Abstract
Purpose
The pressure recording analytical method (PRAM) monitor is a non-invasive pulse contour cardiac output (CO) device that cannot be considered interchangeable with the gold standard for CO estimation. It, however, generates additional hemodynamic indices that need to be evaluated. Our objective was to investigate the performance of a multiparametric predictive score based on a combination of several parameters generated by the PRAM monitor to predict fluid responsiveness.
Methods
Secondary analysis of a prospective observational study from April 2016 to December 2017 in two French teaching hospitals. We included critically ill patients who were monitored by esophageal Doppler monitoring and an invasive arterial line, and received a 250–500 mL crystalloid fluid challenge. The main outcome measure was the predictive score discrimination evaluated by the area under the receiver operating characteristics curve.
Results
The three baseline PRAM-derived parameters associated with fluid responsiveness in univariate analysis were pulse pressure variation, cardiac cycle efficiency, and arterial elastance (P < 0.01, P = 0.03, and P < 0.01, respectively). The median [interquartile range] predictive score, calculated after discretization of these parameters according to their optimal threshold value was 3 [2–3] in fluid responders and 1 [1–2] in fluid non-responders, respectively (P < 0.001). The area under the curve of the predictive score was 0.807 (95% confidence interval, 0.662 to 0.909; P < 0.001).
Conclusion
A multiparametric score combining three parameters generated by the PRAM monitor can predict fluid responsiveness with good positive and negative predictive values in intensive care unit patients.
Résumé
Objectif
Le moniteur PRAM (pressure recording analytical method) est un dispositif non invasif de surveillance du débit cardiaque (DC) fondé sur la mesure de contour de l’onde de pouls qui ne peut être considéré comme interchangeable avec la référence de l’estimation du DC. Cependant, ce dispositif génère des indices hémodynamiques supplémentaires qui doivent être évalués. Notre objectif était d’examiner la performance d’un score prédictif multiparamétrique fondé sur une combinaison de plusieurs paramètres générés par le moniteur PRAM afin de prédire la réponse au remplissage volémique.
Méthode
Analyse secondaire d’une étude observationnelle prospective entre avril 2016 et décembre 2017 dans deux hôpitaux universitaires français. Nous avons inclus des patients en état critique monitorés par un Doppler oesophagien et une ligne artérielle invasive, et ayant reçu un bolus de cristalloïdes de 250–500 mL. Le critère d’évaluation principal était la discrimination du score prédictif telle qu’évaluée par la surface sous la courbe de fonction d’efficacité de l’observateur (ROC).
Résultats
Les trois paramètres de base dérivés du PRAM associés à la réponse au remplissage dans l’analyse univariée étaient la variation de pression différentielle, l’efficacité du cycle cardiaque, et l’élastance artérielle (P < 0,01, P = 0,03, et P < 0,01, respectivement). Le score prédictif médian [écart interquartile], calculé après discrétisation de ces paramètres selon leur valeur seuil optimale, était de 3 [2–3] chez les répondeurs au remplissage et de 1 [1–2] chez les non-répondeurs, respectivement (P < 0,001). La surface sous la courbe du score prédictif était de 0,807 (intervalle de confiance 95 %, 0,662 à 0,909; P < 0,001).
Conclusion
Un score multiparamétrique combinant trois paramètres générés par le moniteur PRAM peut prédire la réponse au remplissage volémique avec de bonnes valeurs prédictives positives et négatives chez les patients à l’unité de soins intensifs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In critically ill patients, fluid resuscitation remains a daily concern.1 Fluid challenge is one of the most frequent interventions in the intensive care unit (ICU),2 but only 50% of patients are fluid responders.3 Measuring the cardiac output (CO) is recommended to evaluate the response to fluid in the most severe patients, especially in case of shock.4 While less invasive devices are generally desirable, many of them have failed to show interchangeability with the gold standard (i.e., pulmonary thermodilution).5 Interchangeability is usually evaluated based on the ability to produce similar values for single parameters, such as stroke volume (SV) or CO.6 Nevertheless, most CO monitoring devices also produce additional parameters that may be relevant and informative—some are directly measured and others are calculated.7 Whether combining several hemodynamic derived parameters can enhance fluid responsiveness prediction is still an unanswered question.
The pressure recording analytical method (PRAM) is a pulse contour-based CO monitor based on invasive arterial pressure monitoring.8 This device allows for continuous beat-to-beat CO measurement and is minimally invasive because it only requires connection to the transducer of an arterial line. Studies evaluating the ability of the PRAM monitor to track changes in CO during a fluid challenge in ICU patients produced conflicting results.9,10,11 Our group recently performed a two-centre study evaluating the performance of the PRAM monitor in critically ill patients and showed insufficient performance to detect changes in CO when compared with esophageal Doppler monitoring (EDM).12 Other direct and indirect baseline hemodynamic parameters are, however, provided by the PRAM monitor. Their usefulness, specifically in predicting fluid responsiveness, has not been adequately studied.
The aim of this post hoc study was to investigate the performance of a multiparametric predictive score based on a combination of several baseline parameters generated by the PRAM monitor to predict fluid responsiveness in a mixed cohort of ICU patients.
Methods
This study is a post hoc analysis of a two-centre prospective observational study. Ethical approval (IRB 00010254-2016-033) was provided by an institutional ethics committee (Comité d’éthique de la Société Française d’Anesthésie-Réanimation, Paris, France, chairman Prof J.-E Bazin) on 14 April 2016. Informed consent was acquired in accordance with French law. This report follows the STARD statement (Standards for Reporting of Diagnostic Accuracy Studies) for diagnostic accuracy studies.13
Patients
The study protocol was described previously.12 The study was conducted in two French surgical ICUs between April 2016 and December 2017. Both sites had used EDM as their primary CO monitor for more than ten years and were trained to use the PRAM monitor. Inclusion criteria were: i) presence of an invasive arterial line; ii) CO monitoring using EDM; iii) decision by the attending physician to perform a fluid challenge; iv) immediate availability of the PRAM device; and v) availability of an investigator trained on both devices. Exclusion criteria were: younger than 18 yr old, pregnancy, cardiac dysrhythmia,14 and poor signal quality for one of the two CO monitoring methods.
Measurements and data collection
Patient characteristics, diagnosis at admission, haemodynamic status, current vasopressor treatment, mechanical ventilation, Simplified Acute Physiology Score II, sequential organ failure assessment, Charlson comorbidity index, and dead or alive status at day 28 were collected.
The PRAM (MostCareUp; Vytech, Padova, Italy) is a beat-to beat CO monitor connected to an invasive arterial line. The SV is estimated from the area under the curve (AUC) of the systolic portion of the arterial pressure and the dynamic impedance of the cardiovascular system. Impedance is derived from an analysis of the arterial waveform signal sampled at 1,000 Hz.7 Baseline parameters recorded by the PRAM also include pulse pressure variation (PPV); SV variation; cardiac cycle efficiency (CCE), which describes hemodynamic performance in terms of energy expenditure15; cardiovascular system impedance (Zt); arterial elastance (Ea), which measures afterload; and maximum pressure development over time (dP/dt), which describes heart contractility. The quality of the arterial pressure signal was assessed before fluid challenge. Once the PRAM was connected to the arterial line with a Y-connector, zeroing at the phlebostatic level was performed and the detection of the dicrotic notch was checked and corrected if necessary.
The EDM (CardioQ-ODM; Deltex; Deltex, Chichester, Sussex, UK) is a beat-to-beat CO monitor measuring the descending aortic blood flow velocity with a 4 MHz continuous ultrasound esophageal probe assuming a fixed angle of 45° with the aorta. The left ventricular SV is internally computed based on the descending aortic blood flow velocity as well as the patient’s age, height, and weight.16 Before inclusion in the study, the quality of the EDM signal was also assessed by a trained investigator and corrected if necessary (optimal wave pattern and absence of diastolic flow). Patient characteristics entered in the device were checked by the physician and the device was set to average SV measurement over 15 cycles.
Fluid challenge
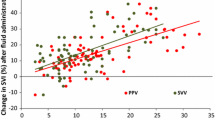
All fluid challenges were performed with a rapidly infused crystalloid solution. The volume of fluid administered (either 250 or 500 mL) was selected by the attending physician. To ensure consistency in timing, a unique simultaneous photograph containing both monitors was taken immediately after the 15 averaging cycles of the EDM, at baseline, one minute after the fluid challenge, and after the signal quality of both devices was checked again. Doses of medications and mechanical ventilation settings were not modified between the two time points. Fluid responsiveness was defined as an increase in the SV as assessed with the EDM of at least 15%.3
Statistical analysis
The overall population was randomly separated into a derivation cohort (two thirds of the fluid challenges) and a validation cohort (one third of the fluid challenges). The multiparametric predictive score was then built in three steps using the derivation cohort.
First, all baseline parameters were evaluated for their ability to discriminate fluid responders from non-responders based on the area under the receiver operating characteristics (ROC) curve. All significant parameters with a P value < 0.05 (PPV, Ea, CCE) were then included in a multiparametric score. The score was then derived as follows: 1) an optimal threshold was identified from the ROC curve for each selected parameter (Youden test); 2) for each parameter, the individual value was discretized (0 or 1) based on the threshold defined in step one; 3) the multiparametric predictive score was defined as the sum of all items, thus ranging from 0 to 3.
The performance of the multiparametric predictive score was studied based on the area under the ROC curve. Sensitivity, specificity, positive and negative likelihood ratio, and positive and negative predictive values were measured for each point of the score. Sensitivity analyses were also performed, including only the first fluid challenge for each patient, and only 250 mL fluid challenges.
Continuous variables are presented as median [interquartile range]. Categorical variables are reported as count (percentage). Continuous variables were compared using the nonparametric Mann Whitney test. Categorical variables were compared using the Chi square or the Fisher exact test as appropriate. A P value < 0.05 was considered to indicate statistical significance.
Results
Patients
Sixty-eight fluid challenges in 49 patients were consecutively included in this study. Patient baseline characteristics are summarized in Table 1. The flow chart of study patients is available in the eFigure (as Electronic Supplementary Material).The vast majority of patients were mechanically ventilated (99%) and septic shock was the most frequent reason for ICU admission (70%). The median dose of norepinephrine was 0.39 [0.23–0.98] µg·kg−1·min−1. Twenty-eight day mortality was 41%. Baseline hemodynamic variables and ventilator settings of each fluid challenge in both cohorts are presented in Table 2.
Predictive score—derivation cohort
In the derivation cohort (n = 45), 30 fluid challenges (66.7%) were classified as fluid responsive, meaning that the patient experienced at least a 15% increase in SV following the fluid challenge as assessed by the EDM. Among the parameters generated by the PRAM monitor, three were found to be significantly predictive of the response to fluid with the following thresholds: PPV > 6% (AUC, 0.741; 95% confidence interval [CI], 0.689 to 0.920); Ea > 1.74 (AUC, 0.740; 95 CI%, 0.593 to 0.856); and CCE < 0.29 (AUC, 0.704, 95% CI, 0.554 to 0.827). Baseline mean arterial pressure, SV, Zt, dP/dt, and dose of norepinephrine were not significantly associated with fluid responsiveness (P = 0.09, P = 0.26, P = 0.12, P = 0.97, and P = 0.38, respectively).
The median value of the predictive score was 3 [2–3] in fluid responders and 1 [1–2] in fluid non-responders respectively (P < 0.001). The ROC curve of the multiparametric predictive score is shown in Fig. 1. The AUC of the predictive score was 0.807 (95% CI, 0.662 to 0.909; P < 0.001; Hosmer-Lemeshow P = 0.44).
Sensitivity was 86.7% (95% CI, 69.3 to 96.2) and specificity 66.7% (95% CI, 38.4 to 88.2) for a score of 2 or greater. The predictive positive value was 72.2% (95% CI, 55.6 to 84.4) for a score of 2 and 80% (95% CI, 51.3 to 93.8) for a score of 3. Positive likelihood ratio was 2.6 (95% CI, 1.3 to 5.4) for a score of 2 and 4.0 (95% CI, 1.1 to 15.2) for a score of 3/3. Negative predictive value and negative likelihood ratio were respectively 100% and 0.0 for a score of 0/3 and 83.3% (95% CI, 65.2 to 93.0) and 0.2 (95% CI, 0.08 to 0.5) for a score of 1/3. Figure 2 displays the percentage of fluid responders at each value of the score.
Predictive score—validation cohort
In the validation cohort (n = 23), nine observations (39.1%) were classified as responsive to fluid. The median predictive score was 3 [1.75–3] in fluid responders and 1.5 [0–2] in fluid non-responders, respectively (P = 0.03). The ROC curve is presented in Fig. 3, the AUC was 0.762 (95% CI, 0.540 to 0.913; P < 0.008).
Sensitivity analysis
In the sensitivity analysis including only the first fluid challenge (n = 49), 32 observations (65.3%) were responsive to fluid. The median predictive score was 2.5 [2–3] in fluid responders and 1 [0–2] in fluid non-responders respectively (P = 0.002). The AUC of the ROC curve was 0.813 (95% CI, 0.675 to 0.910; P < 0.0001). A sensitivity analysis including fluid challenges of 250 mL only was also performed (n = 50), 27 observations (54%) were responsive to fluid. The median score was 3 [2–3] in fluid responders and 1 [0–2] in fluid non-responders, respectively (P = 0.001). The AUC of the ROC curve was 0.804 (95% CI, 0.668 to 0.903; P < 0.0001).
Discussion
In this prospective observational study, a multiparametric predictive score composed of three parameters generated by the PRAM monitor was able to predict fluid responsiveness assessed by a rise in SV using EDM in a population of ICU patients with good positive and negative predictive performance.
In our previous study, the PRAM monitor had insufficient performance to track CO changes induced by a fluid challenge compared with EDM.12 The present study focused on the performance of a combination of parameters generated by the PRAM monitor to predict fluid responsiveness in critically ill patients. The interest of this multiparametric approach relies on the good predictive performance to rule out fluid responsiveness for a score inferior or equal to 1, and to rule in fluid responsiveness when the score is equal or superior to 2.
Numerous dynamic parameters have been evaluated to assess fluid responsiveness, mostly based on heart-lung interactions.17,18,19 Pulse pressure variation, first described in 2000, is widely used in the ICU and the operating room to guide fluid resuscitation.18,20 Nevertheless, this parameter is known to suffer some limitations: the tidal volume must be greater than 8 mL·kg−1,21 and cardiac arrhythmias, spontaneous breathing,22 right ventricular dysfunction,23 and intra-abdominal hypertension24 all must be absent. Thus, this parameter is often not applicable in practice, especially in ICU patients. Indeed, a recent study showed that this parameter can be used in no more than 17% of critically ill patients.25 Echocardiographic indices, such as respiratory variations of the superior and inferior vena cava diameter were also associated with better predictive performance than PPV.26
The concept of our multiparametric predictive score was to combine more than one parameter to optimize predictive performance by leveraging the information conveyed by different physiologic variables. One possible advantage is to reduce the risk of a “grey zone”, as previously described with the PPV. The “grey zone” corresponds to a range of values for which the test should be considered inconclusive.27 We also expect this score to be applicable to a broader population of ICU patients. For instance, the patients included in our study were ventilated with a mean tidal volume of less than 8 mL·kg−1 of ideal body weight. The PRAM monitor produces original hemodynamic parameters in addition to SV that have been, to our knowledge, poorly described in hemodynamic management. Interestingly, two of the parameters that were included in our prediction score (PPV and CCE) were already shown to be associated with fluid responsiveness.28 The PPV threshold identified in our study is lower than the one used in previous studies.18,20 As previously described by De Baker et al.,21 the value of 6% identified in our cohort can be explained by the use of a lower tidal volume.
Our study has limitations. First, the EDM is not considered a gold standard of CO monitoring. It has, however, very low static and dynamic bias and is less invasive than pulmonary thermodilution.16 Although aortic diameter variations could lead to underestimating the CO, its variation after a fluid challenge remains acceptable and the risk of fluid responsiveness misclassification is very low.16 Second, our population is composed of mechanically ventilated patients with severe hemodynamic instability who are in sinus rhythm. The results, therefore, cannot be generalized to all ICU patients. Third, our study was not designed to explore the physiologic significance of the selected variables and further work is needed to confirm and explore the relation between Ea, CCE, and fluid responsiveness. Our results are also not generalizable to other monitoring devices that don’t provide similar hemodynamic indices. Finally, our population was limited in size and we included several fluid challenges performed in the same patient. Nevertheless, fluid challenges had to be separated by at least 12 hr, and the sensitivity analysis limited to the first fluid challenge for each patient showed similar results.
In conclusion, a multiparametric predictive score combining three parameters generated by the PRAM monitor is able to predict fluid responsiveness in ICU patients with good positive and negative predictive values. Further validation of this score in a prospective cohort is needed before it can be implemented at the bedside.
References
Perner A, Hjortrup PB, Arabi Y. Focus on fluid therapy in critically ill patients. Intensive Care Med 2019; 45: 1469-71.
Boulain T, Boisrame-Helms J, Ehrmann S, et al. Volume expansion in the first 4 days of shock: a prospective multicentre study in 19 French intensive care units. Intensive Care Med 2015; 41: 248-56.
Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002; 121: 2000-8.
Cecconi M, Hofer C, Teboul JL, et al. Fluid challenges in intensive care: the FENICE study: a global inception cohort study. Intensive Care Med 2015; 41: 1529-37.
Scheeren TW, Ramsay MA. New developments in hemodynamic monitoring. J Cardiothorac Vasc Anesth 2019; 33(Suppl 1): S67-72.
Montenij LJ, Buhre WF, Jansen JR, Kruitwagen CL, de Waal EE. Methodology of method comparison studies evaluating the validity of cardiac output monitors: a stepwise approach and checklist. Br J Anaesth 2016; 116: 750-8.
Romagnoli S, Franchi F, Ricci Z, Scolletta S, Payen D. The Pressure Recording Analytical Method (PRAM): technical concepts and literature review. J Cardiothorac Vasc Anesth 2017; 31: 1460-70.
Romano SM, Pistolesi M. Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 2002; 30: 1834-41.
Biais M, Cottenceau V, Stecken L, et al. Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med 2012; 40: 1186-91.
Scolletta S, Franchi F, Romagnoli S, et al. Comparison between Doppler-echocardiography and uncalibrated pulse contour method for cardiac output measurement: a multicenter observational study. Crit Care Med 2016; 44: 1370-9.
Franchi F, Falciani E, Donadello K, et al. Echocardiography and pulse contour analysis to assess cardiac output in trauma patients. Minerva Anestesiol 2013; 79: 137-46.
Barthélémy R, Neuschwander A, Dramé F, et al. Monitoring haemodynamic response to fluid-challenge in ICU: comparison of pressure recording analytical method and oesophageal Doppler: a prospective observational study. Eur J Anaesthesiol 2019; 36: 135-43.
Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 2003; 138: W1-12.
Maj G, Monaco F, Landoni G, et al. Cardiac index assessment by the pressure recording analytic method in unstable patients with atrial fibrillation. J Cardiothorac Vasc Anesth 2011; 25: 476-80.
Romano SM. Cardiac cycle efficiency: a new parameter able to fully evaluate the dynamic interplay of the cardiovascular system. Int J Cardiol 2012; 155: 326-7.
Dark PM, Singer M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med 2004; 30: 2060-6.
Jardin F, Farcot JC, Gueret P, Prost JF, Ozier Y, Bourdarias JP. Cyclic changes in arterial pulse during respiratory support. Circulation 1983; 68: 266-74.
Michard F, Boussat S, Chemla D, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000; 162: 134-8.
Perel A, Pizov R, Cotev S. Respiratory variations in the arterial pressure during mechanical ventilation reflect volume status and fluid responsiveness. Intensive Care Med 2014; 40: 798-807.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009; 37: 2642-7.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 2005; 31: 517-23.
De Backer D, Taccone FS, Holsten R, Ibrahimi F, Vincent JL. Influence of respiratory rate on stroke volume variation in mechanically ventilated patients. Anesthesiology 2009; 110: 1092-7.
Mahjoub Y, Pila C, Friggeri A, et al. Assessing fluid responsiveness in critically ill patients: false-positive pulse pressure variation is detected by Doppler echocardiographic evaluation of the right ventricle. Crit Care Med 2009; 37: 2570-5.
Jacques D, Bendjelid K, Duperret S, Colling J, Piriou V, Viale JP. Pulse pressure variation and stroke volume variation during increased intra-abdominal pressure: an experimental study. Crit Care 2011; DOI: https://doi.org/10.1186/cc9980.
Preau S, Dewavrin F, Demaeght V, et al. The use of static and dynamic haemodynamic parameters before volume expansion: a prospective observational study in six French intensive care units. Anaesth Crit Care Pain Med 2016; 35: 93-102.
Vignon P, Repessé X, Bégot E, et al. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 2017; 195: 1022-32.
Cannesson M, Le Manach Y, Hofer CK, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness: a “gray zone” approach. Anesthesiology 2011; 115: 231-41.
Messina A, Romano SM, Bonicolini E, et al. Cardiac cycle efficiency and dicrotic pressure variations: new parameters for fluid therapy: an observational study. Eur J Anaesthesiol 2017; 34: 755-63.
Author contributions
Arthur Neuschwander, Romain Barthélémy, and Romain Pirracchio contributed to the study design and drafted the manuscript. David Ditchi, Fatou Dramé, Maximilien Redouté, and Jules Stern contributed to acquisition of data. Bernard Cholley, Alexandre Mebazaa, and Benjamin Glenn Chousterman revised the work for important intellectual content. Arthur Neuschwander, Romain Barthélémy, and Romain Pirracchio analysed and interpreted data.
Conflict of interest
Romain Barthélémy received travel expenses and unrestricted grants for meeting and congresses from Vygon France.
Funding statement
None.
Editorial responsibility
This submission was handled by Dr. Sangeeta Mehta, Associate Editor, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neuschwander, A., Barthélémy, R., Ditchi, D. et al. Accuracy of a multiparametric score based on pulse wave analysis for prediction of fluid responsiveness: ancillary analysis of an observational study. Can J Anesth/J Can Anesth 67, 1162–1169 (2020). https://doi.org/10.1007/s12630-020-01736-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01736-y