Abstract
In the current work, N,N′-bipyridyl (N- sulfonic acid) (N′- silica-n-propyl) propane mesylate/chloride bonded to Fe3O4 coated with bilayer silica ([BPSSPMCFS]) was used as a novel bi-functional inorganic-organic hybrid magnetic nanocatalyst to manufacture 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-diones under solvent-free conditions. The methods of field emission scanning electron microscopy (FE SEM), energy-dispersive X-ray spectroscopy (EDX), elemental mapping, Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD) analyses, thermal gravimetric analysis (TGA), vibrating sample magnetometry (VSM) and Brunauer-Emmett-Teller (BET) were used to identify this nanohybrid. The [BPSSPMCFS] produced the mentioned derivatives with great selectivity without producing side products, and besides this important feature, which has always been one of the main problems on the way to the synthesis of these compounds, it has significant advantages such as simple synthesis, the use of green media, large surface area, simple separation and workup, very suitable turnover number (TON) and turnover frequency (TOF) values, great reuse for several consecutive cycles without noticeably changing its catalytic activity, and short reaction time. Moreover, the hot filtration technique was used to examine and confirm the heterogeneous nature of [BPSSPMCFS].
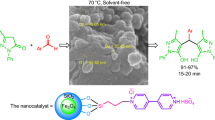
Graphical abstract

Similar content being viewed by others
Data availability
We have confirmed.
References
Ghorbani-Choghamarani A, Mohammadi M, Taherinia Z (2019) (ZrO) 2 Fe 2 O 5 as an efficient and recoverable nanocatalyst in C–C bond formation. J Iran Chem Soc 16:411–421. https://doi.org/10.1007/s13738-018-1522-9
Maleki A, Hajizadeh Z (2019) Magnetic aluminosilicate nanoclay: a natural and efficient nanocatalyst for the green synthesis of 4 H-pyran derivatives. Silicon 11(6):2789–2798. https://doi.org/10.1007/s12633-019-0069-4
Maleki A, Ravaghi P, Aghaei M, Movahed H (2017) A novel magnetically recyclable silver-loaded cellulose-based bionanocomposite catalyst for green synthesis of tetrazolo [1, 5-a] pyrimidines. Res Chem Intermed 43:5485–5494. https://doi.org/10.1007/s11164-017-2941-4
Maleki A (2018) An efficient magnetic heterogeneous nanocatalyst for the synthesis of pyrazinoporphyrazine macrocycles. Polycycl Aromat Compd 38(5):402–409. https://doi.org/10.1080/10406638.2016.1221836
Shaabani A, Soleimani E, Maleki A, Moghimi-Rad J (2008) Rapid synthesis of 3-Aminoimidazo [1, 2-a] pyridines and pyrazines. Synth Commun 38(7):1090–1095. https://doi.org/10.1080/00397910701862931
Nikoorazm M, Ghorbani-Choghamaranai A, Khanmoradi M et al (2018) Synthesis and characterization of Cu(II)-adenine-MCM-41 as stable and efficient mesoporous catalyst for the synthesis of 5-substituted 1H-tetrazoles and 1H-indazolo [1,2-b]phthalazine-triones. J Porous Mater 25:1831–1842. https://doi.org/10.1007/s10934-018-0597-0
Kohzadi H, Soleiman-Beigi M (2020) A recyclable heterogeneous nanocatalyst of copper-grafted natural asphalt sulfonate (NAS@ Cu): characterization, synthesis and application in the Suzuki–Miyaura coupling reaction. New J Chem 44(28):12134–12142. https://doi.org/10.1039/D0NJ01883J
Kohzadian A, Filian H, Kordrostami Z et al (2020) A simple, rapid and effective protocol for synthesis of bis(pyrazolyl)methanes using nickel–guanidine complex immobilized on MCM-41. Res Chem Intermed 46:1941–1953. https://doi.org/10.1007/s11164-019-04073-y
Zare A, Barzegar M (2020) Dicationic ionic liquid grafted with silica-coated nano-Fe3O4 as a novel and efficient catalyst for the preparation of uracil-containing heterocycles. Res Chem Intermed 46:3727–3740. https://doi.org/10.1007/s11164-020-04171-2
Almajidi YQ, Ubaidullah M, Pandit B, Kareem AK, Romero-Parra RM, Bobirjon A, Kadhum WR, Al-Erjan AM, Abosaooda M, Mahmoud AK (2023) Immobilized Ni on TMEDA@ βSiO 2@ αSiO 2@ Fe 3 O 4: as a novel magnetic nanocatalyst for preparation of pyrido [2, 3-d: 6, 5-d′] dipyrimidines. RSC Adv 13(17):11393–11405. https://doi.org/10.1039/D3RA01720F
Maleki A, Kamalzare M (2014) An efficient synthesis of benzodiazepine derivatives via a one-pot, three-component reaction accelerated by a chitosan-supported superparamagnetic iron oxide nanocomposite. Tetrahedron Lett 55(50):6931–6934. https://doi.org/10.1016/j.tetlet.2014.10.120
Esmaeili MS, Khodabakhshi MR, Maleki A, Varzi Z (2021) Green, natural and low cost xanthum gum supported Fe3O4 as a robust biopolymer nanocatalyst for the one-pot synthesis of 2-amino-3-cyano-4 H-pyran derivatives. Polycycl Aromat Compd 41(9):1953–1971. https://doi.org/10.1080/10406638.2019.1708418
Maleki A, Aghaei M, Kari T (2017) Facile synthesis of 7-aryl-benzo [h] tetrazolo [5, 1-b] quinazoline-5, 6-dione fused polycyclic compounds by using a novel magnetic polyurethane catalyst. Polycycl Aromat Compd 39(3):266–278. https://doi.org/10.1080/10406638.2017.1325746
Hajizadeh Z, Valadi K, Taheri-Ledari R, Maleki A (2020) Convenient Cr (VI) removal from aqueous samples: executed by a promising clay-based catalytic system, magnetized by Fe3O4 nanoparticles and functionalized with humic acid. ChemistrySelect 5(8):2441–2448. https://doi.org/10.1002/slct.201904672
Hosseini ES, Mohammadi S, Khodarahmi R, Fouani MH, Tavallaei O, Jaberi KR, Shabaninejad Z, Janfaza S, Yazdian-Robati R, Sajadimajd S (2022) New insights into the biosensing of Parkinson's disease biomarkers: a concise review. Curr Med Chem 29(22):3945–3972. https://doi.org/10.2174/0929867328666211213111812
Sun DH, Lu P, Zhang JL, Liu YL, Ni JZ (2011) Synthesis of the Fe3O4@ SiO2@ SiO2–Tb (PABA) 3 luminomagnetic microspheres. J Nanosci Nanotechnol 11(11):9774–9779. https://doi.org/10.1166/jnn.2011.5265
Niu HY, Li WH, Shi YL, Cai YQ (2011) A core–shell magnetic mesoporous silica sorbent for organic targets with high extraction performance and anti-interference ability. Chem Commun 47(15):4454–4456. https://doi.org/10.1039/C1CC10300H
Yanagisawa T, Shimizu T, Kuroda K, Kato C (1990) The preparation of alkyltrimethylammonium–kanemite complexes and their conversion to microporous materials. Bull Chem Soc Jpn 63(4):988–992. https://doi.org/10.1246/bcsj.63.988
Valadi K, Gharibi S, Taheri-Ledari R, Maleki A (2020) Ultrasound-assisted synthesis of 1, 4-dihydropyridine derivatives by an efficient volcanic-based hybrid nanocomposite. Solid State Sci 101:106141. https://doi.org/10.1016/j.solidstatesciences.2020.106141
Maleki A, Gharibi S, Valadi K, Taheri-Ledari R (2020) Pumice-modified cellulose fiber: an environmentally benign solid state hybrid catalytic system for the synthesis of 2, 4, 5-triarylimidazole derivatives. J Phys Chem Solids 142:109443. https://doi.org/10.1016/j.jpcs.2020.109443
Taheri-Ledari R, Valadi K, Gharibi S, Maleki A (2020) Synergistic photocatalytic effect between green LED light and Fe3O4/ZnO-modified natural pumice: a novel cleaner product for degradation of methylene blue. Mater Res Bull 130:110946. https://doi.org/10.1016/j.materresbull.2020.110946
Soltani SS, Taheri-Ledari R, Farnia SM, Maleki A, Foroumadi A (2020) Synthesis and characterization of a supported Pd complex on volcanic pumice laminates textured by cellulose for facilitating Suzuki–Miyaura cross-coupling reactions. RSC Adv 10(39):23359–23371. https://doi.org/10.1039/D0RA04521G
Kazemi B, Javanshir S, Maleki A, Safari M, Khavasi HR (2012) An efficient synthesis of 4H-chromene, 4H-pyran, and oxepine derivatives via one-pot three-component tandem reactions. Tetrahedron Lett 53(51):6977–6981. https://doi.org/10.1016/j.tetlet.2012.10.046
Filian H, Ghorbani-Choghamarani A, Tahanpesar E (2019) Ni-guanidine@MCM-41 NPs: a new catalyst for the synthesis of 4,4′-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) and symmetric di-aryl sulfides. J Iran Chem Soc 16:2673–2681. https://doi.org/10.1007/s13738-019-01727-x
Kohzadian A, Filian H (2023) Production and characterization of Fe3O4@SiO2@TMEDA-Pd as a very effectual interphase catalyst for the rapid preparation of di-aryl sulfides and pyrido-dipyrimidines. Silicon 15:4539–4554. https://doi.org/10.1007/s12633-023-02372-z
Maleki A, Jafari AA, Yousefi S (2017) Green cellulose-based nanocomposite catalyst: design and facile performance in aqueous synthesis of pyranopyrimidines and pyrazolopyranopyrimidines. Carbohydr Polym 175:409–416. https://doi.org/10.1016/j.carbpol.2017.08.019
Maleki A, Azadegan S (2017) Amine-functionalized silica-supported magnetic nanoparticles: preparation, characterization and catalytic performance in the Chromene synthesis. J Inorg Organomet Polym 27:714–719. https://doi.org/10.1007/s10904-017-0514-z
Maleki A, Movahed H, Ravaghi P (2017) Magnetic cellulose/Ag as a novel eco-friendly nanobiocomposite to catalyze synthesis of chromene-linked nicotinonitriles. Carbohydr Polym 156:259–267. https://doi.org/10.1016/j.carbpol.2016.09.002
Maleki A, Aghaei M, Ghamari N (2016) Facile synthesis of tetrahydrobenzoxanthenones via a one-pot three-component reaction using an eco-friendly and magnetized biopolymer chitosan-based heterogeneous nanocatalyst. Appl Organomet Chem 30(11):939–942. https://doi.org/10.1002/aoc.3524
Jazinizadeh E, Zare A, Sajadikhah SS et al (2022) Synthesis, characterization and application of a magnetically separable nanocatalyst for the preparation of 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) derivatives. Res Chem Intermed 48:5059–5075. https://doi.org/10.1007/s11164-022-04854-y
Naeimi H, Nejadshafiee V, Islami MR (2016) Iron (III)-doped, ionic liquid matrix-immobilized, mesoporous silica nanoparticles: application as recyclable catalyst for synthesis of pyrimidines in water. Microporous Mesoporous Mater 227:23–30. https://doi.org/10.1016/j.micromeso.2016.02.036
Zare A, Kohzadian A, Abshirini Z, Sajadikhah SS, Phipps J, Benamara M, Beyzavi MH (2019) Nano-2-(dimethylamino)-N-(silica-n-propyl)-N, N-dimethylethanaminium chloride as a novel basic catalyst for the efficient synthesis of pyrido [2, 3-d: 6, 5-d′] dipyrimidines. New J Chem 43(5):2247–2257. https://doi.org/10.1039/C8NJ04921A
Naeimi H, Didar A (2017) Facile one-pot four component synthesis of pyrido [2, 3-d: 6, 5-d′] dipyrimidines catalyzed by CuFe2O4 magnetic nanoparticles in water. J Mol Struct 1137:626–633. https://doi.org/10.1016/j.molstruc.2017.02.044
Hajizadeh Z, Maleki A (2018) Poly (ethylene imine)-modified magnetic halloysite nanotubes: a novel, efficient and recyclable catalyst for the synthesis of dihydropyrano [2, 3-c] pyrazole derivatives. Mol Catal 460:87–93. https://doi.org/10.1016/j.mcat.2018.09.018
Maleki A, Azizi M, Emdadi Z (2018) A novel poly (ethyleneoxide)-based magnetic nanocomposite catalyst for highly efficient multicomponent synthesis of pyran derivatives. Green Chem Lett Rev 11(4):573–582. https://doi.org/10.1080/17518253.2018.1547795
Maleki A, Hajizadeh Z, Salehi P (2019) Mesoporous halloysite nanotubes modified by CuFe2O4 spinel ferrite nanoparticles and study of its application as a novel and efficient heterogeneous catalyst in the synthesis of pyrazolopyridine derivatives. Sci Rep 9:5552. https://doi.org/10.1038/s41598-019-42126-9
Maleki A, Eskandarpour V (2019) Design and development of a new functionalized cellulose-based magnetic nanocomposite: preparation, characterization, and catalytic application in the synthesis of diverse pyrano[2,3-c]pyrazole derivatives. J Iran Chem Soc 16:1459–1472. https://doi.org/10.1007/s13738-019-01610-9
Filian H, Kohzadian A, Mohammadi M, Ghorbani-Choghamarani A, Karami A (2020) Pd (0)-guanidine@ MCM-41: a very effective catalyst for rapid production of bis (pyrazolyl) methanes. Appl Organomet Chem 34(6):e5579. https://doi.org/10.1002/aoc.5579
Neyts J, Clercq ED, Singha R, Chang YH, Das AR, Chakraborty SK, Hong SC, Tsay SC, Hsu MH, Hwu JR (2009) Structure− activity relationship of new anti-hepatitis C virus agents: Heterobicycle− coumarin conjugates. J Med Chem 52(5):1486–1490. https://doi.org/10.1021/jm801240d
Stefanachi A, Leonetti F, Pisani L, Catto M, Carotti A (2018) Coumarin: a natural, privileged and versatile scaffold for bioactive compounds. Molecules 23(2):250. https://doi.org/10.3390/molecules23020250
Hwu JR, Singha R, Hong SC, Chang YH, Das AR, Vliegen I, De Clercq E, Neyts J (2008) Synthesis of new benzimidazole–coumarin conjugates as anti-hepatitis C virus agents. Antivir Res 77(2):157–162. https://doi.org/10.1016/j.antiviral.2007.09.003
Suzuki M, Nakagawa-Goto K, Nakamura S, Tokuda H, Morris-Natschke SL, Kozuka M, Nishino H, Lee KH (2006) Cancer preventive agents. Part 5. Anti-tumor-promoting effects of coumarins and related compounds on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Pharm Biol 44(3):178–182. https://doi.org/10.1080/13880200600686491
Garazd YL, Kornienko EM, Maloshtan LN et al (2005) Modified Coumarins. 17. Synthesis and anticoagulant activity of 3,4-Cycloannelated Coumarin D-Glycopyranosides. Chem Nat Compd 41:508–512. https://doi.org/10.1007/s10600-005-0194-8
Anand P, Singh B, Singh N, Karatas MO, Alici B, Cakir U, Cetinkaya E, Demir D, Ergün A, Gençer N, Arslan O (2012) Synthesis and carbonic anhydrase inhibitory properties of novel coumarin derivatives. Bioorg Med Chem 20:1175–1180
Yamahara J, Kozuka M, Sawada T, Fujimura H, Nakano K, Tomimatsu T, Nohara T (1985) Biologically active principles of crude drugs. Anti-allergic principles in" Cnidii monnieri". Chem Pharm Bull 33(4):1676–1680. https://doi.org/10.1248/cpb.33.1676
Yang G, Jin Q, Xu C, Fan S, Wang C, Xie P (2018) Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int J Biol Macromol 106:179–184. https://doi.org/10.1016/j.ijbiomac.2017.08.009
Kayser O, Kolodziej H (1999) Antibacterial activity of simple coumarins: structural requirements for biological activity. Zeitschrift für Naturforschung C 54(3–4):169–174. https://doi.org/10.1515/znc-1999-3-405
Chohan ZH, Shaikh AU, Rauf A, Supuran CT (2006) Antibacterial, antifungal and cytotoxic properties of novel N-substituted sulfonamides from 4-hydroxycoumarin. J Enzyme Inhib Med Chem 21(6):741–748. https://doi.org/10.1080/14756360600810340
Kontogiorgis CA, Hadjipavlou-Litina DJ (2005) Synthesis and antiinflammatory activity of coumarin derivatives. J Med Chem 48(20):6400–6408. https://doi.org/10.1021/jm0580149
Stanchev S, Momekov G, Jensen F, Manolov I (2008) Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem 43(4):694–706. https://doi.org/10.1016/j.ejmech.2007.05.005
Zhao H, Neamati N, Hong H, Mazumder A, Wang S, Sunder S, Milne GW, Pommier Y, Burke TR (1997) Coumarin-based inhibitors of HIV integrase. J Med Chem 40(2):242–249. https://doi.org/10.1021/jm960450v
Pradhan K, Paul S, Das AR (2013) Fe (DS) 3, an efficient Lewis acid-surfactant-combined catalyst (LASC) for the one pot synthesis of chromeno [4, 3-b] chromene derivatives by assembling the basic building blocks. Tetrahedron Lett 54(24):3105–3110. https://doi.org/10.1016/j.tetlet.2013.04.001
Elinson MN, Dorofeev AS, Feducovich SK, Gorbunov SV, Nasybullin RF, Stepanov NO, Nikishin GI (2006) Electrochemically induced chain transformation of salicylaldehydes and alkyl cyanoacetates into substituted 4H-chromenes. Tetrahedron Lett 47(43):7629–7633. https://doi.org/10.1016/j.tetlet.2006.08.053
Sun W, Cama LD, Birzin ET, Warrier S, Locco L, Mosley R, Hammond ML, Rohrer SP (2006) 6H-benzo [c] chromen-6-one derivatives as selective ERβ agonists. Bioorg Med Chem Lett 16(6):1468–1472. https://doi.org/10.1016/j.bmcl.2005.12.057
Stachulski AV, Berry NG, Lilian Low AC, Moores SL, Row E, Warhurst DC, Adagu IS, Rossignol JF (2006) Identification of isoflavone derivatives as effective anticryptosporidial agents in vitro and in vivo. J Med Chem 49(4):1450–1454. https://doi.org/10.1021/jm050973f
Gesson J. P., Fonteneau N., Mondon M., Charbit S., Ficheux H. and Schutze F., Negma-Lerads, 7-carboxy-flavone derivatives preparation method and therapeutic use, US Pat., 6965039B2, 2005
Esmaeilpour M, Sardarian AR, Javidi J (2014) Synthesis and characterization of Schiff base complex of Pd (II) supported on superparamagnetic Fe3O4@ SiO2 nanoparticles and its application as an efficient copper-and phosphine ligand-free recyclable catalyst for Sonogashira–Hagihara coupling reactions. J Organomet Chem 749:233–240. https://doi.org/10.1016/j.jorganchem.2013.10.011
Ghorbani-Choghamarani A, Tahmasbi B, Noori N, Faryadi S (2017) Pd–S-methylisothiourea supported on magnetic nanoparticles as an efficient and reusable nanocatalyst for heck and Suzuki reactions. Comptes Rendus Chimie 20(2):132–139. https://doi.org/10.1016/j.crci.2016.06.010
Aphesteguy JC, Kurlyandskaya GV, De Celis JP, Safronov AP, Schegoleva NN (2015) Magnetite nanoparticles prepared by co-precipitation method in different conditions. Mater Chem Phys 161:243–249. https://doi.org/10.1016/j.matchemphys.2015.05.044
Emtiazi H, Amrollahi MA (2014) An efficient and rapid access to the synthesis of tetrahydrochromeno [4, 3-b] chromene-6, 8-dione derivatives by magnesium perchlorate. S Afr J Chem 67:175–179
Kohzadian A, Zare A (2019) Efficient and highly selective production of 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-diones using a mesoporous silica-based nanocatalyst. Res Chem Intermed 45:5473–5485. https://doi.org/10.1007/s11164-019-03913-1
Ebrahimi SES, Ghadirian P, Emtiazi H et al (2016) Hetero-annulated coumarins as new AChE/BuChE inhibitors: synthesis and biological evaluation. Med Chem Res 25:1831–1841. https://doi.org/10.1007/s00044-016-1626-7
Wei Z, Abbaspour S, Tayebee R (2023) Nickel nanoparticles originated from cressa leaf extract in the preparation of a novel Melem@ Ni-HPA photocatalyst for the synthesis of some chromenes and a preliminary MTT assay on the anticancer activity of the nanocomposite. Polycycl Aromat Compd 43(1):552–571. https://doi.org/10.1080/10406638.2021.2019063
Patil BM, Mali SR, Patil BM, Patil SS (2020) Averrhoa bilimbi in organic transformation: a highly efficient and green biosurfactant for the synthesis of multi-functional chromenes and xanthenes. Curr Sci 118(6):931
Jarrahi M, Tayebee R, Maleki B, Salimi A (2021) One-pot multicomponent green LED photoinduced synthesis of chromeno [4, 3-b] chromenes catalyzed by a new nanophotocatalyst histaminium tetrachlorozincate. RSC Adv 11(32):19723–19736. https://doi.org/10.1039/D1RA00189B
Anaraki-Ardakani H, Ghanavatian R, Akbari M (2013) An efficient one-pot synthesis of tetrahydro-chromeno [4, 3-b] chromene-6, 8-dione and tetrahydro-pyrano [4, 3-b] chromene-1, 9-dione derivatives under solvent-free conditions. World Appl Sci J 22:802–808. https://doi.org/10.5829/idosi.wasj.2013.22.06.333
Ganguly NC, Roy S, Mondal P (2014) Boric acid–catalyzed one-pot access to 7-aryl-benzopyrano [4, 3-b] benzopyran-6, 8-diones under aqueous micellar conditions. Synth Commun 44(3):433–440. https://doi.org/10.1080/00397911.2013.813546
Iniyavan P, Sarveswari S, Vijayakumar V (2015) Microwave-assisted clean synthesis of xanthenes and chromenes in [bmim][PF 6] and their antioxidant studies. Res Chem Intermed 41:7413–7426. https://doi.org/10.1007/s11164-014-1821-4
Chen Z, Zhu Q, Su W (2011) A novel sulfonic acid functionalized ionic liquid catalyzed multicomponent synthesis of 10, 11-dihydrochromeno [4, 3-b] chromene-6, 8 (7H, 9H)-dione derivatives in water. Tetrahedron Lett 52(20):2601–2604. https://doi.org/10.1016/j.tetlet.2011.03.059
Sun XJ, Zhou JF, Zhi SJ (2012) Efficient one-pot synthesis of tetrahydrobenzo [c] xanthene-1, 11-dione derivatives under microwave irradiation. Synth Commun 42(13):1987–1994. https://doi.org/10.1080/00397911.2010.551285
Patil KT, Walekar LS, Undare SS, Kolekar GB, Deshmukh MB, Choudhari PB, Anbhule PV (2016) An efficient one-pot synthesis of tetrahydro-chromeno [4, 3-b] chromene-6, 8-dione and tetrahydro-pyrano [4, 3-b] chromene-1, 9-dione derivatives under solvent-free conditions. Indian J Chem 55:1151–1159
Vajar S, Mokhtary M (2019) Nano-CuFe2O4@ SO3H catalyzed efficient one-pot cyclo-dehydration of dimedone and synthesis of chromeno [4, 3-b] chromenes. Polycycl Aromat Compd 39(2):111–123. https://doi.org/10.1080/10406638.2017.1280516
Zare A, Asvar H, Zarei F, Khalili M, Kordrostami Z, Moosavi-Zare AR, Khakyzadeh V (2016) Synthesis and identification of SO3H-functionalized Phthalimide (SFP) as an efficient catalyst for the condensation of Dimedone with Arylaldehydes. J Appl Chem Res 10(2):59–67 https://dorl.net/dor/20.1001.1.20083815.2016.10.2.6.2
Kauthale SS, Tekale SU, Jadhav KM, Pawar RP (2016) Ethylene glycol promoted catalyst-free pseudo three-component green synthesis of bis (coumarin) s and bis (3-methyl-1-phenyl-1 H-pyrazol-5-ol) s. Mol Divers 20:763–770. https://doi.org/10.1007/s11030-016-9673-z
Irannejad-Gheshlaghchaei N, Zare A, Sajadikhah SS, Banaei A (2018) A novel dicationic ionic liquid as a highly effectual and dual-functional catalyst for the synthesis of 3-methyl-4-arylmethylene-isoxazole-5 (4 H)-ones. Res Chem Intermed 44:6253–6266. https://doi.org/10.1007/s11164-018-3488-8
Shirini F, Langarudi MS, Daneshvar N, Mashhadinezhad M, Nabinia N (2017) Preparation of a new DABCO-based ionic liquid and investigation on its application in the synthesis of benzimidazoquinazolinone and pyrimido [4, 5-b]-quinoline derivatives. J Mol Liq 243:302–312. https://doi.org/10.1016/j.molliq.2017.07.080
Mohammadi K, Shirini F, Yahyazadeh A (2015) 1, 3-Disulfonic acid imidazolium hydrogen sulfate: a reusable and efficient ionic liquid for the one-pot multi-component synthesis of pyrimido [4, 5-b] quinoline derivatives. RSC Adv 5(30):23586–23590. https://doi.org/10.1039/C5RA02198G
Sheikhhosseini E, Yahyazadehfar M (2023) Synthesis and characterization of an Fe-MOF@ Fe3O4 nanocatalyst and its application as an organic nanocatalyst for one-pot synthesis of dihydropyrano [2, 3-c] chromenes. Front Chem 10:984502. https://doi.org/10.3389/fchem.2022.984502
Ezzatzadeh E, Hossaini Z, Rostamian R, Vaseghi S, Mousavi SF (2017) Fe3O4 magnetic nanoparticles (MNPs) as reusable catalyst for the synthesis of Chromene derivatives using multicomponent reaction of 4-Hydroxycumarin basis on Cheletropic reaction. J Heterocyclic Chem 54(5):2906–2911. https://doi.org/10.1002/jhet.2900
Acknowledgements
The authors are grateful to acknowledge the Takin Shimi Sepanta Industries Co, Ilam, Iran.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Riyadh Hasan Mohammed Ali: synthesis of some 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione derivatives (1a-6a) and manuscript editing. Ahmed Hjazi: synthesis of some 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione derivatives (7a-12a) and helping to edit the manuscript. Herlina Uinarni: optimizing the synthesis conditions of 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione derivatives. Sarah Salah Jalal: identifying the structure of the catalyst by means of FT-IR and XRD analyzes. Saurabh Aggarwal: identifying the structure of the catalyst by means of TGA and BET analyzes. Sherzod Shukhratovich Abdullaev: identifying the structure of the catalyst by means of FE-SEM and EDS analyzes. Mohammed Kadhem Abid: identifying the structure of 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione by 1H NMR and 13CNMR, as well as analyzing their resulting data. Abbas F. Almulla: providing part of the required raw materials and helping to edit the manuscript. Ali Alsaalamy: providing part of the required raw materials and designing and drawing schemes. Rohollah Fathollahi: designed and synthesized the catalyst, and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The author's declare that the paper is not be submitted simultaneously to another journal. The submitted work is original and has not been published elsewhere in any form or language, and the authors have no conflict of interest regarding this manuscript. The authors agree to participate in submitting our manuscript to this journal, and agree to the publication of our research data in this journal.
Consent for publication
We have confirmed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, R.H.M., Hjazi, A., Uinarni, H. et al. Design, Preparation and Identification a Mesoporous Bi-functional Organic–inorganic Hybrid Magnetic Catalyst for Selective and Effectual Synthesis 10,11-Dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione Derivatives. Silicon 16, 939–954 (2024). https://doi.org/10.1007/s12633-023-02799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02799-4