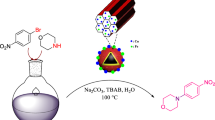
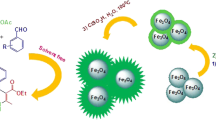
Abstract
Magnetically recoverable Fe3O4@SiO2@Cu–Ni–Fe–Cr LDH was prepared under co-precipitation conditions. Characterization of the mesoporous catalyst was confirmed using Fourier-transformed infrared spectroscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, X-ray diffraction, vibration sample magnetometer, Brunauer–Emmett–Teller, thermogravimetric, differential thermogravimetric analyses and transmission electron microscopy. Reduction of nitroarenes to the corresponding arylamines and one-pot reductive-acetylation of nitroarenes to acetanilides were carried out successfully by nanoparticles of the immobilized Cu–Ni–Fe–Cr layered double hydroxide on silica-coated Fe3O4 in water as a green solvent. All reactions were carried out within 6–22 min affording arylamines and N-arylacetamides in high-to-excellent yields. Reusability of the core–shell nanocatalyst was examined six times without significant loss of its catalytic activity.
Graphical abstract

















Similar content being viewed by others
References
M. Boudart, Chem. Rev. 95, 661 (1995)
N. Mizuno, M. Misono, Chem. Rev. 98, 199 (1998)
M. Heitbaum, F. Glorius, I. Escher, Angew. Chem. Int. Ed. 45, 4732 (2006)
S.M. George, Chem. Rev. 95, 475 (1995)
H. Hattori, Chem. Rev. 95, 537 (1995)
A. Corma, H. Garcia, F.X. Llabres i Xamena, Chem. Rev. 110, 4606 (2010)
M. Gilanizadeh, B. Zeynizadeh, New J. Chem. 42, 8553 (2018)
M.R. Othman, Z. Helwani, W.J.N. Martunus, Fernando, Appl. Organomet. Chem. 23, 335 (2009)
M.B. Gawande, R.K. Pandey, R.V. Jayaram, Catal. Sci. Technol. 2, 1113 (2012)
M. Gilanizadeh, B. Zeynizadeh, Res. Chem. Intermed. https://doi.org/10.1007/s11164-018-3475-0 (2018)
M. Gilanizadeh, B. Zeynizadeh, E. Gholamiyan, Iran. J. Sci. Technol. T. A Sci. https://doi.org/10.1007/s40995-018-0594-9 (2018)
R.G. Chaudhuri, S. Paria, Chem. Rev. 112, 2373 (2012)
V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara, J.M. Basset, Chem. Rev. 111, 3036 (2011)
K. Bakhmutsky, N.L. Wieder, M. Cargnello, B. Galloway, P. Fornasiero, R.J. Gorte, Chem. Sustain. Chem. 5, 140 (2012)
Z. Shu, S. Wang, J. Nanomater (2009) http://dx.doi. https://doi.org/10.1155/2009/340217
F. Niu, L. Zhang, S.Z. Luo, W.G. Song, Chem. Commun. 46, 1109 (2010)
S. Xuan, W. Jiang, X. Gong, Y. Hu, Z. Chen, J. Phys. Chem. C 113, 553 (2009)
R. Cano, D.J. Ramon, M. Yus, J. Org. Chem. 75, 3458 (2010)
M.J. Aliaga, D.J. Ramon, M. Yus, Org. Biomol. Chem. 8, 43 (2010)
G. A.Uheida, E. Salazar-Alvarez, Z. Bjorkman, M. Yu, Muhammed, J. Colloid Interface Sci. 298, 501 (2006)
T. Poursaberi, V. Akbar, S.M.R. Shoja, Iran. J. Chem. Chem. Eng. 34, 41 (2015)
M. Ma, J. Xie, Y. Zhang, Z. Chen, N. Gu, Mater. Lett. 105, 36 (2013)
J. Liu, X. Peng, W. Sun, Y. Zhao, C. Xia, Org. Lett. 10, 3933 (2008)
M. Kotani, T. Koike, K. Yamaguchi, N. Mizuno, Green Chem. 8, 735 (2006)
K.V.S. Ranganath, J. Kloesges, A.H. Schafer, F. Glorius, Angew. Chem. Int. Ed. 49, 7786 (2010)
S.T. Chen, R. Si, T. Eric, J. Jonathan, J.Y. Chen, J. Phys. Chem. C 116, 12969 (2012)
H.D. Cai, K.G. Li, M. Shen, S. Wen, Y. Luo, C. Peng, G. Zhang, X. Shi, J. Mater. Chem. 22, 15110 (2012)
K.V.S. Ranganath, F. Glorius, Catal. Sci. Technol. 1, 13 (2011)
Z. Zhao, J. Liu, F. Cui, H. Feng, L. Zhang, J. Mater. Chem. 22, 9052 (2012)
W. Lu, Y. Shen, A. Xie, X. Zhang, W. Chang, J. Phys. Chem. C 114, 4846 (2010)
A.M. Tafesh, J. Weiguny, Chem. Rev. 96, 2035 (1996)
S.A. Lawrence, Amines: Synthesis, Properties and Applications (Cambridge University Press, Cambridge, 2004)
T.C. Nugent, Chiral Amine Synthesis: Methods, Developments and Applications (Wiley, Weinheim, 2010)
T. Farooqui, A.A. Farooqui, Biogenic Amines: Pharmacological, Neurochemical and Molecular Aspects in the CNS (Nova Science, New York, 2010)
A.M. Birch, S. Groombridge, R. Law, A.G. Leach, C.D. Mee, C. Schramm, J. Med. Chem. 55, 3923 (2012)
M.R. Yazdanbakhsh, A. Mohammadi, E. Mohajerani, H. Nemati, N.H. Nataj, A. Moheghi, E. Naeemikhah, J. Mol. Liq. 151, 107 (2010)
S. Vishnoi, V. Agrawal, V.K. Kasana, J. Agric. Food Chem. 57, 3261 (2009)
J. Seyden-Penne, Reductions by the Alumino and Borohydrides in Organic Synthesis, 2nd edn. (Wiley-VCH, New York, 1997)
A.F. Abdel-Magid, Reductions in Organic Synthesis. ACS Symposium Series, vol. 641 (1996)
P.G. Andersson, I.J. Munslow, Modern Reduction Methods (Wiley-VCH, New York, 2008)
M. Hudlicky, Reductions in Organic Chemistry (Ellis Horwood, Chichester, 1984)
P.S. Rathore, R. Patidar, T. Shripathi, S. Thakore, Catal. Sci. Technol. 5, 286 (2015)
M.B. Gawande, A.K. Rathi, P.S. Branco, I.D. Nogueira, A. Velhinho, J.J. Shrikhande, U.U. Indulkar, R.V. Jayaram, C.A.A. Ghumman, N. Bundaleski, O.M.N.D. Teodoro, Chem. Eur. J. 18, 12628 (2012)
R.K. Rai, A. Mahata, S. Mukhopadhyay, S. Gupta, P.Z. Li, K.T. Nguyen, Y. Zhao, B. Pathak, S.K. Singh, Inorg. Chem. 53, 2904 (2014)
R.V. Jagadeesh, D. Banerjee, P.B. Arockiam, H. Junge, K. Junge, M.M. Pohl, J. Radnik, A. Brückner, M. Beller, Green Chem. 17, 898 (2015)
B. Zeynizadeh, K. Zahmatkesh, J. Chin. Chem. Soc. 50, 267 (2003)
B. Zeynizadeh, M. Zabihzadeh, J. Iran. Chem. Soc. 12, 1221 (2015)
M. Periasamy, M. Thirumalaikumar, J. Organomet. Chem. 609, 137 (2000)
B.H. Kim, R. Han, F. Piao, Y.M. Jun, W. Baik, B.M. Lee, Tetrahedron Lett. 44, 77 (2003)
Y. Jia, Q. Li, X. Wang, H. Wang, X. Liu, J. Shanghai Univ.(Eng.) 10, 277 (2006)
X. Wang, H. Guo, G. Xie, Y. Zhang, Synth. Commun. 34, 3001 (2004)
D.C. Owsley, J.J. Bloomfield, Synthesis 118 (1977)
K.Y. Lee, J.M. Kim, J.N. Kim, Bull. Korean Chem. Soc. 23, 1359 (2002)
R.N. Baruah, Indian J. Chem. 39B, 300 (2000)
A.E. Wahba, J. Peng, M.T. Hamann, Tetrahedron Lett. 50, 3901 (2009)
R.J. Rahaim, R.E. Maleczka, Synthesis 19, 3316 (2006)
M.L. Kantam, R.S. Reddy, K. Srinivas, R. Chakravarti, B. Sreedhar, F. Figueras, C.V. Reddy, J. Mol. Catal. A: Chem. 355, 96 (2012)
E.M. Nahmed, G. Jenner, Tetrahedron Lett. 32, 4917 (1991)
B. Zeynizadeh, D. Setamdideh, Synth. Commun. 36, 2699 (2006)
B. Zeynizadeh, H. Ghasemi, J. Chem. Res. 542 (2006)
Z. Shokri, B. Zeynizadeh, S.A. Hosseini, B. Azizi, J. Iran. Chem. Soc. 14, 101 (2017)
B. Zeynizadeh, I. Mohammadzadeh, Z. Shokri, S.A. Hosseini, J. Colloid Interface Sci. 500, 285 (2017)
Z. Shokri, B. Zeynizadeh, S.A. Hosseini, J. Colloid Interface Sci. 485, 99 (2017)
G.Y. Li, Y.R. Jiang, K.L. Huang, P. Ding, L.L. Yao, Colloids Surf. A 320, 11 (2008)
J.A. Lopez, F. González, F.A. Bonilla, G. Zambrano, M.E. Gómez, Rev. Latin Am. Metal. Mat. 30, 60 (2010)
C. Busetto, G.D. Piero, G. Manara, F. Trifiro, A. Vaccari, J. Catal. 85, 260 (1984)
K. Basu, S. Chakraborty, C. Saha, A. Kumar Sarkar, IOSR J. Appl. Chem. 7, 30 (2014)
X. Liu, Z. Ma, J. Xing, H. Liu, J. Magn. Magn. Mater. 270, 1 (2004)
Y. Zhang, G.M. Zeng, L. Tang, D.L. Huang, X.Y. Jiang, Y.N. Chen, Biosens. Bioelectron. 22, 2121 (2007)
Acknowledgements
The authors gratefully acknowledge the financial support of this work by the research council of Urmia University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gilanizadeh, M., Zeynizadeh, B. Synthesis of magnetic Fe3O4@SiO2@Cu–Ni–Fe–Cr LDH: an efficient and reusable mesoporous catalyst for reduction and one-pot reductive-acetylation of nitroarenes. J IRAN CHEM SOC 15, 2821–2837 (2018). https://doi.org/10.1007/s13738-018-1469-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1469-x