Abstract
Bauxite residue (BR) is the main by-product of the alkaline production of alumina from bauxite containing significant amounts of valuable metals such as scandium that belongs to rare-earth elements (REEs), classified by the European Community as critical raw materials (CRMs). BR is considered a hazardous waste due to its huge volume and high alkalinity making its disposal a serious universal environmental problem. The recovery of scandium from Greek BR can be an excellent approach for waste management and resource efficiency of the waste using environmentally friendly biometallurgical methods. In this work, bioleaching of scandium from bauxite residue using the fungus Aspergillus niger was studied. Bioleaching experiments were performed using the Taguchi experimental design, in batch cultures with BR at various pulp densities (1, 5 and 10%, w/v), sucrose concentrations (40, 90 and 140 g/L) and fungus suspension of 2, 4, and 6% v/v under one-step bioleaching condition and subculturing. The highest Sc recovery equal to 46%, was achieved in 20 days at 1% pulp density. Biosorption phenomena were observed during the leaching process. Lactic, acetic, oxalic and citric were the main organic acids identified.
Graphical Abstract

Similar content being viewed by others
Statement of Novelty
To date, studies investigating the bioleaching procedure for scandium recovery from bauxite residue are scarce in literature and lack information about process optimization by testing different variables. Bioleaching experiments are usually long and data are not shown in detail. Results in our previous studies indicated that bioleaching of scandium using the fungus Aspergillus niger can reach up to 46% recovery. In this study process parameters are optimized and their interactions are evaluated. The importance of subculturing and the synergistic effects of different organic acids produced from the microorganism are highlighted. Biosorption phenomena during the leaching process are also investigated for the first time. Bioleaching of bauxite residue is a promising alternative for scandium recovery and an appropriate application for establishing a circular economic strategy aiming at the valorization of this industrial residue.
Introduction
Alumina production by Bayer method generates the bauxite residue (BR), that is an extremely alkaline solid and fine-grained waste (by-product), representing the main disposal problem in the alumina refining industry [1]. In 2018, the global storage volume of BR had exceeded 4.6 billion tons [2]. Annually, 150 million tons of BR are produced and only less than 4 million tons are used in a productive way [1]. The large volume of BR produced, the high alkalinity (pH 10–12.5) and small particle size (90% < 10 μm), the increased concentrations of potentially harmful elements (PHEs) and its high disposal costs, as well as its lack of industrial applications, constitute an environmental and economic problem that needs to be addressed [3].
ΒR is a very interesting material as it contains various metals such as Cu, Pb, Mn, Ni, Cr, K, Zn, V, Ga, Nb, Zr, Ba, Ta, Cd, Hg, As, Be, Th, U, rare earth elements (REEs) including scandium and non-metals, such as C, S, P and F, some of which are also potentially harmful elements (PHEs) [1, 4]. However, on a global scale the chemical composition of BRs from different alumina refineries varies greatly, presenting a direct dependence on the type of bauxite ore and the operating conditions of the Bayer method [3].
In Greece, in the last few years five million tons of BR have been deposited in land-based disposal sites [1, 5]. In 2021, the company Mytilineos S.A. former Aluminium of Greece S.A., one of the largest European industries in the sector, presented an annual alumina production capacity of 875,000 tons [6]. Greek BR is enriched in elements of high techno-economic interest, such as REEs and scandium, by twice the concentration as compared to the original bauxite ore [4, 7] remaining almost constant during the last 25 years. In particular, the REEs have concentrations of ~ 1 kg/t of dry BR, while Sc2O3, which participates in its composition by 0.02% and is expressed in ~ 120 g Sc/t of dry BR, represents more than 95% of the economic value of REEs in BR [5, 7,8,9].
Regarding to the limitation of BR large volume deposition, the interest is focused to alternative ways of BR exploiting and reuse, with the primary aim of environmental protection, but also the introduction of BR into the economic cycle, taking advantage of its physico-chemical properties features [3].
Many authors investigated Sc recovery from BR, due to its high demand in important applications and its low availability. Hydrometallurgy is the most common method for the recovery of REEs including Sc from BR. Many different mineral and organic acids have been investigated for Sc recovery such as hydrochloric, nitric, sulfuric, phosphoric, acetic and citric in lab scale [4, 10,11,12] and pilot scale [13]. However, Sc maximization in the leachate requires aggressive chemical conditions with high iron co-extraction at the expense of process selectivity [12, 14]. For a selective leaching (low Fe extraction 2–4%) milder conditions are suggested [4, 12] while further iron removal and of other metals and REEs, andindividual separation of metals can be achieved by an innovative combined ion exchange, solvent extraction and liquid chromatography process [13, 15, 16]. Despite their wide application, hydrometallurgical methods include some limitations in their use, which reduce their sustainability [17]. The value and environmental footprint of some acids, such as hydrochloric and nitric are significantly increased, while treatments of this type also produce waste which can be secondary pollutants during their disposal [18,19,20,21].
Other methods that have been investigated for the recovery of Sc from BR, are pyrometallurgical ones. However, these methods require high energy consumption and therefore the researchers prefer the use of hydrometallurgical processes wherever possible [22, 23]. In an alternative approach to hydrometallurgical processes, ionic liquids are used instead of solvents [24, 25]. However, their increased cost and high toxicity suspend their choice [25].
An innovative and upcoming approach for the recovery of critical metals from wastes and wastewaters are biotechnological methods [26, 27]. Biohydrometallurgy such as bioleaching is considered as an environmental-friendly and green technology due to their low cost and low–energy requirements compared to hydrometallurgical methods [17]. Microbial processes are advantageous because of their specificity, energetics and minimal production of new wastes. For BR bioleaching, heterotrophic microorganisms are considered more suitable, because they present benefits such as better adaptation and faster growth in a wide pH range [28], while no inorganic substances such as sulfur (S) can be found in BR, to favor the growth of autotrophic microorganisms [29]. Chemoheterotrophic bacteria and chemoheterotrophic fungi have been studied. Various researchers have investigated the ability of bacteria genes such as Acidiphilium spp., Acetobacter spp., Bacillus spp., Pseudomonas spp. and fungi genes such as Aspergillus spp. and Penicillium spp. in the bioleaching of different solid wastes [30, 31], but the studies on bioleaching of BR and recovery of REEs, as well as the investigation of the factors controlling bioleaching are limited [17, 28, 29, 32].
Despite the easier management of the bacteria, as pointed out by Qu et al. [28], fungi are advantageous in their application, because they can withstand a larger pH range, and in particular in alkaline environments such as those determined by the pH of BR [32]. Furthermore, compared to bacteria, fungi produce a greater variety of organic acids, more suitable to facilitate bioleaching of Sc in batch experiments [22, 32].
For this reason, in the present work, the chemoheterotrophic, filamentous fungus Aspergillus niger was used for the extraction of Sc from Greek BR. This particular microorganism has been widely used in the past in bioleaching experiments and is considered one of the most suitable, mainly due to the secretion of large amounts of metabolites, such as organic acids [31, 33, 34], and their easy adaptation to various environmental conditions of 6–47 °C and pH 1.5–9.8 [35]. A Taguchi experimental design of three variables (BR pulp density i.e., solid to liquid (S/L), medium (sucrose) concentration, initial fungus concentration) at three levels (S/L: 1, 5, 10% w/v, sucrose: 40, 90, 140 g/L and fungus: 2, 4, 6% v/v) was performed in order to study the different bioleaching conditions and their synergy effect on % Sc recovery maximization with no prior pH adjustment. Biomass production in relation to the variation of the pH of the leachate, biosorption phenomena and subculturing processes were also investigated. The organic acids produced during BR bioleaching were determined by HPLC–UV. Finally, a comparison of bacteria bioleaching and the conventional hydrometallurgical methods was carried out.
Materials and Methods
Bauxite Residue Samples
BR was originated from the metallurgical units of the company Mytilineos S.A. in Agios Nikolaos, Boeotia, Greece. The BR has been processed in filter presses after Bayer process with a pH value 11.3 and humidity 26%. The chemical composition of the batch of BR tested is: Fe2O3 (43.5%), Al2O3 (19%), SiO2 (7.3%), TiO2 (5.6%), Na2O (3%), and CaO (9.4%), as determined in previous study [4].
Microorganism and Cultivation Mineral Media
The microorganism used in the experiments of this work is the chemoheterotrophic fungus Aspergillus niger (DSM No. 821), which was purchased as active culture from the ‘Leibniz-Institute DSMZ-German collection of microorganisms and cell cultures’ in Braunschweig (https://www.dsmz.de/).
The cultivation of fungus culture was carried out in two ways: (a) liquid culture in Erlenmeyer flasks and (b) culture in Petri dishes.
For liquid culture, the Aspergillus niger culture was cultivated at 30 °C in a mineral medium (potato dextrose agar) of the following composition: Infusion from potatoes, 1000.0 mL; Glucose, 20.0 g; Agar, 15.0 g. The potato infusion was prepared by boiling 200 g scrubbed and sliced potatoes in 1000 mL water for 1 h. Then, the boiled potato infusion was passed through 16 mesh fine sieve. The medium was sterilized by autoclaving at 121 °C for 15 min. The trace mineral solution was added from a sterile stock solution and was prepared by dissolving the following compounds in a 1.5 g·L−1 nitrilotriacetic acid disodium salt solution (quantities were reported in g·L−1): 1.00 NaCl, 0.50 MnSO4, 0.10 FeSO4·7H2O, 0.10 CoCl2·6H2O, 0.10 CaCl2·2H2O; 0.13 ZnCl, 0.01 CuSO4·5H2O, 0.01 AlK(SO4)2·12H2O, 0.025 Na2MoO4·2H2O and 0.01 H3BO3, as described in [36].
For the cultures in petri dishes, a mineral medium of 500 mL of potato infusion, 10 g of glucose and 7.5 g of agar was used. The solution was autoclaved at 121 °C for 15 min, while the petri dishes were sterilized under UV radiation in a biological safety cabinet (Esco Micro Pte. Ltd., Changi, Singapore). An amount of 10 mL of sterilized mineral medium was added to each petri dish and 1 mL of A. niger was added. The plates were placed in the incubator at temperature of 30 °C. In some cultures, grains of crushed BR were added, for the microscopic study of the growth of the fungus on them.
Bioleaching Experiments and Sampling
Bioleaching experiments were conducted using 250 mL Erlenmeyer flasks containing 120 mL of sucrose medium in an orbital shaking incubator at 30 °C and 100 rpm. Sucrose medium was prepared with the composition (g/L): KNO3 0.5; KH2PO4 0.5; yeast extract 2.0; peptone 2.0 and sucrose of 40, 90 or 140, as described in [37]. Erlenmeyer flasks were covered with hydrophobic cotton and aluminum foil, and autoclaved at 121 °C for 15 min.
The bioleaching process was one-step process: incubating the fungi together with the red mud and medium. Bioleaching was performed using a 2, 4, or 6% v/v of fungus suspension with different S/L ratios (1, 5 or 10% w/v S/L) of sterilized BR (Table 1). The flasks were covered with hydrophobic cotton and placed in an Orbital Shaker—Incubator ES-20 from Biosan Llc. (Riga, Latvia).
No adjustment of pH was carried out. The total bioleaching period was 20 d. Experiments were terminated when there were no significant changes in the Sc recoveries. Sampling was performed by taking 2 mL samples at regular intervals to analyze the pH value, biomass concentration, organic acids and scandium concentration. All the culture media involved in this study were sterilized by autoclaving at 121 °C for 15 min before use. The Sc extraction degree was calculated as described in Kiskira et al. [17].
Taguchi Experimental Design
In order to study as many and different bioleaching conditions as possible, maximize the efficiency, distinguish the important parameter and study the interaction among them, a Taguchi experimental design was carried out, involving three factors at three levels each. The factors tested were sucrose concentration in the medium at 40, 90, 140 g‧L−1, BR pulp density at 1, 5, 10% w/v and initial fungal concentration at 2, 4, 6% v/v. Temperature and agitation were chosen to be kept constant, according to standard conditions suggested by the microorganism culture laboratory. The above design resulted in a system of 9 experiments, with 3 variables and 3 levels, as shown in Table 1, instead of a series of 27 experiments corresponding to the conventional full factorial experimental design.
Statistical processing of Sc concentrations was performed using Minitab Llc software. (State College, PA, USA). Sc concentration and % recovery on the 15th day were the response factors, since maximum performance was obtained on the 15th day for most experiments and was used for optimization. Regression analysis led to the estimation of main effects and the regression equation allowing the estimation of Sc concentrations and % recovery even for values outside the design range. Interval plots, interaction plots and contour plots were generated for all variables at 95% confidence level.
Biosorption Experiments
In addition, fungus was studied for its ability of biosorption. Experiments were carried out in order to investigate other possible bioprocesses or alternative mechanisms of bioleaching of BR. As in the bioleaching experiments, Erlenmeyer flasks were supplemented with nutrient medium with 0.5 KNO3, 0.5 KH2PO4, 2.0 yeast extract, 2.0 peptone (all units are in g L−1), 40, 90 or 140 g·L−1 sucrose and 2, 4 or 6% v/v fungus. In this experimental procedure however, instead of BR, standard scandium solutions of 1 mg/L and 1.5 mg/L Sc were added from Tracecert 92,279 standard solution of 1000 mg/L Sc concentration from FlukaChemie GmbH (Buchs, Switzerland). Sc concentration was monitored.
Analytical Methods
The identification of organic acids was performed with an Agilent Technologies (Santa Clara, USA)1260 Infinity II HPLC, with a column Agilent Hi-plex H of 300 mm × 7.7 mm. The operating condition of HPLC: Eluent: Sulfuric Acid 5 mM (Aquatic), Flow Rate: 0.4 mL/min, Time: 75 min, Column Temperature: 50 °C, Injection Volume: 20 μL, and Detector: DAD (UV at 210 nm). The operating conditions were based on previous experiments of the research team and the chromatographic peaks were evaluated by the use of standard solutions of the expected organic acids. A chemical analysis for Sc (361.383 nm) of the leachate solutions after filtration at 0.45 μm under vacuum was conducted in triplicate after the appropriate dilution using ICP-OES Optima 7000 DV supplied by PerkinElmer (Waltham, MA, USA). The quantitative analysis of Sc was carried out by calibration curves using the standard scandium solution (Tracecert 92,279 of 1000 mg Sc/L from FlukaChemie GmbH), spiked in the culture medium. The measurements of TSS (Total Suspended Solids) and VSS (Volatile Suspended Solids) for biomass determination were carried out according to Standard Methods (WEF, 1995). The determination of the fungus growth of Aspergillus niger was performed by measuring the optical density at 600 nm with an DR6000 UV VIS spectrophotometer (Hach Co., Loveland, CO, USA). The micro-morphology of the samples after sputter coating with gold before and after bioleaching was also examined by a scanning electron microscope (JSM-6380 LV SEM, JEOL Ltd., Tokyo, Japan). A DMLM optical microscope from Leica Microsystems GmbH (Wetzlar, Germany) is used to study the morphology of A. niger and its growth mode in the presence of BR in petri dish culture. The pH was measured using an MP125 (Mettler Toledo Llc., Columbus, OH, USA) digital pH-meter.
Results and Discussion
Microorganism Selection
Table 2 presents the maximum recoveries of Sc and other metals from BR, by bioleaching processes reported so far. In biometallurgical processes, the main parameters that control the performance of the method is the microorganism, its concentration in the bioleaching solution, the pulp density of BR (or solid/liquid ratio—S/L), the composition of the medium, temperature, agitation, pH and duration of bioleaching [17, 29]. Authors investigated the genus of Acetobacter for bioleaching of BR [17, 28]. Qu et al. [28] reported a higher recovery, most probably due to the isolation of Acetobacter sp. from the same BR samples used in the bioleaching experiments. Aspergillus niger and Penicillium tricolor were used in bioleaching experiments, however, the bioleaching time period was long and some data were not shown in detail. Also, a mixed culture collected from a pilot-scale anaerobic digester [38], resulted in 30% recovery of Sc in 10 days [17].
In the present study, fungus Aspergillus niger was selected, as it has been extensively investigated in bioleaching experiments in different ores and wastes. It is considered one of the most appropriate for such applications, due to the production of large quantities of organic acids and its growth in extreme environmental conditions [29, 31, 33, 35]. The morphology of Aspergillus niger shows transparent, filamentous hyphae with smooth walls and septa, which intertwine to form the mycelium and spherical vesicles (conidiophores) at the end of the hyphae, with black, produced conidiospores (spores) [35, 39]. Its length is usually 900–1600 µm, while the diameter of the vesicles is 40–60 µm. The vesicles are not visible, because they are completely covered by one or two rows of phials at the end of which grow the conidiospores, spherical in shape and 3–5 µm in diameter. Macroscopically, it grows in colonies that retain a white to yellow color at their base and a covering of spores with the characteristic black (niger) color [35, 39].
Microorganism Growth
Macroscopic and Microscopic Observations
During the microorganism’s growth, and before the bioleaching experiments, the liquid cultures of the fungus show gradual growth in the form of a layer on the surface of the solution, which completely covers it within the first 5 days. Then and up to the 12th day, the fungal layer grows in thickness, begins to divide and finally settles, possibly due to its weight increase. After the 12th day, the cultures show no appreciable changes. During the subculturing, a faster growth of the fungal layer is observed by at least 2 days. The fungal layer has a characteristic relief and milky to pale yellow color, in agreement with literature [35, 39].
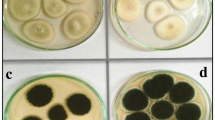
Accordingly, in petri dish cultures, the growth of the microorganism is in the form of a colony and it was observed within the first 8 days, which then stops. Drying of the colony and nutrient material is observed under the temperature of 30 °C, maintained in the incubator. The lower part of the colony in which the hyphae develop shows a whitish color, which turns yellow to brown as drying progresses, while the upper part of the colony develops the conidia of the fungus with their characteristic black (niger) color.
The most important observation of optical microscopy is the morphology presented by A. niger during its growth in a petri dish culture with BR (Fig. 1a), and in different focal depths of the microscope (Fig. 1b, c). Also, the vitreous form of its hyphae, the spherical conidia and some rows of larger conidiospores are shown in Fig. 1d.
A more detailed morphological study of the microorganism is carried out in the scanning electron microscope, where its structural parts are observed (Fig. 2). More specifically, the A. niger being studied consists of smooth, flattened ribbon-shaped hyphae, in only a few cases branched, which present a high density and are entangled with each other forming the mycelium. At the end of the hyphae are placed the conidia, which bear arrays of phials mainly spherical, or in some cases of slightly distorted hemispherical, at the end of which they produce the conidiospores (seeds), which have an ellipsoidal or spherical shape.
Biomass and pH Measurements
The biomass measurements have been carried out with the UV–Vis spectrophotometer to investigate the growth of the fungus. The absorbance readings that were plotted against time as shown in Fig. 3. The cell mass of A. niger without the addition of BR of initial experiments, subculturing experiments and biosorption experiments are presented. In initial experiments, a log phase emerges, with intense growth action, until the 15th day, which is followed by a period of stationary phase, during which the growth of the microorganism has stopped. The fungus growth in subculturing experiment is higher during all period. In biosorption experiments, the growth period of the fungus is shorter, and biomass production is reduced. The standard Sc solution added to this series of cultures is estimated to be suppressive to the microorganism.
The pH value was monitored during the growth period. The metabolic activities of the A. niger resulted in reducing the pH value from 11 to 7. Results showed time periods of relatively small and smooth changes in the range of 7–5, which are however interrupted by sudden, diurnal transitions to higher or lower values, in the range from 7 to 10. Changes in pH are likely due to both bacterial growth and the effect of their metabolic activity.
Determination of Organic Acids
The organic acids determined over the 30-day period include acetic, oxalic, citric, gluconic, succinic, propionic, butyric acid, and smaller quantities of malic, formic and lactic acid. Oxalic acid shows the highest production in the specific time period, while the others showed lower values. Fluctuations in the concentrations of an acid over time are estimated to be probably due to its consumption by other microorganisms, such as bacteria. This is in agreement with other authors that reported the A. niger secreted mainly citric, gluconic, oxalic and acetic acids [37, 40, 41].
Bioleaching Experiments
Effect of pH
The pH of all bioleaching experiments was monitored throughout a 20 day time period. An overall drop of 1.5–2.5 units was observed in all cases, starting from a pH range of 6.0 to 6.6 and ending in a range of 3.8 up to 5.0, depending on the different amounts of BR, fungus, medium and sucrose content. A slight increase, observed within the first two days, was attributed to the growth of acid-consuming bacteria. Final pH value was stabilized after the 13th day.
Biomass Measurements
Biomass growth measurements performed in the bioleaching experiments. The growth of the fungus was recorded for a time period of 20 days. Biomass growth shows a maximum value at 15 days in all experiments, resulting to 42–48 g/L of biomass production. After 15 days the curve is almost constant at around 39–45 g/L biomass, until the end of the bioleaching experiments on 20th day.
Determination of Organic Acids
Figure 4 shows the chromatograph of the organic acids produced in the EXP7 bioleaching experiment (140 g/L sucrose, 1% pulp density, 6% fungus biomass) that resulted in the maximum recovery of Sc, in the 20th day. The metabolites of A. niger in the leaching liquid, such as acetic oxalic, lactic, malic, succinic, formic, propionic and citric acid were determined with the HPLC–DAD system. Higher amount of lactic acid was observed in bioleaching experiment compared to those without BR, due to the addition of sucrose that promotes the lactic acid production via fermentation by A. niger that use sucrose [42]. Other peaks correspond to other metabolites of A. niger such as sugars that were not determined in this study. All experiments showed similar organic acid production.
Determination of Concentrations and Recoveries of Sc
The maximum concentration of 3.12 mg Sc/L was obtained in 15 days with the conditions of EXP9 (140 g/L sucrose, 10% S/L pulp density and 4% fungus concentration). Figure 5a shows the Sc concentrations for all experiments. It is observed that the Sc extraction starts substantially after the 7th day and reaches the maximum yield at 12–15 days, which is in agreement with the increase of the fungal biomass in the same time period, as shown in Fig. 3. After reaching the maximum concentration, for most experiments a decrease in Sc concentration was observed. This phenomenon was not observed when bioleaching experiments have been performed with the use of bacteria, in similar conditions as in Kiskira et al. [17].
a Sc concentrations, b Sc recovery, for the 9 Sc bioleaching experiments, c) % Sc recoveries from EXP4 and the subculturing of EXP4 (Table 1). Experiment number: (Sucrose [g/L], S/L [%], A. niger [%]), EXP1: (40, 1, 2), EXP2: (40, 5, 4), EXP3: (40, 10, 6), EXP4: (90, 1, 4), EXP5: (90, 5, 6), EXP6: (90, 10, 2), EXP7: (140, 1, 6), EXP8: (140, 5, 2), EXP9: (140, 10, 4), EXP4Sub: (90, 1, 4)
Therefore, it is assumed that the fungus A. niger has the ability to uptake Sc as a result of physicochemical interactions of metal ions with the cellular compounds, known as biosorption process [43]. This is in agreement with other studies that investigated the removal of metals from aqueous solution by biosorption with the use of A. niger [43, 44].
The three experiments that showed the highest Sc concentrations correspond to S/L 10%, regardless the A. niger content, demonstrating that the pulp density is the most important parameter for maximum Sc concentration in bioleaching experiments, (EXP9, EXP6, EXP3), in agreement with a previous study [17]. The increase of sucrose concentration, results in the increase of the growth of the microorganism and thus the enhancement of the bioleaching efficiency.
Figure 5b shows the Sc recovery for all 9 experiments. In contrast to the Sc concentrations the maximum Sc recovery values were obtained from experiments with pulp density equal to 1% (EXP7, EXP4 and EXP1). This is in agreement with other studies that recorded higher recoveries at low S/L ratios [17, 28, 29]. The maximum Sc recovery of 46.3% was obtained in EXP7 on the 20th day. The increase of sucrose concentration demonstrates a positive effect on Sc recovery.
Bioleaching was also stimulated by subculturing the A. niger culture in a fresh medium, as suggested by other studies [17, 45]. The experiment with the most significant increase is presented in Fig. 5c. An increase in recovery from 35 to 45% was observed on day 15with the subculturing experiment. In addition, the bioleaching with the subculturing experiment was by about 1–2 days shorter, in agreement with the biomass growth (Fig. 3).
Statistical Analysis of Taguchi Experimental Design
Statistical analysis of all results was performed with Minitab software using as response factors the Sc concentration and recovery achieved on the 15th day of the experiments, representing the highest values in most cases.
The most important factors were the pulp density and the sucrose content (Fig. 6). Sucrose content shows a positive effect in all cases whereas A.niger load does not appear to be of great importance. The effect of pulp density is more significant in case of Sc concentration. Recovery is highly raised at low pulp density while the exact opposite effect applies for Sc concentration. As it is clearly shown by the contour plots (Fig. 7), maximum concentration requires elevated amounts of sucrose at high pulp densities although maximization of recovery occurs at low pulp densities.
Regression analysis and method optimization resulted to an A. niger content of 6%, a sucrose level of 140 g/L and S/L at 1% for optimum recovery and at S/L 10% for optimum concentration. No confirmation experiment was necessary since the optimum values were already included within the design experiments. It must be mentioned though, that as far as the optimum conditions are concerned, the maximum recovery was achieved on the 20th day of the process. Regression equations, for Sc concentration (Eq. 1) and Sc recovery (Eq. 2) allow the estimation of performance for factors values beyond the design range.
where: C Sc: Sc Concentration (mg/L) RSc: Sc Recovery (%) S: sucrose (g/L), R: S/L (%) and A: AspNig (%)
Biosorption Experiments
The ability of A. niger to bind a significant amount of Sc, during its metabolic activity was observed during the biosorption experiments carried out. More specifically, from 1.5 mg/L Sc added to an Erlenmeyer flask together with the fungus, on the 4th day ~ 1.2 mg/L Sc was bound. Then, as shown in Fig. 8, from the 4th to the 12th day a gradual release of Sc in the solution was observed, while again a binding of ~ 1.2 mg/L Sc follows within two days, which was maintained until 20 days. Changes in pH value during the experiment, showed similar trend with the changes in Sc concentrations in the solution. During the period of Sc binding by A. niger the pH decreased to 6, while during the release period it increased up to 10.5. Reduction of A. niger biomass in the presence of Sc (Fig. 3) and different morphology of the fungal layer in the growth flasks were also observed. A more detailed study of the effect of Sc biosorption and/or other bioprocesses parallel to the bioleaching process, is required.
The findings of this work are in agreement with those reported in the literature. Other studies demonstrated the ability of Aspergillus genes for biosorption of metals. The usage of Aspergillus terreus allowed the scandium sorption and also desorption when solubilization of sorbed associated elements was inhibited by high pH values [46], as also observed in the current study. Barros Júnior et al. [44] performed successful sorption experiments using A. niger for cadmium removal. Kapoor and Viraraghavan [43] investigated the ability of A. niger for the biosorpion of several metals, and suggested that carboxylate and amine groups are important in metal ion biosorption of A. niger biomass. Also, Mukhopadhyay et al. [47], reviewed the literature for the feasibility of A. niger for efficient removal of toxic trace metals from industrial wastewater.
Comparison of Bioleaching Methods
Comparison of several leaching methods of Sc from BR, conducted by the Laboratory of Inorganic and Analytical Chemistry of the School of Chemical Engineering (NTUA) was performed (Table 3). The methods compared with the current study, include conventional hydrometallurgical processes with inorganic acids (HNO3, H2SO4, HCl, and H3PO4) and bioleaching experiments with digestate (anaerobic digestion effluent) and chemoheterotrophic bacterium.
The maximum recovery of almost 80% is achieved using inorganic acids within a period of 24 h. It is obvious that biometallurgical treatments do not have the possibility to achieve Sc recoveries in short periods of time, compared to strong inorganic acids. However, this is not a limitation when treatment is designed as a continuous leaching process in a reactor. On other hand, nitric, hydrochloric acids which exhibit the best recoveries have a severe environmental impact restricting their usage. Also, phosphoric acid has moderate acute and chronic toxicity to aquatic life in waters. In the case of H2SO4 that is more environmentally friendly than the other inorganic acids, when the maximum possible Sc recovery is presented (Table 3), regardless of the conditions, it is observed that the bioprocesses are comparable.
In case of bioprocesses comparison, the maximum recoveries for the microorganisms, fungus A. niger and bacterium A. tropicalis were comparable with values at around 40–45%. The bacterium achieved a faster recovery of about 40% within 5 days and then the recovery increased at a slower rate up to 42% in 20 days [17]. In contrast, the fungus performed a slower bioleaching process, achieving 40% recovery on the 15th day. However, bioleaching with the bacterium, required the usage of HCl for pH adjusting prior to the addition of bacterium, since it does not tolerate alkaline environments. When fungus was used, no adjustment of pH prior to the experiment was necessary.
Further research study should be carried out with the fungus A. niger, as it shows remarkable potential for the extraction of Sc at different BR pulp densities. Recovery of other metals from BR, such as CRMs could also be considered. In addition, it is essential to further investigate the phenomenon of biosorption and to recover metals bound by the microorganism. Bioleaching experiments performed with a series of two or more subculturing as well as continuous flow experiments are expected to significantly improve metal recovery, as suggested by other studies [17, 48]. The bioleaching process is amenable to optimization for all parameters to increase its efficiency. Finally, techno-economical assessment is fundamental in order to determine the method’s sustainability.
Conclusions
An environmentally friendly biometallurgical method was investigated to recover Sc from BR with the fungus Aspergillus niger. The maximum recovery of 46% Sc is achieved in 20 days, with 140 g/L sucrose content, 1% BR pulp density and 6% initial fungal concentration. The main organic acids identified in the solutions contributing synergistically, are acetic, oxalic, lactic and citric. As demonstrated by the statistical analysis performed, the most important factor controlling the bioleaching process, is the pulp density. Higher BR pulp density results to higher Sc concentration, despite that maximum recovery is achieved with the lower pulp density. Sucrose content in the medium is also crucial, favoring the growth and activity of A. niger and consequently the recovery of Sc. A sustainable and economically efficient but surely user-friendly recovery of Sc can be performed when bioleaching is performed continuously in a bioreactor, reducing the significant delays of Sc recovery till growth of the fungus reaches the adequate mass. Sustainable recovery is also produced with the continuous addition of sucrose, while removal of biomass is regulated. The reduction of the bioleaching period time is important for a possible future application in full-scale plants.
Data Availability
The data that support the findings of this study are available from the corresponding author, Kyriaki Kiskira, upon request.
References
Reddy, P.S., Reddy, N.G., Serjun, V., et al.: Properties and assessment of applications of red mud (Bauxite Residue): current status and research needs. Waste Biomass Valor. 12, 1185–1217 (2021). https://doi.org/10.1007/s12649-020-01089-z
Tian, T., Zhou, J., Zhu, F., Ye, Y., Guo, Y., Hartley, W., Xue, S.: Effect of amendments on the leaching behavior of alkaline anions and metal ions in bauxite residue. J. Environ. Sci. 85, 74–81 (2019). https://doi.org/10.1016/j.jes.2019.05.005
Wang, S., Jin, H., Deng, Y., Xiao, Y.: Comprehensive utilization status of red mud in China: a critical review. J. Clean. Prod. 289, 125136 (2021). https://doi.org/10.1016/j.jclepro.2020.125136
Ochsenkuehn-Petropoulou, M., Tsakanika, L.-A., Lymperopoulou, T., Ochsenkuehn, K.-M., Hatzilyberis, K., Georgiou, P., Stergiopoulos, C., Serifi, O., Tsopelas, F.: Efficiency of sulfuric acid on selective scandium leachability from bauxite residue. Metals (Basel) 8, 915 (2018). https://doi.org/10.3390/met8110915
Deady, É.A., Mouchos, E., Goodenough, K., Williamson, B.J., Wall, F.: A review of the potential for rare-earth element resources from European red muds: examples from Seydişehir, Turkey and Parnassus-Giona. Greece. Mineral. Mag. 80, 43–61 (2016). https://doi.org/10.1180/minmag.2016.080.052
Annual Report 2021, Mytilineos S.A., Maroussi, 2022. https://www.mytilineos.gr/interactive-document/annual-report-2021-eng/index.html. Accessed 2 May 2022
Ochsenkühn-Petropoulou, M., Lymperopoulou, T., Parissakis, G.: Direct determination of landthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta. 296, 305–313 (1994). https://doi.org/10.1016/0003-2670(94)80250-5
Tsakanika A., Ochsenkühn-Petropoulou, M. and Ochsenkuehn, K.-M.: (2015) Separation of scandium and rare earths elements after red mud leaching by cation-exchange chromatography. In Proceedings of 2015 RedMud Conference | Bauxite Residue Valorisation and Best Practices, Leuven, 5–7 October 2015
Binnemans, K., Jones, P.T., Blanpain, B., Van Gerven, T., Pontikes, Y.: Towards zero-waste valorisation of rare-earth-containing industrial process residues: a critical review. J. Clean. Prod. 99, 17–38 (2015). https://doi.org/10.1016/j.jclepro.2015.02.089
Ochsenkühn-Petropoulou, M., Lymperopoulou, T., Ochsenkühn, K.M., Parissakis, G.: Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta. 319, 249–254 (1996). https://doi.org/10.1016/0003-2670(95)00486-6
Borra, C.R., Pontikes, Y., Binnemans, K., Van Gerven, T.: Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 76, 20–27 (2015). https://doi.org/10.1016/j.mineng.2015.01.005
Tsakanika, L.-A., Panagiotatos, G., Lymperopoulou, T., Chatzitheodoridis, E., Ochsenkühn, K., Ochsenkühn-Petropoulou, M.: Direct phosphoric acid leaching of bauxite residue for selective scandium extraction. Metals (Basel). 12, 228 (2022). https://doi.org/10.3390/met12020228
Ochsenkühn-Petropoulou, M.T., Hatzilyberis, K.S., Mendrinos, L.N., Salmas, C.E.: Pilot-plant investigation of the leaching process for the recovery of scandium from red mud. Ind. Eng. Chem. Res. 41, 5794–5801 (2002). https://doi.org/10.1021/ie011047b
Lymperopoulou, T., Georgiou, P., Tsakanika, L.-A., Hatzilyberis, K., Ochsenkuehn-Petropoulou, M.: Optimizing conditions for scandium extraction from bauxite residue using Taguchi methodology. Minerals. 9, 236 (2019). https://doi.org/10.3390/min9040236
Ochsenkühn-Petropoulou, M., Lymperopoulou, T., Parissakis, G.: Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal. Chim. Acta. 315, 231–237 (1995). https://doi.org/10.1016/0003-2670(95)00309-N
Tsakanika, L., Ochsenkühn-Petropoulou, M., Mendrinos, L.: Investigation of the separation of scandium and rare earth elements from red mud by use of reversed-phase HPLC. Anal. Bioanal. Chem. (2004). https://doi.org/10.1007/s00216-004-2667-1
Kiskira, K., Lymperopoulou, T., Tsakanika, L.-A., Pavlopoulos, C., Papadopoulou, K., Ochsenkühn, K.-M., Lyberatos, G., Ochsenkühn-Petropoulou, M.: Study of microbial cultures for the bioleaching of scandium from alumina industry by-products. Metals (Basel). 11, 951 (2021). https://doi.org/10.3390/met11060951
Psomopoulos, C.S., Kiskira, K., Kalkanis, K., Leligou, H.C., Themelis, N.J.: The role of energy recovery from wastes in the decarbonization efforts of the EU power sector. IET Renew. Power Gener. 16(1), 48–64 (2022). https://doi.org/10.1049/rpg2.12315
Hatzilyberis, K., Tsakanika, L.-A., Lymperopoulou, T., Georgiou, P., Kiskira, K., Tsopelas, F., Ochsenkühn, K.-M., Ochsenkühn-Petropoulou, M.: Design of an advanced hydrometallurgy process for the intensified and optimized industrial recovery of scandium from bauxite residue. Chem. Eng. Process. Intensif. 155, 108015 (2020). https://doi.org/10.1016/j.cep.2020.108015
Alexakis, D.E., Kiskira, K., Gamvroula, D., Emmanouil, C., Psomopoulos, C.S.: Evaluating toxic element contamination sources in groundwater bodies of two Mediterranean sites. Environ. Sci. Pollut. Res. 28, 34400–34409 (2021). https://doi.org/10.1007/s11356-021-12957-z
Kalkanis, K., Alexakis, D.E., Kyriakis, E., et al.: Transforming waste to wealth. Achieving Circular Economy. Circ. Econ. Sust. 2, 1541–1559 (2022). https://doi.org/10.1007/s43615-022-00225-2
Wang, W., Pranolo, Y., Cheng, C.Y.: Metallurgical processes for scandium recovery from various resources: a review. Hydrometallurgy 108, 100–108 (2011). https://doi.org/10.1016/j.hydromet.2011.03.001
Borra, C.R., Blanpain, B., Pontikes, Y., Binnemans, K., Van Gerven, T.: Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2, 28–37 (2016). https://doi.org/10.1007/s40831-015-0026-4
Davris, P., Balomenos, E., Panias, D., Paspaliaris, I.: Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 164, 125–135 (2016). https://doi.org/10.1016/j.hydromet.2016.06.012
Binnemans, K., Jones, P.T.: The twelve principles of circular hydrometallurgy. J. Sustain. Metall. (2022). https://doi.org/10.1007/s40831-022-00636-3
Kiskira, K., Papirio, S., van Hullebusch, E.D., Esposito, G.: Fe(II)-mediated autotrophic denitrification: a new bioprocess for iron bioprecipitation/biorecovery and simultaneous treatment of nitrate-containing wastewaters. Int. Biodeterior. Biodegradation. 119, 631–648 (2017). https://doi.org/10.1016/j.ibiod.2016.09.020
Kiskira, K., Papirio, S., Mascolo, M.C., Fourdrin, C., Pechaud, Y., van Hullebusch, E.D., Esposito, G.: Mineral characterization of the biogenic Fe(III)(hydr)oxides produced during Fe(II)-driven denitrification with Cu. Ni and Zn. Sci. Total Environ. 687, 401–412 (2019). https://doi.org/10.1016/j.scitotenv.2019.06.107
Qu, Y., Li, H., Wang, X., Tian, W., Shi, B., Yao, M., Zhang, Y.: Bioleaching of major, rare earth, and radioactive elements from red mud by using indigenous chemoheterotrophic bacterium Acetobacter sp. Minerals. 9, 67 (2019). https://doi.org/10.3390/min9020067
Qu, Y., Lian, B.: Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 136, 16–23 (2013). https://doi.org/10.1016/j.biortech.2013.03.070
Rathna, R., Nakkeeran, E.: Biological treatment for the recovery of minerals from low-grade ores. In: Current developments in biotechnology and bioengineering, pp. 437–458. Elsevier (2020)
Dusengemungu, L., Kasali, G., Gwanama, C., Mubemba, B.: Overview of fungal bioleaching of metals. Environ. Adv. 5, 100083 (2021). https://doi.org/10.1016/j.envadv.2021.100083
Qu, Y., Li, H., Wang, X., Tian, W., Shi, B., Yao, M., Cao, L., Yue, L.: Selective parameters and bioleaching kinetics for leaching vanadium from red mud using Aspergillus niger and penicillium tricolor. Minerals. 9, 697 (2019). https://doi.org/10.3390/min9110697
Bahaloo-Horeh, N., Mousavi, S.M., Baniasadi, M.: Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 197, 1546–1557 (2018). https://doi.org/10.1016/j.jclepro.2018.06.299
Pathak, A., Kothari, R., Vinoba, M., Habibi, N., Tyagi, V.V.: Fungal bioleaching of metals from refinery spent catalysts: A critical review of current research, challenges, and future directions. J. Environ. Manage. 280, 111789 (2021). https://doi.org/10.1016/j.jenvman.2020.111789
Pitt, J.I., Hocking, A.D.: Ecology of fungal food spoilage. In: Fungi and food spoilage, pp. 3–12. Springer International Publishing, Cham (2022)
Kiskira, K., Papirio, S., Fourdrin, C., van Hullebusch, E.D., Esposito, G.: Effect of Cu, Ni and Zn on Fe(II)-driven autotrophic denitrification. J. Environ. Manage. 218, 209–219 (2018). https://doi.org/10.1016/j.jenvman.2018.04.050
Qu, Y., Li, H., Tian, W., Wang, X., Wang, X., Jia, X., Shi, B., Song, G., Tang, Y.: Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner. Eng. 81, 1–4 (2015). https://doi.org/10.1016/j.mineng.2015.07.022
Michalopoulos, I., Lytras, G.M., Mathioudakis, D., Lytras, C., Goumenos, A., Zacharopoulos, I., Papadopoulou, K., Lyberatos, G.: Hydrogen and methane production from food residue biomass product (FORBI). Waste and Biomass Valorization. 11, 1647–1655 (2020). https://doi.org/10.1007/s12649-018-00550-4
Mohammed H.D.: Culturing and growth requirement of Aspergillus niger. Int. J. Sci. Eng. Res. 10, 1128–1142 (2019)
Bosshard, P.P., Bachofen, R., Brandl, H.: Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environ. Sci. Technol. 30, 3066–3070 (1996). https://doi.org/10.1021/es960151v
Zeng, X., Wei, S., Sun, L., Jacques, D.A., Tang, J., Lian, M., Ji, Z., Wang, J., Zhu, J., Xu, Z.: Bioleaching of heavy metals from contaminated sediments by the Aspergillus niger strain SY1. J. Soils Sediments. 15, 1029–1038 (2015). https://doi.org/10.1007/s11368-015-1076-8
Abedi, E., Hashemi, S.M.B.: Lactic acid production – producing microorganisms and substrates sources-state of art. Heliyon. 6, e04974 (2020). https://doi.org/10.1016/j.heliyon.2020.e04974
Kapoor, A., Viraraghavan, T.: Heavy metal biosorption sites in Aspergillus niger. Bioresour. Technol. 61, 221–227 (1997). https://doi.org/10.1016/S0960-8524(97)00055-2
Barros Júnior, L.M., Macedo, G.R., Duarte, M.M.L., Silva, E.P., Lobato, A.K.C.L.: Biosorption of cadmium using the fungus Aspergillus niger. Brazilian J. Chem. Eng. 20, 229–239 (2003). https://doi.org/10.1590/S0104-66322003000300003
Kiskira, K., Papirio, S., van Hullebusch, E.D., Esposito, G.: Influence of pH, EDTA/Fe(II) ratio, and microbial culture on Fe(II)-mediated autotrophic denitrification. Environ. Sci. Pollut. Res. 24, 21323–21333 (2017). https://doi.org/10.1007/s11356-017-9736-4
Karavaiko, G.I., Kareva, A.S., Avakian, Z.A., Zakharova, V.I., Korenevsky, A.A.: Biosorption of scandium and yttrium from solutions. Biotechnol. Lett. 18, 1291–1296 (1996). https://doi.org/10.1007/BF00129957
Mukhopadhyay, M., Noronha, S.B., Suraishkumar, G.K.: A review on experimental studies of biosorption of heavy metals by Aspergillus niger. Can. J. Chem. Eng. 89, 889–900 (2011). https://doi.org/10.1002/cjce.20460
Kiskira, K., Papirio, S., Pechaud, Y., Matassa, S., van Hullebusch, E.D., Esposito, G.: Evaluation of Fe(II)-driven autotrophic denitrification in packed-bed reactors at different nitrate loading rates. Process Saf. Environ. Prot. 142, 317–324 (2020). https://doi.org/10.1016/j.psep.2020.05.049
Funding
Open access funding provided by HEAL-Link Greece. Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kyriaki Kiskira, Theopisti Lymperopoulou, Ioannis Lourentzatos, Lamprini-Areti Tsakanika, Charalampos Pavlopoulos, Elias Chatzitheodoridis and Maria Ochsenkühn-Petropoulou. The first draft of the manuscript was written by Kyriaki Kiskira, Theopisti Lymperopoulou, Lamprini-Areti Tsakanika and Maria Ochsenkühn-Petropoulou and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiskira, K., Lymperopoulou, T., Lourentzatos, I. et al. Bioleaching of Scandium from Bauxite Residue using Fungus Aspergillus Niger. Waste Biomass Valor 14, 3377–3390 (2023). https://doi.org/10.1007/s12649-023-02116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02116-5