 1.1 μm high) prominences and thinner (≤ 320 μm) pileipellis (Das et al. 2015).
Lactifluus (Pers.) Roussel
We follow the latest treatment and updated account of Lactifluus in De Crop et al. (2017) and Hyde et al. (2017). The new species Lactifluus midnapurensis is introduced in Lactifluus subgen. Lactifluus (Burl.) Hesler & A.H. Sm (Fig. 158).
Phylogram generated from maximum likelihood (− InL = 10343.610778) (RAxML) analysis using a GTR + I + G model of nucleotide evolution based on ITS sequence dataset for 47 Lactifluus and six outgroup sequences (Lactarius scrobiculatus, L. olympianus, L. hatsudak, L. miniatescens, L. fuliginosus, L. tenellus). Maximum likelihood bootstrap support values (left) greater than 50% and Bayesian posterior probabilities (right) greater than 0.50 BYPP are indicated above the nodes. Sequences used in this study mostly have been sampled from a previous study (Stubbe et al. 2010, 2012a, b). The newly described taxon is indicated in blue and ex-type strains are indicated in bold. Classification of the subgenus and sections follows De Crop et al. (2017)
Lactifluus midnapurensis S. Paloi & K. Acharya, sp. nov.
MycoBank number: MB820587; Facesoffungi number: FoF03248, Figs. 159, 160
Etymology: The specific epithet refers to the type locality, Purba Midnapur (India).
Holotype: CAL 1516
Colour codes follow: Methuen handbook of colour (Kornerup and Wanscher 1978).
Pileus 11–48 mm diam., convex to broadly convex with an umbo when young, becoming plain to infundibuliform or applanate with a slight umbo toward centre at maturity, semi-moist to dry when young, dry and shiny at maturity, smooth, becoming slightly wavy and translucent with maturity, margin incurved at young stage, brown (5E4; 6E4) to yellowish brown (5E5) toward centre, brownish grey (5C2; 6D2) at early stage, becoming finally white (1A1) toward margin at maturity, no colour change on bruising, turns orange to reddish orange with guaiacol, negative in phenol, SV and KOH; context 1 mm toward centre, < 1 mm toward margin, white (1A1), no colour change on exposed in air. Partial veil present at the young stage, white (1A1), but disappear when mature. Lamellae ca. 3 mm broad, decurrent, distant, white (1A1), negative in phenol and KOH; edge even, regular, concolorous; lamellae two to three of length, forked toward margin. Stipe 8–43 × 2–5 mm, central when young, becoming central or excentric when mature, semi-moist at early stage, becoming dry and shiny when mature, smooth, grey (4C1) to golden grey (4C2) or brownish grey (4D2) when young, becoming white (1A1) at maturity, turns light yellowish brown on bruising at early stage but no change when mature, orange to orange-red with guaiacol, negative with KOH, phenol and SV; context hollow, white (1A1), no colour change on exposed in air. Latex water-like (transparent), no colour change up to 5 minutes in exposed on air. Odour unknown. Taste mild. Spore print white. Lamellar trama mainly composed of irregularly arranged sphaerocytes, hyphae and lactiflus hyphae. Basidia (43–)46.5–51.5–53.7(–57.2) × (7.1–)8–8.9–10(–11) µm, clavate to subclavate, hyaline, thin-walled, oil droplets present when viewed KOH, 4-spored; sterigmata 3.6–7.2 × 1–2.7 µm. Basidiospores 5.5–6.5–7.1(–8) × (5–)6–6.6–6.9(–7.5) µm, (n = 30) Q = 1.02–1.07–1.12, globose to subglobose, ornamentation amyloid, composed of short (0.3–0.6 µm) and long warts (1.5–2.0 µm), interconnected by a ridge line, forming a complete reticulum, sometimes connected line are shown wing-like pattern in side view, suprahilar spot amyloid or not. Lamellae edge sterile mainly composed of numerous cheilocystidia. Cheilocystidia 27–48.5 × 4.5–7.5 µm, subclavate to subcylindrical with mostly capitate apex, hyaline, thin-walled, oil droplet present when viewed with KOH. Pleuromacrocystidia 43–61 × 7.5–11 µm, rare toward cap margin but abundant toward cap centre, mostly appendiculate or mucronate, hyaline, thin-walled. Pleuropseudocystidia 2–4.5 µm in diam., abundant, cylindrical to subcylindrical with capitate or obtuse apex, dense with cytoplasmic component. Pileipellis a palisade, context made up with unequal, irregular shaped sphaerocytes and loosely arranged hyphal cells, branched, septet; distantly 2-layers, 30.6–46.2 µm deep, subpellis non-gelatinous, composed of tightly arranged globose to subglobose or irregular shaped cell, 11.6–19.7 × 10.7–17.9 µm, thin-walled, 2–4-celled deep; suprapellis composed of erect to sub-erect hyphal cell, 14.3–39.4 × 3.6–7.1 µm, hyaline, thin-walled, 2–3-celled, underling attached with the subpellis region of globose cells, non-gelatinized. Stipitipellis a palisade, 52.2–85.6 µm deep, upper layer composed of thin-walled, septet (up to 4 times) hyphae, 4.5–7.5 µm in diam., hyphal end subulate or obtuse, underling 2–3 globose like cells.
Material examined: INDIA, West Bengal, Purba Midnapur, Kasafaltalya, 21°43′0.552′’ N, 87°30′26.6472″E, S. Paloi & K. Acharya, 23 August 2015, SOUMITRA-22 (CAL 1516, holotype); ibid., 21°43′6.222′’N, 87°30′7.9524′’E, S. Paloi & A.K. Dutta, 27 August 2015, SOUMITRA-40 (CAL 1523).
Habit and habitat: Common, solitary or gregarious, growing under Shorea robusta C.F. Gaertn. tree (Dipterocarpaceae).
GenBank numbers: ITS = KY785175, LSU = KY785177 (CAL 1516, holotype); ITS = KY785176, LSU = KY785178 (CAL 1523).
Notes: The diagnostic features of the newly described species includes small to medium sized (11–48 mm in diam.), convex to infundibuliform or applanate pileus, brownish grey to white that turns reddish orange with guaiacol; distant lamellae, white; slightly excentric stipe; watery latex (transparent); white spore print; mild taste; globose to subglobose basidiospores with a mean size of 7.1 × 6.6 µm, Qm = 1.07, ornamentation composed of warts (up to 2 µm), interconnected with each other forming complete reticulate; pileipellis a palisade type. This combination of characteristics undoubtedly place Lactifluus midnapurensis under subg. Lactifluus sect. Gerardii (Stubbe et al. 2010; De Crop et al. 2017).
Lactifluus midnapurensis appears to be related to L. coniculus (Stubbe & Verbeken) Verbeken, described from Sri Lanka growing in association with Shorea sp. However, L. coniculus differs by cigar brown to snuff brown pileus, solid stipe context, comparatively larger basidiospores (7.1–10.2 × 6.4–8.8 µm) with ornamentation like interconnected conical to triangular, round to acute warts and absence of pleuromacrocystidia (Stubbe et al. 2012b).
Within the same section, among taxa with similar sized basidiospores, colouration of the latex and growth in association to Dipterocarpus plants, Lactifluus limbatus (Stubbe & Verbeken) Stubbe (described from Malaysia) is a phylogenetically close taxon (Fig. 158). Lactifluus limbatus has a larger pileus (30–60 mm), brownish grey towards centre and grey towards margin, greyish brown lamellar edge, spore ornamentation up to 0.8 µm high and absence of pleuromacrocystidia (Stubbe et al. 2012a, b). Lactarius atrovelutinus J.Z. Ying frequently encountered from China and Malaysia, has a dark black-brown pileus, cream (young) to yellow (mature) lamellae, latex that turns pink or brownish pink on drying, presence of fruity or sweet odour, absence of pleuromacrocystidia and very thick pileipellis (80–100 µm; Ying 1991). Lactarius conchatulus Stubbe & Verbeken, described from northern Thailand (alt. 1300 m), has smaller pileus (2–7 mm diam.) that turns pale yellow on bruising, subdecurrent lamellae, and broadly ellipsoid basidiospores (Q = 1.08–1.35) with up to 0.9 µm long ornamentation (Stubbe et al. 2012a, b).
Being a well representative member of the sect. Gerardii; Lactifluus bicolor (Massee) Verbeken, distributed in Malaysia and Singapore, differs from L. midnapurensis in having a larger pileus (20–80 mm diam.), dark brown or fuliginous, longer stipe (10–80 mm), and larger basidiospores (7.5–9.6 × 6.4–8.6 µm) with a ornamentation up to 0.3–0.5 µm high (Massee 1914; Stubbe et al. 2012a, b). A taxon with similar pileus colouration, L. leae Stubbe & Verbeken differs by its very larger pileus (35–120 mm in diam.), somewhat differently sized basidiospores (6.6–12.1 × 5.9–9.1 µm), ornamentation up to 0.5–1 µm high and absence of macrocystidia (Stubbe et al. 2012a, b).
Russula Pers.
Russula is species-rich and one of the most thoroughly monographed genera of fungi that is mainly ectomycorrhizal with a diverse range of plants in deciduous or evergreen, broadleaf or coniferous woods, scrubland and even meadows from tropical to subalpine areas and has a cosmopolitan distribution (Knudsen and Borgen 1982; Singer 1986; Buyck et al. 1996; Rawla 2001; Das and Sharma 2005; Das et al. 2006a, b, Das et al. 2010, Das et al. 2013, Das et al. 2014, 2017a, b; Miller et al. 2012; Li et al. 2013, 2015; Hyde et al. 2016; Ghosh et al. 2016, 2017; Ghosh and Das 2017). Species within this genus are known by the combination of their conspicuous and fleshy basidiomes, colourful fragile pileus, amyloid warty basidiospores, abundant sphaerocytes in a heteromerous trama that can make these fungi brittle, absence of latex and the hyphae that lack clamp connections (Romagnesi 1967; Singer 1986; Sarnari 1998, 2005). Russula has 10 subgenera namely: R. subg. Compactae, R. subg. Heterophyllidia, R. subg. Ingratula, R. subg. Amoenula, R. subg. Incrustatula and R. subg. Russula, R. subg. Archaea, R. subg. Brevipes, R. subg. Malodora and R. subg. Crassotunicata Buyck & V. Hofstetter (Sarnari 1998; Hongsanan et al. 2015; Das et al. 2017b). Here, two novel species (belonging to subg. Incrustatula and Russula) collected from the northwestern part of Indian Himalaya are described with morphological details and phylogenetic placement (Figs. 161, 162).
The ITS sequence of the newly generated Russula species (Russula choptae) plus those acquired from GenBank, UNITE database and relevant literature, were aligned with the help of AliView (Larsson 2014) using default settings. Sequences of ITS were phylogenetically analyzed using Bayesian inference analysis (BI). Bayesian inference was computed independently twice in MrBayes v.3.2.2 (Ronquist et al. 2012). The best-fit substitution model of nucleotide evolution (TIMef) was carried out in MrModeltest 3.7 (Posada and Crandall 1998). Posterior probabilities (PP) were calculated in two simultaneous runs with Markov chain Monte Carlo (MCMC) algorithm (Larget and Simon 1999). Markov chains were run for 1000000 generations, saving a tree every 100th generation. Default settings in MrBayes were used for the incremental heating scheme for the chains (3 heated and 1 cold chain), unconstrained branch length [unconstrained: exponential (10.0)] and uninformative topology (uniform) priors. The analysis was terminated when the average standard deviation of split frequencies fell below 0.01. The first 25% of trees were discarded as burn-in (Hall 2004). The convergence of runs was visually assessed using Trace function in Tracer version 1.6 (Rambaut et al. 2013). The novel species having GenBank no. MG897815 and MG925207 (ITS) are shown in blue and bold on the tree and ex-type strains are in bold. Russula pseudoamoenicolor and R. violeipes are considered as the outgroup taxa
The ITS sequence of the newly generated Russula uttarakhandia plus those acquired from GenBank, UNITE database and relevant literature, were aligned with the help of AliView (Larsson 2014) using default settings. Sequences of ITS were phylogenetically analyzed using Bayesian inference analysis (BI). Bayesian inference was computed independently twice in MrBayes v.3.2.2 (Ronquist et al. 2012). The best-fit substitution model of nucleotide evolution (TIMef) was carried out in MrModeltest 3.7 (Posada and Crandall 1998). Posterior probabilities (PP) were calculated in two simultaneous runs with Markov chain Monte Carlo (MCMC) algorithm (Larget and Simon 1999). Markov chains were run for 1000000 generations, saving a tree every 100th generation. Default settings in MrBayes were used for the incremental heating scheme for the chains (3 heated and 1 cold chain), unconstrained branch length [unconstrained: exponential (10.0)] and uninformative topology (uniform) priors. The analysis was terminated when the average standard deviation of split frequencies fell below 0.01. The first 25% of trees were discarded as burn-in (Hall 2004). The convergence of runs was visually assessed using Trace function in Tracer version 1.6 (Rambaut et al. 2013). The novel species having GenBank no. KY873997 and MF684758 (ITS-rDNA) are shown in blue and ex-type strains are in bold. Russula fellea, R. sanguinea and R. persicina are considered as the outgroup taxa
Russula choptae A. Ghosh & K. Das, sp.nov.
MycoBank number: MB824343; Facesoffungi number: FoF04953, Figs. 163, 164
Russula choptae (AG 16–1186, holotype). a–e Fresh and dissected basidiomes in the field. f, g Radial section through pileipellis showing elements. h Image of basidiospores under SEM. i–k Transverse section through lamellae showing pleurocystidia. l Transverse section through lamellae showing cheilocystidia. Scale barsb = 55 mm, f = 25 µm, g = 10 µm, h = 2 µm, i = 25 µm, j–l = 10 µm
Etymology: Referring to Chopta in Uttarakhand (India), the type locality.
Holotype: CAL 1658
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Basidiomes 34–112 mm in height, small to medium sized. Pileus 6–48 mm in diam., hemispheric when young, convex, plano-convex, expanding to applanate when mature, centre slightly depressed with age, finely wrinkled and areolate at maturity; margin decurved to plane with maturity, entire, slightly tuberculately striate, dry (viscid when moist), peeling 1/4 from the edge, brownish red (9C6–8) at young stage, pale red (9–10A3), pastel red (9–10A4–5) to madder red (9A6–7), intermixed with pink, orange to yellowish tinge of coral red (9B7) at the centre, pinkish white (9A2), pale red to pastel red (9A3–4) towards the margin; pale yellow to pastel yellow (2A3–4) with KOH. Pileus context greyish white (1B1), 2–4 mm thick, unchanging after bruising, turning reddish brown (8D6–7) with guaiacol. Lamellae adnexed to subdecurrent, crowded (10–16/cm), chalky white to yellowish white (1A1–2) with maturity, up to 3 mm thick, narrowing towards the margin, forked near the stipe apex, middle of lamellae and near cap margin, entire, edges concolorous, unchanging when bruised. Lamellulae present in different lengths (up to 5 series). Stipe 37–88 × 6–13 mm, cylindrical to subclavate, central, dry, smooth, brittle, pale red, pastel pink to pink rose (11A3–5) with white (1A1) to yellowish white (1A2) areas or orange white to pale orange (5A2–3) tinge at the base; turning reddish brown (8D6–7) with guaiacol. Stipe context stuffed to hollow, dirty white, unchanging after bruising but reddish brown (8D6–7) and greyish green (27C4–5) with guaiacol and KOH respectively. Taste acrid. Spore print yellowish white. Subhymenium layer up to 28 µm thick, pseudoparenchymatous. Basidia 30–40 × 9–14 µm, 4-spored, cylindrical, subclavate to clavate, sterigmata up to 6 µm long. Basidiospores (6.9–)7–7.4–7.9(–8) × (6–)6.1–6.6–7 µm, [n = 30, Q = (1–)1.06–1.13–1.2(–1.31)], subglobose to broadly ellipsoid, rarely globose and ellipsoid, composed of amyloid warts becoming linked as small crests and thick ridges, forming an incomplete network, intermixed with isolated warts (up to 1 μm) in height; suprahilar plage inamyloid, apiculi up to 2 µm high. Pleurocystidia (35–)45–64–80(–96) × (7–)7.5–10–12(–13) µm, abundant, cylindrical to subclavate with capitate, appendiculate, pointed, mucronate, moniliform or rounded apex, emergent up to 47 µm, completely filled with dense, finely crystalline content in congo red, turning grey-black with sulphovanillin (SV). Lamellar edge fertile with basidia and cystidia. Cheilocystidia (30–)35–45.5–56(–61) × (6–)7–9–11(–12) µm, cylindrical to subclavate with mostly rounded apex, completely filled with dense, finely crystalline content in congo red, turning grey-black with sulphovanillin (SV). Pileipellis orthochromatic in cresyl blue, a trichoderm, up to 260 μm thick, two-layered and sharply delimited from the underlying sphaerocytes of the context; distinctly divided in a 120–160 μm deep, suprapellis of erect to suberect hyphal ends and pileocystidia, containing branched, septate, hyphal endings, and underneath this suprapellis a 140–100 μm deep, subpellis of interwined, more or less horizontally oriented hyphae. Hyphal extremities near the cap margin composed of a single or a few cells only; the terminal cells subcylindrical to cylindrical, with rounded or obtuse tips, measuring (15–)18–24–30(–34) × (2.5–)3–3.5–4(–5) μm; subterminal cells subcylindrical to cylindrical; in the cap centre, terminal cells subcylindrical to cylindrical, with rounded or obtuse tips, measuring (19–)20–25–29(–35) × 3–4–4.5(–5) μm; subterminal cells cylindrical. Pileocystidia near the pileus margin aseptate, short to very long, measuring (2.5–)4–5–6(–7) μm broad, subcylindrical to cylindrical, sometimes with lateral projections, obtuse-rounded or sometimes swollen apex, almost completely filled with dense granular content in congo red, distinctly grey-black in sulfovanillin (SV), without incrustations. Pileocystidia near the pileus centre aseptate, short to very long, measuring (3)4–5–6(–8) μm broad, slightly larger, subcylindrical to cylindrical, sometimes with lateral projections, obtuse-rounded or swollen or sometimes forked apex, almost completely filled with dense granular content in congo red, distinctly grey-black in sulfovanillin (SV), without incrustations. Clamp connections and laticiferous hyphae absent from all tissues.
Material examined: INDIA, Uttarakhand, Rudraprayag District, Chopta, N30°28.997′ E79°10.298′, alt. 2632 m, under Quercus sp., 24 July 2016, A. Ghosh, AG 16-1186 (CAL, holotype; GUH, isotype); ibid., N30°28.693′ E79°11.636′, alt. 2315 m, under Quercus sp., 25 August 2016, A. Ghosh, AG 16-1363; Pabdhar, N30°29.376′ E79°09.673′, alt. 2356 m, under Quercus sp., 25 July 2016, A. Ghosh, AG 16-1206; Bageshwar District, Dhakuri, N30°04.970′ E79°55.134′, alt. 2586 m, under Quercus sp., 2 August 2016, A. Ghosh (AG 16-1269, paratype); Baniyakund, N30°28.998′ E79°10.657′, alt. 2614 m, under Quercus sp., 7 August 2017, A. Ghosh, AG 17-1529; Baniyakund, N30°29.091′ E79°10.400′, alt. 2565 m, under Quercus sp., 8 August 2017, A. Ghosh, AG 17-1549.
Host and habitat: Growing caespitose, under Quercus sp. (Fagaceae) in mixed forests dominated by Quercus, Rhododendron (Ericaceae) and Abies (Pinaceae).
GenBank numbers: ITS = MG897815 (AG 16–1186); ITS = MG925207 (AG 16–1363).
Notes: The combination of macro- and micromorphological features such as, taste being usually acrid or very acrid, epicutis containing well characterized dermatocystidia, yellowish white spore print, often pinkish or lilac stipe, mostly adnate or subdecurrent lamellae and red pilei growing in deciduous forests place Russula choptae under subg. Russula sect. Russula subsect. Sardoninae (Sarnari 1998).
In the field Russula choptae is distinct from other species of this group by its brownish red, pale red, pastel red to madder red finely wrinkled pileus with intermixed of pink, orange to yellowish tinge of coral red at the centre, long and slim stipe with yellowish white (1A2) areas or orange white to pale orange (5A2–3) tinge at the base; lamellae with bifurcate, different lengths at the apex of stipe, lamellulae with different lengths (up to 5 series); stipe cuticle and context turns reddish brown and greyish green with guaiacol and KOH, respectively, and micromorphologically it is separated from allied species by different types of cystidial apex (capitate, appendiculate, pointed, mucronate, moniliform or rounded) and basidiospores with warts that are linked as small crests and thick ridges, forming an incomplete network and intermixed with isolated warts.
Based on the BLASTn search in NCBIs GenBank nucleotide database, the closest hit using ITS sequences (AG 16-1186 and AG 16-1363) is Russula sp. (voucher HKAS 78400 GenBank no. KF002784) collected from China. Unfortunately, there is no micro- and macromorphological details available for this Chinese collection in published literature. Our phylogeny (Fig. 161) shows R. choptae as sister to this unknown Chinese Russula sp.
In subsect. Sardoninae, three species Russula persicina Krombh., R. exalbicans (Pers.) Melzer & Zvára and R. gracillima Jul. Schäff. (also appeared to be close in ITS phylogeny in Fig. 161) resemble R. choptae. However, R. persicina, originally reported from Europe, has larger pileus (40–100 mm), widely spaced rather sinuate lamellae, basidiospores, mostly with isolated warts (0.8 µm) with few crests (Galli 1996; Sarnari 1998; Kränzlin 2005; Knudsen et al. 2012). Russula exalbicans (originally reported from Europe), differs in having larger (50–100 mm diam.), rose-red, pink, vinaceous, rapidly becoming paler to almost entirely greenish white pileus usually with a trace of pink at the extreme margin, firm white stipe which soon becomes grey with age or in wet weather, frequent 0–1-septate dermatocystidia, larger basidiospores (7–10 × 5–7 µm) with low (up to 0.75 µm high) ornamentations and ochre spore print (Pearson 1950; Galli 1996; Sarnari 1998; Kränzlin 2005). Russula gracillima (also reported from Europe), differs from R. choptae in possessing moderately spaced lamellae, presence of abundant, broad (5–10 µm) dermatocystidia and basidiospores with isolated blunt warts (Rayner 1985; Galli 1996; Sarnari 1998; Kränzlin 2005; Knudsen et al. 2012; Kibby 2014). Russula renidens Ruots. et al., another taxon in subsect. Sardoninae and originally reported from Europe, has bright crimson red, blood-red to copper red larger pileus (35–80(–100) mm), sulcate margin with age, some intervening lamellae, cylindric to clavate stipe (40–100 × 10–22 mm), basidiospores with low ornamentation (up to 0.5 µm) consisting of small warts with some clusters or ridges, 0–2-septate and slender to clavate dermatocystidia (Galli 1996; Sarnari 1998; Knudsen et al. 2012).
Five more taxa of this subsection, Russula sanguinea Fr., R. queletii Fr., R. sardonia Fr., R. torulosa Bres. and R. thindii K. Das & S.L. Mill. also differ from R. choptae. Russula sanguinea, originally reported from Europe, has a coniferous habitat especially under pines and elliptical larger basidiospores (7–9 × 6–7 µm), with ornamentation consisting mostly of solitary warts which are somewhat elongated (only a few with connections) (Rayner 1985; Sarnari 1998; Kränzlin 2005; Knudsen et al. 2012). Russula queletii (originally reported from Europe) can be distinguished by presence of a combination of characteristics i.e. vinaceous red to violet pileus and stipe, strong fruity odour and changing of pilear context to light vinaceous red with FeSO4 (Rayner 1985; Saini and Atri 1989; Sarnari 1998; Kränzlin 2005; Knudsen et al. 2012). Similarly, R. sardonia, originally reported from Europe, differs in having dark violaceous purple, reddish purple or livid vinaceous pileus, sometimes with greyish olivaceous or with yellowish patches; solid and firm stipe; slightly larger basidiospores (7–9 × 6–7 μm) and is mycorrhizal with Pinus on acid soil (Sarnari 1998; Knudsen et al. 2012). Russula torulosa (originally reported from Europe) possess purple-violet, purple-red, violet-red or dark red pileus sometimes with lighter ochraceous or olivaceous spots, often blackish at centre; solid and firm stipe; larger basidiospores (7.2–9.5 × 5.6–7.3 µm) with low ornamentations (0.4–0.6 µm) and is found mostly under Pinus sp. (Sarnari 1998; Knudsen et al. 2012). Russula thindii (originally reported from India) is distinct by its larger pileus (38–77 mm diam.), pale yellow spore print and larger basidiospores (7.5–9–10 × 6–7–8 μm), with ornamentation composed mostly of isolated conical to spinoid warts being occasionally connected by fine connectors (Das et al. 2014).
In Eastern Asia, Russula choptae might be confused with R. chiui G.J. Li & H.A.Wen, R. zhejiangensis G.J. Li & H.A. Wen and R. minutula var. minor Bi (originally described from China). However, R. chiui differs in having brightly red tinged pileus, white stipe, septate pileocystidia, densely reticulate larger basidiospores [(8–)8.5–10(–10.5) × 7–8(–8.5) µm] and sterile lamellar edge (Li et al. 2015). Russula zhejiangensis has a purely white stipe, isolated conical to obtuse warts in basidiospores, shorter pleurocystidia (35–74 × 6–11 µm) with mostly rounded or mucronate apex, sterile lamellar edge and 0–3-septate pileocystidia (Li et al. 2011). Russula minutula var. minor, described in Guangdong Province of South China, differs by its white to yellowish gills, white stipe, isolated spinoid basidiospores and shorter pleurocystidia (Bi and Li 1986; Li et al. 2011).
Russula uttarakhandia A. Ghosh & K. Das, sp. nov.
MycoBank number: MB820834; Facesoffungi number: FoF04954, Figs. 165, 166
Russula uttarakhandia (CAL 1537, holotype). a–d Fresh basidiomes in the field. e, f Radial section through pileipellis showing elements. g Basidia. h Basidiospores. i SEM image of basidiospores. j–m Transverse section through lamellae showing pleurocystidia. Scale barsa = 80 mm, e–h = 10 µm, i = 2 µm, j–m = 10 µm
Etymology: Referring to the name of an Indian state, Uttarakhand from where the species was collected.
Holotype: CAL 1537
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Basidiomes 70–90 mm high. Pileus 45–60 mm diam., planoconvex to applanate with slightly depressed centre, margin decurved to plane with maturity, entire, tuberculately striate, surface dry, viscid when moist, gelatinous, glossy, pinkish white or reddish white (10A2) to venetian pink (10A3) and centrally olive yellow (2B6–7) to greyish yellow (2A4–6), discoloring to colourless with KOH, cuticle peeling 1/3 of the radius, chalky white (1–2A1) beneath the cuticle; pileus context chalky white (1–2A1), unchanging on bruising. Lamellae adnate to adnexed, close to rather crowded (7–10 cm at pileus margin), chalky white (1A1), entire, with concolorous edges, unchanging when bruised. Lamellulae present, in 2 series. Stipe 55–70 × 10–12 mm, cylindric, central, dry, smooth, longitudinally striate, brittle, chalky white (1–2A1) turning pink or red rose (11A3–5) with guaiacol; stipe context hollow, chalky white (1–2A1), unchanging when bruised but pink or red rose (11A3–5) sometimes after the applications of guaiacol. Odour indistinctive. Taste mild. Spore print yellowish white (2A2). Hymenophoral trama mainly composed of large nests of sphaerocytes and few hyphal elements. Subhymenium layer up to 15 µm thick, pseudoparenchymatous. Basidia (26–)28.5–33–37(–45) × (9–)12–14–15.6 µm, 4-spored, clavate, sterigmata up to 6 µm long. Basidiospores (6–)6.5–7–8(–9) × (5–)5.5–6–6.8(–7) µm, [n = 40, Q = (1–)1.1–1.2(–1.3)], broadly ellipsoid to subglobose, rarely globose and ellipsoid; ornamentation amyloid, composed of isolated spines (up to 1.1 µm high), suprahilar plage amyloid; apiculus up to 2 µm high. Pleurocystidia (48–)50–59–67(–71) × 7–10–12(–14) µm, cylindrical, subclavate to clavate with capitate, rounded, moniliform, appendiculate (appendages up to 23 µm long) or blunt apex; emergent up to 35 µm, partly filled with heteromorphous, mostly thick coarsely crystalline content in congo red, sulphovanillin (SV) negative. Lamellae edge fertile with basidia. Cheilocystidia not found. Pileipellis up to 65 μm thick, metachromatic in cresyl blue, not sharply delimited from the underlying spherocytes of the context, an ixotrichoderm type, distinctly divided in a 15–18 μm deep and gelatinized suprapellis of erect or ascending hyphal ends and primordial hyphae, gelatinous layer containing branched, septate, hyphal endings, and underneath this suprapellis, 50–47 μm deep, subpellis of interwined, compact, more or less horizontally oriented hyphae. Hyphal extremities near the cap margin composed of a single or a few cells only; the terminal cells subcylindrical, subclavate to clavate with tapered base, measuring (22–)25–31–37(–41) × (3–)4–5–6(–7) μm, with rounded or obtuse tips, subterminal cells cylindrical; in the cap centre, terminal cells slightly shorter in length, subcylindrical, subclavate to clavate with tapered base, measuring (20–)21–26–30(–38) × (3–)4–5–6(–7) μm, with rounded or obtuse tips; subterminal cells cylindrical. Primordial hyphae comparatively longer than the other extremities and often protruding or repent on the cap surface, occurring mostly singly, composed of 5–7–9(–10) μm broad, cylindrical cells, obtuse apex, thick-walled (up to 1 μm thick); incrustations present. Clamp connections and laticiferous hyphae absent from all tissues.
Material examined: INDIA, Uttarakhand, Rudraprayag District, Baniyakund, alt 2634 m, N30°28.914′ E79°10.854′, 17 July 2015, A. Ghosh, AG 15-670 (CAL 1537, holotype); ibid., alt 2560 m, N30°29207′ E79°10.258′, 24 July 2016, A. Ghosh, (AG 16-1190 paratype).
Habitat and distribution: Under Quercus sp. in mixed forests dominated by Quercus, Rhododendron and Abies.
GenBank numbers: ITS = KY873997 (CAL 1537); ITS = MF684758 (AG 16-1190).
Notes: The combination of macro- and micromorphological features such as: epicutis with primordial hyphae, absence of dermatocystidia, mild taste, yellow to ochre spore print, nonpruinose cap and epicutis with clavate hyphal ends undoubtedly place Russula uttarakhandia under subg. Incrustatula, sect. Amethystinae subsect. Chamaeleontinae (Sarnari 1998). In the field R. uttarakhandia is distinct from other known species of this group by its pinkish white or reddish white to venetian pink and centrally olive yellow to greyish yellow gelatinous, glossy pileus; stipe that turns pink or red rose after sometime with application of guaiacol, pale yellowish cream spore print. Micromorphologically, it is separated from allied species by the combination of various shapes of cystidial apex (distinctively appendiculate cystidia with long appendages up to 23 µm long).
Based on the BLASTn search in NCBIs GenBank nucleotide database, the closest hit using ITS sequences of Russula uttarakhandia (collection numbers AG 15-670 and AG 16-1190) is Russula sp. (voucher HMAS 277786 collected from China, GenBank no. LT602960), showing 96% similarity with 98% query coverage. Unfortunately, there is no morphological details available for this Chinese collection in any published literature. Our phylogeny (Fig. 162) clearly shows that R. uttarakhandia is genetically closer and sister to unknown Chinese Russula sp. (LT602960).
In subsection Chamaeleontinae, Russula postiana Romell and R. risigallina (Batsch) Sacc. also appeared to be close in ITS phylogeny with R. uttarakhandia (Fig. 162). But, R. postiana (originally reported from Europe), differs from R. uttarakhandia by its herbage green to yellowish green, smooth, shining pileus; bright ochre yellow lamellae, heavily encrusted primordial hyphae, slightly larger basidiospores (8–10.2 × 7–8 µm) and its occurrence under coniferous trees (Galli 1996; Kibby 2014). Russula risigallina (also originally reported from Europe), differs from R. uttarakhandia by its red or orange pileus often with paler yellow centre (sometimes entirely yellowish opaque), strongly intervened egg yellow to almost orange lamellae and deep yellow spore print (Galli 1996; Sarnari 2005; Kibby 2014). Another taxon of this subsect., R. lutea (Huds.) Gray (also appeared in the BLASTn search), originally reported from Europe, separated from R. uttarakhandia by its entirely yellow, apricot or coral pileus, deep ochre gills and deep ochraceous spore print (Rayner 1985; Kibby and Fatto 1990).
Considering the cap colouration, R. uttarakhandia may also be confused in the field with R. gracillima Jul. Schäff., R. cremeirubra Murrill, R. robertii J. Blum, R. nitida (Pers.) Fr. and R. decipiens (Singer) Svrcek. However, R. gracillima (originally described from Europe) possess moderately spaced lamellae, a little acrid taste, presence of abundant broad dermatocystidia (5–10 µm) and basidiospores with isolated blunt warts (Rayner 1985; Galli 1996; Sarnari 1998; Chou and Wang 2005; Kibby 2014). Russula cremeirubra (originally reported from Florida) has rather distant straw coloured lamellae, basidiospores with amyloid warts connected by fine lines or connectors which combine to form few meshes and broken reticulum, pilear hyphae (2–6 µm broad) with acute to rounded apex (Murrill 1945; Hesler 1960; Kibby and Fatto 1990; Fatto 1998). Russula robertii (originally described from Europe) has 1–2-septate, slender dermatacystidia (3–5 µm broad) and spores with many crests and connectives forming partial to complete reticulum (Sarnari 2005; Kibby 2014). Russula nitida (originally reported from Europe) has pale straw-yellow gills, sometimes with reddish edge near the cap margin, larger basidiospores (8–10.5(–12) × 6.5–8(–9) µm) with rather sharp spines (up to 1.2 µm high), isolated or variously connected, and abundant dermatocystidia with 1–3 septation (Rayner 1985; Galli 1996; Sarnari 2005). Russula decipiens (belonging to subg. Russula), originally reported from Europe has slightly infundibuliform pileus, yellowish white stipe that slightly becomes grey on bruising, acrid taste and presence of aseptate, subclavate to clavate dermatocystidia (Rayner 1985; Sarnari 1998).
In field, Russula uttarakhandia may be confused with R. rajendrae A. Ghosh & K. Das (originally described from India and also collected from Uttarakhand, Rudraprayag District, Baniyakund), but the latter possess an acrid taste, numerous pileocystidia in cuticle and belongs to subg. Russula (Ghosh and Das 2017).
Considering the acystidiate aspect of pileipellis and mild taste (key characters of subg. Incrustatula), Russula uttarakhandia is also similar to other taxa of subg. Incrustatula reported from India viz. R. sharmae K. Das et al., R. dafianus K. Das & J.R. Sharma, R. dhakuriana K. Das et al., R. hookeri S. Paloi et al. and R. kewzingensis K. Das et al. However, R. sharmae (reported for the first time from West District of Sikkim) possess red to scarlet with yellow blotched larger pileus (70–140 mm), absence of lamellulae, smaller basidiospores (6.8–7.3–7.9 × 6–6.6–7 µm) and palisade to trichopalisade type of pileipellis (Das et al. 2013). Russula dafianus (reported from Dafia Dhura, Uttarakhand) has smaller basidiomes (35–45 mm), yellowish white to pale yellow lamellae and encrusted pilear hyphae (Das and Sharma 2005). Russula dhakuriana (reported from Dhakuri, Uttarakhand) has more robust basidiomes (pileus 80–120 mm diam., stipe 50–125 × 20–28 mm), longer basidiospores (6.5–10.2 × 6–7.7 μm) and pleurocystidia (60–115 × 7–12 μm) and cellular nature to the subpellis (Das et al. 2006a). Russula hookeri (reported from Darjeeling Hill, Eastern Himalaya) has small to medium basidiomes (20–35 mm), greyish red to greyish rose pileus with brownish red centre, concolorous stipe; smaller basidiospores (5.4–7.2 × 4.5–6.4 µm) and white spore print (Paloi et al. 2015). Russula kewzingensis (reported from Kewzing, Sikkim Himalaya) has deep and intense red pileus, red-flushed stipe and pseudoparenchymatous nature of subpellis (Das et al. 2017b).
Stereaceae Pilát
The family Stereaceae was proposed by Pilát (1930) with Stereum Hill ex Pers. as generic type. This family is one of the most widespread and diverse in the Russulales, together with Russulaceae. It includes species with basidiome appressed, effuse-reflexed or discoid, rarely stalked; pileus often zoned; monomitic or dimitic hyphal system; hymenophore smooth to tuberculate; basidia and spores hyaline, smooth, amyloid or non-amyloid. The family comprises species that grow in exposed positions, such as dead branches still attached to hardwood or conifer trees, causing white rot (Miller et al. 2006; Larsson 2007).
Aleurodiscus Rabenh. ex J. Schröt.
Aleurodiscus is one of the largest genera in Stereaceae. It was proposed by Rabenhorst (1874) without diagnosis, and validated by Schröter in (1888), with Peziza amorpha Pers. (≡ Aleurodiscus amorphus (Pers.) J. Schröt.) as type species. Pilát (1926) included Aleurodiscus corticioid fungi with large basidia and large spores, as well as variable sterile elements and this concept was largely accepted (Bourdot and Galzin 1912, 1928; Burt 1918). Lemke (1964a, b) considered the genus to be artificial and proposed to exclude species with inamyloid spores, and described the following new genera: Aleurocystidiellum Lemke, Aleurocorticium Lemke and Licrostroma Lemke.
On the basis of morphological and molecular data, Wu et al. (2000) described Acanthofungus Sheng H. Wu et al. with Acanthofungus rimosus Sheng H. Wu et al. as type species, and Neoaleurodiscus Sheng H. Wu with Neoaleurodiscus fujii Sheng H. Wu as type species. From a phylogenetic perspective, Wu et al. (2001) analyzed the limits of Aleurodiscus sensu lato and the monophyly of previously segregated genera, concluding that Aleurocystidiellum and Acanthobasidium are monophyletic. However, subsequent studies (Larsson and Larsson 2003; Miller et al. 2006; Larsson 2007) have shown that Aleurocystidiellum does not belong to Stereaceae. Wu et al. (2001) set the boundaries of Aleurodiscussensu stricto and confirmed that Aleurodiscus sensu lato is paraphyletic.
According to Wu et al. (2001), Aleurodiscus sensu lato includes species with highly variable characteristics; these are not congruent and usually overlap, so the species in Aleurodiscus sensu lato show all possible combinations of spore surface from smooth or ornamented, presence or absence of acanthophyses, and hyphae with clamps or simple-septate. The novel species, Aleurodiscus patagonicus is introduced following the treatment in Wu et al. (2001), Dai and He (2016), and Dai et al. (2017b, c).
Aleurodiscus patagonicus Nogal, Telleria, M. Dueñas & M.P. Martín, sp. nov.
MycoBank number: MB823981; Facesoffungi number: FoF05726, Figs. 167, 168
Aleurodiscus patagonicus (19609Tell., MA-Fungi 90714, holotype). a Basidiome. b Section of basidiome under SEM; hymenial layer (1), basal layer (2). c Probasidia with basal clamp. d Spores under SEM. e, f Basidiospores. Notes scanning electron microscope (SEM) was used after coating basidiome samples in gold with Balzers SCD 004 sputter coater with a Hitachi S-3000 N SEM. Scale barsa = 5 mm, b = 50 μm, c = 10 μm, d–f = 5 μm
Etymology: Named after the Chilean Northern Patagonian region where the holotype and paratypes were collected.
Holotype: 19609Tell., MA-Fungi 90714
Colour codes follow: ISCC-NBS Centroid Colour Charts (Kelly and Judd 1976).
Basidiomes first discoid then confluent; margin determinate, involute; hymenophore smooth to slightly reticulate, pale orange yellow to medium orange yellow (73. p. OY–71. m. OY). Hyphal system monomitic; hyphae thick-walled, 3–6 μm wide; paraphysoid hyphae cylindrical, occasionally branched, with scattered clamps, 3–5 μm wide. Basidia clavate, thin-walled, stalked, with basal clamp, 150–190 × (22 −)24–27 μm, with four sterigmata, 5–7 μm wide. Basidiospores citriform, smooth, thin-walled, hyaline, strongly amyloid, 19–22(−24) × 14–16 μm, Q = 1.38.
Material examined: CHILE, Palena, Comuna Hualaihué, Huinay Reserve, path to Cerro del Tambor, 42°22′44″S, 72°24′25″W, 100 m asl., on unidentified wood, 7 May 2013, M. Dueñas, M.P. Martín & M.T. Telleria, 19609Tell. (MA-Fungi 90714, holotype); ibid., 14537MD (MA-Fungi 90713); Comuna Hualaihué, Base Paula, path of Geyser, on unidentified wood, 42°24′16.1″S, 72°44′0.59″W, 52 msl., 28 May 2012, M. Dueñas, M.P. Martín & M.T. Telleria, 14080MD (MA-Fungi 90711).
Habitat and distribution: On unidentified wood in Valdivian temperate rainforest in Chilean Northern Patagonian region.
Additional material examined: Aleurodiscus limonisporus—AUSTRALIA, Victoria, Cumberland falls, on Nothofagus cunninghamii (Nothofagaceae), 6 June 1954, A. Miller (PDD 16691, isotype); NEW ZEALAND, Bay of Plenty, Te Urewera, Tarapounamu, west of road, on decaying branch, 19 May 2005, B. Paulus & M. Fletcher (PDD 83502); Te Urewera, Tarapounamu, east of road, on decaying wood, 17 May 2005, B. Paulus (PDD 83552); Te Urewera, Tarapounamu, west of road, on fallen branch, 19 May 2005, B. Paulus & M. Fletcher (PDD 83553); Buller, North of Reefton, Perseverance Bridge, 5 May 2001, E. Johannesen (PDD 72991); Paparoa National Park, Bullock Creek Farm, on N. fusca, 26 April 1987, P.K. Buchanan (PDD 55241); Paparoa Ranges, Tiropahi Walk, on N. menziesii, 27 April 1987, P.K. Buchanan (PDD 55264); Victoria Forest Park, east of Maruia, on N. menziesii, 22 April 1986, R.E. Beever (PDD 53413); Canterbury, Arthur’s Pass National Park, Dobson Nature Walk, on Olearia capillaris (Asteraceae), 20 November 1988, P.K. Buchanan (PDD 55021); Jollie’s Bush Reserve, Christchurch, on standing dead tree, 4 July 2010, J.A. Cooper (PDD 95980); Gisborne, Moanui Conservation Area, 38°24′24.84″S, 177°23′58.92″E, on Nothofagus, 13 May 2013, S.R. Pennycook (PDD 97004); Hawke’s Bay, Upper Mohaka River, Kaimanawa Range, on Nothofagus fusca, May 1953, J.M. Dingley (PDD 12600); Nelson, Abel Tasman National Park, Hardwoods Hole, on decaying branch of N. solandri, 15 April 2008, A.J. O’Donnell & B.C. Paulus (PDD 94131); Kahurangi National Park, Flora Saddle, on decaying bark, 14 April 2008, A.J. O’Donnell & B.C. Paulus (PDD 94144); Murchison, on N. fusca, April 1956, S.D. & P.J. Brook (PDD 17122); Southland, Porakino Valley, on bark of N. solandri, 7 May 2012, Lloyd (PDD 96617); Wairarapa, Tararua Forest Park, Mt Holdsworth, Gentle Annie Track, on N. fusca, 10 May 2007, A.J. O’Donnell (PDD 92582); Tararua Forest Park, Mt Holdsworth, Donnelly Flat track, on decaying branch, 10 May 2007, B.C. Paulus (PDD 92616); Tararua Forest Park, Mt Holdsworth track, on decaying wood, 7 May 2007, G. Gates & D. Ratkowsky (PDD 92829); Wellington, Kaimanawa Range, on N. menziesii, April 1955, J.M. Dingley (PDD 15227); Mangatorutoru Stream, on N. solandri var. cliffortioides, March 1948, J.M. Dingley (PDD 7452); Tongariro National Park, on N. solandri var. cliffortioides, February 1951, G.H. Cunningham (PDD 15230); Ohakune, Lake Surprise Track, on Nothofagus sp., April 1935, E.E. Chamberlain (PDD 15229); Whakapapa-iti Stream, Ruapehu, on N. solandri var. cliffortioides, Sep 1955, J.M. Dingley (PDD 15355): Whakapapa-iti Stream, Tongariro National Park, on N. solandri var. cliffortioides, January 1951, J.M. Dingley (PDD 11188); Turangi, near Beggs Pool, Kaimanawa Range, on Weinmannia racemosa (Cunoniaceae), 25 May 1970, J.M. Dingley (PDD 28631); Westland, Granville Forest, Orwell Creek, Ahaura, on N. fusca, 2 April 1963, J.M. Dingley (PDD 20997). Aleurodiscus sp.–Otago Lakes, Beyond Paradise, on Nothofagus small branch, 7 May 2016, P. Catcheside (PDD 110288) [identified as A. limonisporus]. Unidentified–Glacier Burn Track, on Nothofagus, 8 May 2016, A. Chinn (PDD 109766) [identified as A. limonisporus].
GenBank numbers: ITS = MF631175, LSU = MF631191 (MA-Fungi 90711); ITS = MF631176, LSU = MF631192 (MA-Fungi 90713); ITS = MF631177, LSU = MF631193 (MA-Fungi 90714); ITS = MF631152, LSU = MF631178 (PDD 7452); ITS = MF631153 (PDD 12600); ITS = MF631154 (PDD 15229); ITS = MF631155 (PDD 15230); ITS = MF631156, LSU = MF631179 (PDD 16691); ITS = MF631157 (PDD 17122); ITS = MF631158 (PDD 28631); ITS = MF631159 (PDD 53413); ITS = MF631160 (PDD 55021); ITS = MF631161 (PDD 55241); ITS = MF631162 (PDD 72991); ITS = MF631163 (PDD 83502); ITS = MF631164, LSU = MF631180 (PDD 83552); ITS = MF631165, LSU = MF631181 (PDD 92582); ITS = MF631166, LSU = MF631182 (PDD 92616); ITS = MF631167, LSU = MF631183 (PDD 92829); ITS = MF631168, LSU = MF631184 (PDD 94131); ITS = MF631169, LSU = MF631185 (PDD 94144); ITS = MF631170, LSU = MF631186 (PDD 95980); ITS = MF631171, LSU = MF631187 (PDD 96617); ITS = MF631172, LSU = MF631188 (PDD 97004); ITS = MF631173, LSU = MF631189 (PDD 109766); ITS = MF631174, LSU = MF631190 (PDD 110288).
Notes: Phylogenetic analysis based on a combined ITS and LSU sequence dataset (Fig. 169) shows that Aleurodiscus patagonicus clusters within Stereaceae, far away from the Aleurodiscus sensu stricto clade, which includes only A. amorphous (Pers.) J. Schröt. (type species) and A. grantii Lloyd (Wu et al. 2001). Aleurodiscus sensu lato is paraphyletic; sequencing shows species of apparently unrelated genera intermingled with the species of Aleurodiscus sensu lato (Wu et al. 2001, 2010b; Larsson and Larsson 2003; Binder et al. 2005; Miller et al. 2006). In the phylogeny (Fig. 169), A. patagonicus groups in a highly supported clade (99% ML, 99% MP and 1.00 PP), which is sister to the A. limonisporus D.A. Reid clade and this relationship is also highly support (92% ML, 90% MP and 1.00 BYPP).
Bayesian majority rule consensus topology based on a combined ITS and LSU DNA sequence dataset. Two Gloeodontia species were included as the outgroup taxa. Maximum parsimony and maximum likelihood bootstrap support (≥ 50%), and posterior probability (≥ 0.95 BYPP) are indicated above branches. Newly generated sequences are marked in blue and ex-type strains are in bold. Scale bar indicates substitution per site. Sequences were retrieved from EMBL Nucleotide Sequence Database (Cochrane et al. 2015), and they were published in Wu et al. (2001), Larsson and Larsson (2003), Wu et al. (b), Dai and He (2016), Dai et al. (2017a, b)
Aleurodiscus patagonicus is similar to A. limonisporus. Both species have a monomitic hyphal system, cylindrical paraphysoid hyphae, and characteristic citriform smooth spores. However, A. patagonicus differs in the following characteristics: clamps present in paraphysoid hyphae, longer and wider basidia, with basal clamp, and, spores with higher length/width ratio, as well as, in the geographical distribution (Table 3). Aleurodiscus patagonicus is reported from Chilean Northern Patagonia, while A. limonisporus occurs in Australia (Reid 1955) and New Zealand (McKenzie et al. 2000).
Trechisporales K.H. Larsson
Hydnodontaceae Jülich
Hydnodontaceae was described by Jülich (1981) with Hydnodon Banker as generic type, and includes Brevicellicium K.H. Larss. & Hjortstam, Cristelloporia I. Johans. & Ryvarden and Trechispora P. Karst. Hydnodon is now considered as a synonym of Trechispora (Ryvarden 2002). This family was characterized by the resupinate, effused-reflexed or pileate basidiome, hymenophore smooth to hydnoid or poroid, as well as by the ampulate septa, short cylindrical basidia and ornamented spores. The family concept was revised by Larsson (2007), on the basis of morphological and molecular data, and Brevicellicium, Fibriciellum J. Erikss. & Ryvarden, Fibrodontia Parmasto, Luellia K.H. Larss. & Hjortstam, Porpomyces Jülich, Subulicystidium Parmasto, Trechispora and Tubulicium Oberw. were included.
Trechispora P. Karst.
Trechispora was erected by Karsten (1890) to include only one species, Trechispora onusta P. Karst. (= Trechispora hymenocystis). This genus was characterized by its adnate basidiomes, poroid hymenophore, with hyaline and ornamented spores. Trechispora was treated by Bondartsev and Singer (1941), and Rogers (1944, 1951), and the initial description was amended by Liberta (1966) to include species with clamped hyphae, ampulate septa and smooth spores. Liberta (1973), in his monograph, introduced new morphological criteria for species delimitation, such as shape and size of spores, as well as the spore surface (smooth or ornamented), hyphal system (monomitic or dimitic), and hymenophore configuration (smooth, poroid or hydnoid), and this species concept was followed in all subsequent treatments. Larsson (1994, 1995a, b, 1996) gave detailed micromorphological studies, described new taxa, proposed new combinations and recognized the morphology of crystal deposits on subicular hyphae as a reliable character for species delimitation.
Most species of Trechispora have been described from boreal and temperate zones, growing on deeply decayed wood and other debris on the ground, although Matssura and Yashiro (2010) reported an insect-fungus association between a termite species (Reticulotermes termites) and an undescribed trechisporoid fungus (Trechispora sp.) from Japan. Trechispora contains 82 legitimate names (Index Fungorum 2019). According to Hjortstam and Ryvarden (2007), 27 species have been reported from tropical and subtropical areas. Trechispora echinospora sp. nov. is introduced from Equatorial Guinea.
Trechispora echinospora Telleria, M. Dueñas, I. Melo & M.P. Martín, sp. nov.
MycoBank number: MB825275; Facesoffungi number: FoF05727, Fig. 171
Etymology: From Latin “echinus” which means hedgehog and spore; referring to the characteristic ornamented spores with straight prickles
Holotype: 13858Tell., MA-Fungi 82485
Colour codes follow: ISCC-NBS Centroid Colour Charts (Kelly and Judd 1976).
Basidiomes resupinate, effused, confluent, adnate; hymenophore farinaceous to grandinioid, yellowish grey (93. y Grey) to cream (92. y White–89. p. Y). Margin not differentiated and strands not seen. Hyphal system monomitic. Subicular hyphae thin-walled, loosely interwoven, moderately branched, straight, 1–2 µm wide, long-celled, with clamps and ampullate septate, up to 4 µm wide. Sphaerocysts thin-walled, with basal clamp, (11–)15–18 µm diam., sometimes present. Subhymenial hyphae thin-walled, branched, short-celled, isodiametric, up to 6 µm wide, with clamps in all septae. Crystals not seen. Basidia short-cylindrical to urniform, four sterigmata, 13–16(–17) × 5–6 µm, with basal clamp. Spores globose, 4–5 µm (excluding the ornamentation), thin-walled, echinulate with straight prickles up to 1 µm long, sometimes with one oil drop in the protoplasm, Q (L/W) = 1.02.
Material examined: EQUATORIAL GUINEA, Centro Sur Province, road Niefang to Evinayong, next to Nkumekie, on Oxythenantera abyssinica (Poaceae), 7 December 1990, M.T. Telleria, 13858Tell. (MA-Fungi 82485, holotype); ibid., 13861Tell. (MA-Fungi 82486); 13870Tell. (MA-Fungi 91312).
Habitat and distribution: Only known from the type locality in the Continental Region (Mbini) of Equatorial Guinea, West Tropical Africa, on dead stem of bamboo (Oxythenantera, Poaceae)
GenBank numbers: ITS = JX392845, JX392847, LSU = JX392846, JX392848, JX392849 (13858Tell., MA-Fungi 82485, holotype); ITS = JX392853, JX392850, JX392852, LSU = JX392851, JX392854 (13861Tell., MA-Fungi 82486).
Notes: In the framework of our study Telleria et al. (2013), a number of ITS and LSU sequences of Trechispora obtained by our team were deposited in GenBank under Trechispora sp. because many specimens were collected in tropical areas, and require a thorough study of their morphology to clearly identify them to the species level.
In this paper, ITS sequences from 13858Tell. and 13861Tell. collected during our study of the corticoid fungi from Equatorial Guinea (Centro Sur Province), growing on Oxythenantera abyssinica (A.Rich.) Munro, were compared with sequences available in the EMBL/GenBank/DDBJ (Cochrane et al. 2011) and UNITE (Abarenkov et al. 2011; Kõljalg et al. 2013) databases, mainly from Larsson et al. (2004), Lutzoni et al. (2004), Krause et al. (2011), Brazee et al. (2012, 2014), Sjökvist et al. (2012), Telleria et al. (2013), and Ordynets et al. (2015). The sequences were analyzed under maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI). The BI tree is shown in Fig. 170, including the MP and ML bootstrap values, as well as the posterior probabilities. The five sequences from these collections, group together in a highly supported clade (85% MP, 99% ML and 1.00 BYPP), sister group of the clade formed by Trechispora araneosa (Höhn. & Litsch.) K.H. Larss., T. farinacea (Pers.) Liberta, T. hymenocystis (Berk. & Broome) K.H. Larss. and T. mollusca (Pers.) Liberta, although this relationship is not well-supported. These collections from Equatorial Guinea show unique morphological characteristics, such as the spores being globose and echinulate with straight prickles, striking under SEM (Fig. 171). The morphological characteristics and molecular phylogenetic results, its habitat on dead stem of bamboo, and its geographical distribution led us to propose the new species, T. echinospora.
Phylogram generated from Bayesian inference analysis of ITS sequence dataset of Trechispora sequences. Maximum parsimony and maximum likelihood bootstrap support values ≥ 50% (10,000 replicates both), and Bayesian posterior probabilities ≥ 80% are shown above the nodes. The tree is rooted with Brevicellicium exile and B. olivascens. Newly generated sequences are marked in blue and ex-type strains are in bold
Trechispora echinospora (13858Tell., MA-Fungi 82485, holotype). a, b Basidiome dry specimen. c Apical hypha of aculeus. d Basidia. e Spores. f, h Sphaerocysts (SEM). g Spores (SEM) of T. echinospora (13861Tell., MA-Fungi 82486). i Spores (SEM). Scale barsa = 1 cm, b = 5 mm, c–f = 5 µm, g, i = 2.5 µm, h = 10 µm
Subclass Auriculariomycetidae Jülich
Auriculariales J. Schröt.
Auriculariaceae Fr. Ex Lindau
Auriculariaceae, typified by Auricularia mesenterica (Dicks.) Pers. (= Helvella mesenterica Dicks.), belongs to Auriculariales and comprises nine genera and 155 species (Kirk 2017). The family includes species with resupinate, effused-reflexed, hydnoid, cerebriform and pileate basidiomes; thin or thick-walled probasidia and metabasidia, both varying from globose to cylindrical and thin-walled basidiospores that germinate by tubes or producing conidia (Lowy 1971; Martin 1952). The most common genera of Auriculariaceae are Auricularia Bull., Heterochaete Pat., Exidia Fr., Eichleriella Bres., Exidiopsis (Bref.) A. Møller and Hirneolina (Pat.) Bres.
Tremellochaete Raitv.
Tremellochaete was described by Raitviír (1964) with T. japonica (Lloyd) Raitv. (= Exidia japonica Lloyd) as the type species and comprises two legitimate species (Kirk 2017). The genus is characterized by tough-gelatinous basidiomes, often becoming softer with age, varying from tuberculate-erumpent and effused-tuberculate; papillate hymenium, papillae heavily encrusted with irregular, hyaline crystals; metabasidia with 2- or 4-celled; and allantoid-suballantoid, hyaline, aseptate basidiospores. Tremellochaete is similar to Exidia, which has fewer papillae (Raitviír 1964), and has been considered a synonym of the latter. However, Tremellochaete was reinstated after morphological and phylogenetic analyses (Malysheva and Spirin 2017). We follow the treatments and updated accounts in Malysheva and Spirin (2017). The new species, T. atlantica is introduced based on phylogenetic analysis of a combined ITS and LSU sequence dataset coupled with morphological characteristics (Fig. 172).
Phylogenetic tree of the Tremellochaete obtained by analyses of DNA sequences. The phylogenetic tree was generated by partial concatenated analyses of the ITS and LSU sequence dataset. Only species with at least the LSU sequences were considered for the phylogeny. The sequences obtained in this study were aligned with other from GenBank in MEGA6 (Tamura et al. 2013) and edited using the Staden Package 2.0 software (Staden et al. 1998). Sistotrema brinkmannii (Bres.) J. Erikss. was used as outgroup taxa. Prior to phylogenetic analyses, the model of nucleotide substitution was estimated using Topali 2.5 (Milne et al. 2004). Bayesian inference analyses [two runs over 2 × 106 generations with a burn in value of 25% and maximum likelihood (1000 bootstrap)] were performed, respectively, in MrBayes 3.1.2 (Ronquist et al. 2012) and PhyML (Guindon and Gascuel 2003) launched from Topali 2.5, using the GTR + G model. Sequences obtained in this study are in blue. Support values are maximum likelihood (BSML) and Bayesian posterior probability (BYPP). Only support values of at least 50% are shown
Tremellochaete atlantica Alvarenga, sp. nov.
MycoBank number: MB823716; Facesoffungi number: FoF05728, Fig. 173
Etymology: The name refers to the phytophysiognomy where it was collected.
Holotype: URM 90199
Colour codes follow: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Basidiomes yellowish to greyish or brown (1B, 2A, 5E, 4D) when fresh, greyish brown to yellowish brown (3F, 8E) when dry, foliose, 2.5–3 × 3.5 cm, gelatinous, densely papillate, papillae 50–107.5 × 40 μm, encrusted with irregular, hyaline crystals not dissolving in KOH, margin detaching from the substrate, marginal hairs present in tufts, 50–212 μm or absent, subhymenial hyphae with ochraceus, granular content. Basidia with complete septum, 2- or 4-celled, hyaline, ovoid, 9–12(–13) × 6–9(–10) μm, clamped at the base. Basidiospores allantoid to reniform, 7.8–10(–12) × 2–5 μm, thin-walled, IKI-. Hyphal system monomitic, hyphae clamped, thin-walled, hyaline, 1–2 μm in diam. Cystidia absent.
Material examined: BRAZIL, Pernambuco: Igarassu, Refúgio Ecológico Charles Darwin, May 2017, R.L.M. Alvarenga, RLMA 491 (URM 90198); ibid., Pernambuco: Recife, Centro de Biociências, near the Departamento de Zoologia, Universidade Federal de Pernambuco, June 2017, R.L.M. Alvarenga, RLMA 477 (URM 90199 holotype; isotype in O).
GenBank numbers: ITS = MG594382, LSU = MG594384 (URM 90198); ITS = MG594381, LSU = MG594383 (URM 90199).
Notes: Tremellochaete atlantica is recognized by the marginal hairs ranging from 50–212 μm and the heavily encrusted papillae with hyaline, irregular crystals. It is similar to T. japonica and T. nigerima (Viégas) Spirin & V. Malysheva. Tremellochaete japonica (type locality Japan) differs by the darker basidiomes, fewer dark hyphae, smaller papillae (60–75 μm), absence of marginal hairs and slightly smaller basidiospores (9.5–12 × 4–4.5 μm) (Roberts 2006), while T. nigerima (type locality Brazil) has larger metabasidia and basidiospores (20–25 × 10–15 μm and 17–20 × 7–8 μm, respectively) (Viégas 1945).
Tremellochaete hispidula (Lowy) Raitv. (= Exidia hispidula Lowy, from USA) also has marginal hairs (up to 125 μm), but lacks clamps, has larger metabasidia [(7–)8–10 × (10–)12–16] and ovoid to allantoid basidiospores [(9–13(–14.5) × 5–7)] (Lowy 1957). This species, however, is illegitimate, because Exidia hispidula Berk. has priority over E. hispidula Lowy.
Phylogenetic analysis of a combined ITS and LSU sequence dataset shows that Tremellochaete atlantica forms a well-resolved clade in Tremellochaete clade as a sister group to T. japonica, collected in Russia (Fig. 172).
Class Dacrymycetes Doweld
The classification of the families in Dacrymycetes follows Oberwinkler (2014), Zhao et al. (2016) and Shirouzu et al. (2017).
Dacrymycetales Henn.
Dacrymycetaceae J. Schröt.
Dacrymycetaceae was introduced by Schröter (1889), with the genera Calocera (Fr.) Fr., Dacrymyces Nees, Dacryomitra Tul. & C. Tul. and Guepinia Fr. Nowadays, according to Oberwinkler (2014) this family contains ten genera: Arrhytidia Berk. & M.A. Curtis, Calocera, Dacrymyces, Dacryomitra, Dacryonaema Nannf., Dacryopinax G.W. Martin, Ditiola Fr. (= Guepinia), Femsjonia Fr., Guepiniopsis Pat. and Heterotextus Lloyd.
The species included in this family are characterized by the form of basidiome: pustulate, cupulate, clavate, or sometimes branched; gelatinous when fresh and usually yellow or orange. The hyphal system is monomitic, the basidia bifurcate, with two well-developed sterigmata and the spores are usually septate germinating by conidia or germ tubes. All species are wood-decaying and cause brown rot (Oberwinkler 1993). The genera in this family have been delimited according to morphological criteria such as the shape of basidiome, the position of the hymenium, and presence or absence of a specialized cortex (Kobayasi 1939; McNabb 1973; Reid 1974). Phylogenetic studies (Weiß and Oberwinkler 2001; Shirouzu et al. 2007, 2009, 2013, 2017) showed that Calocera, Cerinomyces, Dacrymyces, and Dacryopinax are polyphyletic, suggesting that the traditional taxonomy should be revised.
We follow the treatment and updated accounts in Shirouzu et al. (2017) as well as following Reid (1974) for taking into account Dacrymyces sensu lato. The species of this genus are characterized by the basidiomes being gelatinous when fresh, at first pustular, becoming pulvinate, discoid or turbinate, sometimes stipitate; hymenium amphigenous, or restricted to the upper surface; hyphal system monomitic; hyphidia simple, or somewhat branched, with or without clamp connections; basidia clavate becoming bifurcate; spores cylindrical, ellipsoid, allantoid, ovate or subglobose, becoming variously septate at maturity and germinating by conidia or germ-tubes. The novel species, Dacrymyces invisibilis is introduced based on morphological characteristics coupled with ITS phylogenetic analyses (Fig. 174).
UPGMA tree based on Kimura-2-parameters of ITS sequence dataset of seleceted Dacrymycetales species from Shirouzu et al. (2017). Maximum parsimony and maximum likelihood bootstrap support values ≥ 50% (10,000 replicates) and Bayesian posterior probabilities ≥ 0.95 BYPP are shown above the nodes. The analyses were done without rooting the tree. The 189-246 positions in the alignment to D. invisibilis and D. subantarcticensis are shown. New species is indicated in blue bold
Dacrymyces invisibilis M. Dueñas, Telleria & M.P. Martín, sp. nov.
MycoBank number: MB825361; Facesoffungi number: FoF05729, Figs. 175, 176
Etymology: From Latin “invisibilis” not visible, refers to dry basidiome difficult to see.
Holotype: 14597MD, MA-Fungi 91306.
Basidiomes pustulate at first, pulvinate to discoid, confluent, 0.5–3 mm diam., gelatinous, white, becoming varnish-layered when dry; hymenophore amphigenous, smooth; margin determinate. Hyphal system monomitic. Hyphae thin- to thick-walled, smooth, or incrusted, loosely interwoven, sparsely ramified, 2–4 µm wide, without clamps. Basidia 37–48 × 5–6 µm, hyaline, guttulate, subcylindrical to subclavate, without basal clamp, two sterigmata, 21–30 × 3 µm. Spores (14–)15–18 × 5–6(–7) µm (\( \bar{x} \) = 15.8 × 5.95 μm), hyaline, ellipsoid, 3-septate at maturity, thin-walled, smooth, with oil drops, Q (L/W) = 2.65.
Material examined: CHILE, Los Lagos, Palena, comuna Hualaihué, Hornopirén, Huinay Biological Reserve, behind the hydroelectric power station, next to the beach, 42°22′53.4″S 72°24′55.6″W, on unidentified wood, 9 May 2013, M. Dueñas, M.P. Martín & M.T. Telleria, 14597MD, (MA-Fungi 91306, holotype); ibid., CHILE, Los Lagos, Palena, comuna Hualaihué, Hornopirén, Huinay Biological Reserve, “Cementerio de los Alerces” experimental plot, 42°22′01.5″S 72°24′57.8″W, 50 msl, on unidentified wood, 10 May 2013, M. Dueñas, M.P. Martín & M.T. Telleria, 14617MD (MA-Fungi 91307).
Habitat and distribution: Known from the Valdivian temperate rainforest in the Chilean Northern Patagonian region, growing on unidentified wood.
GenBank numbers: ITS = MH230100 (14597MD, MA-Fungi 91306); ITS = MH230101 (14617MD, MA-Fungi 91307).
Notes: Previous ITS/LSU analyses (data not shown) of Dacrymyces invisibilis, and 119 homologous sequences from Shirouzu et al. (2017), included this taxon with D. subantarcticensis Burds. & Laursen described from New Zealand (Burdsall and Laursen 2004). However, morphological and molecular features clearly separate the two taxa. In this paper, a shorter and more accurate alignment is reported, including the species closest to D. subantarcticensis in Shirouzu et al. (2017): D. aureosporus Shirouzu & Tokum., D. chrysospermus Berk. & M.A. Curtis, D. dictyosporus G.W. Martin, D. minor Peck, D. subalpinus Kobayasi, D. stillatus Nees and Guepiniopsis buccina (Pers.) L.L. Kenn. In the UPGMA tree (Fig. 174) based on Kimura-2-parameters, the genetic distances proposed in Schoch et al. (2012) as barcode analyses, are shown, including the parsimony bootstrap and maximum likelihood support values, as well as the Bayesian posterior probabilities. The two collections from Chile form a well-supported group (81% MP, 78% ML and 1.00 BYPP) indicating that D. invisibilis is distinct from D. subantarcticensis. In addition, in this figure a small part of the alignment (around positions 189 and 246) is included to show some of the variable positions between these two species. Dacrymyces invisibilis and D. subantarcticensis have basidiomes nearly invisible when dry, and thin-walled spores, with three septa, but they differ in the size of basidia and spores, smaller in D. subantarcticensis (20–30 × 5 µm and 10–13 × 4.5–6 µm, respectively). Dacrymyces subalpinus, D. aureosporus, D. chrysospermus and D. dictyosporus form a subclade close to D. subantarcticensis and D. invisibilis; all of them have spores with more than three septa at maturity. Dacrymyces subalpinus and D. aureosporus are known only from Japan (Kobayasi 1939; Shirouzu and Hosoya 2017). Dacrymyces chrysospermus has been widely reported and D. dityosporus is known from Honduras, and Mexico (Lowy 1971).
Mucoromycota Doweld
We follow the latest treatment and updated accounts of Mucoromycota in Tedersoo et al. (2018) and Wijayawardene et al. (2018b).
Mucoromycetes Doweld
The classification of the families in Mucoromycetes follows Tedersoo et al. (2018) and Wijayawardene et al. (2018b).
Mucorales Fr.
Mucoraceae Dumort.
We follow the latest treatment and updated accounts of Mucoraceae in Spatafora et al. (2016), Tibpromma et al. (2017) and Wanasinghe et al. (2018). A high-level classification of Mucoraceae was updated by Tedersoo et al. (2018) based on an evolutionary ecological analysis.
Mucor Fresen.
The genus Mucor was described by Fresenius (1850), and comprises the largest number of species within the Mucorales (Benny et al. 2014). They are readily isolated from soil, fruits, vegetables, stored grains, insects or dung (Benny 2008). Several species are of great interest to the biotechnology industry because of their ability to produce proteolytic enzymes (de Souza et al. 2015), while some species are considered as the causal agents of cutaneous zygomycosis in humans (Ribes et al. 2000). During a study of Mucorales from fecal samples of praying mantis collected from the gardens of Chonnam National University, Gwangju, Korea, a new species of Mucor was isolated and is described here. Recently, only three new Mucor species have been reported in Korea: M. koreanus Hyang B. Lee et al. from tangerine fruit (Li et al. 2016); M. stercorarius Hyang B. Lee et al. from rat faeces (Tibpromma et al. 2017); and M. fluvii Hyang B. Lee et al. from freshwater (Wanasinghe et al. 2018) (Fig. 177).
Phylogenetic tree of Mucor orantomantidis (CNUFC-MID1-1 and CNUFC-MID1-2), and related species based on a maximum likelihood analysis of a combined ITS and LSU sequence dataset. The sequence of Backusella oblongispora was used as outgroup taxon. Numbers at the nodes indicate the bootstrap values (> 50%) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are shown in blue and ex-type strains in bold
Mucor orantomantidis Hyang B. Lee, P.M. Kirk & T.T.T. Nguyen, sp. nov.
Index Fungorum number: IF555163; Facesoffungi number: FoF05733, Fig. 178
Mucor orantomantidis (CNUFC-MID1-1, holotype). a A praying mantis. b Colony in potato dextrose agar. c Colony in synthetic mucor agar. d Colony in malt extract agar. e, f Sporangiophores and sporangia. g, h Columellae with collarette. i Sporangiospores. j–m Zygospores. (e, g–k: LM; f, l, m: SEM). Scale barse–k = 20 μm, l, m = 50 μm
Etymology: The specific epithet “orantomantidis” refers to the origin of the fecal pellet of a praying mantis from which the species was first isolated.
Holotype: CNUFC-MID1-1
Colonies on SMA reaching 38–41 mm diam. at 25 °C after 3 days of incubation, yellowish; reverse yellow brownish. Sporangiophores 6.5–16.5 μm wide, arising from aerial hyphae, simple or sympodially branched, with short or long branches. Sporangia globose to subglobose, multispored, initially light yellow then turning brownish yellow, 29.5–60.5 × 28.5–58.5 μm. Columellae globose to subglobose, ellipsoidal, 25–58.5 × 22.5–45.5 μm. Sporangiospores ellipsoidal, sometimes one side slightly flattened, 8–11.5 × 4.5–6 μm. Chlamydospores abundantly produced in aerial hyphae, in chains or irregular, globose, fusiform, barrel-shaped, or irregular in shape. Zygospores borne near the agar surface, reddish brown when young, later blackish brown, 38.5–60.5 × 31.5–49 μm.
Culture characteristics: The isolate grew over a wide range of temperatures with varying growth rates on SMA, PDA, and MEA of 12.5, 14.5, and 12 mm per 24 h, respectively. Optimal growth was observed around 25–30 °C, slow growth was observed below 10 °C, and no growth at 40 °C.
Material examined: REPUBLIC OF KOREA, Jeonnam Province, garden of Chonnam National University located in Gwangju (35° 09′ 16.99″N, 126° 54′ 56.02″E), from fecal sample of praying mantis, October 2017 (CNUFC-MID1-1, holotype); isotype in Korean Agricultural Culture Collection (KACC, Wanju, Korea); ex-type living culture, JMRC:SF:013605 (deposited at Jena Microbial Resource Collection, University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany and preserved as glycerol stock at -80 °C in the CNUFC).
GenBank number: ITS = MH594737, LSU = MH591457 (CNUFC-MID1-1); ITS = MH594738, LSU = MH591458 (CNUFC-MID1-2).
Notes: The phylogenetic analyses of a combined ITS and LSU sequence dataset (Fig. 177) show that Mucor orantomantidis belongs to M. amphibiorum group as defined by Walther et al. (2013). Mucor orantomantidis is closely related to Mucor sp. ‘hiemalis’. However, M. orantomantidis differs from Mucor sp. ‘hiemalis’ by producing zygospores with equal or unequal suspensors, and by having larger sporangiospores. M. orantomantidis grows and sporulates at 35 °C, whereas, the maximum temperature for Mucor sp. ‘hiemalis’ is around 30 °C. In addition, the strain forms a separate branch from other species of the genus, showing it represents a new species in the phylogenetic tree (Fig. 177).
References
Abarenkov K, Nilsson RH, Larsson KH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T, Sen R, Taylor AF, Tedersoo L, Ursing BM, Vrålstad T, Liimatainen K, Peintner U, Kõljalg U (2011) The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytol 186:281–285
Abdel-Wahab MA, Pang KL, Nagahama T, Abdel-Aziz FA, Gareth Jones EB (2010) Phylogenetic evaluation of anamorphic species of Cirrenalia with the description of eight new genera and four new species. Mycol Prog 9:537–558
Acero FJ, González V, Sánchez-Ballesteros J, Rubio V, Checa J, Bills GF, Salazar O, Platas G, Peláez F (2004) Molecular phylogenetic studies on the Peroneutypa based on rDNA-ITS sequences. Mycologia 96:249–259
Adams GC, Wingfield MJ, Common R, Roux J (2005) Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol 52:1–144
Adams GC, Roux J, Wingfield MJ (2006) Cytospora species (Ascomycota, Diaporthales, Valsaceae): introduced and native pathogens of trees in South Africa. Australas Plant Pathol 35:521–548. https://doi.org/10.1071/AP06058
Agnello C, Baglivo A, Carbone M (2013) Rivalutazione e tipificazione di Peziza sicula alla luce di raccolte effettuate nel Salento (Puglia-Italia). Boll A.M.E.R. 88(1):15–24
Agnello C, Baglivo A, Carbone M (2015) Typification of Peziza sicula. Ascomycete 7(1):30
Agnello C, Alvarado P, Loizides M (2018) Sarcopeziza (Pezizaceae, Ascomycota), a new monotypic genus for Inzenga’s old taxon Peziza sicula. Ascomycete.org 10(4):177–186
Alvarado P, Moreno G, Manjón JL, Sánz MA (2011) Eremiomyces magnisporus (Pezizales), a new species from central Spain. Mycotaxon 118:103–111
Alvarez LV, Groenewald JZ, Crous PW (2016) Revising the Schizoparmaceae: coniella and its synonyms Pilidiella and Schizoparme. Stud Mycol 85:1–34
Anastasiou CJ (1967) Two species of Coprinus from alkali lakes. Can J Bot 45:2213–2222
Anonymous (2004) The online auction color chart. Online Auction Color Co, Palo Alto
Antonín V, Ryoo R, Shin HD (2010) Two new marasmielloid fungi widely distributed in the Republic of Korea. Mycotaxon 112:189–199
Antonín V, Ryoo R, Shin HD (2012) Marasmioid and gymnopoid fungi of the Republic of Korea. 4. Marasmius sect. Sicci. Mycol Prog 11:615–638
Aptroot A (1998) A world revision of Massarina (Ascomycota). Nova Hedwigia 66(1):89–162
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Mapook A, Chukeatirote E (2014) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Lumbsch HT, Matsumura M, Moncada B, Nuankaew S, Parnmen S, de Azevedo Santiago ALCM, Sommai S, Song Y, de Souza CAF, de Souza-Motta CM, Su HY, Suetrong S, Wang Y, Wei SF, Wen TC, Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH (2015a) Fungal Diversity Notes 111–252 taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:1–248
Ariyawansa HA, Phukhamsakda C, Thambugala KM, Bulgakov TS, Wanasinghe DN, Perera RH, Mapook A, Camporesi E, Kang JC, Jones EG, Bahkali AH (2015b) Revision and phylogeny of Leptosphaeriaceae. Fungal Divers 74(1):19–51
Ariyawansa HA, Phillips AJ, Chuang WY, Tsai I (2018) Tzeananiaceae, a new pleosporalean family associated with Ophiocordyceps macroacicularis fruiting bodies in Taiwan. MycoKeys 37:1–17
Babos M, Halász K, Zagyva T, Zöld-Balogh Á, Szegő D, Bratek Z (2011) Preliminary notes on dual relevance of ITS sequences and pigments in Hygrocybe taxonomy. Persoonia 26:99–107
Bakhshi M, Arzanlou M, Groenewald JZ, Quaedvlieg W, Crous PW (2019) Parastagonosporella fallopiae gen. et sp. nov. (Phaeosphaeriaceae) on Fallopia convolvulus from Iran. Mycol Prog 18:203–214
Baral HO (1992) Vital versus herbarium taxonomy: morphological differences between living and dead cells of ascomycetes, and their taxonomic implications. Mycotaxon 44:333–390
Barbosa FR, Silva SS, Fiuza PO, Gusmão LFP (2011) Conidial fungi from the semi-arid Caatinga biome of Brazil. New species and records for Thozetella. Mycotaxon 115:327–334
Barbosa FR, Gusmão LF, Raja HA, Shearer CA (2013) New species and new records of freshwater ascomycetes from Brazil and Costa Rica. Mycologia 105(2):335–343
Barge EG, Cripps CL (2016) New reports, phylogenetic analysis, and a key to Lactarius Pers. in the Greater Yellowstone Ecosystem informed by molecular data. Mycokeys 15:1–58
Barge EG, Cripps CL, Osmundson TW (2016) Systematics of the ectomycorrhizal genus Lactarius in the Rocky Mountain alpine zone. Mycologia 108(2):414–440
Barr ME (1976) Perspectives in the Ascomycotina. Mem N Y Bot Gard 28:1–8
Barr ME (1978) The Diaporthales in North America: with emphasis on Gnomonia and its segregates. Mycol Mem 7:1–232. https://doi.org/10.1002/fedr.19800910313
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1987) New taxa and combinations in the Loculoascomycetes. Mycotaxon 29:501–505
Barr ME (1990) Prodomus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 39:43–184
Bas C (2000) A broader view on Amanita. Boll Gruppo Micol G Bresadola 43:9–12
Benny GL (2008) Methods used by Dr. R. K. Benjamin, and other mycologists, to isolate Zygomycetes. Aliso 26:37–61
Benny GL, Humber RA, Voigt K (2014) Zygomycetous fungi: Phylum Entomophthoromycota and subphyla Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In: McLaughlin DJ, Spatafora JW (eds) Mycota VII, part A, Systematics and evolution. Springer, New York, pp 209–250
Berkeley MJ, Broome CE (1871) The fungi of Ceylon. (Hymenomycetes, from Agaricus to Cantharellus). Bot J Linn Soc 11:494–567
Berlese AN (1902) Icones Fungorum omnium hucusque cognitorum ad usum Sylloges Saccardianae Adcommodatae. Abellini 3(3–4):58
Beyma JFH (1940) Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland). VI. Mitteilung. Antonie van Leeuwenhoek. 6:263–290
Bhatt RP, Tulloss RE, Semwal KC, Bhatt VK, Moncalvo JM, Stephenson SL (2003) Amanitaceae from India. A critically annotated checklist. Mycotaxon 88:249–270
Bi ZS, Li TH (1986) A preliminary note on Russula species from Guangdong, with a new species and a new variety. Guihaia 6(3):193–199
Binder M, Hibbett DS, Larsson KH, Larsson E, Langer E, Langer G (2005) The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Syst Biodivers 3:1–45
Bissett J, Gams W, Jaklitsch W, Samuels GJ (2015) Accepted Trichoderma names in the year 2015. IMA Fungus 6(2):263–295
Blanco-Dios JB (2015) Agaricales of the dunes of Galicia (IV): Marasmiellus ciesanus (Omphalotaceae), a new species found in the National Maritime-Terrestrial Park of the Atlantic Islands of Galicia (Spain). Mycosphere 6(5):585–592
Boerema GH, Kesteren HAV (1964) The nomenclature of two fungi parasitizing brassica. Persoonia 3(1):17–28
Boertmann D (1995) The fungi of northern Europe. The genus Hygrocybe, vol 1. Greve, Denmark
Bondartsev A, Singer R (1941) Zur Systematik der Polyporaceae. Ann Mycol 39:43–65
Boonmee S, D’souza MJ, Luo Z, Pinruan U, Tanaka K, Su H, Bhat JD, McKenzie EHC, Jones EBG, Taylor JE, Phillips AJL, Hirayama K, Eungwanichayapant PD, Hyde KD (2016) Dictyosporiaceae fam. nov. Fungal Divers 80:457–482
Borse BD, Borse KN, Patil SY, Pawara CM, Nemade LC, Patil VR (2016) Freshwater higher fungi of India. Lulu, Com
Both EE (1998) New taxa of boletes and two boletes with identity problems. Bull Buffalo Soc Nat Sci 36:215–232
Bourdot H, Galzin A (1912) Hyménomycètes de France: IV. Corticiés: Aleurodiscus. Bull Soc Mycol Fr 28:349–354
Bourdot H, Galzin A (1928) Contribution à la flore mycologique de la France I. Hyménomycètes de France, Paris
Brahmanage RS, Lu YZ, Bhat DJ, Wanasinghe DN, Yan JY, Hyde KD, Boonmee S (2017) Phylogenetic investigations on freshwater fungi in Tubeufiaceae (Tubeufiales) reveals the new genus Dictyospora and new species Chlamydotubeufia aquatica and Helicosporium flavum. Mycosphere 8(7):917–933
Braun U, Nakashima C, Crous PW, Groenewald JZ, Moreno-Rico O, Rooney-Latham S, Blomquist CL, Haas J, Marmolejo J (2018) Phylogeny and taxonomy of the genus Tubakia s. lat. FUSE 1:41–99
Brazee NJ, Lindner DL, Fraver S, D’Amato AW, D’Amato AM (2012) Wood-inhabiting fungi in aspen-dominated forests managed for biomass in the U.S. Lake States. Fungal Ecol 5(5):600–609
Brazee NJ, Lindner DL, D’Amato AW, Fraver S, Forrester JA, Mladenoff DJ (2014) Disturbance and diversity of woo-inhabiting fungi: effects of canopy gaps and downed debris. Biodivers Conserv 23:2155–2172
Breitenbach J, Kränzlin F (1995) Pilze der Schweiz, vol 4. Verlag Mycologia, Luzern
Bulliard JBF (1788) Herbier de la France. Tome 8:337–384
Burdsall HH (1985) A contribution to the taxonomy of the genus Phanerochaete. Mycol Mem 10:1–165
Burdsall HH Jr, Laursen GA (2004) Fungi of New Zealand’s subantartic islands I: Two new species of Dacrymyces (Basidiomycotina). In: Miller OK, Crips CL (eds) Fungi in forest ecosystems: systematic, diversity and ecology. New York Botanical Garden, Bronx, pp 107–111
Burt EA (1918) The helephoraceae of North America: IX. Aleurodiscus. Ann Mo Bot Gard 5:177–203
Buyck B, Thoen D, Watling R (1996) Ectomycorrhizal fungi of the Guinea-Congo Region. Proc R Soc Edinb 104:313–333
Buyck B, Hofstetter V, Eberhardt U, Verbeken A, Kauff F (2008) Walking the thin line between Lactarius and Russula: the dilemma of Russula sect. Ochricompactae. Fungal Divers 28:15–40
Buyck B, Hofstetter V, Verbeken A, Walleyn R (2010) Proposal 1919: to conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Mycotaxon 111:504–508
Cacialli G, Caroti V, Doveri F (1999) Contributio ad cognitionem coprinorum. A.M.B, Trento
Cai L, Jeewon R, Hyde KD (2005) Phylogenetic evaluation and taxonomic revision of Schizothecium based on ribosomal DNA and protein coding genes. Fungal Divers 19:1–21
Cai L, Jeewon R, Hyde KD (2006a) Phylogenetic investigations of Sordariaceae based on multiple gene sequences and morphology. Mycol Res 110:137–150
Cai L, Jeewon R, Hyde KD (2006b) Molecular systematics of Zopfiella and allied genera: evidence from multi-gene sequence analyses. Mycol Res 110:359–368
Cai Q, Tulloss RE, Tang LP, Tolgor B, Zhang P, Chen ZH, Yang ZL (2014) Multi-locus phylogeny of lethal amanitas: implications for species diversity and historical biogeography. BMC Evol Biol 14:143. https://doi.org/10.1186/1471-2148-14-143
Campbell J, Volkmann-Kohlmeyer B, Gräfenhan T, Spatafora JW, Kohlmeyer J (2005) A re-evaluation of Lulworthiales: relationships based on 18S and 18S rDNA. Mycol Res 109:556–568
Cannon PF (1991) A revision of Phyllachora and some similar genera on the host family Leguminosae. Mycol Pap 163:1–302
Cannon PF, Kirk PM (eds) (2007) Fungal families of the world. CABI, Wallingford
Cantrell SA, Hanlin RT (1997) Phylogenetic relationships in the family Hyaloscyphaceae inferred from sequences of ITS regions, 5.8S ribosomal DNA and morphological characters. Mycologia 89(5):745–755
Cantrell SA, Lodge DJ (2001) Hygrophoraceae (Agaricales) of the Greater Antilles: Hygrocybe subgenus Pseudohygrocybe section Firmae. Mycol Res 105:215–224
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Carlucci A, Raimondo ML, Santos J, Phillips AJ (2012) Plectosphaerella species associated with root and collar rots of horticultural crops in southern Italy. Persoonia Mol Phylogeny Evol Fungi 28:34–48
Carmarán CC, Romero AI, Giussani LM (2006) An approach towards a new phylogenetic classification in Diatrypaceae. Fungal Divers 23:67–87
Castañeda-Ruiz RF, Arnold GRW (1985) Deuteromycotina de Cuba. I. Hyphomycetes. Revista del Jardín Botánico Nacional Universidad de la Habana 6(1):47–67
Castañeda-Ruiz RF, Guarro J, Vel asquez-Noa S, Gene J (2003) A new species of Minimelanolocus and some hyphomycete records from rain forests in Brazil. Mycotaxon 85:231–239
Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002) A preliminary overview of Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94:1017–1031. https://doi.org/10.1080/15572536.2003.11833157
Cesati V, De Notaris G (1863) Schema di classificazione degle sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaerianell’antico significato attribuitoglide Persono. Commentario della Soc Crittogamol Ital 1:177–420
Chaiwan N, Lu YZ, Tibpromma S, Bhat DJ, Hyde KD, Boonmee S (2017) Neotubeufia gen. nov. and Tubeufia guangxiensis sp. nov. (Tubeufiaceae) from freshwater habitats. Mycosphere 8(9):1443–1456
Chang JW, Kao HW, Wang YZ (2010) Molecular Phylogeny of Cercophora, Podospora, and Schizothecium (Lasiosphaeriaceae, Pyrenomycetes). Taiwania 55:110–116
Cheewangkoon R, Groenewald JZ, Verkley GJ, Hyde KD, Wingfield MJ, Gryzenhout M, Summerell BA, Denman S, Toanun C, Crous PW (2010) Re-evaluation of Cryptosporiopsis eucalypti and Cryptosporiopsis-like species occurring on Eucalyptus leaves. Fungal Divers 44(1):89–105
Chen J, Parra LA, Kesel AD, Khalid AN, Qasim T, Ashraf A, Bahkali AH, Hyde KD, Zhao RL, Callac P (2016) Inter- and intra-specific diversity in Agaricus endoxanthus and allied species reveals a new taxon, A. punjabensis. Phytotaxa 252(1):1–16
Chethana KWT, Zhou Y, Zhang W, Liu M, Xing QK, Hyde KD, Yan JY, Li XH (2017) Coniella vitis sp. nov. is the common pathogen of white rot in Chinese vineyards. Plant Dis 101:2123–2136
Chou WN, Wang YZ (2005) Nine Species of Russula (Basidiomycotina) New to Taiwan. Taiwania 50(2):93–100
Cibula WG, Weber NS (1996) Hygrocybe andersonii a new psammophilus Hygrocybe from Horn Island, a Mississippi barrier island. Mycologia 88:514–516
Clements FE, Shear CL (1931) Genera of fungi, 2 vol. H.W. Wilson, New York, pp i–vii
Cochrane G, Karsch-Mizrachi I, Nakamura Y (2011) The international nucleotide sequence database collaboration. Nucleic Acids Res 39:D15–D18
Cochrane G, Karsch-Mizrachi I, Takagi T (2015) Sequence Database Collaboration IN. The international nucleotide sequence database collaboration. Nucleic Acids Res 44(D1):D48–D50
Cooke WB (1953) The genera of Homobasidiomycetes (exclusive of the Gasteromycetes). USDA:1–100
Corner EJH (1950) A monograph of Clavaria and allied genera. Oxford University Press, London
Corner EJH (1970) Supplement to “A monograph of Clavaria and allied genera”. Beih Nova Hedwig 33:1–299
Corner EJH, Bas C (1962) The genus Amanita in Singapore and Malaya. Persoonia 2:241–304
Corner EJ, Thind KS, Dev S (1958) The Clavariaceae of the Mussoorie Hills (India). IX. Trans Br Mycol Soc 41(2):203–206
Crous PW, Braun U, Groenewald JZ (2007a) Mycosphaerella is polyphyletic. Stud Mycol 58:1–32
Crous PW, Schubert K, Braun U, De Hoog GS, Hocking AD, Shin HD, Groenewald JZ (2007b) Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Stud Mycol 58:185–217
Crous PW, Summerell BA, Shivas RG, Burgess TI, Decock CA, Dreyer LL, Granke LL, Guest DI, Hardy GS, Hausbeck MK, Hüberli D, Jung T, Koukol O, Lennox CL, Liew ECY, Lombard L, McTaggart AR, Pryke JS, Roets F, Saude C, Shuttleworth LA, Stukely MJC, Vánky K, Webster BJ, Windstam ST, Groenewald JZ (2012) Fungal Planet description sheets: 107–127. Persoonia 28:138–182
Crous PW, Giraldo A, Hawksworth DL, Vincent R, Paul K, Josep G, Barbara R, Conrad S, Ulrike D, Thippawan T, Johannes G (2014a) The genera of fungi: fixing the application of type species of generic names. IMA Fungus 5:141–160
Crous PW, Wingfield MJ, Schumacher RK, Summerell BA, Giraldo A, Gené J, Guarro J, Wanasinghe DN, Hyde KD, Camporesi E, Garethjones EB, Thambugala KM, Malysheva EF, Malysheva VF, Acharya K, Álvarez J, Alvarado P, Assefa A, Barnes CW, Bartlett JS, Blanchette RA, Burgess TI, Carlavilla JR, Coetzee MPA, Damm U, Decock CA, Denbreeÿen A, Devries B, Dutta AK, Holdom DG, Rooney-Latham S, Manjón JL, Marincowitz S, Mirabolfathy M, Moreno G, Nakashima C, Papizadeh M, Shahzadehfazeli SA, Amoozegar MA, Romberg MK, Shivas RG, Stalpers JA, Stielow B, Stukely MJC, Swart WJ, Tan YP, Vanderbank M, Wood AR, Zhang Y, Groenewald JZ (2014b) Fungal Planet description sheets: 281–319. Persoonia 33:212–289
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun MH, Schumacher RK, Stielow JB, Van der Linde EJ (2015a) The genera of fungi-fixing the application of the type species of generic names—G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6(1):163–198
Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Duenas M, Dutta AK (2015b) Fungal planet description sheets: 320–370. Persoonia 34:167–266
Crous PW, Wingfield MJ, Burgess TI, Hardy GSJ, Crane C, Barrett S, Cano-Lira JF, Le Roux JJ, Thangavel R, Guarro J, Stchigel AM, Martín MP, Alfredo DS, Barber PA, Barreto RW, Baseia IG, Cano-Canals J, Cheewangkoon R, Ferreira RJ, Gené J, Lechat C, Moreno G, Roets F, Shivas RG, Sousa JO, Tan YP, Wiederhold NP, Abell SE, Accioly T, Albizu JL, Alves JL, Antoniolli ZI, Aplin N, Araújo J, Arzanlou M, Bezerra JDP, Bouchara JP, Carlavilla JR, Castillo A, Castroagudín VL, P.C. Ceresini PC, Claridge GF, Coelho G, Coimbra VRM, Costa LA, da Cunha KC, Silva SS, Daniel R, de Beer ZW, Dueñas M, Edwards J, Enwistle P, Fiuza PO, Fournier J, García D, Gibertoni TB, Giraud S, Guevara-Suarez M, Gusmão LFP, Haituk S, Heykoop M, Hirooka Y, Hofmann TA, Houbraken J, Hughes DP, Kautmanová I, Koppel O, Koukol O, Larsson E, Latha KPD, Lee DH, Lisboa DO, Lisboa WS, López-Villalba Á, Maciel JLN, Manimohan P, Manjón JL, Marincowitz S, Marney TS, Meijer M, Miller AN, Olariaga I, Paiva LM, Piepenbring M, Poveda-Molero JC, Raj KNA, Raja HA, Rougeron A, Salcedo I, Samadi R, Santos TAB, Scarlett K, Seifert KA, Shuttleworth LA, Silva GA, Silva M, Siqueira JPZ, Souza-Motta CM, Stephenson SL, Sutton DA, Tamakeaw N, Telleria MT, Valenzuela-Lopez N, Viljoen A, Visagie CM, Vizzini A, Wartchow F, Wingfield BD, Yurchenko E, Zamora JC, Groenewald JZ (2016a) Fungal Planet description sheets: 469–557 Persoonia 37:218–403
Crous PW, Wingfield MJ, Richardson DM, Le Roux JJ, Strasberg D, Edwards J, Roets F, Hubka V, Taylor PWJ, Heykoop M, Martín MP, Moreno G, Sutton DA, Wiederhold NP, Barnes CW, Carlavilla JR, Gené J, Giraldo A, Guarnaccia V, Guarro J, Hernández-Restrepo M, Kolařík M, Manjón JL, Pascoe IG, Popov ES, Sandoval-Denis M, Woudenberg JHC, Acharya K, Alexandrova AV, Alvarado P, Barbosa RN, Baseia IG, Blanchette RA, Boekhout T, Burgess TI, Cano-Lira JF, Čmoková A, Dimitrov RA, Dyakov MY, Dueñas M, Dutta AK, Esteve-Raventós F, Fedosova AG, Fournier J, Gamboa P, Gouliamova DE, Grebenc T, Groenewald M, Hanse B, Hardy GESJ, Held BW, Jurjević Ž, Kaewgrajang T, Latha KPD, Lombard L, Luangsa-ard JJ, Lysková P, Mallátová N, Manimohan P, Miller AN, Mirabolfathy M, Morozova OV, Obodai M, Oliveira NT, Ordóñez ME, Otto EC, Paloi S, Peterson SW, Phosri C, Roux J, Salazar WA, Sánchez A, Sarria GA, Shin HD, Silva BDB, Silva GA, Smith MT, Souza-Motta CM, Stchigel AM, Stoilova-Disheva MM, Sulzbacher MA, Telleria MT, Toapanta C, Traba JM, Valenzuela-Lopez N, Watling R, Groenewald JZ (2016b) Fungal Planet description sheets: 400–468. Persoonia 36:316–458
Crous PW, Wingfield MJ, Burgess TI, Hardy GEStJ, Barber PA, Alvarado P, Barnes CW, Buchanan PK, Heykoop M, Moreno G (2017) Fungal Planet description sheets: 558–624. Persoonia 38:240–384
Crous PW, Schumacher RK, Wingfield MJ, Akulov A, Denman S, Roux J, Braun U, Burgess TI, Carnegie AJ, Váczy KZ, Guatimosim E (2018) New and interesting FUNGI. 1. FUSE 1(1):169–215
Cui YY, Cai Q, Tang LP, Yang ZL, Liu JW (2018) The family Amanitaceae: molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Divers 91:5–230
Dai LD, He SH (2016) New species and new records of Aleurodiscus s.l. (Basidiomycota) in China. Mycol Prog 15:717–730
Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, Bhat DJ, Xu JC, Taylor JE, Hyde KD, Chukeatirote E (2017a) Bambusicolous fungi. Fungal Divers 82:1–105
Dai LD, Wu SH, Karen N, Burdsall HH Jr, He SH (2017b) Two new species of Aleurodiscus s.l. (Russulales, Basidiomycota) on bamboo from tropics. Mycoscience 58:213–220
Dai LD, Zhao Y, Wu SH (2017c) Three new species of Aleurodiscus s.l. (Russulales, Basidiomycota) on bamboos from East Asia. Cryptogam Mycol 38:227–239
Damm U, Fourie PH, Crous PW (2010) Coniochaeta (Lecythophora), Collophora gen. nov. and Phaeomoniella species associated with wood necroses of Prunus trees. Persoonia 24:60–80
Daranagama DA, Hyde KD, Sir EB, Thambugala KM, Tian Q, Samarakoon MC, McKenzie EHC, Jayasiri SC, Tibpromma S, Bhat JD, Liu XZ, Stadler M, Liu X (2018) Towards a natural classification and backbone tree for Graphostromataceae, Hypoxylaceae, Lopadostomataceae and Xylariaceae. Fungal Divers 88:1–65
Das K (2013) Lactarius fennoscandicus (Russulaceae) A new record for India. Nelumbo 55:214–218
Das K, Sharma JR (2005) Russulaceae of Kumaon Himalaya. Botanical Survey of India, Kolkata, p 255
Das K, Verbeken A (2011) Three new species of Lactarius (Russulaceae) from Sikkim, India. Cryptogam Mycol 32(4):365–381
Das K, Miller SL, Sharma JR (2006a) Russula in Himalaya 2: four new taxa. Mycotaxon 95:205–215
Das K, Sharma JR, Atri NS (2006b) Russula in Himalaya 3: a new species of subgenus Ingratula. Mycotaxon 95:271–275
Das K, Van de Putte K, Buyck B (2010) New or interesting Russula from Sikkim Himalaya (India). Cryptogam Mycol 31(4):373–387
Das K, Atri NS, Buyck B (2013) Three new species of Russula (Russulales) from India. Mycosphere 4(4):707–717
Das K, Dowie NJ, Li GJ, Miller SL (2014) Two new species of Russula (Russulales) from India. Mycosphere 5(5):612–622
Das K, Verbeken A, Nuytinck J (2015) Morphology and phylogeny of four new Lactarius species from Himalayan India. Mycotaxon 130:105–130
Das K, Ghosh A, Bhatt RP, Chakraborty D, Hofstetter V, Buyck B (2017a) Fungal biodiversity profiles 41–50. Cryptogam Mycol 38(4):527–547
Das K, Ghosh A, Chakraborty D, Li J, Qiu L, Baghela A, Halama M, Hembrom ME, Mehmood T, Parihar A, Pencakowski B, Bielecka M, Reczynska K, Sasiela D, Singh U, Song Y, Swierkosz K, Szczesniak K, Uniyal P, Zhang J, Buyck B (2017b) Fungal biodiversity profiles 31–40. Cryptogam Mycol 38(3):1–56
Dayarathne MC, Phookamsak R, Ariyawansa HA, Jones EBG, Camporesi E and Hyde KD (2015) Phylogenetic and morphological appraisal of Leptosphaeria italica sp. nov. (Leptosphaeriaceae, Pleosporales) from Italy. Mycosphere 6(5): 634–642
Dayarathne MC, Maharachchikumbura SSN, Jones EBG, Goonasekara ID, Bulgakov TS, Al-Sadi AM, Hyde KD, Lumyong S, McKenzie EHC (2017) Neophyllachora gen nov. (Phyllachorales), three new species of Phyllachora from Poaceae and resurrection of Polystigmataceae (Xylariales). Mycosphere 8(10):1598– 1625
Dayarathne MC, Wanasinghe DN, Jones EG, Chomnunti P, Hyde KD (2018) A novel marine genus, Halobyssothecium (Lentitheciaceae) and epitypification of Halobyssothecium obiones comb. nov. Mycol Prog 17(10):1161–1171
De Almeida DAC, Gusmão LFP, Miller AN (2016) Taxonomy and molecular phylogeny of Diatrypaceae (Ascomycota, Xylariales) species from the Brazilian semi-arid region, including four new species. Mycol Prog 15:1–27
De Crop E, Nuytinck J, Van de Putte K, Wisitrassameewong K, Hackel J, Stubbe D, Hyde KD, Roy M, Halling RE, Moreau PA, Eberhardt E, Verbeken A (2017) A multi-gene phylogeny of Lactifluus (Basidiomycota, Russulales) translated into a new infrageneric classification of the genus. Persoonia 38:58–80
De Gruyter JD, Woudenberg JHC, Aveskamp AA, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
De Koker TH, Nakasone KK, Haarhof J, Burdsall HH Jr, Janse BJ (2003) Phylogenetic relationships of the genus Phanerochaete inferred from the internal transcribed spacer region. Mycol Res 107:1032–1040
De Notaris G (1844) Osservazione su alcuni generi e specie della tribu dei Pirenomiceti sferiacei. G Bot Ital 2:38–55
De Souza PM, Bittencourt MLA, Caprara CC, Freitas M, Almeida RPC, Silveira D, Fonseca YM, Ferreira Filho EX, Pessoa Junior A, Magalhães PO (2015) A biotechnology perspective of fungal proteases. Braz J Microbiol 46:337–346
Dennis RWG (1949) A revision of the British Hyaloscyphaceae, with notes on related European species. Mycol Papers 32:1–97
Desjardin DE, Retnowati A, Horak E (2000) Agaricales of Indonesia: 2. A preliminary monograph of Marasmius from Java and Bali. Sydowia 52:92–193
Devadatha B, Sarma VV, Jeewon R, Wanasinghe DN, Hyde KD, Jones EG (2018) Thyridariella, a novel marine fungal genus from India: morphological characterization and phylogeny inferred from multigene DNA sequence analyses. Mycol Prog 17(7):791–804
Dharne CG (1965) Taxonomic investigations on the discomycetous genus Lachnellula Karst. Phytopathol Z 53(2):101–144
Dissanayake AJ, Phillips AJL, Li XH, Hyde KD (2016) Botryosphaeriaceae: current status of genera and species. Mycosphere 7(7):1001–1073
Dissanayake AJ, Camporesi E, Hyde KD, Yan JY, Li XH (2017) Saprobic Botryosphaeriaceae, including Dothiorella italica sp nov., associated with urban and forest trees in Italy. Mycosphere 8(2):1157–1176
Doilom M, Shuttleworth LA, Roux J, Chukeatirote E, Hyde KD (2015) Botryosphaeriaceae associated with Tectona grandis (teak) in Northern Thailand. Phytotaxa 233(1):1–26
Doilom M, Dissanayake AJ, Wanasinghe DN, Boonmee S, Liu JK, Bhat DJ, Taylor JE, Bahkali AH, McKenzie EHC, Hyde KD (2017) Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers 82:107–182
Donk MA (1933) Revis. niederl. Homob.-Aph. 2. Proefschr., Utrecht (& Meded. bot. Mus. Herb. Utrecht No. 9, & Meded. Nederl. mycol. Ver. 22)
Donk MA (1957) The generic names proposed for Hymenomycetes–VII. “Thelephoraceae” (conclusion). Taxon 6:106–123
Donk MA (1961) Four new families of Hymenomycetes. Persoonia 1(4):405–407
Donk MA (1962) Notes on resupinate Hymenomycetes–VI. Persoonia 2:217–238
Douanla-Meli C (2007) Fungi of Cameroon. Ecological diversity with emphasis on the taxonomy of non-gilled Hymenomycetes from Mbalmayo forest reserve. Biblioth Mycol 202:1–410
Drinkwater LE, Schipanski M, Snapp S, Jackson LE (2017) Ecologically based nutrient management. In: Agricultural systems, 2nd edn. Elsevier, Amsterdam, pp 203–257
Du Z, Hyde KD, Yang Q, Liang YM, Tian CM (2017) Melansporellaceae: a novel family of Diaporthales (Ascomycota). Phytotaxa 305(3):191–200
Duhem B, Buyck B (2007) Edible mushrooms from Madagascar 2. Clavulina albiramea comb. nov. (Cantharellales), an edible clavarioid fungus shared between African miombo and Malagasy Uapaca woodland. Nova Hedwig 85:317–330
Dumortier BC (1829) Analyse des familles des plantes, avec l’indication des principaux genres qui s’y rattachent. J. Casterman, Tornay
Dutta AK, Chandra S, Pradhan P, Acharya K (2014) A new species of Marasmius sect. Sicci from India. Mycotaxon 128:117–125
Ehrenberg CG (1818) Sylvae Mycologicae Berolinenses. Formis Theophili Bruschcke, Berlin (In Latin)
Ekanayaka AH, Hyde KD, Jones EBG, Zhao Q (2018) Taxonomy and phylogeny of operculate discomycetes: Pezizomycetes. Fungal Divers 90(1):161–243
Ellis MB (1971) Dematiaceous hyphomycetes. Key Surrey, England
Ellis MB (1976) More dematiaceous hyphomycetes. Key Surrey, England
Eriksson OE (1981) The families of bitunicate ascomycetes. Opera Bot 60:1–220
Eriksson OE (2001) Outline of Ascomycota. Myconet 6:1–27
Eriksson OE, Hawksworth DL (1993) Notes on ascomycete systematics—Nos 1418–1529. Syst Ascom 11:163–194
Eriksson J, Hjortstam K, Ryvarden L (1978a) The Corticiaceae of North Europe, vol 5. Mycoaciella—Phanerochaete. Fungiflora, Oslo
Eriksson J, Weresub LK, Burdsall HH (1978b) Phanerochaete vs Grandiniella (Corticiaceae, Aphyllophorales). Taxon 27:51–52
Farook VA, Manimohan P (2015) Marasmius odoratus—a new jasmine-scented species of Marasmius section Globulares from India. Phytotaxa 227(3):275–281
Farr DF, Rossman AY (2018) Fungal databases. U.S. National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/. Retrieved May–Nov 2018
Fatto RM (1998) A study of selected Murrill’s Russulas. Mycotaxon 69:487–502
Feng PY, de Hoog GS, Najafzadeh MJ, van den Ende AG, Stielow JB, Badali H, Boeger WA, Vicente VA (2014) Cladophialophora abundans, a novel species of Chaetothyriales isolated from the natural environment. Mycol prog 13(2):381–391
Ferdman Y, Aviram S, Roth-Bejerano N, Trappe JM, Kagan-Zur V (2005) Phylogenetic studies of Terfezia pfeilii and Choiromyces echinulatus (Pezizales) support new genera for southern African truffles: Kalaharituber and Eremiomyces. Myol Res 109(2):237–245
Finlay RD (2008) Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59(5):1115–1126
Firmino AL, Pereira OL (2014) Lembosia bezerrae, a new asterinaceous fungus associated with a terrestrial orchid from Bahia, Brazil. Mycotaxon 127:199–205
Fischer E (1897) Plectacineae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol I. Engelmann, Leipzig
Floudas D, Hibbett DS (2015) Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol 119:679–719
Fresenius G (1850) Beiträge zur Mykologie 1:1–38
Friebes G (2012) A key to the non-lichenicolous species of the genus Capronia (Herpotrichiellaceae). Ascomycete.org 4(3):55–64
Fries EM (1825) as ‘Cytisporei’. Syst. orb. veg. (Lundae) 1:118
Frisvad JC, Samson RA (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol 49:1–174
Galli R (1996) Le Russule. Edinatura, Milano
Gao L, Ma Y, Zhao W, Wei Z, Gleason ML, Chen H, Hao L, Sun G, Zhang R (2015) Three new species of Cyphellophora (Chaetothyriales) associated with sooty blotch and flyspeck. PLoS ONE 10(9):e0136857
Geiser DM, LoBuglio KF, Gueidan C (2015) 5. Pezizomycotina: Eurotiomycetes. In: Systematics and evolution. Springer, Berlin, pp 121–141
Ghimire SR, Hyde KD (2008) Fungal endophytes. In: Plant surface microbiology. Springer, Berlin, pp 281–292
Ghobad-Nejhad M, Liu SL, Langer E, Dai YC (2015) Molecular and morphological evidence reveal a new species in the core Phanerochaete clade (Polyporales). Mycol Prog 14:1–7
Ghosh A, Das K (2017) Russula (Russulaceae) in western Himalaya 1: two new species from subg. Russula. Phytotaxa 323(3):237–252
Ghosh A, Das K, Adhikari S, Bhatt RP (2016) A novel species of Russula (Russulaceae) from Indian Himalaya. Mycosphere 7(6):778–785
Ghosh A, Das K, Bhatt RP (2017) Russula sarnarii sp. nov. (Russulaceae, Basidiomycota) from Indian Himalaya. Curr Res Environ Appl Mycol J 7(1):64–72
Gierczyk B, Kujawa A, Pachlewski T, Szczepkowski A, Wójtowski M (2011) Rare species of the genus Coprinus Pers. s. lato. Acta Mycol 46(1):27–73
Gilbert EJ (1940) Amanitaceae. Icon Mycol 27:1–199
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61(4):1323–1330
Glez-Peña D, Gòmez-Blanco D, Reboiro-Jato M, Fdez-Riverola F, Posada D (2010) ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Res 38:W14–W18
Gnavi G, Garzoli L, Poli A, Prigione V, Burgaud G, Varese GC (2017) The culturable mycobiota of Flabellia petiolata: first survey of marine fungi associated to a Mediterranean green alga. PLoS ONE 12(4):e0175941
Goh TK, Ho WH, Hyde KD, Whitton SR, Umali TE (1998) New records and species of Canalisporium (Hyphomycetes), with a revision of the genus. Can J Bot 76:142–152
Gomes RR, Glienke C, Videira CIR, Lombard L, Groenewald JZ, Crous PW (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31:1–41
Goos RD (1985) The anamorph genus Zalerion. Mycotaxon 23:445–449
Greslebin AL, Rajchenberg M (2003) Diversity of Corticiaceae sens. lat. in Patagonia, Southern Argentina. N Z J Bot 41:437–446
Griffith GW, Easton GL, Jones AW (2002) Ecology and diversity of waxcap (Hygrocybe spp.) fungi. Bot J Scotl 54:7–22
Grum-Grzhimaylo AA, Debets AJM, van Diepeningen AD, Georgieva ML, Bilanenko EN (2013) Sodiomyces alkalinus, a new holomorphic alkaliphilic ascomycete within the Plectosphaerellaceae. Persoonia 31:147–158
Guarro J, Cannon PF, van der Aa HA (1991) A synopsis of the genus Zopfiella (Ascomycetes, Lasiosphaeriaceae). Syst Ascom 10:79–112
Guatimosim E, Firmino AL, Bezerra JL, Pereira OL, Barreto RW, Crous PW (2015) Towards a phylogenetic reappraisal of Parmulariaceae and Asterinaceae (Dothideomycetes). Persoonia 35:230–241
Gueidan C, Aptroot A, da Silva Cáceres ME, Badali H, Stenroos S (2014) A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes, Ascomycota). Mycol Prog 13(4):990. https://doi.org/10.1007/s11557-014-0990-2
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Haelewaters D, Page RA, Pfister DH, Balboa P (2018) Hyperparasites: Morphological and molecular diversity of Laboulbeniales fungi associated with ectoparasitic bat flies (Diptera: Nycteribiidae, Streblidae). Studies of the laboulbeniomycetes: diversity, evolution, and patterns of speciation 179
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hall BG (2004) Phylogenetic trees made easy: a how-to manual, 2nd edn. Sinauer Associates, Sunderland
Halling RE, Nuhn M, Fechner NA, Osmundson TW, Soytong K, Arora D, Hibbett DS, Binder M (2012) Sutorius: a new genus for Boletus eximius. Mycologia 104:951–961
Han JG, Hosoya T, Sung GH, Shin HD (2014) Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biol 118(2):150–167
Hansen K, Læssøe T, Pfister DH (2001) Phylogenetics of the Pezizaceae, with an emphasis on Peziza. Mycologia 93(5):958–990
Hansen K, Læssøe T, Pfister DH (2002) Phylogenetic diversity in the core group of Peziza inferred from ITS sequences and morphology. Mycol Res 106(8):879–902
Hansen K, LoBuglio KF, Pfister DH (2005) Evolutionary relationships of the cup-fungus genus Peziza and Pezizaceae inferred from multiple nuclear genes: RPB2, β-tubulin, and LSUrDNA. Mol Phylogenet Evol 36(1):1–23
Hansford CG (1946) The foliicolous Ascomycetes, their parasites and associated fungi. Mycol Pap 15:1–240
Hashimoto A, Matsumura M, Hirayama K, Tanaka K (2017) Revision of Lophiotremataceae (Pleosporales, Dothideomycetes): Aquasubmersaceae, Cryptocoryneaceae, and Hermatomycetaceae fam. nov. Persoonia 39(1):51–73
Hawksworth DL (1991) The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res 95(6):641–655
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105(12):1422–1432
Hawksworth DL, Lücking R (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectrum. https://doi.org/10.1128/microbiolspec.FUNK-0052-2016
Hawksworth DL, Kirk PM, Sutton BC, Pegler DN (1995) Ainsworth and Bisby ‘s dictionary of the fungi, 8th edn. CAB International, Wallingford
He G, Chen SL, Yan SZ (2016) Morphological and molecular evidence for a new species in Clavulina from southwestern China. Mycoscience 57:255–263
Heilmann-Clausen J, Verbeken A, Vesterholt J (1998) The genus Lactarius, vol 2—fungi of northern Europe. Svampetryk, Danish Mycological Society, Denmark
Henkel TW, Meszaros R, Aime MC, Kennedy A (2005) New Clavulina species from the Pakaraima Mountains of Guyana. Mycol Prog 4:343–350
Henkel TW, Aime MC, Uehling JK, Smith ME (2011) New species and distribution records of Clavulina (Cantharellales, Basidiomycota) from the Guyana Shield. Mycologia 103:883–894
Henkel TW, Obase K, Husbands D, Uehling JK, Bonito G, Aime MC, Smith ME (2016) New Boletaceae taxa from Guyana: Binderoboletus segoi gen. and sp. nov., Guyanaporus albipodus gen. and sp. nov., Singerocomus rubriflavus gen. and sp. nov., and a new combination for Xerocomus inundabilis. Mycologia 108:157–173
Hennebert GL (1968) New species of Spirosphaera. Trans Br Mycol SOC 51:13–24
Hernandez-Restrepo M, Schumacher RK, Wingfield MJ, Ishtiaq A, Cai L, Duong TA, Edwards J, Gene J, Groenewald JZ, Sana J, Khalid AN (2016) FUSE: FUSE 2. Sydowia 68:193–230
Hesler LR (1960) A study of Russula types. Memoirs of the Torrey Botanical Club 21(2):1–59
Hibbett DS, Bauer R, Binder M, Giachini AJ, Hosaka K, Justo A, Larsson E, Larsson KH, Lawrey JD, Miettinen O, Nagy LG, Nilsson RH, Weiss M, Thorn RG (2014) 14 Agaricomycetes. In: McLaughlin D., Spatafora J. (eds) Systematics and evolution. The Mycota (A comprehensive treatise on fungi as experimental systems for basic and applied research), vol 7A. Springer, Berlin, pp 373–429
Hirayama K, Hashimoto A, Tanaka K (2014) A new species, Lophiostoma versicolor, from Japan (Pleosporales, Dothideomycetes). Mycosphere 5(3):411–417
Hirayama K, Tanaka K (2011) Taxonomic revision of Lophiostoma and Lophiotrema based on reevaluation of morphological characters and molecular analyses. Mycoscience 52:401–412
Hjortstam K, Ryvarden L (2007) Checklist of corticioid fungi (Basidiomycotina) from the tropics, subtropics and the southern hemisphere. Syn Fung 22:27–146
Hjortstam K, Roberts P, Spooner B (2009) Corticoid fungi from the Kimberley Region, Western Australia. Kew Bull 64:353–368
Holmskjold T (1790) Beata ruris otia fungis danicis, vol. 1. Copenhagen, Denmark
Honan AH, Desjardin DE, Perry BA, Horak E, Baroni TJ (2015) Towards a better understanding of Tetrapyrgos (Basidiomycota, Agaricales): new species, type studies, and phylogenetic inferences. Phytotaxa 231(2):101–132
Hong SB, Cho HS, Shin HD, Frisvad JC, Samson RA (2006) Novel Neosartorya species isolated from soil in Korea. Int J Syst Evol Microbiol 56:477–486
Hongsanan S, Hyde KD (2017) Phylogenetic placement of Micropeltidaceae. Mycosphere 8(10):1930–1942
Hongsanan S, Li YM, Liu JK, Hofmann T, Piepenbring M, Bhat JD, Boonmee S, Doilom M, Singtripop C, Tian Q, Mapook A (2014) Revision of genera in Asterinales. Fungal divers 68(1):1–68
Hongsanan S, Hyde KD, Bahkali AH, Camporesi E, Chomnunti P, Ekanayaka H, Gomes AAM, Hofstetter V, Jones EBG, Pinho DB, Pereira OL, Tian Q, Wanasinghe DN, Xu JC, Buyck B (2015) Fungal biodiversity profiles 11–20. Cryptogam Mycol 36(3):355–380
Hongsanan S, Sánchez-Ramírez S, Crous PW, Ariyawansa HA, Zhao RL, Hyde KD (2016) The evolution of fungal epiphytes. Mycosphere 7(11):1690–1712
Hongsanan S, Maharachchikumbura SS, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84(1):25–41
Horne WT (1905) A new species of Lembosia. Bull Torrey Bot Club 32:69–71
Horwath WR (2017) The role of the soil microbial biomass in cycling nutrients. A Paradigm Shift in Terrestrial Biogeochemistry, In Microbial Biomass, pp 41–66
Hosagoudar VB, Archana GR, Dan M (2009) Maheshwaramyces, a new genus of the family Lembosiaceae. Indian J Sci Technol 2:12–13
Hosen MI, Feng B, Wu G, Zhu XT, Li YC, Yang ZL (2013) Borofutus, a new genus of Boletaceae from tropical Asia: phylogeny, morphology and taxonomy. Fungal Divers 58:215–226
Hosen MI, Li TH, Aminuzzaman FM, Islam MR (2016) Hygrocybe umbilicata sp. nov., with first generic report for Bangladesh and its phylogenetic placement. Phytotaxa 280:70–76
Hosoya T, Sasagawa R, Hosaka K, Sung GH, Hirayama Y, Yamaguchi K, Toyama K, Kakishima M (2010) Molecular phylogenetic studies of Lachnum and its allies based on the Japanese material. Mycoscience 51(3):170–181
Houbraken J, Samson RA (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol 70:1–51
Houbraken J, Frisvad JC, Samson RA (2011) Taxonomy of Penicillium section Citrina. Stud Mycol 70:53–138
Huang S, Maharachchikumbura SSN, Jeewon R, Bhat DJ, Phookamsak R, Hyde KD, Al-Sadi AM, Kang J (2018) Lecanicillium subprimulinum (Cordycipitaceae, Hypocreales), a novel species from Baoshan, Yunnan. Phytotaxa 348(2):99–108
Huhndorf SM, Fernández FA (2005) Teleomorph-anamorph connections: Chaetosphaeria raciborskii and related species, and their Craspedodidymum-like anamorphs. Fungal Divers 19:23–49
Huhndorf SM, Miller AN, Fernández FA (2004) Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia 96(2):368–387
Hyde KD, Soytong K (2008) The fungal endophyte dilemma. Fungal Divers 33(163173):2
Hyde KD, Kang JC, Kong RYC (1996) Fungi from palms. XXX. Notes on Amphisphaeria species described from palms and a description of A. umbrina. Nova Hedwig 63:101–108
Hyde KD, Goh TK, Taylor JE, Fröhlich J (1999) Byssosphaeria, Chaetosphaeria, Niesslia and Ornatispora gen. nov., from palms. Mycol Res 103:1423–1439
Hyde KD, Bussaban B, Paulus B, Crous PW, Lee S, Mckenzie EH, Photita W, Lumyong S (2007) Diversity of saprobic microfungi. Biodivers Conserv 16(1):7–35
Hyde KD, Jones EBG, Liu JK, Ariyawansa HA, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, De Hoog S, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ekanayaka AH, Gibertoni TB, Góes-Neto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, de Santiago ALCMA, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar AM, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev S, Buyck B, da Silva GA, de Lima CLF, de Oliveira RJV, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytövuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lücking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JZ, Zhu L (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270
Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Chethana KWT, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He MQ, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kusˇan I, Lee H, Li J, Lin CG, Liu NG, Lu YZ, Luo ZL, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Abdel-Aziz FA, Li WJ, Senanayake IC, Shang QJ, Daranagama DA, De Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castañeda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones GEB, Kang JC, Karunarathna SC, Lim YW, Liu JK, Liu ZY, Plautz HL Jr, Lumyong S, Maharachchikumbura SSN, Matocˇec N, Mckenzie EHC, Meśič A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su HY, Suetrong S, Tkalčec Z, Vizzini A, Wen TC, Wisitrassameewong K, Wrzosek M, Xu JC, Zhao Q, Zhao RL, Mortimer PE (2017) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers 87:1–235
Hyde KD, Al-Hatmi AM, Andersen B, Boekhout T, Buzina W, Dawson TL, Eastwood DC, Jones EG, de Hoog S, Kang Y, Longcore JE (2018a) The world’s ten most feared fungi. Fungal Divers 85:1–34
Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchimbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang Q, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat DJ, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, McKenzie EHC, Wen TC, Yan JY, Zhao Q (2018b) Mycosphere notes 169–224. Mycosphere 9:271–430
Index Fungorum (2019) Index Fungorum. http://www.indexfungorum.org/names/Names.asp
Inzenga G (1869) Funghi Siciliani. Centuria seconda. Stabilimento F. Lao, Palermo
Jaklitsch WM (2015) Savoryellaceae Jaklitsch & Réblová. Index Fungorum 209:1
Jaklitsch WM, Voglmayr H (2016) Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Stud Mycol 85:35–64
Jaklitsch WM, Baral HO, Lücking R, Lumbsch HT (2016a) Ascomycota. In: Frey W (ed) Syllabus of plant families-Adolf Engler’s Syllabus der Pflanzenfamilien, 13th edn. Borntraeger, Stuttgart
Jaklitsch WM, Gardiennet A, Voglmayr H (2016b) Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 37:82–105
Jayasiri SC, Hyde KD, Jones EBG, McKenzie EHC, Jeewon R, Phillips AJL, Bhat DJ, Wanasinghe DN, Liu JK (Jack), Lu YZ, Kang JC, Xu JC, Karunarathna SC (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10(1):1–186
Jayawardena RS, Purahong W, Zhang W, Wubet T, Li X, Liu M, Zhao W, Hyde KD, Liu J, Yan J (2018) Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers 90(1):1–84
Jayawardena RS, Hyde KD, Jeewon R, Ghobad-Nejhad M, Wanasinghe DN, Liu NG, Phillips AJL, Oliveira-Filho JRC, da Silva GA, Gibertoni TB, Abeywikrama P, Carris LM, Chethana KWT, Dissanayake AJ, Hongsanan S, Jayasiri SC, McTaggart AR, Perera RH, Phutthacharoen K, Savchenko KG, Shivas RG, Thongklang N, Dong W, Wei DP, Wijayawardena NN, Kang JC (2019) One stops shop II: taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50 (2019) Fungal Divers 94(1):41–129
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7:1669–1677
Jeewon R, Yeung SY, Hyde KD (2009) A novel phylogenetic group within Thozetella Chaetosphaeriaceae): a new taxon based on morphology and DNA sequence analyses. Can J Microbiol 55(6):680–687
Jiang HB, Hyde KD, Jayawardena RS, Doilom M, Xu JC, Phookamsak R (2019) Taxonomic and phylogenetic characterizations reveal two new species and two new records of Roussoella (Roussoellaceae, Pleosporales) from Yunnan. China, Mycol Prog (proof)
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH, Abdel-Wahab MA, Boekhout T, Pang KL (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Jülich W (1981) Higher taxa of Basidiomycetes. Biblioth Mycol 85:1–185
Justo A, Miettinen O, Floudas D, Ortiz-Santana B, Sjökvist E, Lindner DL, Nakasone K, Niemelä T, Larsson KH, Ryvarden L, Hibbett DS (2017) A revised family-level classification of the the Polyporales (Basidiomycota). Fungal Biol 121(9):798–824
Kämmerer A, Besl H, Bresinsky A (1985) Omphalotaceae fam. nov. und Paxillaceae, ein chemotaxonomischer Vergleich zweier Pilzfamilien der Boletales. Plant Syst Evol 150:101–117
Karsten PA (1873) Mycologia fennica. Pars secunda. Pyrenomycetes. Bidrag till Kännedom av Finlands Natur och Folk 23:1–252
Karsten PA (1889) Kritsk öfvergit af Finlands Basidswampar (Basidiomycetes, Gastero- & Hymenomycetes). Bidr Känn Finl Nat Folk 48:1–470
Karsten PA (1890) Fragmenta mycologica XXIX. Hedwigia 29:147–149
Karunarathna SC, Chen J, Mortimer PE, Xu JC, Zhao RL, Callac P, Hyde KD (2016) Mycosphere Essay 8: a review of genus Agaricus in tropical and humid subtropical regions of Asia. Mycosphere 7(4):417–439
Karunarathna A, Papizadeh M, Senanayake IC, Jeewon R, Phookamsak R, Goonasekara ID, Wanasinghe DN, Wijayawardene NN, Amoozegar MA, Shahzadeh Fazeli SA, Camporessi E (2017) Novel fungal species of Phaeosphaeriaceae with an asexual/sexual morph connection. Mycosphere 8:1818–1834
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kelly KL, Judd DB (1976) COLOR. Universal language and dictionary of names. National Bureau of Standards, Special Publications 440
Kendrick B (2000) The fifth kingdom. Hackett Publishing, Indianapolis
Kepler R, Ban S, Nakagiri A, Bischoff J, Hywel-Jones N, Owensby CA, Spatafora JW (2013) The phylogenetic placement of hypocrealean insect pathogens in the genus Polycephalomyces: an application of One Fungus One Name. Fungal Biol 117:611–622
Kepler RM, Luangsa-Ard JJ, Hywel-Jones NL, Quandt CA, Sung GH, Rehner SA, Aime MC, Henkel TW, Sanjuan T, Zare R, Chen M, Li Z, Rossman AY, Spatafora JW, Shrestha B (2017) A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 8(2):335–353
Kerrigan RW (2016) Agaricus of North America. Mem N Y Bot Gard 114:1–574
Khan ZU, Gené J, Ahmad S, Cano J, Al-Sweih N, Joseph L, Chandy R, Guarro J (2013) Coniochaeta polymorpha, a new species from endotracheal aspirate of a preterm neonate, and transfer of Lecythophora species to Coniochaeta. Antonie Van Leeuwenhoek 104(2):243–252
Kibby G (2014) The genus Russula in Great Britain: with synoptic keys to species. Published by the Author
Kibby G, Fatto RM (1990) Key to the species of Russula in northeastern North America, 3rd edn. Kibby-Fatto Enterprises, Somerville
Kirk PM (2017) Species Fungorum (version January 2016). In: Roskov Y, Abucay L, Orrell T, Nicolson D, Bailly N, Kirk PM, Bourgoin T, DeWalt RE, Decock W, De Wever A, Nieukerken E van, Zarucchi J, Penev L (eds) Species 2000 & ITIS Catalogue of Life, 30 June 2017. Digital Resource. www.catalogueoflife.org/col. Species 2000: Naturalis, Leiden. ISSN 2405-8858
Kirk PM (continuously updated) Index Fungorum. www.indexfungorum.org. Accessed 19 Apr 2018
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth & Bisby’s dictionary of the fungi, 10th edn. CABI, Wallingford
Knapp DG, Kovács GM, Zajta E, Groenewald JZ, Crous PW (2015) Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 35:87–100
Knudsen H, Borgen T (1982) Russulaceae in Greenland. In: G. Laursen & J.F. Ammirati (eds) Arct Alp Mycol 1:216–238
Knudsen H, Ruotsalainen J, Vauras J (2012) Russula Pers. In: Knudsen H, Vesterholt J (eds) Funga Nordica. Nordsvamp, Copenhagen, pp 144–186
Kobayasi Y (1939) On the Dacrymyces-group (Fungorum ordinis Tremellalium strudia monographica III). Sci Rep Tokyo Bunrika Daigaku Sect B 4:105–128
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AF, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson K-H (2013) Towards a unified paradigm for sequence-based identification of Fungi. Mol Ecol 22:5271–5277
Komura DL, De Oliveira JJ, Moncalvo JM, Margaritescu S, Zartman CE (2016) Marasmius calvocystidiatus sp. nov. and M. horridulus (Marasmiaceae): characterization of two unusual species from central Amazonia. Phytotaxa 280(3):222–240
Kornerup A, Wanscher JH (1963) Methuen handbook of colour. Eyre Methuen Ltd., London
Kornerup A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Eyre Methuen Ltd., London
Kraichak E, Huang JP, Nelsen M, Leavitt SD, Lumbsch HT (2018) A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot J Linn Soc 188(3):233–249
Kränzlin F (2005) Fungi of Switzerland: Russulaceae, vol 6. Verlag Mycologia, Luzern, Switzerland
Krause C, Garnica S, Bauer R, Nebel M (2011) Aneuraceae (Metzgeriales) and tulasnelloid fungi (Basidiomycota): a model for early steps in fungal simbiosis. Fungal Biol 115:839–851
Krug JC, Jeng RS (1984) Hapsidomyces, a new genus of the Pezizaceae with ornamented ascospores. Mycologia 76(4):748–751
Kruys A, Huhndorf SM, Miller AN (2015) Coprophilous contributions to the phylogeny of Lasiosphaeriaceae and allied taxa within Sordariales (Ascomycota, Fungi). Fungal Divers 70:101–113
Kuhnert R, Oberkofler I, Peintner U (2012) Fungal growth and biomass development is boosted by plants in snow-covered soil. Microb Ecol 64(1):79–90
Kuhnert E, Fournier J, Persoh D, Luangsa-ard JJD, Stadler M (2014) New Hypoxylon species from Martinique and new evidence on the molecular phylogeny of Hypoxylon based on ITS rDNA and b-tubulin data. Fungal Divers 64:181–203
Kuhnert E, Surup F, Sir EB, Lambert C, Hyde KD, Hladki AI, Romero AI, Stadler M (2015) Lenormandins A–G, new azaphilones from Hypoxylon lenormandii and Hypoxylon jaklitschii sp. nov., recognised by chemotaxonomic data. Fungal Divers 71:165–184
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Larget B, Simon DL (1999) Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol Biol Evol 16:750–759
Larsson KH (1994) Poroid species in Trechispora and use of calcium oxalate crystals for species identification. Mycol Res 98:1153–1172
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30(22):3276–3278
Larsson KH (1995a) Taxonomy of Trechispora farinacea and proposed synonyms I. Species with grandinioid or hydnoid hymenophore. Symb Bot Upsal 30:101–118
Larsson KH (1995b) Taxonomy of Trechispora farinacea and proposed synonyms II. Species with smooth hymenophore. Nordic J Bot 16:73–82
Larsson KH (1996) New species and combinations in Trechispora (Corticiaceae, Basidiomycotina). Nordic J Bot 16:83–98
Larsson KH (2007) Re-thinking the classification of corticioid fungi. Mycol Res 111:1040–1063
Larsson E, Larsson KH (2003) Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 95:1037–1065
Larsson KH, Larsson E, Kõljalg U (2004) High phylogenetic diversity among corticioid homobasidiomycetes. Mycol Res 108:983–1002
Le HT, Nuytinck J, Verbeken A, Saisamorn L, Desjardin DE (2007) Lactarius in Northern Thailand: 1. Lactarius subgenus Piperites. Fungal Divers 24:173–224
Leelavathy KM, Manimohan P, Arnolds EJM (2006) Hygrocybe in Kerala State, India. Persoonia 19:101–151
Lemke PA (1964a) The genus Aleurodiscus (sensu stricto) in North America. Can J Bot 42:213–282
Lemke PA (1964b) The genus Aleurodiscus (sensu lato) in North America. Can J Bot 42:723–768
Letourneau A, Seena S, Marvanová L, Bärlocher F (2010) Potential use of barcoding to identify aquatic hyphomycetes. Fungal Divers 40(1):51–64
Léveillé JH (1845) Champignons exotiques. Ann Sci Nat Bot 3:8–71
Li GJ, Li SF, Wen HA (2011) Russula zhejiangensis sp. nov. from East China. Cryptogam Mycol 32:127–133
Li GJ, Zhao Q, Zhao D, Yue SF, Li SF, Wen HA, Liu XZ (2013) Russula atroaeruginea and R. sichuanensis spp. nov. from southwest China. Mycotaxon 124:137–188
Li GJ, Zhao D, Li SF, Wen HA (2015) Russula chiui and R. pseudopectinatoides, two new species from southwestern China supported by morphological and molecular evidence. Mycol Prog 14:33
Li GJ, Hyde KD, Zhao RN, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, Crop ED, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Góes-Neto A, Gorczak M, Haitjema GH, Hapuarachchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jerônimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lücking R, Lumbsch HT, Lumyong S, Leaño EM, Marano AV, Matsumura M, McKenzie EHC, Mongkolsamrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphanphoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspé O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasák J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Yang SD, Hu Y, Zhang JF, Zhao J, Zhou LW, Persˇoh D, Phillips AJL, Maharachchikumbura SSN (2016) Fungal Diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237
Liberta AE (1966) On Trechispora. Taxon 15:317–319
Liberta AE (1973) The genus Trechispora (Basidiomycetes, Corticiaceae). Can J Bot 51:1871–1892
Liew EC, Aptroot A, Hyde KD (2000) Phylogenetic significance of the pseudoparaphyses in Loculoascomycete taxonomy. Mol Phylogenetics Evol 16(3):392–402
Link JHF (1809) Observationes in ordines plantarum naturales. Dissertatio I. Magazin der Gesellschaft Naturforschenden Freunde Berlin (in Latin) 3:3–42
Liu SL, He SH (2016) Phanerochaete porostereoides, a new species in the core clade with brown generative hyphae from China. Mycosphere 7:648–655
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Liu F, Hu DM, Cai L (2012a) Canlarium duplumascospora gen. et. sp nov and Jobellisia guangdongensis sp. nov. from freshwater habitats in China. Mycologia 104:1178–1186
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI (2012b) Towards a natural classification of Botryosphaeriales. Fungal Divers 57(1):149–210
Liu JK, Phookamsak R, Dai DQ, Tanaka K, Jones EBG, Xu JC, Chukeatirote E, Hyde KD (2014) Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa 181:1–33
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, D’souza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe DN, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015a) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu XY, Udayanga D, Luo ZL, Chen LJ, Zhou DQ, Su HY, Hyde KD (2015b) Backbone tree for Chaetothyriales with four new species of Minimelanolocus from aquatic habitats. Fungal Biol 119:1046–1062
Liu JK, Hyde KD, Jeewon R, Phillips AJ, Maharachchikumbura SS, Ryberg M, Liu ZY, Zhao Q (2017a) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers 84(1):75–99
Liu N, Hongsanan S, Yang J, Bhat DJ, Liu J, Jumpathong J, Liu Z (2017b) Periconia thailandica (Periconiaceae), a new species from Thailand. Phytotaxa 323(3):253–263
Liu JK, Lu YZ, Cheewangkoon R, To-Anun C (2018) Phylogeny and morphology of Helicotubeufia gen. nov., with three new species in Tubeufiaceae from aquatic habitats. Mycosphere 9(3):495–509
Liyanage KK, Khan S, Brooks S, Mortimer PE, Karunarathna SC, Xu J, Hyde KD (2018) Morpho-molecular characterization of two Ampelomyces spp. (Pleosporales) strains mycoparasites of powdery mildew of Hevea brasiliensis. Front Microbiol 9:12
Locquin M (1984) Mycologie générale et structural 1–551
Lodge DJ, Ovrebo CL (2008) First records of Hygrophoraceae from Panama including a new species of Camarophyllus and a new veiled species in Hygrocybe section Firmae. Fungal Divers 32:69–80
Lodge DJ, Pegler DN (1990) Hygrophoraceae of the Luquillo Mountains of Puerto Rico. Mycol Res 94:443–456
Lodge DJ, Matheny PB, Cantrell SA, Moncalvo JM, Vilgalys R, Redhead S (2006) Delineating the Hygrophoraceae: character myths vs. gene trees (poster). Inoculum 57(4):27
Lodge DJ, Padamsee M, Matheny PB, Aime MC, Cantrell SA, Boertmann D, Kovalenko A, Vizzini A, Dentinger BTM, Kirk PM, Ainsworth AM, Moncalvo J-M, Vilgalys R, Larsson E, Lücking R, Griffith GW, Smith ME, Norvell LL, Desjardin DE, Redhead SA, Ovrebo CL, Lickey EB, Ercole E, Hughes KW, Courtecuisse R, Young A, Binder M, Minnis AM, Lindner DL, Ortiz-Santana B, Haight J, Læssøe T, Baroni TJ, Geml J, Hattori T (2014) Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Divers 64:1–99
Lofgren LA, LeBlanc NR, Certano AK, Nachtigall J, LaBine KM, Riddle J, Broz K, Dong Y, Bethan B, Kafer CW, Kistler HC (2018) Fusarium graminearum: pathogen or endophyte of North American grasses? New Phytol 217(3):1203–1212
Lowy B (1957) A new Exidia. Mycologia 49(6):899–902
Lowy B (1971) Tremellales. Flora Neotrop 6:1–153
Lowy B (1976) New Tremellales from Panama. Mycologia 68:1103–1108
Lu YZ, Boonmee S, Liu KJ, Hyde KD, McKenzie EH, Eungwanichayapant PD, Kang JC (2018a) Multi-gene phylogenetic analyses reveals Neohelicosporium gen. nov. and five new species of helicosporous hyphomycetes from aquatic habitats. Mycol Prog 17(5):631–646
Lu YZ, Liu JK, Hyde KD, Jeewon R, Kang JC, Fan C, Boonmee S, Bhat DJ, Luo ZL, Lin CG, Eungwanichayapant PD (2018b) A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers 92(1):131–344
Lumbsch HT, Huhndorf SM (eds) (2007) Outline of Ascomycota–2007. Myconet 13:1–58
Lumbsch HT, Huhndorf SM (2010) Myconet Volume 14. Part One. Outline of Ascomycota—2009. Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life Earth Sci 1:1–64
Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY (2018) Lignicolous freshwater fungi from China II: novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 9(3):444–461
Luttrell ES (1951) Taxonomy of the Pyrenomycetes. Univ Mo Stud 24(3):1–120
Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim YW, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91:1446–1480
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, Dsouza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu JK, Liu Z-Y, Norphanphoun C, Pang KL, Perera RH, Senanayake IC, Shang Q, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat JD, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao Y, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang J, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen T, Wijayawardene NN (2016) Families of Sordariomycetes. Fungal Divers 79:1–317
Maharachchikumbura SSN, Larignon P, Al-Sadi AM, Li Z-Y (2017) Characterization of Neopestalotiopsis, Pestalotiopsis and Truncatella species associated with grapevine trunk diseases in France. Phytopathol Mediterr 55(3):380–390
Maharachchikumbura SSN, Ariyawansa HA, Wanasinghe DN, Dayarathne MC, Al-Saady NA, Al-Sadi AM (2019) Phylogenetic classification and generic delineation of Hydeomyces desertipleosporoides gen. et sp. nov. (Phaeosphaeriaceae) from Jebel Akhdar Mountain in Oman. Phytotaxa 391(1):28–38
Mahdizadeh V, Safaie N, Goltapeh EM, Asef MR, Hosseini SM, Callac P (2016) Agaricus section Xanthodermatei in Iran. Phytotaxa 247(3):181–196
Malloch D, Cain RF (1971) New cleistothecial Sordariaceae and a new family, Coniochaetaceae. Can J Bot 49:869–880
Malysheva V, Spirin V (2017) Taxonomy and phylogeny of the Auriculariales (Agaricomycetes, Basidiomycota) with stereoid basidiocarps. Fungal Biol 121:689–715
Mapook A, Hyde KD, Dai DQ, Li J, Jones EG, Bahkali AH, Boonmee S (2016) Muyocopronales, ord. nov. (Dothideomycetes, Ascomycota) and a reappraisal of Muyocopron species from northern Thailand. Phytotaxa 265(3):225–237
Mardones M, Trampe-Jaschik T, Oster S, Elliott M, Urbina H, Schmitt I, Piepenbring M (2017) Phylogeny of the order Phyllachorales (Ascomycota, Sordariomycetes): among and within order relationships based on five molecular loci. Persoonia 39:74–90
Mardones M, Trampe-Jaschik T, Hofmann TA, Piepenbring M (2018) Contribution to the phylogeny and a new species of Coccodiella (Phyllachorales). Mycol Prog 17(1–2):205–213
Marin-Felix Y, Groenewald JZ, Cai L, Chen Q, Marincowitz S, Barnes I, Bensch K, Braun U, Camporesi E, Damm U, De Beer ZW (2017) Genera of phytopathogenic fungi: GOPHY 1. Stud Mycol 86:99–216
Martin GW (1952) Revision of the North Central Tremellales. State Univ Iowa Stud Nat Hist 19(3):1–122
Massee GE (1914) Fungi exotici, XVII. Bulletin of Miscellaneous Information of the Royal Botanical Gardens Kew: 72–76
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol Phylogenet Evol 35:1–20
Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, Ge Z-W, Yang Z-L, Slot JC, Ammirati JF, Baroni TJ, Bougher NL, Hughes KW, Lodge DJ, Kerrigan RW, Seidl MT, Aanen DK, DeNitis M, Daniele GM, Desjardin DE, Kropp BR, Norvell LR, Hibbett DS (2006) Majorclades of Agaricales: a multi-locus phylogenetic over view. Mycologia 98(6):984–997
Matssura K, Yashiro T (2010) Parallel evolution of termite-egg mimicry by sclerotium-forming fungi in distant termite groups. Biol J. Linnean Soc 100:531–537
McKenzie EHC, Buchanan PK, Johnston PR (2000) Checklist of fungi on Nothofagus species in New Zealand. N Z J Bot 38:635–720
McNabb RFR (1973) Taxonomic studies in the Dacrymycetaceae VIII. Dacrymyces Nees ex Fries. N Z J Bot 11:461–524
Melo I, Cardoso J, Dueñas M, Salcedo I, Telleria MT (2012) Peniophora aluticolor (Fungi, Basidiomycota), an orphaned species restudied. Nova Hedwigia 94:437–440
Miadlikowska J, Kauff F, Högnabba F, Oliver JC, Molnár K, Fraker E, Gaya E, Hafellner J, Hofstetter V, Gueidan C, Otálora MA (2014) A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Mol Phylogenet Evol 79:132–168
Miettinen O, Spirin V, Vlasák J, Rivoire B, Stenroos S, Hibbett D (2016) Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). Mycokeys 17:1–46
Miller JH (1933) Some new species of Hypoxylon. Mycologia 25:321–329
Miller AN, Huhndorf SM (2005) Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi). Mol Phylogenet Evol 35:60–75
Miller SL, Larsson E, Larsson KH, Verbeken A, Nuytinck J (2006) Perspectives in the new Russulales. Mycologia 98:960–970
Miller SL, Aime MC, Henkel TW (2012) Russulaceae of Pakaraima Mountain of Guyana 2. New species of Russula and Lactifluus. Mycotaxon 121:233–253
Milne I, Wright F, Rowe G, Marshal DF, Husmeier D, McGuire G (2004) TOPALi: software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20:806–1807
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJ, Larsson E, Baroni TJ, Thorn RG, Jacobssong S, Clémençond H, Miller OK Jr (2002) One hundred and seventeen clades of euagarics. Mol Phylogenet Evol 23(3):357–400
Monteiro JS, Castañeda-Ruiz RF, Gusmão LFP (2016) Thozetella coronata and T. ypsiloidea spp. nov. from the Brazilian Amazon forest. Mycotaxon 131(3):605–611
Moreno G, Heykoop M (1998) Type studies in the genus Coprinus (Coprinaceae, Agaricales)–Coprinus xerophilus a new record in Europe. Persoonia 17(1):97–111
Morin L, Shivas RG, Piper MC, Tan YP (2010) Austropleospora osteospermi gen. et sp. nov. and its host specificity and distribution on Chrysanthemoides monilifera ssp. rotundata in Australia. Fungal Divers 40(1):65–74
Müller E, Petrini O, Fisher PJ, Samuels GJ, Rossman AY (1987) Taxonomy and anamorphs of the Herpotrichiellaceae with notes on generic synonymy. Trans Br Mycol Soc 88(1):63–74
Munk A (1953) The system of the Pyrenomycetes. Dansk Bot Arkiv 15(2):1–163
Munk A (1957a) Danish Pyrenomycetes. A preliminary flora. Dansk Bot Arkiv 17:1–491
Munk A (1957b) On relations between ecologic and taxonomic aspects in pyrenomycetes. Beih Sydowia 1:9–13
Murrill WA (1945) More fungi from Florida. Lloydia 8:263–290
MycoBank (2019) http://www.mycobank.org/Biolomics.aspx?Table=Mycobank&Page=200
Nag Raj TR (1976) Miscellaneous microfungi. I. Can J Bot 54(12):1370–1376
Nagy L (2007) Notes on taxa of Coprinus subsection Alachuani from Hungary. Osterr Z Pilzkd 16:167–180
Nakasone KK (2008) Type studies of corticioid Hymenomycetes described by Bresadola. Cryptog Mycol 29:231–257
Nannfeldt JA (1932) Studien über die Morphologie und Systematik der nicht-lichenisierten inoperculaten Discomyceten. Nova Acta Regiae Soc Sci Ups Ser 4(8):1–368
Nasr S, Bien S, Soudi MR, Alimadadi N, Fazeli SAS, Damm U (2018) Novel Collophorina and Coniochaeta species from Euphorbia polycaulis, an endemic plant in Iran. Mycol Prog 17(6):755–771
Nawawi A, Kuthubutheen AJ (1989) Canalisporium, a new genus of lignicolous Hyphomycetes from Malaysia. Mycotaxon 34:475–487
Nepel M, Voglmayr H, Schönenberger J, Mayer VE (2014) High diversity and low specificity of Chaetothyrialean fungi in carton galleries in a Neotropical ant–plant association. PLoS ONE 9(11):e112756
Noordeloos ME (1995) Tribus Collybieae. In: Bas C, Kuyper TW, Noordeloos ME, Vellinga EC (eds) Flora Agaricina Neerlandica 3. A.A. Balkema, Rotterdam, pp 123–129
Norphanphoun C, Doilom M, Daranagama DA, Phookamsak R, Wen TC, Bulgakov TS, Hyde KD (2017) Revisiting the genus Cytospora and allied species. Mycosphere 8:51–97. https://doi.org/10.5943/mycosphere/8/1/7
Norphanphoun C, Raspé O, Jeewon R, Wen TC, Hyde KD (2018) Morphological and phylogenetic characterisation of novel Cytospora species associated with mangroves. MycoKeys 38:93–120
Nuhn ME, Binder M, Taylor AFS, Halling RE, Hibbett D (2013) Phylogenetic overview of the Boletineae. Fungal Biol 117:479–511
O’Donnell K (1993) Fusarium and its near relatives. CAB International, Wallingford
Oberwinkler F (1993) Genera in a monophyletic group: the Dacrymycetales. Mycol Helv 6:35–72
Oberwinkler F (2014) Dacrymycetes. In Esser K, McLaughin DJ, Spataphora JS (eds) The fungi, vol IVB, pp 317–325
Olariaga I, Salcedo I (2012) New combinations and notes in clavarioid fungi. Mycotaxon 121:37–44
Olariaga I, Begoña MJ, Garcıá-Etxerbarria K, Salcedo I (2009) Species delimitation in the European species of Clavulina (Cantharellales, Basidiomycota) inferred from phylogenetic analyses of ITS region andmorphological data. Mycol Res 113:1261–1270
Ordynets A, Larsson KH, Langer E (2015) Two new Trechispora species from La Réunion Island. Mycol Prog 14:1–11
Paloi S, Dutta AK, Acharya K (2015) A new species of Russula (Russulales) from Eastern Himalaya, India. Phytotaxa 234(3):255–262
Parbery DG (1967) Studies on graminicolous species of Phyllachora Nke in Fckl. V. A taxonomic monograph. Aust J Bot 15:271–375
Parmasto E (1968) Conspectus systematis corticiacearum. Inst Zool Bot Acad Scient Estonicae, Tartu
Parra LA (2013) Agaricus L. Allopsalliota, Nauta & Bas, Fungi Europaei, vol 1A. Candusso Edizioni s.a.s. Alassio
Parra LA, Angelini C, Ortiz-Santana B, Mata G, Billette C, Rojo C, Chen J, Callac P (2018) The genus Agaricus in the Caribbean. Nine new taxa mostly based on collections from the Dominican Republic. Phytotaxa 345(3):219–271
Patouillard N (1904) Contribution à l’histoire naturelle de la Tunisie. Notes mycologiques. Bull Soc Hist Nat. Autun. 17:143–156
Pearce CA, Hyde KD (1994) The genus Phyllachora from Australia: observations on taxa from Callistemon species. Mycol Res 98:1393–1401
Pearson AA (1950) The Genus Russula. The Naturalists Univ. of Leeeds, London
Peck CH (1900) New species of fungi. Bull Torrey Bot Club 27:14–21
Pegler DN (1986) Agaric flora of Sri Lanka. Kew Bull Add Ser XII, Her Majesty’s Stationary Office, London
Pegler DN, Fiard JP (1978) Hygrocybe sect. Firmae (Agaricales) in tropical America. Kew Bull 32:297–312
Perdomo H, García D, Gené J, Cano J, Sutton DA, Summerbell Guarro J (2013) Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia 105(2):398–421
Perera RH, Maharachchikumbura SSN, Bhat JD, Al-Sadi AM, Liu JK, Hyde KD, Liu ZY (2016) New species of Thozetella and Chaetosphaeria and new records of Chaetosphaeria and Tainosphaeria from Thailand. Mycosphere 7:1301–1321
Pérez-De-Gregorio MA, Vizzini A, Contu M, Roqué C, Ercole E (2011) Marasmiellus celebanticus (Agaricales, Omphalotaceae), a new species of Marasmiellus sect. Candidi collected in the Mediterranean area. Phytotaxa 25:49–59
Petersen RH (1967) Notes of clavarioid fungi VI. Two new species and notes on the origin of Clavulina. Mycologia 59:39–47
Petersen RH (1983) Notes on clavarioid fungi XVIII. A preliminary outline of Clavulina in Southeastern Australia. Nova Hedwigia 37:19–35
Petersen RH (1985) Notes on clavarioid fungi XX. New taxa and distributional records in Clavulina and Ramaria. Mycologia 77:903–919
Petersen RH (1988a) The clavarioid fungi of New Zealand. DSIR Science Information Publishing, Wellington
Petersen RH (1988b) Notes on clavarioid fungi XXII. Three interesting South American collections. Mycologia 80:571–576
Petrak F (1923) Beiträge zur Pilzflora von Sternberg in Mähren. Ann Mycol 21:107–132
Pfister DH, Matočec N, Kušan I (2009) Integrated studies in the classification of the Pezizaceae. Re-evaluation of the genus Pachyella with a new segregate genus Adelphella. Mycol Monten 11:7–17
Phillips R (1990) Der Kosmos-Pilzatlas. Franckh-Kosmos Verlag, Stuttgart, p 288
Phillips AJL, Alves A, Correia A, Luque J (2005) Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97:513–529
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Phillips AJL, Hyde KD, Alves A, Liu JKJ (2018) Families in Botryosphaeriales: a phylogenetic, morphological and evolutionary perspective. Fungal Divers 94: 1–22
Phookamsak R, Liu JK, Manamgoda DS, Wanasinghe DN, Ariyawansa H, Mortimer PE, Chukeatirote E, McKenzie EH, Hyde KD (2014a) Epitypification of two bambusicolous fungi from Thailand. Cryptogam Mycol 35(3):239–256
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa HA, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014b) Revision of Phaeosphaeriaceae. Fungal Divers. 68:159–238
Phookamsak R, Manamgoda DS, Li WJ, Dai DQ, Singtripop C, Hyde KD (2015a) Poaceascoma helicoides gen et sp. nov., a new genus with scolecospores in Lentitheciaceae. Cryptogam Mycol 36(2):213–224
Phookamsak R, Norphanphoun C, Tanaka K, Dai DQ, Luo ZL, Liu JK, Su HY, Bhat DJ, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2015b) Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov and Astrosphaeriellopsis, gen. nov. Fungal Divers 74:143–197
Phookamsak R, Wanasinghe DN, Hongsanan S, Phukhamsakda C, Huang SK, Tennakoon DS, Norphanphoun C, Camporesi E, Bulgakov TS, Promputtha I, Mortimer PE, Xu JC, Hyde KD (2017) Towards a natural classification of Ophiobolus and ophiobolus-like taxa; introducing three novel genera Ophiobolopsis, Paraophiobolus and Pseudoophiobolus in Phaeoshaeriaceae (Pleosporales). Fungal Divers 87:299–339
Phookamsak R, Lu YZ, Hyde KD, Jeewon R, Li J, Doilom M, Boonmee S, Promputtha I (2018) Phylogenetic characterization of two novel Kamalomyces species in Tubeufiaceae (Tubeufiales). Mycol Prog 17(5):647–660
Phukhamsakda C, Ariyawansa HA, Phookamsak R, Chomnunti P, Bulgakov TS, Yange JB, Bhat DJ, Bahkalih AH, Hyde KD (2015) Muriphaeosphaeria galatellae gen. et sp. nov. in Phaeosphaeriaceae (Pleosporales). Phytotaxa 227(1):55–65
Phukhamsakda C, Hongsanan S, Ryberg M, Ariyawansa HA, Chomnunti P, Bahkali AH, Hyde KD (2016) The evolution of Massarineae with Longipedicellataceae fam. nov. Mycosphere 7(11):1713–1731
Phukhamsakda C, Bhat DJ, Hongsanan S, Tibpromma S, Yang JB, Promputtha I (2017) Magnicamarosporium diospyricola sp. nov (Sulcatisporaceae) from Thailand. Mycosphere 8(4):512–520
Phukhamsakda C, Bhat DJ, Hongsanan S, Xu JC, Stadler M, Hyde KD (2018) Two novel species of Neoaquastroma (Parabambusicolaceae, Pleosporales) with their phoma-like asexual morphs. MycoKeys 34:47
Pilát A (1926) Monographie der mitteleuropeischen Aleurodisineen. Ann Mycol 24:203–230
Pilát A (1930) Monographie der europaischen Stereaceen. Hedwigia 70:10–132
Pitt JI (1979) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London
Pitt JI, Hocking AD (2009) Fungi and food spoilage, 3rd edn. Springer, New York
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14(9):817–818
Preuss CGT (1851) Übersicht untersuchter Pilze, besonders aus der Umgegend von Hoyerswerda. Linnaea 24:99–153
Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EH, Hyde KD, Jeewon R (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb Ecol 53(4):579–590
Promputtha I, Hyde KD, McKenzie EH, Peberdy JF, Lumyong S (2010) Can leaf degrading enzymes provide evidence that endophytic fungi becoming saprobes? Fungal Divers 41(1):89–99
Quaedvlieg W, Binder M, Groenewald JZ, Summerell BA, Carnegie AJ, Burgess TI, Crous PW (2014) Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33:1
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rabenhorst, GL (1874) Fungi europaea exsiccate. Cent. XIX, No. 1824. Hedwigia 13:184
Raitviír A (1964) Izv. Akad Nauk Estonsk SSR 13:29
Raitviir A (2002) A revision of the genus Dasyscyphella (Hyaloscyphaceae, Helotiales). Polish Bot J 47(2):227–241
Raitviir A (2004) Revised Synopsis of the Hyaloscyphaceae. Scripta Mycol 20:1–133
Rambaut A, Suchard M, Drummond A (2013) Tracer, v1.6. http://tree.bio.ed.ac.uk/software/tracer/
Rappaz F (1992) Phomatospora berkeleyi, P. arenaria and their Sporothrix anamorphs. Mycotaxon 45:323–330
Raspé O, Vadthanarat S, De Kesel A, Degreef J, Hyde KD, Lumyong S (2016) Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycol Prog 15:38
Rawla GS (2001) Himalayan species of Russula Pers. Ex S.F. Gray. In: Pande PC, Samant SS (eds) Plant diversity of the Himalaya. Gyanodaya Prakashan, Nainital, pp 1–48
Rayner RW (1970) A mycological colour chart. Commonwealth Mycological Institute, Kew, p 34
Rayner RW (1985) Keys to the British species of Russula, 3rd edn. British Mycological Society, Cambridge, p 99
Réblová M (2000) The genus Chaetosphaeria and its anamorphs. Stud Mycol 45:149–168
Réblová M, Barr ME, Samuels GJ (1999) Chaetosphaeriaceae, a new family for Chaetosphaeria and its relatives. Sydowia 51:49–70
Réblová M, Untereiner WA, Réblová K (2013) Novel Evolutionary Lineages Revealed in the Chaetothyriales (Fungi) based on Multigene Phylogenetic Analyses and Comparison of ITS Secondary Structure. PLoS ONE 8(5):e63547
Réblová M, Miller AN, Rossman AY, Seifert KA, Crous PW, Hawksworth DL, Abdel-Wahab MA, Cannon PF, Daranagama DA, De Beer ZW, Huang SK, Hyde KD, Jayawardena RS, Jaklitsch W, Jones EBG, Ju YM, Judith C, Maharachchikumbura SSN, Pang KL, Petrini LE, Raja HA, Romero AI, Shearer C, Senanayake IC, Voglmayr H, Weir BS, Wijayawardene NN (2016) Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus 7(1):131–153
Redhead SA, Vilgalys R, Moncalvo JM, Johnson J, Hopple JS Jr (2001) Coprinus Pers. and the disposition of Coprinus species sensu lato. Taxon 50:203–241
Rehner SA (2001) Primers for elongation factor 1-alpha (EF1-alpha). http://www.aftol.org/pdfs/EF1primer.pdf
Reid DA (1955) New or interesting records of Australasian basidiomycetes. Kew Bull 10:631–648
Reid DA (1974) A monograph of the British Dacrymycetales. Trans Br Mycol Soc 62(3):404–433
Retnowati A (2012) Taxonomic study of the genus Marasmiellus Murrill in Java and Bali. Doctoral Thesis. University of Indonesia
Ribes JA, Vanover-Sams CL, Baker DJ (2000) Zygomycetes in human disease. Clin Microbiol Rev 13:236–301
Roberts P (1999) Clavarioid fungi from Korup National Park, Cameroon. Kew Bull 54:517–539
Roberts P (2006) Caribbean heterobasidiomycetes: 2. Jamaica. Mycotaxon 96:83–107
Rogers DP (1944) The genera Trechispora and Galzinia (Thelephoraceae). Mycologia 36:70–103
Rogers DP (1951) Trechispora and Pellicularia. Mycologia 43:111
Rogerson CT (1970) The hypocrealean fungi (ascomycetes, Hypocreales). Mycologia 62(5):865–910
Romagnesi H (1967) Les Russules d' Europe et d' Afrique du nord. Bordas, Paris, p 998
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Rossman AY, Farr DF, Castlebury LA (2007) A review of the phylogeny and biology of the Diaporthales. Mycoscience 48:135–144
Rossman AY, Adams GC, Cannon PF, Castlebury LA, Crous PW, Gryzenhout M, Jaklitsch WM, Mejia LC, Stoykov D, Udayanga D, Voglmayr H (2015) Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6(1):145–154
Ryvarden L (2002) A note on the genus Hydnodon Banker. Syn Fung 15:31–33
Ryvarden L, Melo I (2014) Poroid fungi of Europe. Synopsis Fung 31:1e455
Saccardo PA (1878) Fungi Italici autographice delineati a Prof. P.A. Saccardo. Patavii 1878. Fascicoli V.-VIII. Michelia 1(3):326–350
Saccardo PA (1880a) Conspectus generum fungorum Italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium systemate sporologico dispositorum. Michelia 2(6):1–38
Saccardo PA (1880b) Fungi Gallici lecti a cl. viris P. Brunaud, Abb. Letendre, A. Malbranche, J. Therry, vel editi in Mycotheca Gallica C. Roumeguèri. Series II. Michelia 2(6):39–135
Saccardo PA (1882) Sylloge Fungorum I: 198
Saccardo PA (1883a) Sylloge Fungorum. Omnium hucusque cognitorum 2:658–661
Saccardo PA (1883b) Sylloge Pyrenomycetum, vol. II. Sylloge Fungorum 2:1–813
Saccardo PA (1892) Supplementum Universale, Pars II. Discomyceteae-Hyphomyceteae. Syll Fung 10:1–964
Sádlíková M, Kout J (2017) A new Phanerochaete (Polyporales, Basidiomycota) with brown subicular hyphae from Thailand. Mycosphere 8:1024–1030
Saini SS, Atri NS (1989) North Indian Agaricales XI-section Russula Pers. of Genus Russula Pers. in India. Indian J Mycol Plant Pathol 19:44–49
Samarakoon MC, Hyde KD, Promputtha I, Hongsanan S, Ariyawansa HA, Maharachchikumbura SSN, Daranagama DA, Stadler M, Mapook A (2016) Evolution of Xylariomycetidae (Ascomycota: Sordariomycetes). Mycosphere 7(11):1746–1761
Samarakoon MC, Gafforov Y, Liu NG, Maharachchikumbura SSN, Bhat JD, Liu JK, Promputtha I, Hyde KD (2018) Combined multi-gene backbone tree for the genus Coniochaeta with two new species from Uzbekistan. Phytotaxa 336(1):43–58
Samson RA, Houbraken J, Thrane U (2010) Food and indoor fungi. CBS KNAW Biodiversity Center, Utrecht
Samuels GJ (2006) Trichoderma: systematics, the sexual state, and ecology. Phytopathology 96(2):195–206
Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O (2002) Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94(1):146–170
Samuelson DA (1978) Asci of the Pezizales. I. The apical apparatus of iodine-positive species. Can J Bot 56(16):1860–1875
Sarnari M (1998) Monografia Iillustrata Del Genere Russula in Europa. Tomo Primo, Italy: 799
Sarnari M (2005) Monografia Illustrata Del Genere Russula in Europa. Tromo Secondo, Italy, 2:762
Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, Fleetwood DJ, Haws DC, Moore N, Oeser B, Panaccione DG, Schweri KK, Voisey CR, Farman ML, Jaromczyk JW, Roe BA, O’Sullivan DM, Scott B, Tudzynski P, An Z, Arnaoudova EG, Bullock CT, Charlton ND, Chen L, Cox M, Dinkins RD, Florea S, Glenn AE, Gordon A, Güldener U, Harris DR, Hollin W, Jaromczyk J, Johnson RD, Khan AK, Leistner E, Leuchtmann A, Li C, Liu JG, Liu J, Liu M, Mace W, Machado C, Nagabhyru P, Pan J, Schmid J, Sugawara K, Steiner U, Takach JE, Tanaka E, Webb JS, Wilson EV, Wiseman JL, Yoshida R, Zeng Z (2013) Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet 9(2):e1003323
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W and Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS 109(16):6241–6246
Schröter J (1888) Pilze. Erste Hälfe. In: Cohn F (ed) Krypt.-Fl. Schlesien 3. J.U. Kern Verlag (Max Müller), Breslau (1889)
Schröter J (1889) Pilze. Erste Hälfte. In: F. Cohn Kryptogamen-Flora von Schlesien 3 (1), [ii–iv], 1–814. J.U. Kern’s Verlag, Germany
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87(3):787–799
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat JD, Jones EBG, McKenzie EHC, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S, Li WJ, Shang QJ, Stadler M, Wijayawardene NN, Xiao YP, Norphanphoun C, Li Q, Liu XZ, Bahkali AH, Kang JC, Wang Y, Wen TC, Wendt L, Xu JC, Camporesi E (2015) Towards unravelling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144
Senanayake IC, Crous PW, Groenewald JZ, Maharachchikumbura SSN, Jeewon R, Phillips AJL, Bhat JD, Perera RH, Li QR, Li WJ, Tangthirasunun N, Norphanphoun C, Karunarathna SC, Camporesi E, Manawasighe IS, Al-Sadi AM, Hyde KD (2017) Families of Diaporthales based on morphological and phylogenetic evidence. Stud Mycol 86:217–296
Senanayake IC, Jeewon R, Chomnunti P, Wanasinghe DN, Norphanphoun C, Karunarathna A, Pem D, Perera RH, Camporesi E, McKenzie EHC, Hyde KD (2018) Taxonomic circumscription of Diaporthales based on multigene phylogeny and morphology. Fungal Divers 93(1):241–443
Senthilarasu G, Kumaresan V, Singh SK (2010) A new species of Hygrocybe in section Firmae from Western Ghats, India. Mycotaxon 111:301–307
Senwanna C, Phookamsak R, Doilom M, Hyde KD, Cheewangkoon R (2017) Novel taxa of Diatrypaceae from Para rubber (Hevea brasiliensis) in northern Thailand; introducing a novel genus Allocryptovalsa. Mycosphere 8:1835–1855
Shang QJ, Hyde KD, Phookamsak R, Doilom M, Bhat DJ, Maharachchikumbura SS, Promputtha I (2017) Diatrypella tectonae and Peroneutypa mackenziei sp. nov. (Diatrypaceae) from northern Thailand. Mycol Prog 16:463–476
Shang QJ, Hyde KD, Jeewon R, Khan S, Promputtha I, Phookamsak R (2018) Morpho-molecular characterization of Peroneutypa (Diatrypaceae, Xylariales) with two novel species from Thailand. Phytotaxa 356(1):1–8
Sharma JR, Das K (2003) New and interesting species of Lactarius from India. Mycotaxon 88:377–385
Shearer CA, Crane JL, Chandra Reddy KR (1990) Studies in Leptosphaeria. Lectotypification of Sphaeria doliolum. Mycologia 82:496–500
Shirouzu T, Hosoya T (2017) Type study of Japanese Dacrymycetes described by Yosio Kobayasi: redescriptions of five species and a new name proposal. Mycoscience 58:129–136
Shirouzu T, Hirose D, Tokumasu S (2007) Sequence analyses of the 28S rRNA gene D1/D2 region suggest Dacrymyces (Heterobasidiomycetes, Dacrymycetales) is polyphyletic. Mycoscience 48:388–394
Shirouzu T, Hirose D, Tokumasu S (2009) Taxonomic study of Japanese Dacrymycetes. Persoonia 23:16–34
Shirouzu T, Hirose D, Oberwinkler F, Shimomura N, Maekawa N, Tokumasu S (2013) Combined molecular and morphological data for improving phylogenetic hypothesis in Dacrymycetes. Mycologia 105(5):1110–1125
Shirouzu T, Hosaka K, Nam KO, Weir BS, Johnston PR, Hosoya T (2017) Phylogenetic relationships of eight new Dacrymycetes collected from New Zealand. Persoonia 38:156–169
Shivas RG, Marney TS, Tan YP, Abell SE (2016) Fungal planet description sheets: 469–557. Persoonia 37:218–403
Shoemaker RA (1976) Canadian and some extralimital Ophiobolus species. Can J Bot 54:2365–2404
Shoemaker RA (1984) Canadian and some extralimital Leptosphaeria species. Can J Bot 62:2688–2729
Silva M, Pereira OL (2008) Black mildew disease of the neotropical orchid Epidendrum secundum caused by Lembosia epidendri sp. nov. from Minas Gerais, Brazil. Mycotaxon 104:385–390
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Singer R (1973) The genera Marasmiellus, Crepidotus and Simocybe in the Neotropics. Nova Hedwig Beih 44:1–517
Singer R (1976) Marasmieae (Basidiomycetes-Tricholomataceae). Flora Neotrop Monogr 17:1–347
Singer R (1986) The Agaricales in Modern Taxonomy. Bishen Singh Mahendra Pal Singh, Dehradun
Sjökvist E, Larsson E, Eberhardt U, Ryvarden L, Larsson KH (2012) Stipitate steroid basidiocarps have evolved multiple times. Mycologia 104:1046–1055
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:90–106
Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Lombard L, Wingfield MJ, Postma A, Burgess T, Crous PW (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 76:31–49
Sogonov MV, Castlebury LA, Rossman AY, Mejía LC, White JF (2008) Leaf-inhabiting genera of the Gnomoniaceae, Diaporthales. Stud Mycol 62:1–79
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, O’Donnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108:1028–1046
Spegazzini C (1908) Fungi aliquot paulistani. Revista del Museo de La Plata 15:7–48
Spirin V, Volobuev S, Okun M, Miettinen O, Larsson KH (2017) What is the type of Phanerochaete (Polyporales, Basidiomycota)? Mycol Prog 16:171–183
Sri-Indrasutdhi V, Boonyuen N, Suetrong S, Chuaseeharonnachai C, Sivichai S, Jones EG (2010) Wood-inhabiting freshwater fungi from Thailand: Ascothailandia grenadoidia gen. et sp. nov., Canalisporium grenadoidia sp. nov. with a key to Canalisporium species (Sordariomycetes, Ascomycota). Mycoscience 51(6):411–420
Staden R, Beal KF, Bonfield JK (1998) The Staden Package. Comput Methods Mol Biol 132: 115–130. In: Misener S, Krawetz SA (eds) Bioinformatics methods and protocols. The Humana Press, Totowa
Stöver BC, Müller KF (2010) TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 11:7
Stubbe D, Nuytinck J, Verbeken A (2010) Critical assessment of the Lactarius gerardii species complex (Russulales). Fungal Biol 114(2–3):271–283
Stubbe D, Le HT, Wang XH, Nuytinck J, Van de Putte K, Verbeken A (2012a) The Australasian species of Lactarius subgenus Gerardii (Russulales). Fungal Divers 52(1):141–167
Stubbe D, Wang XH, Verbeken A (2012b) New combinations in Lactifluus. 2. L. subgenus Gerardii. Mycotaxon 119:483–485
Su H, Hyde KD, Maharachchikumbura SSN, Ariyawansa HA, Luo Z, Promputtha I, Tian Q, Lin C, Shang QJ, Zhao YC, Chai HM, Liu XY, Bahkali AH, Bhat DJ, McKenzie EHC, Zhou DQ (2016) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal divers 80:375–409
Su L, Deng H, Niu YC (2017) Phylogenetic analysis of Plectosphaerella species based on multi-locus DNA sequences and description. Mycol Prog 16:823–829
Sun JZ, Liu XZ, McKenzie EHC, Jeewon R, Liu JK, Zhang XL, Zhao Q, Hyde KD (2019) Fungicolous fungi: terminology, diversity, distribution, evolution and species checklist. Fungal Divers. https://doi.org/10.1007/s13225-019-00422-9
Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Sutton BC (1977) Coelomycetes VI. Nomenclature of generic names proposed for coelomycetes. Mycol Pap 141:1–253
Suwannarach N, Kumla J, Lumyong S (2016) Pseudoplagiostoma dipterocarpi sp. nov., a new endophytic fungus from Thailand. Mycoscience 57(2):118–122
Sydow H (1939) Fungi Aequatorienses (series prima). Ann Mycol 37:275–438
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729
Tan YS, Desjardin DE, Perry BA, Vikineswary S, Noorlidah A (2009) Marasmius sensu stricto in Peninsular Malaysia. Fungal Divers 37:9–100
Tanaka K, Harada Y (2003a) Pleosporales in Japan (2): the genus Lophiotrema. Mycoscience 44(2):115–121
Tanaka K, Harada Y (2003b) Pleosporales in Japan (3). The genus Massarina. Mycoscience 44:173–185
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tang LP, Cai Q, Lee SS, Buyck B, Zhang P, Yang ZL (2015) Taxonomy and phylogenetic position of species of Amanita sect. Vaginatae s.l. from tropical Africa. Mycol Prog 39:1–15
Tedersoo L, Sánchez-Ramírez S, Kõljalg U, Bahram M, Döring M, Schigel D, May T, Ryberg M, Abarenkov K (2018) High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers 90(1):135–159
Telleria MT, Melo I, Dueñas M, Larsson K-H, Martín MP (2013) Molecular analyses confirm Brevicellicium in Techisporales. IMA Fungus 4:21–28
Tennakoon DS, Hyde KD, Phookamsak R, Wanasinghe DN, Camporesi E, Promputtha I (2016) Taxonomy and phylogeny of Juncaceicola gen. nov. Phaeosphaeriaceae, Pleosporinae, Pleosporales. Cryptogam Mycol 37(2):135–156
Tennakoon DS, Phookamsak R, Wanasinghe DN, Yang JB, Lumyong S, Hyde KD (2017) Morphological and phylogenetic insights resolve Plenodomus sinensis (Leptosphaeriaceae) as a new species. Phytotaxa 324(1):73–82
Thacker JR, Henkel TW (2004) New species of Clavulina from Guyana. Mycologia 96:650–657
Thambugala KM, Singtripop C, Chunfang Y, Mckenzie EHC, Liu ZY, Chukeatirote E, Hyde KD (2014) Towards a natural classification of Dothideomycetes 7: the genera Allosoma, Austropleospora, Dangeardiella, Griggsia and Karschia (Dothideomycetes genera incertae sedis). Phytotaxa 181(1):34–46
Thambugala KM, Hyde KD, Tanaka K, Tian Q, Wanasinghe DN, Ariyawansa HA, Jayasiri SC, Boonmee S, Camporesi E, Hashimoto A, Hirayama K, Schumacher RK, Promputtha I, Liu ZY (2015) Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Divers 74:199–266
Thambugala KM, Wanasinghe DN, Phillips AJL, Camporesi E, Bulgakov TS, Phukhamsakda C, Ariyawansa HA, Goonasekara ID, Phookamsak R, Dissanayake A, Tennakoon DS, Tibpromma S, Chen YY, Liu ZY, Hyde KD (2017) Mycosphere notes 1-50: grass (Poaceae) inhabiting Dothideomycetes. Mycosphere 8(4):697–796
Thiers HD, Ammirati JF (1982) New species of Amanita from western North America. Mycotaxon 15:155–166
Thind KS (1961) The Clavariaceae of India. Indian Council of Agricultural Research, New Delhi
Thind KS, Sharda RM (1984) Three new species of clavarioid fungi from Himalayas. Indian Phytopathol 37:234–240
Thongklang N, Nawaz R, Khalid AN, Chen J, Hyde KD, Zhao R, Parra LA, Hanif M, Moinard M, Callac P (2014) Morphological and molecular characterization of three Agaricus species from tropical Asia (Pakistan, Thailand) reveals a new group in section Xanthodermatei. Mycologia 106(6):1220–1232
Tibpromma S, McKenzie EHC, Karunarathna SC, Mortimer PE, Xu J, Hyde KD, Hu DM (2016) Muyocopron garethjonesii sp. nov. (Muyocopronales, Dothideomycetes) on Pandanus sp. Mycosphere 7:1480–1489
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo Santiago ALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li JF, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Meiras-Ottoni A, Meŝič A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, Plautz HL Jr, Ariyawansa HA, Lee H, Kuśan I, Song J, Sun J, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikśìk M, Samarakoon MC, Wijayawardene NN, Kim NK, Matocčec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Svetasheva STY, Nguyen TTT, Antonìn V, Li WJ, Wang Y, Indoliya Y, Tkalčec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, Luangsa-ard J, Xu JC, Yan J, Chuan KJ, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen TC, Karunarathna SC (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261
Tibpromma S, Hyde KD, McKenzie EHC, Bhat DJ, Phillips AJL, Wanasinghe DN, Samarakoon MC, Jayawardena RS, Dissanayake AJ, Tennakoon DS, Doilom M, Phookamsak R, Tang AMC, Xu JC, Mortimer PE, Promputtha I, Maharachchikumbura SSN, Khan S, Karunarathna SCK (2018) Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Divers 92:1–160
Tode HJ (1791) Fungi Mecklenburgenses selecti. Fasc. II, Generum novorum appendicem, Lüneburg
Trakunyingcharoen T, Lombard L, Groenewald JZ, Cheewangkoon R, To-anun C, Alfenas AC, Crous PW (2014) Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5(2):391–414
Trappe JM, Castellano MA (2007) Clavulina lilliputiana, a diminutive new species from Tasmania. Australas Mycol 25:87–89
Troy GC, Panciera DL, Pickett JP, Sutton DA, Gene J, Cano JEF, Guarro J, Thompson EH, Wickes BL (2013) Mixed infection caused by Lecythophora canina sp. nov. and Plectosphaerella cucumerina in a German shepherd dog. Medical Mycol 51:455–460
Tulasne LR, Tulasne C (1863) Selecta Fungorum Carpologia, Tomus Secundus. Xylariei-Valsei-Sphaeriei 2:1–319
Tulloss RE (2005) Amanita-distribution in the Americas, with comparison to eastern and southern Asia and notes on spore character variation with latitude and ecology. Mycotaxon 93:189–231
Tulloss RE, Yang ZL (2016) Studies in the Amanitaceae. www.amanitaceae.org. Accessed 2 Dec 2016
Tulloss RE, Ovrebo CL, Halling RE (1992) Studies on Amanita (Amanitaceae) from Andean Colombia. Mem N Y Bot Gard 66:1–46
Tulloss RE, Kuyper TW, Vellinga EC, Yang ZL, Halling RE, Geml J, Sánchez-Ramírez S, Gonçalves SC, Hess J, Pringle A (2016) The genus Amanita should not be split. Amanitaceae 1(3):1–16
Uehling JK, Henkel TW, Aime MC, Vilgalys R, Smith ME (2012a) New species of Clavulina (Cantharellales, Basidiomycota) with resupinate and effused basidiomata from the Guiana Shield. Mycologia 104:547–556
Uehling JK, Henkel TW, Aime MC, Vilgalys R, Smith ME (2012b) New species and distribution records for Clavulina (Cantharellales, Basidiomycota) from the Guiana Shield, with a key to the lowland neotropical taxa. Fungal Biol 116:1263–1274
Uljé CB (2005) 1. Coprinus Pers. In: Noordeloos ME, Kuyper TW, Vellinga EC (eds) Flora Agaricina Neerlandica 6. Taylor & Francis, Boca Raton, pp 22–109
Uniyal P, Das K, Bhatt RP, Singh U, Mehmood T (2017) Lactarius kumaonensis, a novel species in Russulaceae from Uttarakhand, India. Nord J Bot 35(6):724–729
Untereiner WA (2000) Capronia and its anamorphs: exploring the value of morphological and molecular characters in the systematics of the Herpotrichiellaceae. Stud Mycol 45:141–148
Untereiner WA, Gueidan C, Orr MJ, Diederich P (2011) The phylogenetic position of the lichenicolous ascomycete Capronia peltigerae. Fungal Divers 49(1):225–233
Vadthanarat S, Raspé O, Lumyong S (2018) Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys 29:63–80
Van Niekerk JM, Groenewald JZE, Verkley GJM, Fourie PH, Wingfield MJ, Crous PW (2004) Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycol Res 108:283–303
van de Bogart F (1976) The genus Coprinus in Western North America. Part I: Section Coprinus. Mycotaxon. Mycotaxon 4(1):233–275
Vellinga EC (2004) Genera in the family Agaricaceae: evidence from nrITS and nrLSU sequences. Mycol Res 108(4):354–377
Venturella G (2005) L’iconografia micologica di Giuseppe Inzenga. ISPE Archimede S.r.l, Palermo
Verbeken A, Nuytinck J (2013) Not every milkcap is a Lactarius. Scr Bot Belg 51:162–168
Verbeken A, Horak E, Desjardin DE (2001) Agaricales in Indonesia. 3. New records of the genus Lactarius (Basidiomycota, Russulales) from Java. Sydowia 53(2):261–289
Vesterholt J (2012) Coprinopsis P. Karst. In: Knudsen H, Vesterholt J (eds) Funga Nordica. Nordsvamp, Copenhagen, pp 672–687
Viégas AP (1945) Alguns Fungos do Brasil VI: Dacryomycetaceae–Tremellaceae. Bragantia 5:239–251
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Visagie CM, Houbraken J, Frisvad JC, Hong SB, Klaassen CHW, Perrone G, Seifert KA, Varga J, Yaguchi T, Samson RA (2014a) Identification and nomenclature of the genus Penicillium. Stud Mycol 78:343–371
Visagie CM, Seifert KA, Houbraken J, Samson RA, Jacobs K (2014b) Diversity of Penicillium section Citrina within the fynbos biome of South Africa, including a new species from a Protea repens infructescence. Mycologia 106:537–552
Voglmayr H, Park MJ, Shin HD (2011) Spiroplana centripeta gen. & sp. nov., a leaf parasite of Philadelphus with a remarkable aeroaquatic conidium morphology. Mycotaxon 116:203–216
Volobuev S, Okun M, Ordynets A, Spirin V (2015) The Phanerochaete sordida group (Polyporales, Basidiomycota) in temperate Eurasia, with a note on Phanerochaete pallida. Mycol Prog 14:1–13
von Arx JA, Müller E (1954) Die Gattungen der amerosporen Pyrenomyceten. Beitrage zur Kryptogamenflora der Schweiz 11(1):434
von Arx JA, Müller E (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol 9:1–159
von Höhnel F (1918) Dritte vorlaufige Mitteilung mycologischer Ergebnisse (Nr. 201–304). Berichte der Deutschen Botanischen Gesellschaft 36:309–317
Wäli PP, Huhtinen S, Pino-Bodas R, Stenroos S (2014) Three common bryophilous fungi with meristematic anamorphs and phylogenetic alliance to Teratosphaeriaceae, Capnodiales. Fungal Biol 118(12):956–969
Walther G, Pawlowska J, Alastruey-Izquierdo A, Wrzosek M, Rodriguez-Tudela JL, Dolatabadi S, Chakrabarti A, de Hoog GS (2013) DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia 30:11–47
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Hyde KD (2014a) An exciting novel member of Lentitheciaceae in Italy from Clematis vitalba. Cryptogam Mycol 35(4):323–337
Wanasinghe DN, Jones EG, Camporesi E, Boonmee S, Karunarathna SC, Thines M, Mortimer PE, Xu J, Hyde KD (2014b) Dematiopleospora mariae gen. sp. nov., from Ononis spinosa in Italy. Cryptogam Mycol 35(2):105–171
Wanasinghe DN, Jones EBG, Camporesi E, Dissanayake AJ, Kamolhan S, Mortimer PE, Xu JC, Abd-Elsalam K, Hyde KD (2016) Taxonomy and phylogeny of Laburnicola gen. nov. and Paramassariosphaeria gen. nov. (Didymosphaeriaceae, Massarineae, Pleosporales. Fungal Biol 120(11):1354–1373
Wanasinghe DN, Hyde KD, Konta S, To-Anun C, Jones EG (2017) Saprobic Dothideomycetes in Thailand: Neoaquastroma gen. nov. (Parabambusicolaceae) introduced based on morphological and molecular data. Phytotaxa 302(2):133–144
Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Jones EBG, Tibpromma S, Tennakoon DS, Dissanayake AJ, Jayasiri SC, Gafforov Y, Camporesi E, Bulgakov TS, Ekanayake AH, Perera RH, Samarakoon MC, Goonasekara ID, Mapook A, Li WJ, Senanayake IC, Li JF, Norphanphoun C, Doilom M, Bahkali AH, Xu JC, Mortimer PE, Tibell L, Tibell S, Karunarathna SC (2018) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers 89:1–236
Wang M, Jiang X, Wu W, Hao Y, Su Y, Cai L, Xiang M, Liu X (2015) Psychrophilic fungi from the world’s roof. Persoonia 34:100–112
Wang RX, Luo ZL, Hyde KD, Bhat DJ, Su XJ, Su HY (2016) New species and records of Dictyocheirospora from submerged wood in north-western Yunnan, China. Mycosphere 7(9):1357–1367
Wang X, Cai W, Ende AG, Zhang J, Xie T, Xi L, Li X, Sun J, Hoog S (2018) Indoor wet cells as a habitat for melanized fungi, opportunistic pathogens on humans and other vertebrates. Sci Rep 8(1):7685
Wannathes N, Desjardin DE, Hyde KD, Perry BA, Lumyong S (2009) A monograph of Marasmius (Basidiomycota) from Northern Thailand based on morphological and molecular (ITS sequences) data. Fungal Divers 37:209–306
Wartchow F (2012) Clavulina incrustata, a new species from Pernambuco, Brazil. Cryptogam Mycol 33:105–113
Wehmeyer LE (1975) The pyrenomycetous fungi. Mycol Mem 6:1–250
Weiß M, Oberwinkler F (2001) Phylogenetic relationships in Auriculariales and related groups—hypotheses derived from nuclear ribosomal DNA sequences. Mycol Res 105(4):403–415
Wendt L, Sir EB, Kuhnert E, Heitkämper S, Lambert C, Hladki AI, Romero AI, Luangsa-ard JJ, Srikitikulchai P, Peršoh D, Stadler M (2018) Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol Prog 17(1–2):115–154
White TJ, Bruns TD, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic, San Diego, pp 315–322
Wijayawardene NN, Hyde KD, Bhat DJ, Goonasekara ID, Nadeeshan D, Camporesi E, Schumacher RK, Wang Y (2015) Additions to Brown Spored Coelomycetous Taxa in Massarinae, Pleosporales: introducing Phragmocamarosporium gen. nov. and Suttonomyces gen. nov. Cryptogam Mycol 36:213–224
Wijayawardene NN, Hyde KD, Wanasinghe DN, Papizadeh M, Goonasekara ID, Camporesi E, Bhat DJ, McKenzie EHC, Phillips AJL, Diederich P, Tanaka K, Li WJ, Tangthirasunun N, Phookamsak R, Dai D-Q, Dissanayake AJ, Weerakoon G, Maharachchikumbura SNN, Hashimoto A, Matsumura M, Bahkali AH, Wang Y (2016) Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers 77:1–316
Wijayawardene NN, Hyde KD, Rajeshkumar KC, Hawksworth DL, Madrid H, Kirk PM, Braun U, Singh RV, Crous PW, Kukwa M, Lucking R, Kurtzman CP, Yurkov A, Haelewaters D, Aptroot A, Lumbsch HT, Timdal E, Ertz D, Etayo J, Phillips AJL, Groenewald JZ, Papizadeh M, Selbmann L, Dayarathne MC, Weerakoon G, Jones EBG, Suetrong S, Tian Q, Castanéda-Ruiz RF, Bahkali AH, Pang KL, Tanaka K, Dai DQ, Sakayaroj J, Hujslová M, Lombard L, Shenoy BD, Suija A, Maharachchikumbura SSN, Thambugala KM, Wanasinghe DN, Sharma BO, Gaikwad S, Pandit G, Zucconi L, Onofri S, Egidi E, Raja HA, Kodsueb R, Caceres MES, Perez-Ortega S, Fiuza PO, Monteiro JS, Vasilyeva LN, Shivas RG, Prieto M, Wedin M, Olariaga I, Lateef AA, Agrawal Y, Fazeli SAS, Amoozegar MA, Zhao GZ, Pfliegler WP, Sharma G, Oset M, Abdel MA, Takamatsu S, Bensch K, Silva NI, De Kesel A, Karunarathna A, Boonmee S, Pfister DH, Lu YZ, Luo ZL, Boonyuen N, Daranagama DA, Senanayake IC, Jayasiri SC, Samarakoon MC, Zeng XY, Doilom M, Quijada L, Rampadarath S, Heredia G, Dissanayake AJ, Jayawardana RS, Perera PH, Tang LZ, Phukhamsakda C, Hernández-Restrepo M, Ma XY, Tibpromma S, Gusmao LFP, Weerahewa D, Karunarathna SC (2017a) Notes for genera: Ascomycota. Fungal Divers 86:1–594
Wijayawardene NN, Hyde KD, Tibpromma S, Wanasinghe DN, Thambugala KM, Tian Q, Wang Y (2017b) Towards incorporating asexual fungi in a natural classification: checklist and notes 2012–2016. Mycosphere 8(9):1457–1554
Wijayawardene NN, Hyde KD, Lumbsch HT, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018a) Outline of Ascomycota: 2017. Fungal Divers 88:167–263
Wijayawardene NN, Pawłowska J, Letcher PM, Kirk PM, Humber RA, Schüßler A, Wrzosek M, Muszewska A, Okrasińska A, Istel Ł, Gęsiorska A, Mungai P, Lateef AA, Rajeshkumar KC, Singh RV, Radek R, Walther G, Wagner L, Walker C, Wijesundara DSA, Papizadeh M, Dolatabadi S, Shenoy BD, Tokarev YS, Lumyong S, Hyde KD (2018b) Notes for genera: basal clades of Fungi (including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Neocallimastigomycota, Olpidiomycota, Rozellomycota and Zoopagomycota). Fungal Divers 92:43–129
Willis KJ (ed) (2018) State of the World’s Fungi 2018. Royal Botanic Gardens, Kew
Wilson AW, Desjardin DE (2005) Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 97:667–679
Winter G (1884) Rabenhorst’s Kryptogamen-Flora. Pilze Ascomyceten 1(2):1–192
Winter G (1887) Pilze, Ascomyceten. In: Rabenhorst’s Kryptogamen Flora von Deutschland, Oesterreich und der Schweiz [i–vi] 1:1–928
Wong SW, Hyde KD, Ho WH, Stanley SJ (1998) Tamsiniella labiosa gen. et sp.nov., a new freshwater ascomycete from submerged wood. Can J Bot 76:332–337
Wu SH, Boidin J, Chien CY (2000) Acanthofungus rimosus gen. et sp. nov., with reevaluation of the related genera. Mycotaxon 76:153–161
Wu SH, Hibbett DS, Binder M (2001) Phylogenetic analyses of Aleurodiscus s.l. and allied genera. Mycologia 93:720–731
Wu SH, Nilsson HR, Chen CT, Yu SY, Hallenberg N (2010a) The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phleboid clade of Polyporales (Basidiomycota). Fungal Divers 42:107–118
Wu SH, Wang DM, Yu SY (2010b) Neoaleurodiscus fujii, a new genus and new species found at the timberline in Japan. Mycologia 102:217–223
Wu X, Schoch CL, Boonmee S, Bahkali AH, Chomnunti P, Hyde KD (2011) A reappraisal of Microthyriaceae. Fungal Divers 51:189–248
Wu G, Feng B, Xu J, Zhu XT, Li Y-C, Zeng NK, Hosen MI, Yang ZL (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers 69:93–115
Wu G, Zhao K, Li YC, Zeng NK, Feng B, Halling RE, Yang ZL (2015) Four new genera of the fungal family Boletaceae. Fungal Divers 81:1–24
Wu G, Li YC, Zhu XT, Zhao K, Hang LH, Cui YY, Li F, Xu JP, Yang ZL (2016) One hundred noteworthy boletes from China. Fungal Divers 81:25–188
Xia JW, Ma YR, Li Z, Zhang XG (2017) Acrodictys-like wood decay fungi from southern China, with two new families Acrodictyaceae and Junewangiaceae. Sci Rep 7:78–88
Yang ZL (1997) Die Amanita-Arten von Südwestchina. Bibl Mycol 170:1–240
Yang ZL (2000) Species diversity of the genus Amanita (Basidiomycetes) in China. Acta Bot Yunnanica 22:135–142
Yang ZL (2004) Two new species of Amanita (Basidiomycota) from China. In: Agerer R, Piepenbring M, Blanz P (eds) Frontiers in Basidiomycote Mycology. IHW Verlag, Eching, pp 315–324
Yang ZL (2005) Flora fungorum sinicorum, vol 27, Amanitaceae. Science Press, Beijing (in Chinese)
Yang ZL (2015) Atlas of the Chinese species of Amanitaceae. Science Press, Beijing (in Chinese)
Yang ZL, Weiss M, Oberwinkler F (2004) New species of Amanita from the eastern Himalaya and adjacent regions. Mycologia 96(3):636–646
Yang T, Groenewald JZ, Cheewangkoon R, Jami F, Abdollahzadeh J, Lombard L, Crous PW (2017) Families, genera and species of Botryosphaeriales. Fungal Biol 121:322–346
Yang H, Hyde KD, Karunarathna SC, Deng C, Gu CH, Yang SA, Zhang ZC (2018a) New species of Camptophora and Cyphellophora from China, and first report of sexual morphs for these genera. Phytotaxa 343(2):149–159
Yang J, Liu JK, Hyde KD, Jones EG, Liu ZY (2018b) New species in Dictyosporium, new combinations in Dictyocheirospora and an updated backbone tree for Dictyosporiaceae. MycoKeys 36:83
Yang J, Maharachchikumbura SSN, Liu JK, Hyde KD, Jones EBG, Al-Sadi AM, Liu Z-Y (2018c) Pseudostanjehughesia aquitropica gen. et sp. nov. and Sporidesmium sensu lato species from freshwater habitats. Mycol Prog 17:591–616
Ying JZ (1991) Studies on the genus Lactarius S.F. Gray from China I. New taxa of Lactarius. Acta Mycol Sin 10:190–199
Young AM, Mills AK (2002) The Hygrophoraceae of Tasmania. Muelleria 16:3
Yuan ZW, Pei MH, Hunter T, Royle DJ (1998) Eudarluca caricis, the teleomorph of the mycoparasite Sphaerellopsis filum, on blackberry rust phragmidium violaceum. Mycol Res 102:866–868
Yuan Y, Chen JJ, He SH (2017) Geliporus exilisporus gen. et comb. nov., a xanthochroic polypore in Phanerochaetaceae from China. Mycoscience 58(3):197–203
Zare R, Gams W (2016) More white Verticillium-like anamorphs with erect conidiophores. Mycol Prog 15:993–1030
Zare R, Gams W, Starink-Willemse M, Summerbell RC (2007) Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwig 85:463–489
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015) The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol Monogr 85(2):133–155
Zhan G, Tian Y, Wang F, Chen X, Guo J, Jiao M, Huang L, Kang Z (2016) A novel fungal hyperparasite of Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. PLoS ONE 9(11):e111484
Zhang N, Wang Z (2015) 3 Pezizomycotina: Sordariomycetes and Leotiomycetes. In: Systematics and evolution. Springer, Berlin, pp 57–88
Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009a) Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009b) Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers 38:225–251
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012) Pleosporales. Fungal Divers 53:1–221
Zhang M, He W, Wu JR, Zhang Y (2016) Two new species of Spencermartinsia (Botryosphaeriaceae, Botryosphaeriales) from China. Mycosphere 7:942–949
Zhang H, Dong W, Hyde KD, Maharachchikumbura SSN, Hongsanan S, Jayarama Bhat D, Al-Sadi AM, Zhang D (2017a) Towards a natural classification of Annulatascaceae-like taxa: introducing Atractosporales ord. nov. and six new families. Fungal Divers 85:75–110
Zhang JF, Liu JK, Hyde KD, Yang W, Liu ZY (2017b) Fungi from Asian Karst formations II. Two new species of Occultibambusa (Occultibambusaceae, Dothideomycetes) from karst landforms of China. Mycosphere 8(4):550–559
Zhang JF, Liu JKJ, Ran HY, Khongphinitbunjong K, Liu ZY (2018) A new species and new record of Lophiotrema (Lophiotremataceae, Dothideomycetes) from karst landforms in southwest China. Phytotaxa 379(2):169–179
Zhao GZ, Yu P, Liu XZ (2013) Cancellidium and Canalisporium (hyphomycetes) from China. Nova Hedwig 96(1–2):221–236
Zhao K, Wu G, Feng B, Yang ZL (2014a) Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycol Prog 13:1127–1136
Zhao K, Wu G, Yang ZL (2014b) A new genus, Rubroboletus, to accommodate Boletus sinicus and its allies. Phytotaxa 188:61–77
Zhao RL, Zhou JL, Chen J, Margaritescu S, Sánchez-Ramírez S, Hyde KD, Callac P, Parra LA, Li GJ, Moncalvo JM (2016) Towards standardizing taxonomic ranks using divergence times-a case study for reconstruction of the Agaricus taxonomic system. Fungal Divers 78:239–292
Zhao RL, Li GJ, Sánchez-Ramírez S, Stata M, Yang ZL, Wu G, Dai YC, He SH, Cui BK, Zhou JL, Wu F, He MQ, Moncalvo JM, Hyde KD (2017) A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Divers 84(1):43–74
Zhou JL, Su SY, Su HY, Wang B, Callac P, Guinberteau J, Hyde KD, Zhao RL (2016) A description of eleven new species of Agaricus sections Xanthodermatei and Hondenses collected from Tibet and the surrounding areas. Phytotaxa 257(2):99–121
Zhu HY, Tian CM, Fan XL (2018) Multigene phylogeny and morphology reveal Cytospora spiraeae sp. nov. (Diaporthales, Ascomycota) in China. Phytotaxa 338(1):49–62
Zhuang WY (2001) Higher fungi of tropical China. Mycotaxon Limited, Ithaca
Acknowledgements
We express sincere appreciations to the Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, China and the Centre of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, Thailand for providing the facilities of taxonomic laboratory. We also thank to the director Jun-Bo Yang and Plant Germplasm and Genomics Center in Germplasm Bank of Wild Species, Kunming Institute of Botany for the molecular laboratory support. Prof. Dr. Shaun Pennycook is thanked for his essential nomenclatural review. We gratefully thank Prof. Dr. Jian-Kui Liu, Drs. Dimuthu S. Manamgoda, Dhanushka Udayanga, Komsit Wisitrassameewong, Nalin N. Wijayawardene, Philippe Callac and Miss Na Zhou for valuable suggestions and general assistance. Rungtiwa Phookamsak thanks CAS President’s International Fellowship Initiative (PIFI) for Young Staff 2019–2021 (grant number 2019FY0003), the Research Fund from China Postdoctoral Science Foundation (Grant No. Y71B283261), the Yunnan Provincial Department of Human Resources and Social Security (Grant No. Y836181261), and National Science Foundation of China (NSFC) project code 31850410489 for financial research support. Kevin D. Hyde thanks the Foreign Experts Bureau of Yunnan Province, Foreign Talents Program (2018; Grant No. YNZ2018002), Thailand Research grants entitled Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans (Grant No: RSA5980068), the future of specialist fungi in a changing climate: baseline data for generalist and specialist fungi associated with ants, Rhododendron species and Dracaena species (Grant No: DBG6080013), Impact of climate change on fungal diversity and biogeography in the Greater Mekong Subregion (Grant No: RDG6130001). Jianchu Xu thanks the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (Grant No. QYZDY-SSW-SMC014). Peter E Mortimer would like to thank the National Science Foundation of China and the Chinese Academy of Sciences for financial support under the following grants: 41761144055, 41771063 and Y4ZK111B01. Olivier Raspé is grateful to the Fonds de la Recherche Scientifique-FNRS (Belgium) for travel grants. Samantha C. Karunarathna thanks CAS President’s International Fellowship Initiative (PIFI) for funding his postdoctoral research (Grant No. 2018PC0006) and the National Science Foundation of China (NSFC, project code 31750110478). Dhanushaka N. Wanasinghe would like to thank CAS President’s International Fellowship Initiative (PIFI) for funding his postdoctoral research (Grant No. 2019PC0008). E.B. Gareth Jones is supported under the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Kingdom of Saudi Arabia. Aniket Ghosh, Kanad Das, Priyanka Uniyal, Rajendra P. Bhatt, Tahir Mehmood, and Upendra Singh are grateful to the Head, Department of Botany & Microbiology & USIC Dept. HNB Garhwal University, Srinagar Garhwal and thank to the Director, Botanical Survey of India, Kolkata for providing all kinds of facilities during the present study and UGC for providing fellowship to Aniket Ghosh, Priyanka Uniyal and Tahir Mehmood. K. P. Deepna Latha acknowledge the Kerala State Council for Science, Technology and Environment (KSCSTE) in the form of a PhD fellowship (Grant No. 001/FSHP/2011/CSTE) and the Principal Chief Conservator of forests, Kerala State, for granting permission (No. WL10- 4937/2012, dated 03-10-2013) to collect agarics from the forests of Kerala. K. N. Anil Raj thanks the Council of Scientific & Industrial Research (CSIR), New Delhi, India, in the form of an award of CSIR Research Associateship (09/043(0178) 2K17 dated: 31/03/2017). Mao-Qiang He and Rui-Lin Zhao thank the National Natural Science Foundation of China (Project ID: 31470152 and 31360014) and the Foundation of Innovative Group of Edible Mushrooms Industry of Beijing (Project ID:BAIC05-2017). Mingkwan Doilom would like to thank the 5th batch of Postdoctoral Orientation Training Personnel in Yunnan Province and the 64th batch of China Postdoctoral Science Foundation. Tatiana Baptista Gibertoni and Renato Lúcio Mendes Alvarenga acknowledge Dr. Viacheslav Spirin for the taxonomic and morphological advises, CNPq for the Ph.D scholarship of RLMA (140283/2016-1), Pós-Graduação em Biologia de Fungos (UFPE, Brazil), Capes (Capes-SIU 008/13), CNPq (PQ 307601/2015-3) and FACEPE (APQ 0375-2.03/15) for funding the research. Margarita Dueñas, M. Teresa Telleria and María P. Martín acknowledge financial support from the Agreement ENDESA and San Ignacio de Huinay Foundations and Consejo Superior de Investigaciones Científicas, CSIC (Projects No. 2011HUIN10, 2013CL0012, 2014CL0011), the AECID (Agencia Española de Cooperación Internacional para el Desarrollo) and Plan Nacional I + D+i project no. CGL2015–67459–P; to Reinhard Fitzek (San Ignacio del Huinay Foundation, Chile) for his invaluable help during fieldwork, and M. Glenn (Seton Hall University, US) for revising the text and Miguel Jerez and Yolanda Ruiz León for SEM technical assistance. Sandra Nogal-Prata was supported by a Predoctoral Grant from the Ministerio de Economía y Competitividad (Spain) (BES-2016-077793). Armin Mešić and Zdenko Tkalčec thank Croatian Science Foundation for their partial support under the project HRZZ-IP-2018-01-1736 (ForFungiDNA) and are grateful to Milan Čerkez for collecting specimens of coprinoid taxa for this study. Hyang Burm Lee was supported by the Graduate Program for the Undiscovered Taxa of Korea, and the Project on Survey and Discovery of Indigenous Fungal Species of Korea funded by NIBR and Project on Discovery of Fungi from Freshwater and Collection of Fungarium funded by NNIBR of the Ministry of Environment (MOE), and in part carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development (PJ013744), Rural Development Administration, and BK21 PLUS program funded by Ministry of Education, Republic of Korea. Mubashar Raza would like to thank the CAS-TWAS for the PhD Fellowship. Sanjay K. Singh, Paras Nath Singh, Shiwali Rana and Frank Kwekucher Ackah thank Director, MACS, Agharkar Research Institute, Pune, India for providing facilities. Shiwali Rana and Frank Kwekucher Ackah thank UGC (Junior Research Fellowship) and DST, Govt. of India (CV Raman Fellowship for African Researchers), respectively. Gen-Nuo Wang, Huang Zhang, Wei Dong and Xian-Dong Yu thank the National Natural Science Foundation of China (Project ID: NSF 31500017). Bandarupalli Devadatha and V. Venkateswara Sarma thank The Ministry of Earth sciences, Govt. of India (Sanction order: MOES/36/OO1S/Extra/40/2014/PC-IV dt.14.1.2015) for a funding of the project, T, District Forest Office, Tiruvarur, Tamil Nadu and PCCF (Head of Forest Force), Chennai, Tamil Nadu Forest Department for providing permission to collect samples from Muthupet mangroves, and Department of Biotechnology, Pondicherry University is thanked for providing the facilities. Myung Soo Park, Seung-Yoon Oh and Young Woon Lim thank the Marine Bio Resource Bank Program of the Ministry of Ocean & Fisheries, Korea. Olinto Pereira thanks the CAPES, CNPq and FAPEMIG for financial support. Neven Matočec, Ivana Kušan and Margita Jadan express their gratitude to Livio Lorenzon, Enrico Bizio and Raffaella Trabucco (MCVE) for their kind help with loan of Sarcopeziza sicula type material; parts of their research were financed by Public Institutions Sjeverni Velebit National Park and Paklenica National Park. Dong-Qin Dai and Li-Zhou Tang thank the National Natural Science Foundation of China (No. NSFC 31760013, NSFC 31260087, NSFC 31460561), the Scientific Research Foundation of Yunnan Provincial Department of Education (2017ZZX186) and utilization of endophytes and the Thousand Talents Plan, Youth Project of Yunnan Provinces. Ting-Chi Wen, Chada Norphanphoun and Shi-Ke Huang are grateful to the National Natural Science Foundation of China (No. 31760014) and the Science and Technology Foundation of Guizhou Province (No. [2017]5788). Monika C. Dayarathne thanks to Thailand Research Fund (TRF) Grant No MRG6080089 for financial research support. Napalai Chaiwan thanks The Royal Golden Jubilee Ph. D. Program (PHD60K0147) under Thailand Research Fund, for financial research supports on project entitle “Fungi on limestone outcrops from southern Thailand to lower himalyas”. Saranyaphat Boonmee and Sirinapa Konta would like to thank the National Research Council of Thailand (Grant No. 61215320023, 61215320013) and the Thailand Research Fund (Grant No. TRG6180001) for research financial support. Saisamorn Lumyong would like to thank the Thailand Research Fund (RTA 5880006) and Chiang Mai University for partially support this research work. Itthyakorn Promputtha would like to thank Chiang Mai University for partially supported this research work. Lei Cai acknowledges China-Thailand Joint Lab on Microbial Biotechnology (Most KY201701011) for financial support. PhD students from Mae Fah Laung and Chiang Mai Universities thank the Mushroom Research Foundation for research financial support and PhD Fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Phookamsak, R., Hyde, K.D., Jeewon, R. et al. Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Diversity 95, 1–273 (2019). https://doi.org/10.1007/s13225-019-00421-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-019-00421-w