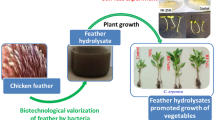
Abstract
Purpose
The green biorefinery approach is an alternative to the chemical method of production of enzyme-rich hydrolysate from waste biomass. Specifically, feather and coconut oil cake (COC) hydrolysate manifests the generation of bioproducts like dietary fibers, biofertilizers, bioplastics, esters, nanoparticles, etc.
Methods
We scaled up the production of haloextremozyme-rich hydrolysates using Haloferax lucentensis GUBF-2 MG076878 in an economical bioreactor for valuation as biofertilizer for rice growth and biosynthesis of silver nanoparticles (AgNPs), showing antimicrobial activity.
Results
The strain solubilizes 10% feather and COC with 78.75 ± 0.717% and 88.35 ± 0.654% degradation after 20 days, respectively. Moreover, tangential filtration aided co-concentration of both protease and lipase from feather/COC hydrolysate to >70% yield. SEM of feather/COC hydrolysate depicted particle size ranging 100-10000 nm. FTIR evidenced the functional groups of >C=O, -CH, -NH, -CH3, OH, and COO−. Priming seeds of rice with feather hydrolysate resulted in 100% germination energy and higher vigor index (1214), alongside increased shoot length (43.2 ± 0.58) under saline conditions. Biosynthesized AgNPs showed absorption maxima at 440 nm and vibrations of -CH, -OH, -NH, >C=C, and >C=O functionality in FTIR. AFM depicted the semi-oval morphology of AgNPs with a maximum height of 22 nm. Also, the presence of silver was confirmed by SEM-EDAX. AgNPs exhibited antimicrobial activity against human pathogens as; C. albicans>S. aureus>S. pyogenes>E. coli ATCC 8439>P.vulgaris, and S.typhi.
Conclusion
Conclusively, the production of haloextremozyme-rich feather and COC hydrolysate via the biorefinery approach employing Haloferax lucentensis GUBF-2; is lucrative, considering waste valorization and biosynthesis of bioproducts in biotechnological applications.
Graphic Abstract







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Consent for publication
Not applicable.
References
Santamaria-Fernandez, M., Ytting, N.K., Lübeck, M., Uellendahl, H.: Potential nutrient recovery in a green biorefinery for production of feed, fuel and fertilizer for organic farming. Waste Biomass Valor. 11, 5901–5911 (2020). https://doi.org/10.1007/s12649-019-00842-3
Singh, K., Garg, S.K., Kalla, A., Bhatnagar, A.: Oilcakes as protein sources in supplementary diets for the growth of Cirrhinus mrigala (Ham.) fingerlings: Laboratory and field studies. Bioresour Technol. 86, 283–291 (2003). https://doi.org/10.1016/S0960-8524(02)00120-7
Sobucki, L., Ferraz, R., Elci, R., Gustavo, G., Douglas, B., Kaiser, R., Daroit, D.J.: Feather hydrolysate as a promising nitrogen - rich fertilizer for greenhouse lettuce cultivation. Int J Recycling Org Waste Agric. 8, 493–499 (2019). https://doi.org/10.1007/s40093-019-0281-7
Ramachandran, S., Patel, A.K., Nampoothiri, K.M., Francis, F., Nagy, V., Szakacs, G., Pandey, A.: Coconut oil cake - a potential raw material for the production of α-amylase. Bioresour. Technol. 93, 169–174 (2004). https://doi.org/10.1016/j.biortech.2003.10.021
Nurdiawati, A., Suherman, C., Maxiselly, Y., Akbar, M.A., Purwoko, B.A., Prawisudha, P., Yoshikawa, K.: Liquid feather protein hydrolysate as a potential fertilizer to increase growth and yield of patchouli (Pogostemon cablin Benth) and mung bean (Vigna radiata). Int J Recycling Org Waste Agric. 8, 221–232 (2019). https://doi.org/10.1007/s40093-019-0245-y
Zheng, Y., Tian, H., Li, Y., Wang, X., Shi, P.: Effects of carboxymethylation, hydroxypropylation and dual enzyme hydrolysis combination with heating on physicochemical and functional properties and antioxidant activity of coconut cake dietary fibre. Food Chem. 336, 127688 (2021). https://doi.org/10.1016/j.foodchem.2020.127688
Gaonkar, S.K., Furtado, I.J.: Valorization of low-cost agro-wastes residues for the maximum production of protease and lipase haloextremozymes by Haloferax lucentensis GUBF-2 MG076878. Process Biochem. 101, 72–88 (2020). https://doi.org/10.1016/j.procbio.2020.10.019
Govinden, G., Puchooa, D.: Isolation and characterization of feather degrading bacteria from Mauritian soil. Afr. J. Biotech. 11, 13591–13600 (2012). https://doi.org/10.5897/ajb12.1683
Ghosh, A., Chakrabarti, K., Chattopadhyay, D.: Degradation of raw feather by a novel high molecular weight extracellular protease from newly isolated Bacillus cereus DCUW. J. Ind. Microbiol. Biotechnol. 35, 825–834 (2008). https://doi.org/10.1007/s10295-008-0354-5
Kaewsalud, T., Yakul, K., Jantanasakulwong, K., Tapingkae, W., Watanabe, M., Chaiyaso, T.: Biochemical characterization and application of thermostable-alkaline keratinase from Bacillus halodurans SW-X to valorize chicken feather wastes. Waste Biomass Valor. (2020). https://doi.org/10.1007/s12649-020-01287-9
Lateef, A., Adelere, I.A., Asafa, T.B., Beukes, L.S.: Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. Int Nano Lett (2015). https://doi.org/10.1007/s40089-014-0133-4
Tamreihao, K., Mukherjee, S., Khunjamayum, R., Devi, L.J., Asem, R.S., Ningthoujam, D.S.: Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J Baisc Microbiol (2019). https://doi.org/10.1002/jobm.201800434
Bhange, K., Chaturvedi, V., Bhatt, R.: Ameliorating effects of chicken feathers in plant growth promotion activity by a keratinolytic strain of Bacillus subtilis PF1. Bioresour Bioprocess. (2016). https://doi.org/10.1186/s40643-016-0091-y
Rai, S.K., Mukherjee, A.K.: Optimization for production of liquid nitrogen fertilizer from the degradation of chicken feather by iron-oxide (Fe3O4) magnetic nanoparticles coupled β-keratinase. Biocatal. Agric. Biotechnol. 4, 632–644 (2015). https://doi.org/10.1016/j.bcab.2015.07.002
Paul, D., Lade, H.: Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain (2014). https://doi.org/10.1007/s13593-014-0233-6
Bose, A., Pathan, S., Pathak, K.: Keratinolytic protease production by Bacillus amyloliquefaciens 6B using feather meal as substrate and application of feather hydrolysate as organic nitrogen input for agricultural soil. Waste Biomass Valor. (2014). https://doi.org/10.1007/s12649-013-9272-5
Khot, M., Gupta, R., Barve, K., Zinjarde, S., Govindwar, S., RaviKumar, A.: Fungal production of single cell oil using untreated copra cake and evaluation of its fuel properties for biodiesel. J. Microbiol. Biotechnol. 25, 459–463 (2015). https://doi.org/10.4014/jmb.1407.07074
Sabu, A., Sarita, S., Pandey, A., Bogar, B., Szakacs, G., Soccol, C.R.: Solid-state fermentation for production of phytase by Rhizopus oligosporus. Appl Biochem Biotechnol Part A Enzyme Eng Biotechnol 102–103, 251–260 (2002)
Govarthanan, M., Lee, G.W., Park, J.H., Kim, J.S., Lim, S.S., Seo, S.K., Cho, M., Myung, H., Kamala-Kannan, S., Oh, B.T.: Bioleaching characteristics, influencing factors of Cu solubilization and survival of Herbaspirillum sp. GW103 in Cu contaminated mine soil. Chemosphere. 109, 42–48 (2014). https://doi.org/10.1016/j.chemosphere.2014.02.054
Roopan, S.M., Elango, G.: Exploitation of Cocos nucifera a non-food toward the biological and nanobiotechnology field. Ind. Crops Prod. 67, 130–136 (2015). https://doi.org/10.1016/j.indcrop.2015.01.008
Kalishwaralal, K., Deepak, V., Ram Kumar Pandian, S.B., Kottaisamy, M., BarathManiKanth, S., Kartikeyan, B., Gurunathan, S.: Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B Biointerf 77, 257–262 (2010). https://doi.org/10.1016/j.colsurfb.2010.02.007
Patil, S., Fernandes, J., Tangasali, R., Furtado, I.: Exploitation of Haloferax alexandrinus for biogenic synthesis of silver nanoparticles antagonistic to human and lower mammalian pathogens. J. Cluster Sci. 25, 423–433 (2014). https://doi.org/10.1007/s10876-013-0621-0
Srivastava, P., Bragança, J., Ramanan, S.R., Kowshik, M.: Synthesis of silver nanoparticles using haloarchaeal isolate Halococcus salifodinae BK3. Extremophiles 17, 821–831 (2013). https://doi.org/10.1007/s00792-013-0563-3
Oren, A.: Industrial and environmental applications of halophilic microorganisms. Environ. Technol. 31, 825–834 (2010). https://doi.org/10.1080/09593330903370026
Amoozegar, M.A., Siroosi, M., Atashgahi, S., Smidt, H., Ventosa, A.: Systematics of haloarchaea and biotechnological potential of their hydrolytic enzymes. Microbiology (United Kingdom). 163, 623–645 (2017). https://doi.org/10.1099/mic.0.000463
Zhou, Y., Fang, X., Zhang, R., Qin, S., Wang, J., Lu, J.: Salt-tolerant microorganisms treating hypersaline organic wastewater and the microbial population dynamics. Energy Sour Part A Recovery Util Environ Effects. 38, 2854–2859 (2016). https://doi.org/10.1080/15567036.2015.1107923
Etesami, H., Beattie, G.A.: Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol. (2018). https://doi.org/10.3389/fmicb.2018.00148
Bhambure, A.B., Mahajan, G.R., Kerkar, S.: Salt tolerant bacterial inoculants as promoters of rice growth and microbial activity in coastal saline soil. Proc Nat Acad Sci India Sect B Biol Sci. 88, 1531–1538 (2018). https://doi.org/10.1007/s40011-017-0901-9
Yadav, A.N., Gulati, S., Sharma, D., Singh, R.N., Rajawat, M.V.S., Kumar, R., Dey, R., Pal, K.K., Kaushik, R., Saxena, A.K.: Seasonal variations in culturable archaea and their plant growth promoting attributes to predict their role in establishment of vegetation in Rann of Kutch. Biologia 74, 1031–1043 (2019). https://doi.org/10.2478/s11756-019-00259-2
Yadav, A.N., Verma, P., Kaushik, R., Dhaliwal, H.S., Saxena, A.K.: Archaea endowed with plant growth promoting attributes. EC Microbiology 8(6), 294–298 (2017)
Yadav, A.N., Sharma, D., Gulati, S., Singh, S., Dey, R., Pal, K.K., Kaushik, R., Saxena, A.K.: Haloarchaea endowed with phosphorus solubilization attribute implicated in phosphorus cycle. Sci. Rep. 5, 1–10 (2015). https://doi.org/10.1038/srep12293
Dave, B.P., Anshuman, K., Hajela, P.: Siderophores of halophilic archaea and their chemical characterization. Indian J. Exp. Biol. 44, 340–344 (2006)
Saxena, A.K., Kaushik, R., Yadav, A.N., Gulati, S., Sharma, D.: Role of Archaea in sustenance ofplants in extreme saline environments. Proc 56th Ann Conf Assoc Microbiol India Int Symp B Emerg Discoveries Microbiol. (2015). https://doi.org/10.13140/RG.2.1.2073.9925
Srivastava, P., Braganca, J.M., Kowshik, M.: In vivo synthesis of selenium nanoparticles by Halococcus salifodinae BK18 and their anti-proliferative properties against HeLa cell line. Biotechnol. Prog. 30, 1480–1487 (2014). https://doi.org/10.1002/btpr.1992
Costa, M.I., Álvarez-Cerimedo, M.S., Urquiza, D., Ayude, M.A., Hoppe, C.E., Fasce, D.P., De Castro, R.E., Giménez, M.I.: Synthesis, characterization and kinetic study of silver and gold nanoparticles produced by the archaeon Haloferax volcanii. J. Appl. Microbiol. 129, 1297–1308 (2020). https://doi.org/10.1111/jam.14726
Rodrigo-Baños, M., Garbayo, I., Vílchez, C., Bonete, M.J., Martínez-Espinosa, R.M.: Carotenoids from Haloarchaea and their potential in biotechnology. Marine Drugs 13, 5508–5532 (2015)
Gaonkar, S.K., Furtado, I.J.: Characterization of extracellular protease from the haloarcheon Halococcus sp. strain GUGFAWS-3 (MF425611). Curr Microbiol 77, 1024–1034 (2020). https://doi.org/10.1007/s00284-020-01896-6
Tamreihao, K., Devi, L.J., Khunjamayum, R., Mukherjee, S., Ashem, R.S., Ningthoujam, D.S.: Biofertilizing potential of feather hydrolysate produced by indigenous keratinolytic Amycolatopsis sp. MBR 40 for rice cultivation under field conditions. Biocatalys Agric Biotechnol. 10, 317–320 (2017). https://doi.org/10.1016/j.bcab.2017.04.010
Govarthanan, M., Seo, Y.S., Lee, K.J., Jung, I.B., Ju, H.J., Kim, J.S., Cho, M., Kamala-Kannan, S., Oh, B.T.: Low-cost and eco-friendly synthesis of silver nanoparticles using coconut (Cocos nucifera) oil cake extract and its antibacterial activity. Artificial Cells Nanomed Biotechnol. 44, 1878–1882 (2016). https://doi.org/10.3109/21691401.2015.1111230
Govarthanan, M., Cho, M., Park, J., Jang, J., Yi, Y., Kamala-kannan, S., Oh, B.: Cottonseed oilcake extract mediated green synthesis of silver nanoparticles and its antibacterial and cytotoxic activity. J Nanomat (2016). https://doi.org/10.1155/2016/7412431
Islam, M., Masum, S., Rayhan, K., Haque, Z.: Antibacterial activity of crab-chitosan against Staphylococcus aureus and Escherichia coli. J Adv Scientific Res. 2, 63–66 (2011). https://doi.org/10.5897/AJMR2016.7908
Dei Piu’, L., Tassoni, A., Serrazanetti, D.I., Ferri, M., Babini, E., Tagliazucchi, D., Gianotti, A.: Exploitation of starch industry liquid by-product to produce bioactive peptides from rice hydrolyzed proteins. Food Chem 155, 199–206 (2014). https://doi.org/10.1016/j.foodchem.2014.01.055
Kim, J.M., Choi, Y.M., Suh, H.J.: Preparation of feather digests as fertilizer with Bacillus pumilis KHS-1. J. Microbiol. Biotechnol. 15, 472–476 (2005)
Cao, Z., Lu, D., Luo, L.: Composition analysis and application of degradation products of whole feathers through a large scale of fermentation. Environ Sci Pollut 19, 2690–2696 (2012). https://doi.org/10.1007/s11356-012-0763-x
Deshavath, N.N., et al.: The cost-effective stirred tank reactor for cellulase production from alkaline-pretreated agriculture waste biomass. In: Ghosh, S. (ed.) Utilization and management of bioresources. Springer, Singapore (2018)
De Castro, R.E., Maupin-Furlow, J.A., Giménez, M.I., Herrera Seitz, M.K., Sánchez, J.J.: Haloarchaeal proteases and proteolytic systems. FEMS Microbiol. Rev. 30, 17–35 (2006). https://doi.org/10.1111/j.1574-6976.2005.00003.x
Li, X., Yu, H.Y.: Characterization of an organic solvent-tolerant lipase from Haloarcula sp. G41 and its application for biodiesel production. Folia Microbiologica. 59, 455–463 (2014). https://doi.org/10.1007/s12223-014-0320-8
Akmoussi-Toumi, S., Khemili-Talbi, S., Ferioune, I., Kebbouche-Gana, S.: Purification and characterization of an organic solvent-tolerant and detergent-stable lipase from Haloferax mediterranei CNCMM 50101. Int. J. Biol. Macromol. 116, 817–830 (2018). https://doi.org/10.1016/j.ijbiomac.2018.05.087
Sharma, S., Gupta, A., Chik, S.M.S.T., Kee, C.G., Mistry, B.M., Kim, D.H., Sharma, G.: Characterization of keratin microparticles from feather biomass with potent antioxidant and anticancer activities. Int. J. Biol. Macromol. 104, 189–196 (2017). https://doi.org/10.1016/j.ijbiomac.2017.06.015
Eslahi, N., Dadashian, F., Nejad, N.H.: An Investigation on Keratin Extraction from Wool and Feather. Preparat Biochem Biotechnol. (2013). https://doi.org/10.1080/10826068.2013.763826
Pedram Rad, Z., Tavanai, H., Moradi, A.R.: Production of feather keratin nanopowder through electrospraying. J Aerosol Sci. 51, 49–56 (2012). https://doi.org/10.1016/j.jaerosci.2012.04.007
Subaşı, B.G., Casanova, F., Capanoglu, E., Ajalloueian, F., Sloth, J.J., Mohammadifar, M.A.: Protein extracts from de-oiled sunflower cake: structural, physico-chemical and functional properties after removal of phenolics. Food Biosci. (2020). https://doi.org/10.1016/j.fbio.2020.100749
Sritrakul, N., Nitisinprasert, S., Keawsompong, S.: Copra meal hydrolysis by the recombinant β-mannanase KMAN-3 and MAN 6.7 expressed in Escherichia coli. 3 Biotech. 10, 1–7 (2020). https://doi.org/10.1007/s13205-019-2005-0
Drozłowska, E., Łopusiewicz, Ł, Mężyńska, M., Bartkowiak, A.: Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules. (2020). https://doi.org/10.3390/biom10010153
Goswami, D., Thakker, J.N., Dhandhukia, P.C.: Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food & Agriculture. (2016). https://doi.org/10.1080/23311932.2015.1127500
Jones, D.L., Kielland, K.: Soil biology & biochemistry amino acid, peptide and protein mineralization dynamics in a taiga forest soil. Soil Biol. Biochem. 55, 60–69 (2012). https://doi.org/10.1016/j.soilbio.2012.06.005
Bewley, J.D.: Seed germination and dormancy. Plant Cell 9(7), 1055–1066 (1997). https://doi.org/10.1105/tpc.9.7.1055
Negm, N.A., Tawfik, S.M., Abd-elaal, A.A.: Synthesis, characterization and biological activity of colloidal silver nanoparticles stabilized by gemini anionic surfactants. J. Ind. Eng. Chem. (2014). https://doi.org/10.1016/j.jiec.2014.05.015
Hamouda, R.A., Hussein, M.H., Abo-elmagd, R.A., Bawazir, S.S.: Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Scientific Rep. (2019). https://doi.org/10.1038/s41598-019-49444-y
Preetha, D., Arun, R., Kumari, P., Aarti, C.: Synthesis and characterization of silver nanoparticles using cannonball leaves and their cytotoxic activity against Mcf-7 cell line. J Nanotechnol. (2013). https://doi.org/10.1155/2013/598328
Shetty, R., Kumar, B.S., Kumar, Y.S.: Characterization of silver nanoparticles synthesized by using marine isolate characterization of silver nanoparticles synthesized by using marine isolate Streptomyces albidoflavus. J Microbiol Biotechnol. 22, 614–621 (2012). https://doi.org/10.4014/jmb.1107.07013
Amooaghaie, R., Reza, M., Azizi, M.: Ecotoxicology and environmental safety synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with che- mical silver nanoparticles. Ecotoxicol. Environ. Saf. 120, 400–408 (2015). https://doi.org/10.1016/j.ecoenv.2015.06.025
Pletikapić, G., Žutić, V., Vinković Vrček, I., Svetličić, V.: Atomic force microscopy characterization of silver nanoparticles interactions with marine diatom cells and extracellular polymeric substance. J Mol Recognit 25, 309–317 (2012)
Morones, J.R., Elechiguerra, J.L., Camacho, A., Holt, K., Kouri, J.B., Ram, J.T., Yacaman, M.J.: The bactericidal effect of silver nanoparticles. Nanotechnology (2005). https://doi.org/10.1088/0957-4484/16/10/059
Pal, S., Tak, Y.K., Song, J.M.: Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle ? a study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73, 1712–1720 (2007). https://doi.org/10.1128/AEM.02218-06
Young, E., Yong, J., Son, J., Yeun, S., Ji, P., Yoo, Y., Nam, Y., Seong, C., Jeong, Y.: Improved biosynthesis of silver nanoparticles using keratinase from Stenotrophomonas maltophilia R13: reaction optimization, structural characterization, and biomedical activity. Bioprocess Biosyst. Eng. 41, 381–393 (2018). https://doi.org/10.1007/s00449-017-1873-0
Acknowledgements
Authors thank Mr. Naveen Gaonkar, Project Assistant, BITS PILANI, Goa- India, for providing saline soil and Rice (Khorgut) from his field of Panchawadi-Village, Shiroda-Goa. S.K Gaonkar, thank Dr. Vivekanand Gobre for helping in doing AFM analysis at the School of Chemical Sciences, Goa University. S. K Gaonkar also thanks Mr. Aniketh Gaonkar, Research Scholar, School of Physical and Applied Sciences for helping in the setting up of bioreactor. S. K. Gaonkar acknowledges Research studentship, Goa University (Grant No. GU/Acad-PG/Ph.D./Res.Stud./2017-18/211).
Additional Information
Authors dedicate the paper to Prof. Joe D'Souza, Professor in Microbiology on the occasion of his 70 Birthday.
Funding
Grant No. GU/Acad-PG/Ph.D./Res.Stud./2017-18/211.
Author information
Authors and Affiliations
Contributions
Author SKG designed, performed the experiments, analyzed the data, and drafted the manuscript. IJF helped in the data analysis and corrected the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaonkar, S.K., Furtado, I.J. Biorefinery-Fermentation of Agro-Wastes by Haloferax lucentensis GUBF-2 MG076878 to Haloextremozymes for use as Biofertilizer and Biosynthesizer of AgNPs. Waste Biomass Valor 13, 1117–1133 (2022). https://doi.org/10.1007/s12649-021-01556-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01556-1