Abstract
Background
Tumor necrosis results from failure to meet the requirement for rapid proliferation of tumor, related to unfavorable prognosis in colorectal cancer (CRC). However, previous studies used traditional microscopes to evaluate necrosis on slides, lacking a simultaneous phase and panoramic view for assessment. Therefore, we proposed a whole-slide images (WSIs)-based method to develop a necrosis score and validated its prognostic value in multicenter cohorts.
Methods
Necrosis score was defined as the proportion of necrosis in the tumor area, semi-quantitatively classified into 3-level score groups by the cut-off of 10% and 30% on HE-stained WSIs. 768 patients from two centers were enrolled in this study, divided into a discovery (N = 445) and a validation (N = 323) cohort. The prognostic value of necrosis score was evaluated by Kaplan–Meier curves and the Cox model.
Result
Necrosis score was associated with overall survival, with hazard ratio for high vs. low in discovery and validation cohorts being 2.62 (95% confidence interval 1.59–4.32) and 2.51 (1.39–4.52), respectively. The 3-year disease free survival rates of necrosis-low, middle, and high were 83.6%, 80.2%, and 59.8% in discovery cohort, and 86.5%, 84.2%, and 66.5% in validation cohort. In necrosis middle plus high subgroup, there was a trend but no significant difference in overall survival between surgery alone and adjuvant chemotherapy group in stage II CRC (P = .075).
Conclusion
As a stable prognostic factor, high-level necrosis evaluated by the proposed method on WSIs was associated with unfavorable outcomes. Additionally, adjuvant chemotherapy provide survival benefits for patients with high necrosis in stage II CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
After years of exploration and practice, substantial advances have been made in early diagnosis, treatment, and prognosis of colorectal cancer (CRC). However, it remains a disease with high morbidity and mortality [1, 2]. The increasing tendency in the number of patients with CRC motivates continued research on prognostic factors. Presently, numerous clinicopathological factors for predicting prognosis enable to identify and screen the CRC patients at high risk, but there is still ill-defined evidence [3, 4]. The characteristic of a malignant tumor is that the cell has unlimited proliferation ability. Tumor necrosis results from the inability to meet the requirement for the rapid proliferation of tumor cells [5, 6]. The extent of necrosis reveals the degree of hypoxia in the tumor [7]. In addition, increased cellular hypoxia in solid tumors also affects metastatic potential and prognosis [8]. At present, tumor necrosis assessment has been successfully applied in renal cancer [9, 10], lung cancer [11], breast cancer [12], upper tract urothelial carcinoma [13], and CRC [14]. Therefore, necrosis as a potential marker of prognostic biomarker deserves exploration and validation. However, most current studies were single-center studies, and biases in patient selection inevitably exist. Because an instructive prognostic indicator could refine and update tumor-node-metastasis (TNM) stage and have a straight impact on the cancer care, multicenter validation of its prognostic value is warranted before applying the necrosis score to clinical practice.
On the other hand, with the progress of treatment, the prognosis of CRC has improved to some extent [15]. Treatment options for CRC still heavily relies on TNM stage [16]. Undoubtedly, TNM is the most important pathological classification in all international CRC guidelines. Nevertheless, clinical outcomes of patients with the same stage could be heterogeneous, due to the differences in clinical and molecular phenotypes, patterns of genetic damage, and host immune responses [17]. Currently, one of the most critical clinically relevant requirements is lack of sufficient predictive biomarkers that can identify patients at high risk, particularly in stage II CRC patients. These high-risk patients may benefit from adjuvant chemotherapy [15]. Therefore, exploring biomarkers such as necrosis to identify high risk CRC patients in stage II will allow use of adjuvant chemotherapy in a select subgroup of high risk patients to improve their prognosis.
There are two aims involved for this study. First, we proposed a necrosis score using HE-stained whole-slide images (WSIs) and validated its prognostic value in two CRC cohorts. Predictive competence of necrosis score in curative effect of adjuvant chemotherapy in stage II CRC was further investigated.
2 Materials and methods
2.1 Patient cohort
The inclusion criteria were patients with histologically confirmed stage I–III CRC who underwent surgical resection with curative intent and had paraffin-embedded tumor samples available. The discovery cohort consists of CRC patients from Shanxi Cancer Hospital and the validation cohort from The Sixth Affiliated Hospital of Sun Yat-sen University (Jan 2014 to Dec 2014). This study was approved by the Research Ethics Committees of the respective hospitals, with the need for informed consent waived for this retrospective study. Samples were excluded if the patients had other tumors, received neo-adjuvant therapy (radiotherapy, chemotherapy), follow-up information missing, death within 30 days of surgery, or HE-stained WSIs unavailable. The sample size for the analysis was based on pathological evaluation availability.
Clinicopathological characteristics information was collected from medical records, including age, sex, tumor location (colon or rectum), grade, TNM stage, carcinoembryonic antigen (CEA) level (cutoff = 5 ng/mL, normal = 0–5 ng/mL), microsatellite instability (MSI) status, and post-surgery treatment (surgery alone or adjuvant chemotherapy, selected by clinicians according to whether there were high-risk factors). Supplementary Table 1 shows the distributions of post-surgery treatment of stage II CRC patients. TNM stage was performed according to the Union for International Cancer Control (UICC) guideline [18]. MSI status was determined by four microsatellite markers (MLH1, MSH2, MSH6, and PMS2) if available. Clinical follow-up information was retrieved from the patient's electronic medical record and telephone communication. The prespecified primary endpoint was overall survival (OS), referring to the time from diagnosis to death for any reason. Disease-free survival (DFS), the secondary endpoint of interest, was defined as the date of the first event of cancer recurrence was used. Tumor recurrence is defined as the local recurrence, first distal metastasis or without events but with death. If no recurrence occurred, DFS was calculated as the period until the date of last follow-up.
2.2 Evaluation of necrosis score
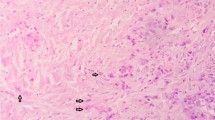
The pathologist sampled representative areas in tumor center and edge. Surgical specimens were formalin-fixed, paraffin-embedded, cut into 4 μm slices, and then stained with HE. These HE-stained slides were all scanned by digital whole-slide scanning systems (NanoZoomer S60 C13210-01, Hamamatsu, Japan; SQS-120P, Shengqiang, China) at 40 × magnification (resolution: 0.20–0.23 μm/pixel). Evaluators, blinded to the patient's clinical information and outcome, examined all available WSIs of the primary tumor. Simultaneous opening of all slices of the same patient on HE-stained WSIs. The existence of necrosis was carefully recognized, which is characterized by agglomerated cells forming condensates containing nuclear and cytoplasmic fragments [19]. Similar to previously criteria [14], the extent of necrosis was semi-quantitatively assessed at low magnification (× 5) and recorded as either low (absent and < 10% of the tumor area), middle (10–30% of the tumor area), or high (≥ 30% of the tumor area) based on all available WSIs. To test the interrater agreement, a random subset of 100 cases from the study population was selected, and necrosis score were independently scored by two authors (HFY and KZ). The rest of WSIs were assessed by one author (HFY).
2.3 Statistical analysis
Clinicopathological characteristics were compared by Student t-test for a continuous variable or Chi-square test for a category variable. Cohen’s Kappa was calculated as a statistical measure of the interrater agreement. Kaplan–Meier curves were plotted to determine the difference in survival rates among different groups, and log-rank tests were used to calculate P values. The multivariate Cox proportional hazard model was used to examine the associations between factors with OS and DFS. For multivariate analysis, univariate variables with P < 0.05 were selected. Hazard ratio (HR) with 95% confidence interval (CI) was calculated by using the Cox model. The discrimination performance of factors was assessed using Harrell's C-statistics (C-index) with 95% CI. Patients with missing data, such as available HE-stained WSIs and follow-up information were excluded, and the imputation method was not used in the analysis. All statistical analyses were conducted in an R environment (version 4.0.3), with statistical significance set at 0.05.
3 Results
3.1 Patients
The overall workflow of our study is illustrated in Fig. 1. Tumor samples were collected from 768 patients from two centers with UICC TNM stage I–III CRC. Among them, 445 patients (232 males and 213 females; mean age 59.2 ± 11.9 years) formed the discovery cohort, with a median follow-up time of 7.67 (interquartile range [IQR], 7.33–7.92) years; 323 patients (180 males and 143 females; mean age 60.1 ± 13.0 years) formed the validation cohort, with a median follow-up time of 7.17 (IQR, 6.96–7.43) years. A comparison of clinicopathologic characteristics between the two cohorts is displayed in Table 1.
Study workflow. a Evaluators examined all available HE-stained WSIs of the primary tumor and scored each of them according to the evaluation criteria, and finally obtained the final necrosis score. b The extent of tumor necrosis was semi-quantitatively assessed at low magnification (× 5) and recorded as either low (absent and < 10% of the tumor area), middle (10% − 30% of the tumor area), or high (≥ 30% of the tumor area). c The discovery cohort (N = 445) consists of CRC patients from SXCH, and the validation cohort (N = 323) from SYSU6. d Kaplan–Meier plots for all patients according to necrosis score and multivariate analysis for OS. HE hematoxylin and eosin, WSIs whole-slide images, CRC colorectal cancer, SXCH Shanxi Cancer Hospital, SYSU6 The Sixth Affiliated Hospital of Sun Yat-sen University
In the discovery cohort, 314 (70.6%) patients were grouped as necrosis-low, and 86 (19.3%) as middle, and 45 (10.1%) as high. 220 (68.1%) cases were classified as a low score, 66 (20.4%) as a middle score, and 37 (11.5%) as a high score in the validation cohort. In a random subset of 100 patients, there was good agreement between the two observers, having a Cohen’s Kappa value of 0.73.
3.2 Association of necrosis score with clinicopathologic characteristics
We further described the characteristics of patients by necrosis score categories in Table 2. In the discovery cohort, in necrosis-high group, tumors were more often located in colon (colon vs. rectum: 68.9% vs. 31.1%, P < 0.001), and as compared to stage I, stage III has a higher proportion (stage I vs. III: 2.2% vs. 55.6%, P < 0.05). While in necrosis-low group, the proportion of normal CEA was higher (normal vs. abnormal: 81.8% vs. 18.2%, P = 0.03). We as well observed similar characteristics trends in the validation cohort of TNM stage (0% vs. 62.2%, P < 0.05) and CEA (75.7% vs. 24.3%, P = 0.006). There was no significant difference in location, but it was more common in patients with low grade in necrosis-low group (high vs. low: 5.3% vs. 94.7%, P = 0.04).
3.3 Correlation of T and N categories with necrosis score
Only T and N categories were considered. Chi-square test was performed for T and N categories and necrosis score. T and N categories were significantly related to necrosis score. Necrosis-high was associated with a high level of T and N categories (T category, P < 0.0001; N category, P = 0.002). All T1 (N = 16) and 88.4% of the T2 (N = 138) patients were necrosis-low. In the T3 group (N = 377), this percentage decreased to 68.2%; in the T4 group (N = 237), it was only 58.6%. For the N category, the necrosis-low percentage was 73.0% in the N0 group (N = 466), 66.2% in the N1 group (N = 204) and 60.2% in the N2 group (N = 98) (Fig. 2).
Correlation of T and N categories with necrosis score. a The proportion of necrosis-high group increased with the increase of T category (Chi-square test, P < 0.0001). b The higher the necrosis score, the higher the proportion of high-ranking T category. c The proportion of necrosis-high group increased with the increase of N category (Chi-square test, P = 0.002). d The higher the necrosis score, the higher the proportion of high-ranking N category
3.4 Prognostic value of necrosis score
Necrosis-low group was associated with favorable OS (discovery cohort, P = 0.0003; validation cohort, P = 0.002; Fig. 3a, b). With necrosis score increased, the 5-year OS rates decreased from 89.0% in necrosis-low group to 68.7% in necrosis-high group in discovery cohort. Similar trends were observed in the validation cohort (92.1–69.7%). Unadjusted HR for high vs. low in discovery and validation cohorts was 2.62 (95% CI 1.59–4.32, P < 0.0001) and 2.51 (1.39–4.52, P = 0.002), respectively (Table 3).
Obtained results showed that necrosis score was significantly correlated with DFS, with unadjusted HR for high vs. low being 2.38 (95% CI 1.47–3.87, P < 0.0001) in discovery cohort and 2.49 (1.46–4.26, P = 0.001) in validation cohort (Supplementary Table 2). The 3-year DFS rates declined with necrosis score increased. In necrosis-low group, the 3-year DFS rates was 83.6%, decreased to 59.8% in necrosis-high group (P < 0.0001; Fig. 3c). In the validation cohort, these findings were confirmed. In validation cohort, they were 86.5% in necrosis-low group, compared with 66.5% in high group (P = 0.003; Fig. 3d).
3.5 Survival analysis of necrosis score stratified with clinicopathological characteristics
In addition, we examined whether necrosis score could be applied to subgroups of patients with clinicopathological characteristics. In DFS and OS, based on stratification by age (≥ 60), sex (female and male), grade-low, location (colon and rectum), and stage III, the score still had a statistically significant impact on the prognosis. (All P < 0.05; Supplementary Figs. S1–S5). The score had no statistically significant impact on the prognosis stratified by the other clinicopathological characteristics (All P > 0.05).
3.6 Necrosis score as an independent prognostic factor for OS and DFS
We performed univariate analyses on age, sex, TNM stage, location, CEA level, tumor grade, and necrosis score. In univariate Cox analyses, characteristics that reached significance for OS were age, grade, TNM stage, and necrosis score (Table 3, all P < 0.05). In the multivariate analysis, necrosis score was an independent prognostic marker for improved OS (discovery cohort: adjusted HR for high vs. low 1.86, 95% CI 1.12–3.08, P = 0.02; validation cohort: 2.09, 1.12–3.91, 0.02, Table 3). We identified age, grade, CEA level, TNM stage, and necrosis score as independent predictors for DFS (Supplementary Table 2, all P < 0.05). In multivariate analysis, necrosis score was still associated with DFS, independent of age, CEA level, grade, and TNM stage (discovery cohort: adjusted HR for high vs. low 1.71, 95% CI 1.05–2.79, P = 0.03; validation cohort: 2.00, 1.13–3.53, 0.02, Supplementary Table 2).
To evaluate the added prognostic value of necrosis score, we developed three Cox models: stage, necrosis score, and combined them (Table 4). In the discovery cohort, the C-index for necrosis score to predict OS was 0.578 (95% CI 0.532–0.625). The addition of TNM stage to necrosis score increased for predicting the OS from 0.578 to 0.706. Similar to the validation cohort, the C-index increases to 0.627 (0.561–0.692) after combining the above two. The C-index for DFS of necrosis score was 0.572 (0.528–0.616) and raised to 0.705 (0.666–0.744) by adding stage in the discovery cohort. Similarly, the C-index for DFS was 0.565 (0.510–0.620) when including only necrosis score and 0.628 (0.568–0.688) when adding stage in the validation cohort.
Starting from the logistic model containing all prognostic factors, we removed some statistically insignificant factors (P > 0.05) and kept the resulting model as the basis for the nomogram. In the discovery cohort, nomograms incorporating the respective independent prognostic factors of OS and DFS were established (Supplementary Fig. S6).
3.7 Survival analysis of necrosis score stratified with MSI status
We further examined that based on stratification by MSI, the score had a statistically significant impact on the prognosis for OS (P = 0.005) and DFS (P = 0.02) (Supplementary Fig. S7). As necrosis score, only a marginally statistically significant was found among microsatellite instability (MSS) individuals in DFS (P = 0.06; Supplementary Fig. S7d), while the difference was no longer presented in OS (P = 0.15; Supplementary Fig. S7c).
3.8 Association of necrosis score with adjuvant chemotherapy in stage II CRC
In stage II CRC, the OS and DFS were stratified, accounting for necrosis score, including necrosis-low, middle, high, necrosis-low plus middle and necrosis-middle plus high groups. The obtained findings exhibited that in necrosis-middle plus high group, there was a marginal difference in OS between surgery alone group and adjuvant chemotherapy group (78.4% vs. 86.3%, P = 0.80; Fig. 4e), while the difference was no longer present in DFS (78.4% vs. 80.2%, P = 0.32; Supplementary Fig. S8e). In stratified to necrosis-low, middle, high, and necrosis-low plus middle groups, OS and DFS had no difference between the two groups (all P > 0.05, OS: Fig. 4a–d; DFS: Supplementary Fig. S8a–d).
4 Discussion and conclusion
In this study, we found that the necrosis score was a stable prognosis factor in two independent cohorts of CRC, independent of TNM stage and other clinicopathological factors, and had the potential ability to predict the curative effect of adjuvant chemotherapy in stage II CRC.
Necrosis has been receiving much attention in the prognosis prediction of CRC [14, 20, 21]. Increased hypoxia due to abnormal rates of cell division, development of solid tumor masses that disrupt vascular penetration, resulting in decreased oxygen diffusion [22]. Tumor necrosis results from the inability to meet the requirement for the rapid proliferation of tumor cells [5, 6]. Necrosis was the final outcome of various pathophysiological processes after the body adapted and regulated. During the avascular phase, tumor mainly relies on the diffusion of surrounding tissues to acquire nutrients and excrete metabolites [23], thereby limiting its growth. During the vascular phase, angiogenesis and production of angiogenic factors were fundamental for tumor progression in form of growth, invasion, and metastasis [24]. New vessels adapted to hypoxia caused by rapid proliferation by conveying oxygen and nutrients. However, blood flow in many tumors is disorganized and variable [25]. First, the arteriovenous pressure difference decreases, and the viscous and geometric resistance increases in tumor, which will exert a compressive effect on blood vessels [26], which will increase blood flow resistance and damage the tumor blood supply. Secondary, compared with normal tissues, functional lymphatic capillaries were absent from the interior of solid tumors [27], which leads to increased interstitial fluid pressure within them [28]. Increased interstitial fluid pressure inhibits the distribution of larger molecules through convection [29] and compresses blood vessels, causing blood to shift from the tumor center to the periphery, resulting in decreased blood supply to the tumor center. These indicated that even though neovascularization could weaken the effects of hypoxia, there were various factors that could affect neovascularization. High tumor necrosis means that even after adaptation and regulation, the body still could not reverse the effect of hypoxia, suggesting worse prognosis. The above explained the results of our work. A high proportion of necrosis corresponded to the low DFS and OS in the two cohorts. The 5-year OS rate of patients in necrosis-high group was much lower than that in necrosis-low group (68.7% vs. 89.0%), consistent with previous studies [14]. The 3-year DFS rate was recorded for 83.6% of patients with a low score and 59.8% with high. The results showed that necrosis score achieved comparable prognostic performance. Multivariate analysis also further confirmed. Validation of necrosis score quantified in HE-stained WSIs by two independent cohorts can support the value of necrosis score for stratifying the risk of CRC patients.
TNM stage remained one of the most clinically applied prognostic factors in CRC, including the depth of local invasion into the bowel wall and the infiltration of regional lymph nodes [30, 31]. As expected, the percentage of necrosis-high group grows with the increase in T and N categories. T category increased with the increase of tumor volume and the extent of adjacent tissue involvement. The larger the tumor volume or the more profound the invasion, the upper the T category and the more prominent tumor necrosis [32]. Additionally, as the tumor grows, the body is uncompensated for the tumor’s blood supply requirement, and necrosis occurs [5, 6]. In other words, with the increase of tumor size or infiltration, the blood supply could not meet the needs of tumor growth, and the chance of tumor necrosis would increase. As shown in the results, with the increase in T category, the proportion of necrosis-low group decreased, with 88.4% of the T2 patients were necrosis-low group. In the T4 group, this percentage decreased to only 58.6%. Lymph nodes metastasis indicated that the tumor has strong metastatic ability and a great degree of aggressiveness, suggesting a poor prognosis for the patient [33]. Tumor cells break away from the primary tumor, invade the basement membrane [34], infiltrate and grow in the surrounding stroma and involve the lymphatic vessels, survive in the lymphatic vessels, and are metastasized to the lymph nodes to form metastatic tumors [35, 36]. In our result, the proportion of positive lymph nodes (N+) increased with the increase of necrosis score. The great extent of necrosis indicated the rapid proliferation of tumor cells and the powerful metastatic potential, suggesting that necrosis score has the potential to speculate on regional lymph node metastasis.
The stage of CRC was assigned according to TNM stage system of UICC, which provided treatment guidelines. Consensus has been reached for the treatment of stage I CRC with surgery alone and chemotherapy in addition to surgery for stage III/IV CRC, but the overall benefit of adjuvant chemotherapy after resection for stage II CRC remains unclear [37]. MSI was not only an independent prognostic factor for stage II CRC [38] but also a predictor of the efficacy of adjuvant chemotherapy. According to the latest ESMO and CSCO guidelines, adjuvant chemotherapy was not recommended, but observation and follow-up for patients with stage II CRC without high-risk factors such as MSI-H [16, 39]. For stage II MSI-H CRC patients, current studies found that patients treated with 5-FU single-agent adjuvant chemotherapy had no survival benefit [40, 41]. However, in the MSI-H stratified analysis, it was found that there were significant differences in OS (P = 0.005) and DFS (P = 0.02) with different necrosis scores. Patients with necrosis-high had a lower survival rate than those with necrosis-low, suggesting that necrosis in patients with MSI-H could further risk stratified, and the treatment of the stage II population still needed to be stratified and analyzed to achieve a more individualized treatment. On the other hand, we also observed that in patients with stage II CRC, in necrosis-middle plus high group, it was found that there was a trend but no significant difference between surgery alone group and adjuvant chemotherapy group (78.4% vs. 86.3%, P = 0.075). It was speculated that adjuvant chemotherapy could improve the survival rate with a high proportion of necrosis. Therefore, according to necrosis score, we speculate that patients with stage II MSI-H CRC could be further selected suitably for adjuvant chemotherapy. Further studies are needed to verify whether patients with high necrosis score are a high-risk factor for adjuvant chemotherapy in stage II MSI-H CRC to provide more accurate and individualized treatment for patients in the future.
One of the limitations of our study is that this analysis is retrospective and may be susceptible to bias introduced by certain risk factors. Therefore, it is necessary to conduct prospective studies to verify the effectiveness of necrosis score in routine clinical applications. Secondly, in this study, WSIs was used to ameliorate differences in reporting among pathologists and the difficulty of scaling up the evaluation process under a microscope. But in our study, necrosis score was still a not full-quantified method that was inconvenient to popularize widely in daily work. Therefore, using image segmentation to quantify tumor necrosis automatically is one of our future research ambitions.
In conclusion, we used a necrosis score for semi-quantitative using HE-stained WSI in CRC. We found evidence that necrosis score has a stable prognostic value in CRC, with a higher necrosis score being more unfavorable for overall survival. Necrosis score might provide vital assistance for changes in risk stratification in stage II CRC patients to perform adjuvant chemotherapy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Khan K, Rata M, Cunningham D, Koh DM, Tunariu N, Hahne JC, Vlachogiannis G, Hedayat S, Marchetti S, Lampis A, Damavandi MD, Lote H, Rana I, Williams A, Eccles SA, Fontana E, Collins D, Eltahir Z, Rao S, Watkins D, Starling N, Thomas J, Kalaitzaki E, Fotiadis N, Begum R, Bali M, Rugge M, Temple E, Fassan M, Chau I, Braconi C, Valeri N. Functional imaging and circulating biomarkers of response to regorafenib in treatment-refractory metastatic colorectal cancer patients in a prospective phase II study. Gut. 2018;67(8):1484–92.
Patel NV, Ghaleb AM, Nandan MO, Yang VW. Expression of the tumor suppressor Krüppel-like factor 4 as a prognostic predictor for colon cancer, Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2010;19(10):2631–8.
Zhao K, Li Z, Yao S, Wang Y, Wu X, Xu Z, Wu L, Huang Y, Liang C, Liu Z. Artificial intelligence quantified tumour-stroma ratio is an independent predictor for overall survival in resectable colorectal cancer. EBioMedicine. 2020;61: 103054.
Zhao M, Yao S, Li Z, Wu L, Xu Z, Pan X, Lin H, Xu Y, Yang S, Zhang S, Li Y, Zhao K, Liang C, Liu Z. The Crohn’s-like lymphoid reaction density: a new artificial intelligence quantified prognostic immune index in colon cancer. Cancer Immunol Immunothera CII. 2022;71(5):1221–31.
Yee PP, Li W. Tumor necrosis: a synergistic consequence of metabolic stress and inflammation. BioEssays News Rev Mol Cell Dev Biol. 2021;43(7):e2100029.
Zhang X, Chen L. The recent progress of the mechanism and regulation of tumor necrosis in colorectal cancer. J Cancer Res Clin Oncol. 2016;142(2):453–63.
Rhee H, Chung T, Yoo JE, Nahm JH, Woo HY, Choi GH, Han DH, Park YN. Gross type of hepatocellular carcinoma reflects the tumor hypoxia, fibrosis, and stemness-related marker expression. Hep Intl. 2020;14(2):239–48.
Ling YH, Chen JW, Wen SH, Huang CY, Li P, Lu LH, Mei J, Li SH, Wei W, Cai MY, Guo RP. Tumor necrosis as a poor prognostic predictor on postoperative survival of patients with solitary small hepatocellular carcinoma. BMC Cancer. 2020;20(1):607.
Zigeuner R, Hutterer G, Chromecki T, Imamovic A, Kampel-Kettner K, Rehak P, Langner C, Pummer K. External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. Eur Urol. 2010;57(1):102–9.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–400.
Swinson DE, Jones JL, Richardson D, Cox G, Edwards JG, O’Byrne KJ. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: correlation with biological variables. Lung Cancer (Amsterdam, Netherlands). 2002;37(3):235–40.
Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79(5–6):991–5.
Lin KC, Jan HC, Hu CY, Ou YC, Kao YL, Yang WH, Ou CH. Tumor necrosis with adjunction of preoperative monocyte-to-lymphocyte ratio as a new risk stratification marker can independently predict poor outcomes in upper tract urothelial carcinoma. J Clin Med. 2021;10(13):2983.
Pollheimer MJ, Kornprat P, Lindtner RA, Harbaum L, Schlemmer A, Rehak P, Langner C. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41(12):1749–57.
Okugawa Y, Toiyama Y, Toden S, Mitoma H, Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR, Goel A. Clinical significance of SNORA42 as an oncogene and a prognostic biomarker in colorectal cancer. Gut. 2017;66(1):107–17.
Yuan Y, Wang X, Chen G, Wang Y, Sheng W, Li X, Zhou A, Zhang Z, Li G, Cai S, Xu R, Li J, Zhang S. Updates in version 2019 of CSCO guidelines for colorectal cancer from version 2018. Chinese J Cancer Res Chung-kuo Yen Cheng Yen Chiu. 2019;31(3):423–5.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (London, England). 2019;394(10207):1467–80.
Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25(6):1454–5.
Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS, Zincke H, Blute ML, Cheville JC, Kwon ED. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104(3):511–20.
Jakubowska K, Koda M, Grudzińska M, Kisielewski W, Lomperta K, Famulski W. Neutrophil infiltration combined with necrosis in the primary tumor is a useful prognostic indicator for three-year disease-free survival time in patients with colorectal cancer. Oncol Lett. 2022;23(6):199.
Richards CH, Roxburgh CS, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99(2):287–94.
Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7(1):10.
Mantzaris NV, Webb S, Othmer HG. Mathematical modeling of tumor-induced angiogenesis. J Math Biol. 2004;49(2):111–87.
Ribatti D, Pezzella F. Overview on the different patterns of tumor vascularization. Cells. 2021;10(3):639.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Sevick EM, Jain RK. Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Can Res. 1989;49(13):3506–12.
Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Can Res. 2000;60(16):4324–7.
Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13.
Netti PA, Hamberg LM, Babich JW, Kierstead D, Graham W, Hunter GJ, Wolf GL, Fischman A, Boucher Y, Jain RK. Enhancement of fluid filtration across tumor vessels: implication for delivery of macromolecules. Proc Natl Acad Sci USA. 1999;96(6):3137–42.
Chen K, Collins G, Wang H, Toh JWT. Pathological features and prognostication in colorectal cancer. Curr Oncol (Toronto, Ont). 2021;28(6):5356–83.
Wiggers T, Arends JW, Schutte B, Volovics L, Bosman FT. A multivariate analysis of pathologic prognostic indicators in large bowel cancer. Cancer. 1988;61(2):386–95.
Park JH, van Wyk H, Roxburgh CSD, Horgan PG, Edwards J, McMillan DC. Tumour invasiveness, the local and systemic environment and the basis of staging systems in colorectal cancer. Br J Cancer. 2017;116(11):1444–50.
Yang J, Lu Z, Li L, Li Y, Tan Y, Zhang D, Wang A. Relationship of lymphovascular invasion with lymph node metastasis and prognosis in superficial esophageal carcinoma: systematic review and meta-analysis. BMC Cancer. 2020;20(1):176.
Palmer TD, Ashby WJ, Lewis JD, Zijlstra A. Targeting tumor cell motility to prevent metastasis. Adv Drug Deliv Rev. 2011;63(8):568–81.
Ross CR, Brennan-Laun SE, Wilson GM. Tristetraprolin: roles in cancer and senescence. Ageing Res Rev. 2012;11(4):473–84.
Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–95.
Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–19.
Erie DA, Weninger KR. Single molecule studies of DNA mismatch repair. DNA Repair. 2014;20:71–81.
Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annal Oncol. 2013;24(Suppl 6):64–72.
de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 2010;28(20):3380–7.
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57.
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFF1201003), Regional Innovation and Development Joint Fund of National Natural Science Foundation of China (U22A20345), Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application (2022B1212010011), National Natural Science Foundation of China (82271941, 82071892), the National Science Foundation for Young Scientists of China (82202267, 81702322), Project Funded by China Postdoctoral Science Foundation (2021M700897), High-level Hospital Construction Project (DFJHBF202105, DFJH201914), and the Natural Science Foundation of Guangdong Province (2022A1515011252).
Funding
This work was supported by the National Key R&D Program of China (2021YFF1201003), Regional Innovation and Development Joint Fund of National Natural Science Foundation of China (U22A20345), Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application (2022B1212010011), National Natural Science Foundation of China (82271941, 82071892), the National Science Foundation for Young Scientists of China (82202267, 81702322), Project Funded by China Postdoctoral Science Foundation (2021M700897), High-level Hospital Construction Project (DFJHBF202105, DFJH201914), and the Natural Science Foundation of Guangdong Province (2022A1515011252).
Author information
Authors and Affiliations
Contributions
Conception and design: HFY, KZ, YFC, and ZYL. Acquisition of data: YTW, YXZ, and SY. Analysis and interpretation of data: KZ, CHL, and YFC. Drafting the article or revising it critically for important intellectual content: HFY, ZYL, CHL and KZ. Provided discussion, critical feedback and manuscript editing: CHL, KZ, and ZYL. Final approval of the version to be published: all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committees of Shanxi Cancer Hospital (202106), and the Institutional Review Board of the Sixth Affiliated Hospital of Sun Yat-sen University (2019ZSLYEC-169) in accordance with the Declaration of Helsinki (as revised in 2013), with the need for informed consent waived for this retrospective study. The manuscript was not presented at a podium or poster meeting presentation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ye, H., Wang, Y., Yao, S. et al. Necrosis score as a prognostic factor in stage I–III colorectal cancer: a retrospective multicenter study. Discov Onc 14, 61 (2023). https://doi.org/10.1007/s12672-023-00655-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00655-w