Abstract
Groundwater chemistry is mainly governed by lithological variations, space and resident time. In addition, hydrogeochemical characteristics of groundwater in the lithological contact zones are too complex. Hence, Cretaceous–Tertiary (KT) boundary from Ariyalur district, Tamilnadu, India, was selected for this study to identify the hydrogeochemistry of groundwater. This study includes 284 groundwater samples from four different seasons (pre-monsoon, post-monsoon, southwest monsoon and northeast monsoon). Groundwater samples were collected and analysed for major cations and anions, including physical parameters using standard procedures. High electrical conductivity (EC) showed the longer residence time of groundwater in hard rock region at the central and southern part of the study area. Ca2+, Na+, Cl− and HCO3− are the dominant ions in all the four seasons. The seasonal composition migration was observed from Na–Ca–Cl–HCO3 type to Na–Mg–Cl–HCO3 type, and Ca-HCO3 is the predominant water type in piper plot. Interpretation of data reveals that the groundwater quality was unsuitable for domestic and irrigation purposes during pre- and southwest monsoon seasons. Rock–water interaction and dissolution of minerals are the main sources of groundwater chemistry. Agriculture activities during monsoonal seasons also play a role in controlling the hydrogeochemistry of groundwater in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dissolved ions in groundwater aid to recognize the major geochemical processes and help to determine the utility of water for various purposes. In regulating the contribution of the various geochemical factors during every stage shows a complex process. The vast extent of the ecosphere's irrigation and agriculture relies on subsurface water. The groundwater quality of a region is determined by precipitation, geological materials, geographic, land-use geomorphology and accessibility of recharge sources. The dependence on surface water in many regions had increased rapidly due to insufficient surface water sources, non-perennial rivers, and frequent monsoon failures. In the recharge zone, hydrochemistry is affected by water chemistry and by various reactions before recharge and reactions between the groundwater and aquifer matrix (Li et al. 2008). Chemical characteristics of the adjoining rocks, the qualitative and measurable characteristics of moving water bodies, and the human interaction with the subsurface aquifers govern the utility of water. The spatial variability of water chemistry results from rock–water interaction under different subsurface migration conditions (Prasanna et al. 2013).
Scientists have recognized many other factors that influence groundwater chemistry, such as the amount of pores in the aquifer, waste water penetration from shallow salt water, water level rise and seawater incursion (Araguas 2003; Carol et al. 2009; Senthilkumar et al. 2017; Acharya et al. 2017). Several authors have claimed that geological heterogeneities influence hydrogeochemical processes on different scales (Singh et al. 2013; Chidambaram et al. 2010; Adithya et al. 2016; Thilagavathi et al. 2017; Olofinlade et al. 2018; Devarajet al. 2018, 2020). In recent decades, groundwater quality concerns have become a major concern, and groundwater quality assessment and health risk assessment have been extensively studied around the world (Zhang et al. 2020a, b; Adimalla and Qian 2021; Adimalla et al. 2020a, b). This present study is more significant due to the difference in the adjoining lithologies, intensity of weathering, water level fluctuation, surface water–groundwater interaction, permeability and porosity of these aquifers (Archaean, Cretaceous, Tertiary and Quaternary).
Hydrogeochemical methods and the study of principle component analysis provide confidence in the degree of water–rock interactions and processes of mixing (Xu et al. 2019a, b, c; Adimalla 2020; Adimalla et al. 2020a, b). There are few studies on the hydrogeochemical status to determine the dissolved ion concentration in the adjoining region of the study area (Mehra et al. 2016; Thivya et al. 2015, 2018; Saravanan et al. 2016; Loganathan and Ahamed 2017; Kumar et al. 2017). Irrigation water quality plots are the major keys to classify the groundwater quality (Zhang et al. 2019; Adimalla 2019). An investigation has been done to identify the hydrogeochemical characterization of groundwater (shallow aquifer) for irrigation purpose (Xu et al. 2019a, b, c).
The study about groundwater chemistry and its relation with local geology are important in groundwater management (Xu et al. 2019a, b, c). Also, Groundwater is one of the key sources of drinking water, and its use and protection as drinking water faces challenges as water tables decline (Zhang et al. 2020a, b). In order to recognize the geochemical processes accountable for the discrepancy in hydrogeochemical characters of groundwater along Cretaceous–Tertiary boundary, many studies have previously been conducted globally, though hydrogeochemical research on groundwater to ascertain the suitability for different purposes are scarce especially along the KT boundary of Trichinopoly, Tamil Nadu, India. Therefore, the current study has attempted with an intense sampling campaign representing four different seasons and adopting even distribution, to determine the water quality for various purposes, its spatial and temporal variation and to identifying chief hydrogeochemical process in the region.
Study area
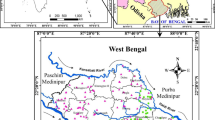
The major city, Ariyalur is in the central portion of Trichinopoly district in southern Tamil Nadu, India (Fig. 1). The present study region lies between the latitude of 78° 808"E and 79° 275"E, the longitudes 11°449"N and 10°974"N representing survey of India toposheets 58-I-15, 58-I-16, 58-M-3 and 58-M-4. The mean elevation of the area is 50 m above mean sea level. Physiographically the north-western portion is elevated and slopes towards the south-east with a spatial extent of 1774 Km2. Temperature varies from 21 to 40 °C in January and June, respectively. Average yearly rainfall of 1096 mm and the highest amount of rainfall is generally recorded during NEM (i.e. 45.9% of total rainfall), followed by SWM (39.6%), winter (2.8%) and summer (11.7%). The river Vellar is the major drainage flowing from the northern part and; another minor tributary is River Marudiyar moving transversely from southwest to south-east. The river Marudiyar is ephemeral and there is no flow during non-monsoon periods.
The geology the region is covered by charnockites, sandstones, migmatite gneiss and calcareous stones. The surface marine structure is characteristic of the Cauvery basin's cretaceous succession with a rich Albian-Maastrichtian era faunal sequence (Rangaraju et al. 1993). Lithological, there are 3 foremost groups classified as Ariyalur, Uttatur and Trichinopoly formations. The Ariyalur formation is the major outcrop than the other two (Sastry et al. 1972), and it outcrops on the eastern and northeastern part of the Ariyalur district. It is the most prominent fossiliferous cretaceous formations of the Cauvery basin in the south India and characterizes the Mesozoic progression. Tectonics, paleontology, stratigraphy, paleoclimate and topographic sequence have been addressed and inferred by various scholars (Banerji 1979; Sundaram and Rao 1986; Ramanathan 1979; Ramasamy and Banerji 1991; Govindan et al. 1996; Madhavaraju and Ramasamy 1999, 2001; Ayyasami 2006; Devaraj et al. 2018, 2020). The major land-cover and land-use features are agricultural terrestrial, water supplies, leftover land, scrubland and farm/plantation. The spatial coverage of these features shows, aquatic bodies (21.45%), left over terrestrial (6.88%), farmstead/coppice (6.29%) and scrubland (3.1%) representing minor portion. Agricultural land occupies 62.17% of the entire area (Devaraj et al. 2018).
Groundwater in weathered and fractured zones in semi-confined conditions depending on the interconnection between the weaker structures, and its development. In Ariyalur region, thickness of aquifer varies from 15 to 35 MBGL. The Alluvium formation is good water bearing region with a highest thickness of 37 m and this formation is very porous and permeable zone. Water table in the study area fluctuates from 10.0 to 15.0 m MBGL in the hard rock aquifers.
Materials and methods
The samples representing the different litho units and land use were collected during four different season (n = 284) South West monsoon (SWM), Pre-monsoon (PRM), North East monsoon (NEM) and Post-monsoon (POM) (Fig. 1). Subsequently the samples were sealed and transported for analysis to the laboratory; they were stored at a temperature of 4 °C and filtered before analysis with a 0.45-micron filter. And the samples were analysed for chemical constituents as per the standard procedure (APHA 1995). The instruments and their specifications which were used for hydrogeochemical parameters are given (Table 1). In situ analysis such as electrical conductivity (EC), pH, total dissolved solids (TDS) and (HCO3) bicarbonate were measured in the sampling site and cross-checked again in the laboratory. The samples obtained were analysed in the laboratory for important cations and anions. Titrimetric method was adopted for determining Ca, Mg, HCO3, and Cl. Na and K were determined by flame photometer (ELICO CL 378).
Fluoride was measured by the ion electrode (Orion 94–09, 96–09). UV-double beam spectrophotometer (DR 6000, HACH) was used to analyse NO3, PO4, SO4 and H4SiO4 adopting standard techniques (APHA 1995, 1998). Each sample was analysed three times and the average was accounted. The precision of major ion analysis was calculated by using Eq. 1 (Freeze and Cherry 1979) by determining ionic balance error (IBE), and it was found that values fell within 5–10% (Fig. 2).
The detailed specifications of the analytical methodology are provided in the table. Values greater than the limit of detection are diluted and measured subsequently multiplied by the dilution factor. The PCA (Factor analysis) and the Pearson correlation analysis were determined by Social Sciences Statistical Package (SPSS) version 17.0. Vertical mapper along with Map info software (Professional 8.5) was used to prepare the spatial distribution maps. The calculation on water quality indices was obtained by the programme 'WATCLAST' (Chidambaram et al. 2003). The software includes Ca2+, Mg2+, K+, Na+, HCO3−, Cl−, SO42−, H4SiO4, PO42− (mg/l) concentrations, and other parameters, including TDS, EC and pH.
Result and discussion
Water chemistry
pH
The pH of the water governs the solubility and thereby the geochemical equilibrium. The pH is a metric of the availability (activity) of hydrogen ions (H+). The hydrogen ion is very small and can penetrate and destroy mineral structures so that dissolved components are added to the groundwater. pH varies from acidic to alkaline in nature, ranging from 5.54 to 8.41 (Table 2). In PRM, lowest pH was observed and highest during SWM.
Electrical conductivity (EC)
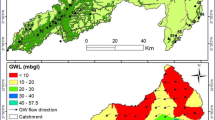
Higher the salt content, greater the electrical current flow would be. Electrical conductance is directly related to the abundance of charged ionic compounds (Hem 1985). The study area has electrical conductivity (EC) ranging from 326 to 15,550 μs/cm. Compared with other seasons, the highest EC value observed during SWM, it may be due to dissolution or leaching of the aquifer content, saline water influence or anthropogenic sources (Devaraj et al. 2018).
The attribute values of the specific locations were used to study the spatial variation. The general pattern of the dissolved ions in water is reflected by the spatial data of electrical conductivity. This provides us initially with first-hand data on the geochemically active regimes (Chidambaram et al. 2000; Anandhan et al. 2005; Srinivasamoorthy et al. 2004). The spatial variation of electrical conductivity with respect to samples from different seasons shows distribution of the higher values in the central part of the study area especially near the lithological contact and the hard rock region (Fig. 3).
Higher values of EC during PRM were observed along the SW and central portion of the study area. The EC ranges from 326 to 15,550 μs/cm during SWM and higher values were noted in the southern and NW part of the study area. The spatial distribution during NEM shows higher values in the NW region of the study area and in POM higher EC concentration was noted in the southern and SW parts. Season distribution of the values shows that the spatial coverage of EC values > 3000 μs/cm was observed in NEM. The higher EC values stretch along the Vellar River during NEM due to the dissolution and infiltration of domestic sewage and landfill leachates (Saxena et al. 2003). In general, seasonal variation of EC spatial representation shows that the southern part of the study area (PRM, SWM, NEM and POM) has higher values. The categorization of the seasonal variation in samples with respect to season is observed in Table 3. Also the fluctuation of EC irrespective with seasons showed in (Fig. 4) according to Richards (1954).
EC classification based on Richards (1954) irrespective of seasons
The percentage of groundwater samples in each category based on this classification indicates that 36.6% of PRM samples, 80% of SWM, 26.7% of NEM and 18.3% of POM samples represent “Fresh” category. Greater percentage of samples belonging to “Brackish” category was observed during PRM, followed by SWM, NEM and POM.
Total dissolved solids (TDS)
TDS is known as the residue of filtered water samples after evaporation. Bicarbonates, sulphate, chloride, calcium, magnesium, sodium, potassium, silica and nitrate are the major dissolved solids. TDS varies between 200 and 8710 mg/l with an average of 1626 mg/l during NEM. NEM shows higher TDS followed by SWM, POM and PRM, indicating groundwater dilution during the monsoon season. Samples were categorized into four classes based on Carroll (1962). Almost 47.8% of the SWM and POM samples represent “Fresh water” category and lesser percentage of samples represented this category during NEM (Table 4). Nearly 54.9% of the PRM samples fall in the “Fresh water” category.
Hydrogeochemical parameters
The average concentration of ions is observed in the order as depicted in Table 5.The calcium concentration ranges between 8 and 380 mg/l with an average of 113.35 mg/l; it is observed to be higher during POM. Mg2+ varies between 4.80 and 242.40 mg/l, with an average of 55.90 mg/l and highest value was observed during PRM (Chidambaram 2000). The leaching of magnesium from mafic rocks, or even hypersthene found in charnockite, is primarily the source of magnesium in groundwater. Mg is important for plant and animal nutrition and serves as the key source of water hardness is magnesium, along with calcium in groundwater (Matthess 1982). The sodium concentration ranged from 11 to 2471 mg/l in the samples, with an average of 358.6 mg/l. Sodium concentration is higher in SWM and lower in NEM, suggesting the contribution from the sodium feldspar weathering along with dissolution and anthropogenic sources. In most fresh water aquifers, potassium is less in groundwater due to its mobility (Hens 1985) or due to ion exchange processes (Chidambaram 2000). K+ varies between 0.20 and 352 mg/l and higher concentrations were noted in SWM, averaging 44.72 mg/l. Potassium is well within the prescribed limit and in most of the samples, with few anomalies due to urban landfill and fertilizer leaching, regardless of the seasons.
Cl− ranges from 30.5 to 4165 mg/l in samples, and higher concentrations were noted in SWM samples with an average of 458.85 mg/l. Some locations show that the chloride in groundwater originates from both natural and anthropogenic sources, exceeding the allowable limit. The use of inorganic fertilizers, leachate from landfills, and septic tank effluent and runoff may be due to this anomalies (Freeze and Cherry 1979). In water, alkalinity is the indicator of its neutralization power (Chapelle et al. 1987), bicarbonate in water was attributed to H2CO3 dissociations. It is also produced due to the organic decomposition of atmospheric CO2 released. Bicarbonate varies between 89 and 1134.6 mg/l, averaging 494.5 mg/l. Average HCO3− values were observed to be in the following order of dominance POM, PRM, NEM and SWM, suggesting the contribution of the chemical weathering of silicate and carbonate as major source (Mondal and Singh 2004). The nitrate accumulation in groundwater is primarily due to the infiltration of sewage and leaching of animal dung and leakage from septic tanks through the soil to groundwater (Chidambaram 2000). Nitrogen is also from the household, fertilizer or nitrogen fixing bacteria. The nitrate concentration ranged from Below Detection Limit (BDL) to as high as 475 mg/l during PRM. In the monsoon season and also by agricultural activity, they accumulated and decomposed. Sulphate is generally found in small amounts (Singh et al. 1994) in groundwater, with the maximum acceptable limit is 400 mg/l (WHO 2004). The SO42− concentration varies between BDL and 18.4 mg/l and the mean concentration is 4.5 mg/l, with higher values observed during SWM. The dissolved sulphate ion contribution may also be attributable mineral dissolution, and other sources or due to bacterial fixation, fertilisers, and other anthropogenic sources (Anandhan 2005; Chidambaram et al. 2012).
The phosphate concentration ranges from BDL to 22.9 mg/l with higher concentration observed during NEM. PO42−has fluctuated without a clear pattern, regardless of space and time. Groundwater fluoride toxicity is a result of many variables, such as fluorine-bearing mineral availability and solubility, temperature, Ca2+ and HCO3−, pH concentration etc (Chandra et al. 1981). Groundwater with higher fluoride is common globally, and it is governed by composition minerals and physical properties of the aquifer matrix, pH, temperature, and the interaction with other ions (Tahaikt et al. 2008). In groundwater, F varies from 4 to 0.01 mg/l, least was observed during SWM and the higher values of F− were during PRM, NEM and POM. Silica is an integral component of nearly all minerals. The silica concentration in the region varies from 5.2 to 242 mg/l with an average of 174 mg/l. In SWM, higher H4SiO4 concentrations were observed and least during PRM, NEM and POM, suggesting dissolution from source rock.
Irrigation quality
Groundwater suitability for agriculture purpose is primarily governed by Sodium Adsorption Ratio, Sodium percent, and Residual Sodium carbonate. Apart from these parameters, total sodium concentration and EC are considered as significant (Wilcox 1955).
Sodium adsorption ratio
The appropriateness of groundwater for irrigation depends on the impact on both plants and the soil and their mineral constituents in water. The effects of salts on soils have an indirect influence on plant growth due to changes in soil composition, permeability, and aeration. The alkali hazard is stated as “Sodium Adsorption Ratio” (SAR) and is commonly utilized to determine irrigation water quality. If the water quality is high in Na+ and low in Ca2+, the ion exchange sites that become saturated with Na+ damage the soil structure due to the dispersion of the clay particles that decreases plant growth. The SAR is determined by using the following formula (Richard 1954).
In (Table 6), the classification is provided by categorizing it into excellent, good, reasonable, and bad. All samples were found to fall into excellent categories in all seasons, with the exception of a few PRM and SWM samples.
Salinity hazard
The water salinity hazard as calculated by electric conductivity (EC) is extremely powerful parameter in determining the utility of water on crop productivity. Salinization can be caused by water with high electrical conductivity (Ghafoor et al. 1990, 1993). High salinity is unsafe water and is harmful to plants. Soils with high overall salinity levels are known as saline soils. High salt concentrations in the soil can result in a state of "physiological" drought. The salinity of water is normally determined by either TDS (total dissolved solids) or EC (electrical conductivity).
Salinity is also increases concentration of specific ions and osmotic pressure for growth and controls the plant yield (Fracois 1989). Studies of waters are categorized into four key groups (Richard 1954) based on electrical conductivity. The USSL groundwater classification for irrigation purposes (Fig. 5) was used by the United State Salinity Laboratory to acquire the irrigation water classification. This plot exhibits that majority of samples are represented in categories C3-S2, C3-S3, and C4-S4 (high salinity hazard) during the PRM and SWM and other seasonal samples of NEM, POM vary from C3-S1 and C4-S1 (low to very high salinity risk) of which the highest salinity is observed in PRM and SWM may be due to the leaching of secondary salts.
Sodium percentage
Classification of sodium percent is categorized as excellent, fine, acceptable, questionable, and unsuitable (Wilcox 1955). The content of Na is an important factor for evaluating appropriateness for farming purposes. The combination of sodium with CO3 contributes for the development of alkaline soils, and the combination of Na with Cl leads to the formation of saline soils. The development of these soil types hinders the plant growth (Karmegam et al. 2010; Thivya et al. 2013; Devaraj et al. 2016). An upper limit of 60% of sodium in water is permitted for agricultural purposes. The higher percentage of sodium is mainly owing to greater water residence time, dissolution of minerals, and due to the leaching of the fertilizers applied (Qiyan and Baoping 2002; SubbaRao et al. 2002). Na percent is classified into two categories, as safe and dangerous (Table 6). Groundwater classification for agricultural purpose using Na% was determined by,
Similarly, in PRM, 26% of samples, 18% of samples during SWM, 98% of NEM samples and 100% of samples during POM fall into the safe category. Most of the samples from PRM and SWM are unsuitable for irrigation purposes.
The Wilcox diagram displays against Na % and EC values (Fig. 8). The figure demonstrates approximately, 85% of the groundwater samples are fall into excellent to good condition, 13% of the samples fall in the permissible to doubtful condition and 2% sample belongs to the good to a permissible field.
Residual sodium carbonate (RSC)
The excess carbonate will increase the precipitation tendency of CaCO3 in the sediments, which in turn will enhance the Na concentration in water. In addition, it is possible that Na will be controlled during the ion dispersion process (Emerson and Bakker 1973). RSC also impacts the absorption nutrients by the plants (Kanwar and Chaudhry 1968). Thus, the alkaline land affects the utility of groundwater for irrigation.
RSC is categorized into three groups as “Good”, “Medium”, and “Poor”, according to Richard (1954) (Table 6). 72% of samples fall in good category during PRM, 12% fall in medium and 15% fall in the poor category. In SWM, 51% of samples fall into good and 14% fall in poor categories. During NEM, 35% of samples represent “good”, 95% in “medium” and 4 percent of samples reflect a “poor” class. In POM, 89% of samples fall in “good category”, 6% in “moderate” and 6% of samples are poor.
Corrosivity ratio
Water is being brought through metal pipelines for many purposes to the study area. The appropriateness of the groundwater for transport is based on the sample's corrosive nature. The value of < 1 is safe and the value of > 1 is dangerous. The CR is calculated by the following formula;
61% of samples during PRM, 52% of samples of SWM, 46% of samples of NEM, and 20% of samples in POM are in safe category.
Permeability index
A water quality infiltration problem is reported when the penetration rate for the water decreases or even after the rainfall event water stays on the soil surface for a long period of time or infiltrates too slowly to provide the crop with enough water to sustain appropriate yields. Doneen (1948) derived the permeability index (PI) parameter of determining the suitability of water for irrigation as;
The Permeability Index is an important factor for evaluating the soil-related quality of irrigation water for agricultural improvement (Thilagavathi et al. 2012; Adimalla and Venkatayogi 2018). If there is a micronutrient deficiency, it can lead to an increase in toxicity associated with HCO3. The Permeability Index of the groundwater sample calculates the cumulative intensity of the total cation content of Na and HCO3. Permeability Index values were plotted along with sodium adsorption ratio. Most samples irrespective of the seasons represent Class I (Figs. 6, 7 and 8) representing the permissible category, with only a few samples PRM, SWM and NEM and SWM in Class II and one SWM sample representing Class III, (poor category). It is important to note that there is a linear distribution in Class I between SAR and PI and it increases in Class II and III.
Modified Doneen’s plot for determining the appropriateness of groundwater for different seasons (after Manivannan 2011)
Chloro-alkaline indices
The “Chloro-Alkaline Indices” (Schoeller 1977) unravels the geochemical interaction between aquifer matrix and the groundwater media during residence time in the aquifer and along the groundwater flow.
and
Chloro-Alkaline indices are either be ‘positive’ or ‘negative’; it exhibits an exchange of Mg + Ca in water to that of Na + K in aquifer matrix or the reverse of sodium / potassium from rock.
To explain the groundwater metasomatism, Schoeller (1977) suggested “Index of Base Exchange” (IBE). There are some minerals capable of adsorption and cation exchange with those in groundwater (clay minerals, glauconite, zeolite and organic substances). The exchange of cations plays a major role in the Na + K in the groundwater. The positive ratio reflects the probability of Ca + Mg (water) exchange to Na + K (mineral) as 84%, 85%, 7% and 20% of samples are represent this process during PRM, SWM, NEM and POM respectively.
Piper plot
According to the seasonal variation, the water type of the sample migrates corresponding to the variation in composition with respect to seasons (Fig. 9), Na–Ca–Cl–HCO3 (PRM) to Na–Cl (SWM) to Ca–HCO3 (NEM) to Mixed Na–Mg–Cl–HCO3 (POM). In order to understand groundwater flow and geochemistry (Dalton and Upchurch 1978) is planned to delineate variability. In the POM season, sample persistence in the field of Ca2+-Mg2+-HCO3− type can be seen indicating natural recharge phase, mainly owing to interaction between groundwater and the aquifer matrix, Chidambaram 2000, Drever 1997). Majority of the POM samples show dominance of Ca2+–Na+–HCO3−–Cl-facies. Due to irrigation and over-exploitation processes, these samples can be affected by farming processes (Thivya et al. 2013) during this period. Subsequently, in PRM and SWM, Na+–Ca2+–HCO3−–Cl− type was inferred due to the integrated process like ion exchange process and natural weathering (Thivya et al. 2015). Lesser Ca2+ and Mg2+ in the groundwater samples reflect ion exchange of Na+ from aquifer matrix during pre-monsoon season.
Statistical analysis
Correlation matrix
Correlation analysis and PCA were adopted for the statistical interpretation. This PCA analysis is used to identify the source of a particular ion by correlating each other (Chidambaram et al. 2014). The correlation shows that there is an excellent correlation observed between Ca–Mg, Ca–Na, Ca–Cl, Mg–Cl, Na–Cl and SO4 in PRM. There is a weak association between other parameters like pH, F, PO4, SO4 and H4SiO4. Cl displays a strong correlation with Ca, Mg and HCO3, suggesting secondary salt leaching, and chemical weathering suggest major correlation of HCO3 with Ca, Mg and pH (Chidambaram et al. 2008). The weak positive association between SO4, PO4, NO3 and H4SiO4 demonstrates the effect of farming activities.
The connection between Ca and Mg, Na, Cl, SO4, EC, between Mg and Na, SO4, EC, between Na and Cl, NO3, SO4, EC, between Cl and SO4, EC, between NO3 and EC also between SO4 and EC, show strong to excellent correlation during SWM (Table 8) suggesting the processes of leaching and weathering. Chloride demonstrates a strong association with Mg, and Na reiterating the process of secondary salt leaching. Chemical weathering is demonstrated by the important association of HCO3 with Mg and K (Srinivasamoorthy et al. 2009). The effect of dilution may be due to a weak positive correlation between SO4, PO4, NO3 and other ions.
There is a strong correlation between Ca and Cl, Mg, EC; Mg with Cl; EC with Na and Cl (Table 7) during NEM. There is a weak correlation with all the other ions between SO4, PO4 and H4SiO4. Cl displays a strong correlation with Ca, Mg, Na and HCO3, suggesting secondary salt leaching and correlation of HCO3 with other ions (Ca, Mg, Na and K) reflect chemical weathering (Karmegam et al. 2012).
There is a strong correlation between Ca and Na, Cl, HCO3, PO4, EC; Mg with Na, Cl and EC; Na with Cl, HCO3, PO4 and EC; Cl with PO4, EC, HCO3 and PO4 showing a positive correlation with EC. Cl shows a good correlation with Ca, Mg, Na and HCO3 suggesting secondary salt leaching and correlation between HCO3 and Ca, Mg, Na, and K suggests chemical weathering. A strong correlation with Ca, Cl, HCO3, Na, K and PO4 suggests the impact of anthropogenic sources on the system.
Factor analysis (pre-monsoon)
FA has resulted in four important variables that explain 64.2% of the pre-monsoon dataset’s total data variability (TDV). The ion association in Factor I represents Ca, Mg, Na, and Cl with a TDV of 30.9%, suggesting secondary salt leaching. This aspect clearly demonstrates that the influence of domestic sewage source (Ruiz et al. 1990; Voudouris et al. 1997). This may also be due to the influence of high Na+, as calcareous and dolomite has Na+ as a result of deposition during the shallow marine conditions and due to the existence of skeletal remains (Billings and Ragland 1968). In the hydrogeochemical setting, factor II indicates (Table 9) the effect of Na, NO3 and SO4. The loading of nitrate and sulphate concentration is primarily due to the weathering of the gypsum found in the cretaceous formation. Factor III with 11.3% of total data variability demonstrates positive loading of K, PO4 indicating human influence such as septic tanks, residential water softeners, and fertilizer (Vengosh and Keren 1996). Factor IV shows F and HCO3 enrichments with TDV 7.87% (Table 9). The loading of HCO3 and F is due to the high alkaline water, favouring release mechanism and enhancing the mobility of F ions in the groundwater.
Factor analysis (southwest monsoon)
Four factors with eigenvalue greater than 1 were extracted during this season. The total data variability of 39.9% was observed for first factor with loadings of EC, Ca, Mg, Na, Cl, SO4 suggesting secondary salt leaching (Tables 8, 9). Factor II (11.2% TDV) has a loading of F and HCO3. Higher positive loading of F and HCO3 and negative loading of pH and temperature show that the pH of the carbonate rocks regulates the release of F during this season. Factor III shows 8.8% TDV with loadings of H4SiO4 and temperature reflecting the thermodynamic constrains on the release of silica to the groundwater. 7.7% of TDV with positive loading of PO4 and K was observed in factor IV, which suggests anthropogenic sources as discussed in the previous season (Vengosh and Keren 1996).
Factor analysis (northeast monsoon)
The season data shows four prominent factors and the Factor I is expressed by a TDV of 31.3% with a positive loading of electrical conductivity, Ca2+, Mg2+, Na+ and Cl measured in groundwater suggest that the adsorbed or other types of solid solution within the geological matrix join the aqueous media upon dissolution of the matrix. It could also be because that the Cl in groundwater is leached from the sediments deposited in marine environments. However, the connate sea water may be washed out due to several reasons within marine sediments, including the aquifer properties like permeability and porosity, the sedimentary basin structure, and distance from the region of recharge (Johns 1968). A TDV of 11.8% was observed for factor II (Table 9) with a positive NO3−loading suggesting mixing of domestic wastewater or other anthropogenic activity. The positive loading of Na and K suggesting intense weathering in Factor III (Prasanna et al. 2009). The positive loadings of F and pH were observed as fourth factor; similar process was discussed in previous season.
Factor analysis (post-monsoon)
The maximum number of factors was extracted in this season due to the geochemical complexity with a TDV of 72.43%. Ca, Na, K, Cl HCO3, PO4 and EC (Table 9) reflect Factor 1 and as discussed earlier the factor explains the dissolution of secondary salts. Mg, Cl, EC with 11.5% of TDV is loaded in Factor II, which represents diffuse groundwater pollution due to agricultural practices (especially in the areas utilizing dolomite as neutralizers, agricultural additives). SO4 and temperature, indicating the temperature controlled factor, are expressed by factor III with 9.2% TDV. The pH shows the dominance in the factor IV representing the base ion exchange and Factor V was loaded with H4SiO4 reflecting silicate dissolution during this season (Chidambaram et al. 2008).
Conclusion
In the study area, the chemical composition of groundwater is altered by hydrogeochemical processes and thus varies with space and time. The four-season spatial representation of the EC shows that higher concentrations were found in the central and southern parts of the studied region chiefly covered by hard rock region, reflecting greater residence time. Thus, the spatial distribution of the EC also reflects the influence of lithology variation. Since the study area is dominated by NEM monsoon rainfall, the flow in the river improves weathering and recharge along with domestic sewage and even along the river course due to the landfill leachates. The Piper plot reveals there is a compositional migration was observed by Ca–HCO3 type in the predominant monsoon and the POM season. The utility of water for different purposes classified by adopting different indices show that PRM and SWM groundwater quality are generally poor with higher dissolved ions. Majority of the samples were inferred to be unsuitable for agricultural and domestic purposes during PRM and SWM. The major sources irrespective of the season were inferred to be the leaching and dissolution apart from silicate weathering and fluoride dissolution. Lithologically, the presence of clay in the sedimentary region has favoured the ion exchange process. There was an interplay of the anthropogenic factor on the hydrogeochemistry of the region, especially, during the monsoon periods due to the onset of the agricultural activities. Thus, the study of the groundwater in four prominent seasons provides a synoptic overview of the hydrogeochemical process in the study area, which can help in the sustainable management of the water resource.
References
Acharya T, Kumbhakar S, Prasad R (2017) Delineation of potential groundwater recharge zones in the coastal area of north-eastern India using geoinformatics. Sustain Water Resour Manag 5:533–540. https://doi.org/10.1007/s40899-017-0206-4
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expos Health 11(2):109–123. https://doi.org/10.1007/s12403-018-0288-8
Adimalla N (2020) Controlling factors and mechanism of groundwater quality variation in semiarid region of South India: an approach of water quality index (WQI) and health risk assessment (HRA). Environ Geochem Health 42(6):1725–1752. https://doi.org/10.1007/s10653-019-00374-8
Adimalla N, Qian H (2021) Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: a case study from the semi-urban region of South India. Ecotoxicol Environ Saf 207:111277. https://doi.org/10.1016/j.ecoenv.2020.111277
Adimalla N, Venkatayogi S (2018) Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl Water Sci 8:44. https://doi.org/10.1007/s13201-018-0682-1
Adimalla N, Qian H, Li P (2020a) Entropy water quality index and probabilistic health risk assessment from geochemistry of groundwaters in hard rock terrain of Nanganur County. South India Geochem 80(4):125544. https://doi.org/10.1016/j.chemer.2019.125544
Adimalla N, Qian H, Nandan MJ (2020b) Groundwater chemistry integrating the pollution index of groundwater and evaluation of potential human health risk: a case study from hard rock terrain of south India. Ecotoxicol Environ Saf 206:111217. https://doi.org/10.1016/j.ecoenv.2020.111217
Adithya VS, Chidambaram S, Tirumalesh K (2016) Assessment of sources for higher Uranium concentration in ground waters of the Central Tamilnadu, India. IOP Conf Ser Mater Sci Eng 121:012009. https://doi.org/10.1088/1757-899x/121/1/012009
Allan freeze, R., Cherry J.A, (1979) Groundwater, Prentice-Hall Inc., Englewood Cliffs, N.J recharge in parts of Sokoto Basin. Nigeria J Environ Hydrol 14:1–17
Anandhan P (2005) Hydrogeochemical studies in and around Neyveli mining region, Tamilnadu. Thesis, India
APHA (1998) Standard methods: for the examination of water and waste- water. American Public Health Association, Washington
Aragu´as LJ (2003) Proceedings of 18th salt water intrusion meeting. In: IGME. pp 365–371
Banerji RK (1979) On the occurrence of Tertiary algal reefs in the Cauvery basin and their stratigraphic relationship. Geol Surv India Misc., pp 181–196
Bertrand A, Nathalie D, Cédric D (2006) In: International symposium Aquifers Systems Management. France
Carroll RWH, Pohll GM, Hershey RL (2009) An unconfined groundwater model of the Death Valley Regional Flow System and a comparison to its confined predecessor. J Hydrol 373:316–328. https://doi.org/10.1016/j.jhydrol.2009.05.006
Champ DR, Gulens J, Jackson RE (1979) Oxidation–reduction sequences in ground water flow systems. Can J Earth Sci 16:12–23. https://doi.org/10.1139/e79-002
Chandra SJ, Thergaonkar VP, Sharma R (1981) Water quality and dental fluorosis. Ind J Pub Health 25:47–51
Chapelle FH, Zelibor JL, Grimes DJ, Knobel LRL (1987) Bacteria in deep coastal plain sediments of Maryland: a possible source of CO2 to groundwater. Water Resour Res 23:1625–1632. https://doi.org/10.1029/wr023i008p01625
Chidambaram S (2000) Hydrogeochemical studies of groundwater in Periyar district, Tamilnadu. Thesis, India
Chidambaram S, Ramanathan AL, Vasudevan S (2004) Fluoride removal studies in water using natural materials : technical note. Water SA. https://doi.org/10.4314/wsa.v29i3.4936
Chidambaram S, Vijayakumar V, Srinivasamoorthy K, Anandhan P, Prasanna MV, Vasudevan S (2007) A study on variation in Ionic Composition of Aqueous System in different lithounits around Perambalur region, Tamil Nadu. Geol Soc India 70:1061–1069
Chidambaram S, Ramanathan AL, Anandhan P, Srinivasamoorthy K, Prasanna MV, Vasudevan S (2008) A statistical approach to identify the hydrogeochemically active regimes in ground waters of Erode district, Tamilnadu. Asian J Water Environ Pollut 5:123–135
Chidambaram S, Ramanathan AL, Prasanna MV (2009) Study on the hydrogeochemical characteristics in groundwater, post- and pre-tsunami scenario, from Portnova to Pumpuhar, southeast coast of India. Environ Monit Assess 169:553–568. https://doi.org/10.1007/s10661-009-1196-y
Chidambara S, John Peter A, Prasanna MV, Karmegam U, Balaji K, Ramesh R, Paramaguru P, Pethaperua S (2010) A study on the impact of landuse pattern in the groundwater quality in and around Madurai region South India-using GIS techniques. Online J Earth Sci 4(1):27–31. https://doi.org/10.3923/ojesci.2010.27.31
Chidambaram S, Karmegam U, Prasanna MV, Sasidhar P (2012) A study on evaluation of probable sources of heavy metal pollution in groundwater of Kalpakkam region, South India. Environmentalist 32:371–382. https://doi.org/10.1007/s10669-012-9398-1
Chidambaram S, Prasad MB, Prasanna MV (2014) Evaluation of metal pollution in groundwater in the industrialized environs in and around Dindigul, Tamilnadu, India. Water Qual Expo Health 7:307–317. https://doi.org/10.1007/s12403-014-0150-6
Chidambaram S, Ramanathan AL, Srinivasamoorthy K, and Anandhan P (2003) WATCLAST A computer program for hydrogeochemical studies. Recent trends in hydrogeochemistry (case studies from surface and subsurface waters of selected countries). Capital Publishing, New Delhi, New Delhi
Dalton MG, Upchurch SB (1978) Interpretation of hydrochemical facies by factor analysis. Ground Water 16:228–233. https://doi.org/10.1111/j.1745-6584.1978.tb03229.x
Devaraj N, Chidambaram S, Gantayat RR (2018) An insight on the speciation and genetical imprint of bicarbonate ion in the groundwater along K/T boundary, South India. Arab J Geosci. https://doi.org/10.1007/s12517-018-3622-3
Devaraj N, Chidambaram S, Vasudevan U (2020) Determination of the major geochemical processes of groundwater along the Cretaceous-Tertiary boundary of Trichinopoly, Tamilnadu, India. Acta Geochimica 39:760–781. https://doi.org/10.1007/s11631-020-00399-2
Devraj N, Chidambaram S, Thivya C, Thilagavathi R (2016) A study on the hydrogeochemical processes groundwater quality of Ariyalur region, Tamilnadu. Int J Curr Res Dev 4:1–13
Emerson W, Bakker AC (1973) The comparative effect of exchangeable Ca, Mg and Na on some soil physical properties of red brown earth soils. 2. The spontaneous dispersion of aggregates in water. Aust J Soil Res 11:151–152
Frankm E (1950) Significance of carbonates in irrigation waters. Soil Sci 69:123–134. https://doi.org/10.1097/00010694-195002000-00004
Ghafoor AMA, Shoaib M (1993) Characterization of tube well and hand pump waters in the Faisalabad tehsil Pakistan. J Soil Sci 8:1–2
Ghafoor A, Ullah F, Abdullah M (1990) Use of high Mg brackish water for reclamation of saline sodic soil. 1. Soil improvement. Pakistan J Agricu Sci 27:294–298
Govindan A, Ravindran CN, Rangaraju MK (1996) Cretaceous stratigraphy and planktonic foraminiferal zonation of Cauvery Basin, South India. Memoir Geol Soc India 37:155–187
Gronberg JA, Pucci Jr., and Birkelo BA (1991) Hydrogeologic framework of the Potomac-Raritan-Magothy aquifer system, northern Coastal Plain of New Jersey. New jersey geological survey special Bulletin. https://doi.org/10.3133/wri904016
James l Drever (1997) The geochemistry of natural waters, 3rd edn. Prentice Hall
Johns MW (1968) Geochemistry of groundwater from upper cretaceous-lower tertiary sand aquifers in South-Western Victoria, Australia. J Hydrol 6:337–357. https://doi.org/10.1016/0022-1694(68)90054-1
Kanwar JS, Chaudhry ML (1968) Effect of Mg on the uptake of nutrients from the soil. J Res Pb Agric Uni 3:309–319
Karmegam U, Chidamabram S, Sasidhar P, Manivannan R, Manikandan S, Anandhan P (2010) Geochemical characterization of groundwaters of shallow coastal aquifer in and around Kalpakkam, South India. Res J Environ Earth Sci 2:170–177
Karmegam U (2012) A study on the hydrogeochemical modeling of coastal aquifers in and around Kalpakkam. Thesis
Kumar M, Ramanathan AL, Tripathi R (2017) A study of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater of Chhaprola Industrial Area, Gautam Buddha Nagar, Uttar Pradesh, India. Chemosphere 166:135–145. https://doi.org/10.1016/j.chemosphere.2016.09.086
Li S, Wenzhi SG, Han LH, Zhang Q (2008) Water quality in relation to land use and land cover in the upper Han River Basin, China. CATENA 75:216–222. https://doi.org/10.1016/j.catena.2008.06.005
Loganathan K, Ahamed AJ (2017) Multivariate statistical techniques for the evaluation of groundwater quality of Amaravathi River Basin: South India. Appl Water Sci 7:4633–4649. https://doi.org/10.1007/s13201-017-0627-0
Madhavaraju J, Ramasamy S (1999) Rare earth elements in limestones of Kallankurichchi Formation of Ariyalur Group. J Geol Soc India 54:291–301
Madhavaraju J, Ramasamy S (2001) Clay mineral assemblages and rare earth element distribution in the sediments of Ariyalur Group, Tiruchirapalli District, Tamil Nadu -Implication for paleoclimate. Jour Geol Soc India 58:69–77
Manivannan R, Chidambaram S, Anandhan P et al (2011) Study on the Significance of Temporal Ion Chemistry in Groundwater of Dindigul District, Tamilnadu, India. E J Chem 8:938–944. https://doi.org/10.1155/2011/632968
Mattheß G (1982) The properties of groundwater. Wiley, New York
Mehra M, Oinam B, Singh CK (2016) Integrated assessment of groundwater for agricultural use in Mewat District of Haryana, India using geographical information system (GIS). J Indian Soc Remote Sens 44:747–758. https://doi.org/10.1007/s12524-015-0541-6
Mishra A (2014) In: Proc. of the 11th Kovacs Colloquium. Ed. Gordon Young, IAHS Publ, France
Mondal NC, Singh VS (2004) A new approach to delineate the groundwater recharge zone in hard rock terrain. In: current science. National Geophysical Research Institute, Hyderabad, Andra pradesh, p 10
Olofinlade WS, Daramola SO, Olabode OF (2018) Hydrochemical and statistical modeling of groundwater quality in two constrasting geological terrains of southwestern Nigeria. Model Earth Syst Enviro 4:1405–1421. https://doi.org/10.1007/s40808-018-0486-1
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2009) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90. https://doi.org/10.1007/s10661-009-1092-5
Prasanna MV, Nagarajan R, Chidambaram S et al (2013) Drip water Geochemistry of Niah Great Cave, NW Borneo, Malaysia: a base line study. Carbonates Evaporites 29:41–54. https://doi.org/10.1007/s13146-013-0164-3
Qiyan F, Baoping H (2002) Hydrogeochemical simulation of water-rock interaction under water flood recovery in Renqiu Oilfield, Hebei Province, China. Chin J Geochem 21:156–162. https://doi.org/10.1007/bf02873773
Ramanathan S (1979) Tertiary formations of South India. Geol Surv India Misc Publ 45:165–180
Ramasamy S, Banerji RK (1991) Geology, petrography and stratigraphy of pre-Ariyalur sequence in Tiruchirapalli District, Tamil Nadu. J Geol Soc India 37:577–594
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. U.S. Dept. of Agriculture, Washington, D.C.
Ryznar JW (1944) A new index for determining amount of calcium carbonate scale formed by a water. J Am Water Works Assoc 36:472–483. https://doi.org/10.1002/j.1551-8833.1944.tb20016.x
Saravanan K, Srinivasamoorthy K, Gopinath S et al (2016) Investigation of hydrogeochemical processes and groundwater quality in Upper Vellar sub-basin Tamilnadu, India. Arab J Geosci. https://doi.org/10.1007/s12517-016-2369-y
Saxena V, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43:731–736. https://doi.org/10.1007/s00254-002-0672-2
Senthilkumar S, Gowtham B, Sundararajan M et al (2017) Impact of landuse on the groundwater quality along coastal aquifer of Thiruvallur district, South India. Sustain Water Resour Manag 4:849–873. https://doi.org/10.1007/s40899-017-0180-x
Singh KP (1994) Temporal changes in the chemical quality of ground water in Ludhiana area. Curr Sci 66:375–378
Singh SK, Srivastava PK, Pandey AC, Gautam SK (2013) Integrated assessment of groundwater influenced by a confluence river system: concurrence with remote sensing and geochemical modelling. Water Resour Manage 27:4291–4313. https://doi.org/10.1007/s11269-013-0408-y
Srinivasamoorthy K (2004) Hydrogeochemistry of groundwater in Salem District, Tamilnadu. Thesis, India
Srinivasamoorthy K, Nanthakumar C, Vasanthavigar M et al (2009) Groundwater quality assessment from a hard rock terrain, Salem district of Tamilnadu, India. Arab J Geosci 4:91–102. https://doi.org/10.1007/s12517-009-0076-7
Subba Rao N, Prakasa Rao J, John Devadas D, Srinivasa Rao KV, Krishna C, Nagamalleswara Rao B (2002) Hydrogeochemistry and groundwater quality in a developing urban environment of a semi-arid region, Guntur, Andhra Pradesh. J Geol Soc Ind 59:159–166
Sundaram R, Rao PS (1986) Lithostratigraphy of cretaceous and paleocene rocks of Trichinopoly District of Tamil Nadu, South India. Record Geolog Surv India 115:9–23
Tahaikt M, Ait Haddou A, El Habbani R et al (2008) Comparison of the performances of three commercial membranes in fluoride removal by nanofiltration. Contin Op Desalination 225:209–219. https://doi.org/10.1016/j.desal.2007.07.007
Thilagavathi R, Chidambaram S, Prasanna MV et al (2012) A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl Water Sci 2:253–269. https://doi.org/10.1007/s13201-012-0045-2
Thilagavathi R, Chidambaram S, Thivya C et al (2016) Dissolved organic carbon in multilayered aquifers of Pondicherry Region (India): spatial and temporal variability and relationships to major ion chemistry. Nat Resour Res 26:119–135. https://doi.org/10.1007/s11053-016-9306-3
Thilagavathi R, Chidambaram S, Thivya C, Prasanna MV, Keesari T, Pethaperumal S (2017) Assessment of groundwater chemistry in layered coastal aquifers using multivariate statistical analysis. Sustain Water Res Manage 3(1):55–69. https://doi.org/10.1007/s40899-017-0078-7
Thivya C, Chidambaram S, Thilagavathi R et al (2013) Identification of the geochemical processes in groundwater by factor analysis in hard rock aquifers of Madurai District, South India. Arab J Geosci 7:3767–3777. https://doi.org/10.1007/s12517-013-1065-4
Thivya C, Chidambaram S, Rao MS et al (2015) Assessment of fluoride contaminations in groundwater of hard rock aquifers in Madurai district, Tamil Nadu (India). Appl Water Sci 7:1011–1023. https://doi.org/10.1007/s13201-015-0312-0
Thivya C, Chidambaram S, Thilagavathi R et al (2018) Short-term periodic observation of the relationship of climate variables to groundwater quality along the KT boundary. J Clim Change 4:77–86. https://doi.org/10.3233/jcc-1800015
Vengosh A, Keren R (1996) Chemical modifications of groundwater contaminated by recharge of treated sewage effluent. J Contam Hydrol 23:347–360. https://doi.org/10.1016/0169-7722(96)00019-8
WHO (2004) Guidelines for drinking water quality. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Dept. of Agriculture, Washington, D.C.
Xu P, Feng W, Qian H, Zhang Q (2019a) Hydrogeochemical characterization and irrigation quality assessment of shallow groundwater in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16:1492. https://doi.org/10.3390/ijerph16091492
Xu P, Li M, Qian H et al (2019b) Hydrochemistry and geothermometry of geothermal water in the central Guanzhong Basin, China: a case study in Xi’an. Environ Earth Sci. https://doi.org/10.1007/s12665-019-8099-1
Xu P, Zhang Q, Qian H et al (2019c) Characterization of geothermal water in the piedmont region of Qinling Mountains and Lantian-Bahe Group in Guanzhong Basin, China. Environ Earth Sci. https://doi.org/10.1007/s12665-019-8418-6
Zhang Q, Xu P, Qian H (2019) Assessment of groundwater quality and human health risk (HHR) evaluation of nitrate in the Central-Western Guanzhong Basin, China. Int J Environ Res Public Health 16:4246. https://doi.org/10.3390/ijerph16214246
Zhang Q, Xu P, Qian H (2020a) Groundwater quality assessment using improved water quality index (WQI) and human health risk (HHR) evaluation in a semi-arid Region of Northwest China. Expos Health 12:487–500. https://doi.org/10.1007/s12403-020-00345-w
Zhang Q, Xu P, Qian H, Yang F (2020b) Hydrogeochemistry and fluoride contamination in Jiaokou Irrigation District, Central China: assessment based on multivariate statistical approach and human health risk. Sci Total Environ 741:140460. https://doi.org/10.1016/j.scitotenv.2020.140460
Funding
No funding was availed for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
This article submitted with consent of all the authors.
Human and animal rights
Also this research work involves only human participants not animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Natesan, D., Sabarathinam, C., Kamaraj, P. et al. Impact of monsoon shower on the hydrogeochemistry of groundwater along the lithological contact: a case study from South India. Appl Water Sci 12, 36 (2022). https://doi.org/10.1007/s13201-021-01538-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01538-7