Abstract
In recent years, groundwater pollution has become increasingly a serious environmental problem throughout the world due to increasing dependency on it for various purposes. The Damodar Fan Delta is one of the agriculture-dominated areas in West Bengal especially for rice cultivation and it has a serious constraint regarding groundwater quantity and quality. The present study aims to evaluate the groundwater quality parameters and spatial variation of groundwater quality index (GWQI) for 2019 using the fuzzy analytic hierarchy process (FAHP) method. The 12 water quality parameters such as pH, TDS, iron (Fe−) and fluoride (F−), major anions (SO42−, Cl−, NO3−, and HCO3−), and cations (Na+, Ca2+, Mg2+, and K+) for the 29 sample wells of the study area were used for constructing the GWQI. This study used the FAHP method to define the weights of the different parameters for the GWQI. The results reveal that the bicarbonate content of 51% of sample wells exceeds the acceptable limit of drinking water, which is maximum in the study area. Furthermore, higher concentrations of TDS, pH, fluoride, chloride, calcium, magnesium, and sodium are found in few locations while nitrate and sulfate contents of all sample wells fall under the acceptable limits. The result shows that 13.79% of the samples are excellent, 68.97% of the samples are very good, 13.79% of the samples are poor, and 3.45% of the samples are very poor for drinking purposes. Moreover, it is observed that very poor quality water samples are located in the eastern part and the poor water wells are located in the northwestern and eastern part while excellent water quality wells are located in the western and central part of the study area. The understanding of the groundwater quality can help the policymakers for the proper management of water resources in the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater, a naturally occurring vital resource is overexploited nowadays in many parts of the world to meet the growing human demand for drinking, agriculture, urban, and industrial purposes (Deepa and Venkateswaran 2018; Mahammad and Islam 2021). Groundwater is a purer form of water as it is always clear, colourless, and odorless and maintains a relatively constant temperature compared to surface water (Fatoba et al. 2017). Therefore, two/third of the world’s population uses groundwater alone to meet their necessary demands (Adimalla and Taroor 2020). The groundwater extraction in India is maximum than any other countries of the world utilizing for irrigation (89%), domestic (9%), and industrial (2%) purposes (Margat and van der Gun 2013; Ahada and Suthar 2018). Unfortunately, in India, the deterioration of groundwater quality is increasing rapidly due to overexploitation of groundwater without a balanced recharge, uncontrolled uses of agrochemicals and fertilizers that percolate into the aquifer system (Wagh et al. 2017). Besides, industrial wastewater, municipal solid waste, and domestic wastewater also add water pollutants. Moreover, geology, chemical weathering of rocks, quality of recharge water, and water-rock interaction of an area have a greater influence on the hydrochemical characteristics of the groundwater (Trabelsi et al. 2012). Consequently, the contaminated groundwater affects human health, the balance of the aquatic ecosystem, economic development, and social prosperity as well (Milovanovic 2007; Zahedi et al. 2017). Therefore, the periodic monitoring of hydrochemical characteristics of groundwater and hydraulic parameters of aquifer holding the groundwater is required for the proper planning and management of groundwater (Fatoba et al. 2017).
Numerous studies have been carried out throughout the world using the groundwater quality parameters to assess the suitability of groundwater for irrigation, drinking, and domestic purposes (Salifu et al. 2017; Kumari et al. 2019; Barik and Pattanayak 2019; Srivastava 2019; Singh et al. 2020; Kurdi and Eslamkish 2017; Duraisamy et al. 2019; Egbueri et al. 2020; Khan and Jhariya 2017; Tiwari et al. 2017; Abdullah et al. 2019). Srivastava and Parimal (2020) have studied the hydrochemistry of groundwater and used the various weathering indices to assess the suitability of water for irrigation purposes. Anbazhagan and Nair (2004) have used the geographical information system (GIS) to represent the spatial variation of various geochemical elements in Panvel Basin, Maharashtra, India. Several multivariate statistical techniques such as cluster analysis (CA), factor analysis (FA), and principal component analysis (PCA) have been employed by many researchers to identify the significant parameters of groundwater quality (Abdelaziz et al. 2020). Zheng et al. (2016) applied CA, discriminate analysis (DA), PCA, and FA to evaluate the surface water quality and categorized the physicochemical parameters of water quality in the Second Songhua River basin in China. Bhuiyan et al. (2016) used multivariate statistics along with a geostatistical technique for the analysis and interpretation of complex datasets of groundwater of the southeastern coastal region of Bangladesh. They also indicated the pollution sources that are responsible for variation in physicochemical parameters and metal contents in groundwater systems.
To assess the surface water and groundwater quality, several approaches have been studied during the last few decades. The water quality index (WQI) tool can be used to assess water quality by transforming a huge number of parameters into a single index (Tyagi et al. 2013; Minh et al 2019; Abdelaziz et al. 2020). The WQI method was first introduced by Horton (1965) by using ten parameters of water quality. Furthermore, the new WQI developed by Brown et al. (1970) is similar to Horton (1965) which was based on weights to individual parameters (Tyagi et al. 2013). However, numerous modifications of water quality indices viz. Weight Arithmetic Water Quality Index (WAWQI), National Sanitation Foundation Water Quality Index (NSFWQI), Canadian Council of Ministers of the Environment Water Quality Index (CCMEWQI), Oregon Water Quality Index (OWQI), etc. have been formulated by several organizations (Tyagi et al. 2013). The Damodar Fan Delta is one of the agriculture-dominated areas in West Bengal especially for rice cultivation and it has a serious constraint regarding groundwater quality and quality. Several studies related to groundwater quality especially in arsenic contamination have been carried out covering the present study area (Acharyya and Shah 2007; Pal and Mukherjee 2009, 2010). Moreover, there has been an increase in the number of semi-critical community development (C.D.) blocks in the Damodar Fan Delta (DFD). In 2004, only two semi-critical C.D. blocks (Memari II and Pandua) were situated in the DFD, whereas in 2013, 13 semi-critical C.D. blocks (Kalna II, Memari II, Raina I, Chanditala I, Chanditala II, Dhaniakhali, Jangipara, Khanakul I, Pandua, Polba-Dadpur, Pursurah, Singur, and Tarakeswar) was located in the DFD (CGWB 2006, 2017). Therefore, the stages of groundwater development and its consequences on agricultural practices have been stressed by various scholars (Das et al. 2021; Majumder and Sivaramakrishnan 2014). However, the suitability of groundwater for drinking purposes in the context of the present study area has not been attempted so far. Therefore, the main objectives of the present study are as follows.
-
1.
To analyse the groundwater quality parameters of the Damodar fan delta.
-
2.
To assess the water quality index for drinking purposes using the fuzzy-AHP MCDM technique.
Study area
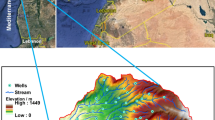
Damodar fan delta (DFD) consists of two alluvial fans -Memari fan trending toward the east and Tarakeswar fan trending toward the south (Acharyya and Shah 2007; Mallick and Niyogi1972; Niyogi 1975). It extends from 22° 31′ 09″ N to 23° 20′ 00″ N latitude and 87° 49′ 00″ E to 88° 29′ 33″ E longitude comprising an area of ~ 3206 km2 (Fig. 1). It lies in the interfluves of Hooghly River located in the east and the Damodar River located in the west and surrounded by Kusumgram fan in the north. The DFD is a younger deltaic plain characterized by the Holocene deposit (Acharyya and Shah 2007). The Damodar River, popularly known as the ‘Sorrow of Bengal,’ is an important western tributary of the Ganga River (Rudra 2010).
Geologically, the study area is a part of the Bengal basin which is a structural depression surrounded by the Chotanagpur plateau to the west, Rajmahal trap to the north, and Chattagram-Tripura hills to the east (Rudra 2010). The Damodar Fan Delta is located in the stable shelf zone of the Bengal basin (Sengupta 1972). The study area is located in the alluvial plain of West Bengal. The elevation of the study area ranges from 7 m (near Amta) to 37 m (near Barddhaman town). The slope of the study area is almost gentle. The general slope trends toward the east and the southeast. In the study area, the climate is characterized by tropical humid to sub-humid type. The maximum temperature is 31.80 °C, which is recorded in May whereas the minimum temperature is 19.85 °C recorded in December (Bhattacharyya 2011). On average, annual rainfall amounts to 1600 mm with its concentration in the monsoon period (Bhattacharyya 2011). The study area reveals the four types of soil texture—very fine, fine, fine loamy, and coarse loamy (NBSS & LUP 1992). Agriculture is the mainstay of the economy with rice as the main crop of the study area. Purba Barddhaman district located in the study area is known as the ‘rice bowl’ of West Bengal for huge production (Dutta 2012).
Data sets
The groundwater quality data for the present study of April 2019 were collected from the Central Ground Water Board (CGWB), the Government of India. The 12 groundwater physical–chemical parameters such as TDS, F−, Cl−, Fe−, NO3−, pH, SO42−, Ca2+, Mg2+, Na+, K+ and HCO3− of 29 wells have been analyzed using a robust methodology. The depth of groundwater level of wells varies from 1 to 18.25 m. The sample wells are of 3 types, such as dug well (DW), tube well (TW), and piezometric well (PW). The error of ion balance has been computed for the water parameters of 29 sample wells. The ion balance error of all the sample wells in the present study falls within ±10 % indicating a good accuracy of analysis. Moreover, to assess the land use and land cover of the study area, a supervised classification has been made using linear imaging self-scanning (LISS IV) images of the National Remote Sensing Council (NRSC) with 5 m resolution, dated December 2014. Apart from that, the borehole data 6 locations were collected from the department of public health engineering (PHE), the Government of West Bengal to portray the sub-surface lithological compositions.
Methodology
The fuzzy analytic hierarchy process (FAHP) was developed to weight criteria in decision-making by using the output of the experts’ opinions. The weighted value was assigned by pair-wise comparison for each of the 12 groundwater quality parameters. The experts compared the parameters by pair-wise variables comparison using fuzzy triangular number scales. The FAHP process of weighting was assigned in four steps and GWQI was then calculated. The inverse distance weighting (IDW) interpolation has been used to display the results of the GWQI (Fig. 2).
The fuzzy-AHP pair-wise comparison approach
Generally, GWQI is developed using the four steps including the selection of parameters, obtaining the sub-index value, assigning weights of the water quality parameters, and aggregating the sub-indices to produce the final water quality score (Abbasi and Abbasi 2012). Basically, techniques of assigning weights of the water quality parameters are classified into two broad categories—(a) statistical-based objective methods and (b) participatory-based subjective methods (OECD 2008). In the first category, the weights are assigned based on the statistical analysis of the data of water quality parameters whereas, in the second category, the weights are determined using the judgment of experts, policymakers, and practitioners from different agencies of a certain area (Sutadian et al. 2017). Several studies used CA and PCA to find out the identical parameters and to define the weights for the development of GWQI (Boateng et al. 2016; Badeenezhad et al. 2020). However, PCA can only reduce the dimensionality of large data sets based on the variation of variables (Minh et al 2019). The entropy method has been applied to determine the weights of water parameters (Gorgij et al. 2017). The analytic hierarchy process (AHP) multi-criteria decision-making (MCDM) technique has also been used by many researchers to generate the weights of water quality parameters in the GWQI (Chakraborty and Kumar 2016; Sutadian et al. 2017; Sarkar and Majumder 2021). However, AHP does not rely solely on human decisions (Haider et al. 2017). Therefore, FAHP has been applied by the researchers as it is more accurate to give interval judgment than fixed value judgments. It also reduces uncertainty in assigned relative weight (Minh et al. 2019). The fuzzy set was first developed by Zadeh (1965) and combined with Saaty’s priority theory to reduce human ambiguity (Bellman and Zadeh 1970).
In the present study, the FAHP technique has been used to achieve relative weights of groundwater quality parameters for the development of GWQI. In the present study, geometric mean method proposed by Buckley (1985) has been used. The process of FAHP was divided into four steps—(a) hierarchy construction development, (b) pair-wise comparisons represented by fuzzy numbers, (c) the fuzzy triangular number calculation, and (d) fuzzy weights.
-
Step I: Hierarchy Construction Development
The first level was the overall objective to determine the quantification of the potential of groundwater resources; the second level was the comparison of water quality parameters (Fig. 3) (Minh et al. 2019). The fuzzy triangular number was used as a scale which was transferred from linguistic terms corresponding to Saaty’s scale (1980) in Table 1 through pair-wise comparison matrices. The higher weighting of a parameter shows the high importance of that parameter. Finally, the groundwater quality was assessed based on classes of groundwater quality index (GWQI).
-
Step II: The pair-wise comparisons represented by fuzzy numbers
Decision-making was based on the opinions of five experts in the present study. The fuzzy triangular number scales were used to compare between two parameters and find out the more important parameter. The parameters were compared by transferring them from linguistic terms to fuzzy numbers. The pair-wise contribution matrix is expressed in Eq. 1.
where \({\tilde{a }}_{ij}\) measure denotes a pair of criteria i and j, let \(\tilde{1 }\) be (1, 1, 1), when i equal j (i.e., \(i=j\)); if \(\stackrel{\sim }{1,}\) \(\stackrel{\sim }{2,}\) \(\tilde{3 }, \tilde{4 },\) \(\tilde{5 },\) \(\stackrel{\sim }{6,}\) \(\tilde{7 }\), \(\tilde{8 }\), \(\tilde{9 }\) measure that criterion i is relatively important to criterion j and then \({\tilde{1 }}^{-1},\) \({\tilde{2 }}^{-1},\) \({\tilde{2 }}^{-1},\) \({\tilde{3 }}^{-1}\), \({\tilde{4 }}^{-1},\) \({\tilde{5 }}^{-1},\) \({\tilde{6 }}^{-1}\), \({\tilde{7 }}^{-1},\) \({\tilde{8 }}^{-1},\) \({\tilde{9 }}^{-1}\) measure that criterion j is relatively important to criterion i.
-
Step III: Determine the Fuzzy Triangular Number
The geometric mean method proposed by Buckley (1985) was used to determine the criterion’s fuzzy geometric mean (Eq. 2).
where \({\tilde{a }}_{in}\) is fuzzy comparison value of criterion i to criterion n; therefore, \({\tilde{r }}_{i}\) is the geometric mean of fuzzy comparison value of criterion i to each criterion.
-
Step IV: Fuzzy weighting
The final fuzzy weights were calculated following Eq. 3.
where \({\tilde{w }}_{i}\)= \({lw}_{i}, {mw}_{i}, {uw}_{i}\), where \({lw}_{i},{mw}_{i},\) \({uw}_{i}\) stand for the lower, middle, and upper values of the fuzzy weight of the criterion i, respectively.
Water quality index
The water quality index provides a reliable picture about groundwater and surface water quality mostly for domestic uses and it is easily understandable to decision-makers about the quality and possible uses of any waterbody (Hamlat and Guidoum 2018). The GWQI includes three steps—(a) defining relative weights, (b) quality rating scale, and (c) sub-index of the parameters.
Step I: Relative weight \((Wi)\) of the parameters has been calculated using the weighted arithmetic GWQI method (Eq. 4)
where \(Wi\) is the relative weight, \(wi\) is the weight of each parameter, and n is the number of parameters.
Step II: Quality rating scale (\(Qi\)) is calculated by dividing the concentration value for each of the quality parameters in each water sample to the standard concentration values for drinking water which were specified by the Bureau of Indian Standard (BIS) (2012, 2015) and World Health Organization (WHO 2011) (Eq. 5).
where \(Qi\) is the quality rating, \({C}_{i}\) is the concentration of each parameter in the water sample, and \({S}_{i}\) is drinking water standard for each parameter.
Step III: The sub-index value is calculated for each chemical parameter (Eq. 6)
where\( {Sl}_{i}\) is the sub-index of i parameter, \({Q}_{i}\) is the quality rating scale based on the concentration of i parameter, and \({W}_{i}\) is the relative weight.
Step IV: Water quality index (WQI) is calculated following the above calculations (Eq. 7). The sum of sub-indices of each of the water samples defines the WQI value. As the WQI has been used in the context of assessing groundwater quality, the index has been denoted as GWQI for the present work.
Results
Spatial variation of groundwater parameters
The pH value of the groundwater denotes whether the water is acidic or alkaline. The low value of pH indicates acidic water whereas the high value represents alkaline water (Boateng et al. 2016). According to the BIS (2012), the acceptable limit of pH is 6.5–8.5 for drinking purposes. Typically it has no direct influence on human health but it can influence the solubility of many salts and determine the level of contaminants in water resources (Khosravi et al. 2017). The pH value of the groundwater in the study area ranges from 7.42 to 9.73 with an average value of 8.08 (Fig. 4). The spatial distribution of the pH value depicts that ~ 10% of the wells contain more than the acceptable limit of pH concentration in the study area (Fig. 5a).
The TDS is an essential parameter to determine the suitability of water for drinking and irrigation purposes (Wagh et al. 2019; Sarkar and Islam 2019). The bulk of total dissolved solids include bicarbonates, sulfates, and chloride of calcium, magnesium, sodium, potassium, silica, potassium chloride, nitrate, and boron (Pradhan and Pirasteh 2011). The TDS value is found to fluctuate from 104 to 1281 mg/L and the average value was 538.72 mg/L (Table 4). Only ~ 4% of the sample wells contain acceptable limits of turbidity in the present study area. Based on the TDS concentration, Carroll (1962) classified water into four types such as freshwater (0–1000 ppm), brackish water (1000–10,000 ppm), saline water (10,000–100,000 ppm), and brine water (> 100,000 ppm). Besides, 29 sample sites fall in the freshwater category whereas two sample sites fall in the brackish water category in the study area. The spatial variation of TDS shows that the maximum concentration of the TDS is located in the eastern part of the study area (Fig. 5b).
Iron (Fe−) in groundwater can be derived from geological, industrial, domestic discharge, or mining industries (Karakuş 2019). The iron concentration in the groundwater ranges from 0 to 6.97 mg/L and the average value is 0.68 mg/L (Table 1). From the analysis, it is found that ~ 7% of sample wells of the study area occupy the acceptable limit of iron concentration provided by BIS (2012). The spatial variation map depicts that maximum iron concentration is found in the western part of the study area (Fig. 5c).
Fluoride (F−) occurs as natural elements in groundwater in the Indian sub-continents (Ahada and Suthar 2018). Mukherjee and Singh (2018) reported that the higher concentration of F− in groundwater is attributed to geogenic sources mainly from country rocks containing fluorine-bearing minerals (apatite, fluorite, biotite, muscovite, and hornblende). The F− concentration in the study area differs from 0 to 1.22 mg/L with an average value of 0.29 (Fig. 5d).
According to WHO (2011), the acceptable limit of sodium (Na+) in drinking water is 200 mg/L. In the present study, Na+ concentration value ranges from 9 to 290 mg/L with a mean value of 85.24 mg/L. About 7% of groundwater sample contains > 200 mg/L (Fig. 6a). The high concentration of Na+ occurs in groundwater due to the weathering of silicate minerals from rocks and the solubility of salt present in the soil as a result of evaporation, human activities, and agricultural activities (Kumar et al. 2015). Furthermore, according to the WHO (2011) standard, the acceptable limit of potassium (K+) is 12 mg/L. In this study, K+ content varies between 1 and 210 mg/L with a mean value of 23.62 mg/L. According to the results, ~ 41% of groundwater samples show potassium content > 10 mg/L (Fig. 6b).
In the study, calcium (Ca2+) content varies from 6 to 130 mg/L with a mean value of 36.69 mg/L (Fig. 6c). As compared to the analytical results with BIS (2012), ~ 14% of groundwater samples are located above the threshold limit. The main sources of Ca2+ in drinking water come from geological units, agricultural wastes, and industrial wastes (Kumaravel et al. 2014). Moreover, magnesium (Mg2+) is another important contributor to water hardness and its contribution remarkably influences the chemistry of groundwater (Ahada and Suthar 2018). In the study, Mg2+ concentration ranges from 4 mg/L to 50 mg/L with a mean value of 24.07 mg/L (Fig. 6d). According to BIS standard (2012), ~ 27.59% sample exceeds the acceptable limit of magnesium concentration.
A high concentration of sulfate (SO42−) in drinking water may cause a laxative effect on the human body system (Kumar et al. 2015). According to BIS (2012) standard, the acceptable limit of SO42− is 200 mg/L. In the present study, the SO42− concentration value varies from 0 to 103 mg/L with a mean value of 15.07 mg/L. From the results, it is found that all samples had an SO42− value fall within the acceptable limit (Fig. 7a). Furthermore, chloride (Cl−) ion is often naturally available in chlorine form in groundwater and it has very low mobility in water (Khosravi et al. 2017). The presence of chloride in groundwater is due to weathering, leakage of soil sediments, minerals, as well as urban and industrial wastewaters into water resources (Kumar et al. 2015). Moreover, Cl− contents in the present study vary between 14 and 493 mg/L with a mean value of 127.34 mg/L. According to BIS standard (2012), the acceptable limit of Cl− in drinking water is 250 mg/L. The results of the study reveal that ~ 10% of groundwater samples exceed the threshold value of Cl− concentration (Fig. 7b). In the study area, the amount of nitrate (NO3−) content in the groundwater ranges from 0 to 23 mg/L, with a mean value of 4.48 mg/L. According to BIS (2012), the acceptable limit of NO3− concentration in drinking water is 45 mg/L. The results of the study reveal that all the samples had the nitrate value falling within the acceptable limit (Fig. 7c).
The presence of bicarbonate (HCO3−) in natural water is influenced by the level of soluble carbon dioxide, temperature, pH, cations, and some soluble salts (Khosravi et al. 2017). The concentration of HCO3− in groundwater is usually higher than that of surface water (Kumar et al. 2015). According to BIS (2015), the acceptable limit of HCO3− concentration in drinking water is 244 mg/L. The concentration of HCO3− in the study area ranges from 61 to 616 mg/L with a mean value of 274.17 mg/L. The result shows that ~ 51% of the sample exceeds the acceptable limit of HCO3− concentration that depicts the poor quality of drinking water (Fig. 7d, Tables 2, 3, 4).
Groundwater suitability for drinking purpose using GWQI
The GWQI summarizes a significant number of parameters of groundwater quality in a general method into a single number, and it is a helpful technique to assess and manage the groundwater resources. The value of GWQI for groundwater quality ranges from 35.52 to 273.02 with an average value of 81.87. However, the GWQI has been classified as excellent water (< 50), good water (50–100), poor water (100–200), very poor water (200–300), and unsuitable for drinking (> 300) (Wagh et al. 2017; Hamlat and Guidoum 2018; Minh et al. 2019). The GWQI in the study has been classified into four classes (Fig. 8).
If the GWQI is less than 50, the water has excellent quality. Moreover, the index ranging from 50–100 indicates good quality of water, the index in the range of 100 to 200 indicates poor water quality and > 200 represents very poor quality for drinking purposes in the present study area. The result shows that 13.79% of the total sample wells (4 wells) are the excellent quality while the 3.45% (1 well) sample is of very poor quality. The results also show that 68.97% of the samples (20 wells) are registered as very good quality and 13.79% (4 wells) samples as poor water quality for drinking purposes in the present study area. The spatial distribution of the GWQI reveals that very poor quality water well (W22) is located in the eastern part of the study area. The location of the poor water wells (W1, W4, W20, and W25) is concentrated in the northwestern part of the Barddhaman town, the southern part of the study area in the Haripal C.D. block and the Pandua C.D. block. The excellent water quality wells (W5, W10, W19, and W28) are located in the western and central parts of the study area. Besides, good water quality wells are located in other parts of the study area.
Discussion
The weightage analysis of the 12 groundwater quality parameters based on FAHP depicts that TDS, F−, Fe−, and Cl− are considered as the major elements which affect GWQI with weights of 0.27, 0.19, 0.14, and 0.1, respectively. The LULC distribution of the study area has a significant effect on the GWQI. In the northwestern part, well 1 and well 3 fall in the poor water quality zone which is covered by the built-up area near the Barddhaman town (Fig. 9a). In the southern part of the study area, one well (W20) falls in poor water quality zone due to the high concentration of TDS and HCO3− and Fe−, which reveals the high salinity of the groundwater. This may be due to the mixing of saline water (sourced from the Bay of Bengal in the south through the Hooghly River) with the groundwater (Sarkar et al. 2021) (Fig. 9a).
Furthermore, sub-surface lithological compositions play an important role in water quality. The subsurface lithologs have been encountered in the 6 boreholes of the study area (Fig. 9b). The lithological layers consist of clay, sand in various textures from very fine coarse with different colours including white, grey, black, yellow, and brown along with kankar, gravels, and pebbles. Grey clay consists of a high concentration of organic matter representing the flood sediments whereas brown sand reveals the greater concentration of illite, siderite, as well as iron-oxyhydroxidecoated grains in the Damodar River floodplain (Pal and Mukherjee 2010). In well 22, the GWQI is very poor due to the high concentration of iron. In the borehole (BH5) located at the sample well site W22, the blackish clay has been found from the depth of 6.1–27.45 m overlain by fine grey sand from the depth of 3.05–6.1 m and topsoil up to 3.05 m depth. The groundwater in well 19 is excellent due to the low concentration of Fe. The borehole (BH1) consists of fine brown sand from the depth of 15–64 m overlain by coarse brown sand from the depth of 9–15 m, black clay from the depth of 3–9 m, and topsoil up to the depth of 3 m from the surface. Therefore, it reveals that blackish clay is associated with the iron concentration in groundwater.
Besides, the influence of geological compositions of rocks, climatic conditions, and anthropogenic controls on groundwater quality is important to assess the hydrochemical behavior of groundwater in an area. In the present study, the Piper plot and Gibbs plot have been applied to ascertain the types of weathering, the influence of rock, precipitation and evaporation, etc. that influence groundwater composition.
Piper plot proposed by Piper (1944) has been used to determine the geochemical classification and hydrochemical evolution of groundwater in the study area (Fig. 10a). The difference in milliequivalent percentage between alkaline earth (Ca2+ + Mg2+) and alkali metals have been plotted on the X-axis and the milliequivalent percentage difference between weak acidic (HCO3−) anions and strong acidic anions (Cl− + SO42−) have been plotted on the Y-axis. The resultant diagram portrays 8 classes. It shows that ~ 45% of the sample wells fall in the magnesium bicarbonate type while ~ 14% of sample wells fall into the sodium chloride type. Besides, only one-sample well (W4) has been found in the Calcium chloride type. Apart from that ~ 35% of sample wells fall into mixed type. The results of the Piper diagram represent that majority of the sample wells (~ 66%) are categorized as alkaline earth while the remaining wells fall into the alkalies. Moreover, it also represents that 20 sample wells (~ 69%) fall into the weak acids category while the remaining wells fall into the strong acids. The results indicate the dominance of alkaline earth and weak acids of all the samples due to the interaction between the alkaline earth and alkali metals that originate from soil or rock interactions with strong acidic anions and weak acidic anions in groundwater.
The Gibbs plot is widely used to determine the relationship between water composition and lithological characteristics of the aquifer (Kumar et al. 2015). It represents the source of chemical constituents into three distinct fields such as precipitation, rock, and evaporation dominant (Gibbs 1970). The ratios of anions and cations, i.e., Na+/(Na+ + Ca2+) (Fig. 10b) and Cl−/(Cl− + HCO3−) (Fig. 10c) of sample wells are plotted against the relative value of TDS. The Gibbs plot of the present study indicates that the majority of the sample (~ 52%) falls in the evaporation dominant field. It is observed that many of the sample wells are located near agricultural area and evaporation increases salinity by increasing of Cl− and Na+ in relation to the increase in TDS. In addition, anthropogenic inputs such as agricultural fertilizers, and irrigation also influence the evaporation by the increase in Na+ and Cl−, and thus, TDS is increased (Wagh et al. 2019). In the present study area, rice is the most dominant crop which is grown in autumn (Aus), winter (Aman), and summer (Boro). Rice is followed by jute, potato, wheat, oilseeds, etc. The Boro crop requires more irrigation water from government canals, wells, and minor irrigation schemes as it is grown in the summer period. Nitrogen, phosphate, and potash fertilizers are dominantly used in the agricultural field (District Statistical Officer 2021). Therefore, the agricultural activity in the study area has a greater influence on groundwater quality. The Gibbs plot also shows ~ 48% of the samples are located in the rock dominant field of the diagram. The dominance of the rock-water interaction field reveals the interaction between rock chemistry and the chemistry of the percolated waters underground (Kumar et al. 2015).
Conclusion
In the study area, groundwater is an important source of water for drinking, domestic and irrigation purposes. Therefore, the present study used 12 physiochemical parameters of 29 sample wells to analyze and evaluate the quality of groundwater for drinking purposes. Besides, spatial variation of water quality parameters and water quality index were analyzed in the GIS environment. The FAHP technique was used to calculate the weights of the parameters for the GWQI. The results show that the bicarbonate content of 51% of sample wells exceeds the acceptable limit of drinking water, which is maximum in the study area. Furthermore, higher concentrations of TDS, pH, fluoride, chloride, calcium, magnesium, and sodium are found in few locations of the study area. The results also depict that iron and potassium concentration is maximum located in the eastern part of the study area, which is, respectively, 21 and 23.23 times higher than the maximum acceptable limit. The results demonstrate that nitrate and sulfate contents of all sample wells fall within the acceptable limits. The result shows that 13.79% of the sample are excellent while 3.45% of the samples are very poor. The results also show 68.97% of the samples are of very good quality and 13.79% of the samples of poor water quality for drinking purposes. From the results, it is observed that very poor quality water is located in the eastern part, and the poor water well is located in the northwestern part of the study area. Besides, excellent water quality wells are located in the western and central part and good water quality wells are located rest of the study area.
The FAHP-based GWQI has successfully been applied to assess the groundwater quality for drinking purposes in the DFD. It is pertinent to mention here that water-stressed conditions due to the exploitation of the groundwater at an accelerating rate for irrigation and pollution of groundwater due to anthropogenic inputs such as fertilizer poses threat to the supply of safe drinking water at an adequate quantity. This study has demonstrated that the spatial variability in the groundwater quality in the DFD with its major driving forces. Therefore, this study would help policymakers and stakeholders to find strategies for planning and management of groundwater quality at the local level.
References
Abbasi T, Abbasi SA (2012) Water quality indices. Elsevier, Amsterdam
Abdelaziz S, Gad MI, El Tahan AHM (2020) Groundwater quality index based on PCA: Wadi El-Natrun. Egypt J Afric Earth Sci 172:103964
Abdullah TO, Ali SS, Al-Ansari NA, Knutsson S (2019) Hydrogeochemical evaluation of groundwater and its suitability for domestic uses in Halabja Saidsadiq Basin. Iraq Water 11(4):690
Acharyya SK, Shah BA (2007) Arsenic-contaminated groundwater from parts of Damodar fan-delta and west of Bhagirathi River, West Bengal, India: influence of fluvial geomorphology and Quaternary morphostratigraphy. Environ Geol 52(3):489–501
Adimalla N, Taloor AK (2020) Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwater quality index (GWQI) techniques. Groundw Sustain Dev 10:100288
Ahada CP, Suthar S (2018) Assessing groundwater hydrochemistry of Malwa Punjab, India. Arab J Geosci 11(2):1–15
Anbazhagan S, Nair AM (2004) Geographic information system and groundwater quality mapping in Panvel Basin, Maharashtra, India. Environ Geol 45(6):753–761
Badeenezhad A, Tabatabaee HR, Nikbakht HA, Radfard M, Abbasnia A, Baghapour MA, Alhamd M (2020) Estimation of the groundwater quality index and investigation of the affecting factors their changes in Shiraz drinking groundwater, Iran. Groundw Sustain Dev 11:100435
Barik R, Pattanayak SK (2019) Assessment of groundwater quality for irrigation of green spaces in the Rourkela city of Odisha, India. Groundw Sustain Dev 8:428–438
Bellman RE, Zadeh LA (1970) Decision-making in a fuzzy environment. Manag Sci 17(4):B-141–B-164. https://doi.org/10.1287/mnsc.17.4.B141
Bhattacharyya K (2011) The Lower Damodar River, India: understanding the human role in changing fluvial environment. Springer, Berlin
Bhuiyan MAH, Bodrud-Doza M, Islam AT, Rakib MA, Rahman MS, Ramanathan AL (2016) Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environ Earth Sci 75(12):1–23
BIS (2012) 10500: 2012 Indian standard drinking water-specification (second revision). Bureau of Indian Standards, New Delhi
BIS (2015) Indian standard drinking water specifcation. second revision, pp 2–6
Boateng TK, Opoku F, Acquaah SO, Akoto O (2016) Groundwater quality assessment using statistical approach and water quality index in Ejisu-Juaben Municipality, Chana. Environ Earth Sci 75(6):1–14
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index-do we dare? Water and sewage works 117(10):339–343
Buckley JJ (1985) Fuzzy hierarchical analysis. Fuzzy Sets Syst 17(3):233–247
Carroll D (1962) Rainwater as a chemical agent of geologic processes: a review. US Government Printing Office, Washington, DC
CGWB (2006) Dynamic Ground Water Resources of India. Faridabad: Ministry of Water Resources, River Development & Ganga Rejuvenation Government of India
CGWB (2017). Dynamic Ground Water Resources of India. Faridabad: Ministry of Water Resources, River Development & Ganga Rejuvenation Government of India
Chakraborty S, Kumar RN (2016) Assessment of groundwater quality at a MSW landfill site using standard and AHP based water quality index: a case study from Ranchi, Jharkhand, India. Environ Monit Assess 188(6):335
Das J, Rahman AS, Mandal T, Saha P (2021) Exploring driving forces of large-scale unsustainable groundwater development for irrigation in lower Ganga River basin in India. Environ Dev Sustain 23(5):7289–7309. https://doi.org/10.1007/s10668-020-00917-5
Deepa S, Venkateswaran S (2018) Appraisal of groundwater quality in upper Manimuktha sub basin, Vellar river, Tamil Nadu, India by using Water Quality Index (WQI) and multivariate statistical techniques. Model Earth Syst Environ 4(3):1165–1180
District Statistical Officer (2021) Agricultural Information, Burdwan, http://bardhaman.nic.in/agri/agriculture.htm. Accessed on 26 March 2021
Duraisamy S, Govindhaswamy V, Duraisamy K, Krishinaraj S, Balasubramanian A, Thirumalaisamy S (2019) Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environ Geochem Health 41(2):851–873
Dutta AK (2012) Rice trade in the ‘rice bowl of Bengal’: Burdwan 1880–1947. Indian Econ Soc Hist Rev 49(1):73–104
Egbueri JC, Ameh PD, Ezugwu CK, Onwuka OS (2020) Evaluating the environmental risk and suitability of hand-dug wells for drinking purposes: a rural case study from Nigeria. Int J Environ Anal Chem 1–21. https://www.tandfonline.com/doi/abs/10.1080/03067319.2020.1800000
Fatoba JO, Sanuade OA, Hammed OS, Igboama WW (2017) The use of multivariate statistical analysis in the assessment of groundwater hydrochemistry in some parts of southwestern Nigeria. Arab J Geosci 10(15):1–11
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Gorgij AD, Kisi O, Moghaddam AA, Taghipour A (2017) Groundwater quality ranking for drinking purposes, using the entropy method and the spatial autocorrelation index. Environ Earth Sci 76(7):269
Haider H, Al-Salamah IS, Ghumman AR (2017) Development of groundwater quality index using fuzzy-based multicriteria analysis for Buraydah, Qassim, Saudi Arabia. Arab J Sci Eng 42(9):4033–4051
Hamlat A, Guidoum A (2018) Assessment of groundwater quality in a semiarid region of Northwestern Algeria using water quality index (WQI). Appl Water Sci 8(8):1–13
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Karakuş CB (2019) Evaluation of groundwater quality in Sivas province (Turkey) using water quality index and GIS-based analytic hierarchy process. Int J Environ Health Res 29(5):500–519
Khan R, Jhariya DC (2017) Groundwater quality assessment for drinking purpose in Raipur City, Chhattisgarh using water quality index and geographic information system. J Geol Soc India 90(1):69–76
Khosravi R, Eslami H, Almodaresi SA, Heidari M, Fallahzadeh RA, Taghavi M et al (2017) Use of geographic information system and water quality index to assess groundwater quality for drinking purpose in Birjand City, Iran. Desalin Water Treat 67(1):74–83
Kumar SK, Logeshkumaran A, Magesh NS, Godson PS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5:335–343. https://doi.org/10.1007/s13201-014-0196-4
Kumaravel S, Gurugnanam B, Bagyaraj M, Venkatesan S, Suresh M, Chidambaram S et al (2014) Mapping of groundwater quality using GIS technique in the east coast of Tamilnadu state and Pondicherry union territory, India. Magnesium (Mg) 30:30–150
Kumari MKN, Sakai K, Kimura S, Yuge K, Gunarathna MHJP (2019) Classification of groundwater suitability for irrigation in the ulagalla tank cascade landscape by gis and the analytic hierarchy process. Agronomy 9(7):351
Kurdi M, Eslamkish T (2017) Hydro-geochemical classification and spatial distribution of groundwater to examine the suitability for irrigation purposes (Golestan Province, north of Iran). Paddy Water Environ 15(4):731–744
Mahammad S, Islam A (2021) Assessing the groundwater potentiality of the Gumani River Basin, India, using geoinformatics and analytical hierarchy process. Groundw Soc Appl Geospatial Technol 161–187. https://link.springer.com/chapter/10.1007%2F978-3-030-64136-8_8
Majumder A, Sivaramakrishnan L (2014) Ground water budgeting in alluvial Damodar fan delta: a study in semi-critical Pandua block of West Bengal, India. Int J Geol Earth Environ Sci 4(3):23–37
Mallick S, Niyogi D (1972) Application of Geomorphology in Groundwater Prospecting in the Alluvial Plains around Burdwan, West Bengal.
Margat J, Van der Gun J (2013) Groundwater around the world: a geographic synopsis. CRC Press, Boca Raton
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River. Southeastern Europe Desalin 213(1–3):159–173
Minh HVT, Avtar R, Kumar P, Tran DQ, Ty TV, Behera HC, Kurasaki M (2019) Groundwater quality assessment using fuzzy-AHP in an Giang Province of Vietnam. Geosciences 9(8):330
Mukherjee I, Singh UK (2018) Groundwater fluoride contamination, probable release, and containment mechanisms: a review on Indian context. Environ geochem health 40(6):2259–2301
National Bureau of Soil Survey and land Use Planning (1992) Soil of West Bengal for Optimising Land Use. Indian Council of Agricultural Research, Nagpur
Niyogi D (1975) Quaternary Geology of the coastal plain of West Bengal and Orissa. Indian J Earth Sci 2:51–61
OCED (2008) Handbook on constructing composite indicators: methodology and user guide. OECD Publishing, Durham
Pal T, Mukherjee PK (2009) Study of subsurface geology in locating arsenic-free groundwater in Bengal delta, West Bengal, India. Environ Geol 56(6):1211–1225
Pal T, Mukherjee PK (2010) Search for groundwater arsenic in Pleistocene sequence of the Damodar River flood plain. West Bengal Indian J Geosci 64(1–4):109–112
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914–928
Pradhan B, Pirasteh S (2011) Hydro-chemical analysis of the ground water of the basaltic catchments: upper Bhatsai region, Maharastra. Open Hydrol J 5(1)
Rudra K (2010) Banglar Nadikatha. Sahitya Sansad, Kolkata
Saaty T (1980) The analytic hierarchy process (AHP) for decision making. In Kobe, Japan
Salifu M, Aidoo F, Hayford MS, Adomako D, Asare E (2017) Evaluating the suitability of groundwater for irrigational purposes in some selected districts of the Upper West region of Ghana. Appl Water Sci 7(2):653–662
Sarkar B, Islam A (2019) Assessing the suitability of water for irrigation using major physical parameters and ion chemistry: a study of the Churni River, India. Arab J Geosci 12:637. https://doi.org/10.1007/s12517-019-4827-9
Sarkar B, Islam A, Majumder A (2021) Seawater intrusion into groundwater and its impact on irrigation and agriculture: evidence from the coastal region of West Bengal. India. Reg Stud Mar Sci 44:101751. https://doi.org/10.1016/j.rsma.2021.101751
Sarkar K, Majumder M (2021) Application of AHP-based water quality index for quality monitoring of peri-urban watershed. Environ Dev Sustain 23:1780–1798. https://link.springer.com/article/10.1007/s10668-020-00651-y
Sengupta S (1972) Geological framework of the Bhagirathi-Hooghly basin. The Bhagirathi-Hooghly Basin, 3–8
Singh KK, Tewari G, Kumar S (2020) Evaluation of groundwater quality for suitability of irrigation purposes: a case study in the Udham Singh Nagar, Uttarakhand. J Chem 2020:1–15. https://doi.org/10.1155/2020/6924026
Srivastava SK (2019) Assessment of groundwater quality for the suitability of irrigation and its impacts on crop yields in the Guna district, India. Agric water manag 216:224–241
Srivastava AK, Parimal PS (2020) Source rock weathering and groundwater suitability for irrigation in Purna alluvial basin, Maharashtra, central India. J Earth Syst Sci 129(1):1–18
Sutadian AD, Muttil N, Yilmaz AG, Perera BJC (2017) Using the Analytic Hierarchy Process to identify parameter weights for developing a water quality index. Ecol Ind 75:220–233
Tiwari K, Goyal R, Sarkar A (2017) GIS-based spatial distribution of groundwater quality and regional suitability evaluation for drinking water. Environ Process 4(3):645–662
Trabelsi R, Abid K, Zouari K (2012) Geochemistry processes of the Djeffara palaeogroundwater (Southeastern Tunisia). Quatern Int 257:43–55
Tyagi S, Sharma B, Singh P, Dobhal R (2013) Water quality assessment in terms of water quality index. Am J Water Resour 1(3):34–38
Wagh V, Panaskar D, Mukate S, Lolage Y, Muley A (2017) Groundwater quality evaluation by physicochemical characterization and water quality index for Nanded Tehsil, Maharashtra, India. In: Proceedings of the 7th international conference on biology, environment and chemistry, IPCBEE (vol 98)
Wagh VM, Mukate SV, Panaskar DB, Muley AA, Sahu UL (2019) Study of groundwater hydrochemistry and drinking suitability through Water Quality Index (WQI) modelling in Kadava river basin, India. SN Appl Sci 1(10):1–16
WHO (2011) WHO guidelines for drinking-water quality, 4th edn. World Health Organization, New York
Zadeh LA (1965) Fuzzy sets. Inf Control 8:338–353
Zahedi S, Azarnivand A, Chitsaz N (2017) Groundwater quality classification derivation using multi-criteria-decision-making techniques. Ecol Ind 78:243–252
Zheng LY, Yu HB, Wang QS (2016) Application of multivariate statistical techniques in assessment of surface water quality in Second Songhua River basin, China. J Central South Univ 23(5):1040–1051
Funding
This work was supported by the University Grants Commission, Govt. of India (Grant No. 19806 (NET-JUNE 2015 dated 23, JUNE 2016) awarded to the first author to carry out his PhD research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical standards
The authors declare that they will follow the guidelines of this journal for integrity of the scientific record.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahammad, S., Islam, A. Evaluating the groundwater quality of Damodar Fan Delta (India) using fuzzy-AHP MCDM technique. Appl Water Sci 11, 107 (2021). https://doi.org/10.1007/s13201-021-01408-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01408-2