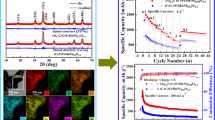
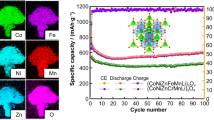
Abstract
Incorporating four cations into a single-phase oxide is beneficial for maintaining structural stability during Li+ insertion/desertion because of the produced entropy-dominated phase stabilization effects. However, medium-entropy oxides exhibit inherently poor electron and ion conductivity. As such, in this work, a single-phase medium-entropy oxide of NixCuyCozMn1–x–y–zO (named as NCCM@oxides(H2)) is prepared by modified-NiCuCoMn alloy through the epitaxial-growing-based self-combustion and hydrogen reduction. During hydrogen reduction, some Cu ions are reduced to elemental Cu (defined as Cu0), which is distributed among the metal oxides, while generating extensive oxygen vacancies around Cu. The synergetic effect between nanoporous metal-core oxide-shell structure and enriched oxygen/Cu0 vacancies greatly enhances the electronic/ionic conductivity. In addition, the lattice of single-phase quaternary metal oxides has the configuration entropy stability, which enables the rock-salt structure to remain stable during repeated conversion reactions. Benefiting from the above-mentioned merits, the anode for Li-ion batteries with entropy-stabled NCCM@oxides(H2) composite shows a high specific capacity of 699 mAh·g−1 at 0.1 A·g−1 and ultra-stable cycling stability, which maintains 618 and 489 mAh·g−1 at 0.1 and 1.0 A·g−1 after 200 cycles, respectively. This is the first use of this novel and simple strategy for modifying medium-entropy oxides, which paves the way for the development of high-entropy oxides as high-performance electrodes.
Graphical abstract

摘要
基于中熵氧化物的构型熵相稳定效应, 单相固溶氧化物中掺杂四个金属阳离子有利于保持结构稳定, 因此在锂离子嵌入/脱出过程中表现出良好的电化学性能。但是中熵氧化物电子和离子导电性相对较差的缺点依然亟待解决。在此项工作中, 以改性NiCuCoMn合金为基底, 通过可控自燃烧外延生长和氢气还原的处理, 制备出NixCuyCozMn1-x–y-zO的单相中熵氧化物 (命名为NCCM@oxides(H2)) 。在氢气还原的过程中, 部分铜离子被还原成单质铜, 并分布在中熵氧化物的晶格中, 同时在单质铜周围产生大量的氧空位。纳米多孔金属氧化物的核-壳结构与丰富的Cu0/氧空位之间的协同效应将大大提高电子/离子导电性, 并在充放电过程中保持结构稳定。基于上述优点, NCCM@oxides(H2)复合材料的锂离子电池负极展现出高比容量和长循环寿命, 其中在电流密度为 0.1 A·g−1时具有 699 mAh·g−1的初始稳定比容量, 并且在1.0 A·g−1大电流密度时经过 200 次循环后能够保持489 mAh·g−1的比容量, 这是首次使用该策略对中熵氧化物进行修饰, 这为开发高性能锂离子电池的高熵氧化物负极材料提供了新的思路。








Similar content being viewed by others
References
Cavers H, Molaiyan P, Abdollahifar M, Lassi U, Kwade A. Perspectives on improving the safety and sustainability of high voltage lithium-ion batteries through the electrolyte and separator region. Adv Energy Mater. 2022;12(23):2200147. https://doi.org/10.1002/aenm.202200147.
Zhang ZJ, Zhao J, Qiao ZJ, Wang JM, Sun SH, Fu WX, Zhang XY, Yu ZY, Dou YH, Kang JL, Yuan D, Feng YZ, Ma JM. Nonsolvent-induced phase separation-derived TiO2 nanotube arrays/porous Ti electrode as high-energy-density anode for lithium-ion batteries. Rare Met. 2021;40(2):393. https://doi.org/10.1007/s12598-020-01571-6.
Yan L, Zong LS, Zhang ZJ, Li JX, Wu HZ, Cui ZY, Kang JL. Oxygen vacancies activated porous MnO/graphene submicron needle arrays for high-capacity lithium-ion batteries. Carbon. 2022;190:402. https://doi.org/10.1016/j.carbon.2022.01.035.
Eftekhari A. High-energy aqueous lithium batteries. Adv Energy Mater. 2018;8(24):1801156. https://doi.org/10.1002/aenm.201801156.
Guo XB, Wang JC, Li GJ, Gao B, Bie CY, Zhang YP. Preparation and lithium-ion storage properties of vanadium nitride/nano silicon/carbon composite microspheres. Chin J Rare Metals. 2022;46(6):829. https://doi.org/10.13373/j.cnki.cjrm.XY2109002.
Sun L, Liu YX, Shao R, Wu J, Jiang RY, Jin Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Materials. 2022;46:482. https://doi.org/10.1016/j.ensm.2022.01.042.
Dong WJ, Xu JJ, Wang C, Lu Y, Liu XY, Wang X, Yuan XT, Wang Z, Lin TQ, Sui ML, Chen IW, Huang FQ. A robust and conductive black tin oxide nanostructure makes efficient lithium-ion batteries possible. Adv Mater. 2017;29(24):1700136. https://doi.org/10.1002/adma.201700136.
Du WR, Du XF, Ma MB, Huang S, Sun XF, Xiong LL. Polymer electrode materials for lithium-ion batteries. Adv Funct Mater. 2022;32(21):2110871. https://doi.org/10.1002/adfm.202110871.
Zhang ZJ, Li WJ, Chou SL, Han C, Liu HK, Dou SK. Effects of carbon on electrochemical performance of red phosphorus (P) and carbon composite as anode for sodium ion batteries. J Mater Sci Technol. 2021;68(30):140. https://doi.org/10.1016/j.jmst.2020.08.034.
Liu SD, Kang L, Jun SC. Challenges and strategies toward cathode materials for rechargeable potassium-ion batteries. Adv Mater. 2021;33(47):2004689. https://doi.org/10.1002/adma.202004689.
Zhao SQ, Guo ZQ, Yan K, Wan SW, He FR, Sun B, Wang GX. Towards high-energy-density lithium-ion batteries: strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mater. 2021;34:716. https://doi.org/10.1016/j.ensm.2020.11.008.
Zhang Q, Zeng YP, Ling CS, Wang L, Wang ZY, Fan TE, Wang H, Xiao JR, Li XY, Qu BH. Boosting fast sodium ion storage by synergistic effect of heterointerface engineering and nitrogen doping porous carbon nanofibers. Small. 2022;18(13):2107514. https://doi.org/10.1002/smll.202107514.
Liu JX, Wang JQ, Ni YX, Zhang K, Cheng FY, Chen J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater Today. 2021;43:132. https://doi.org/10.1016/j.mattod.2020.10.028.
Zhang SF, Zhang ZJ, Li HW, Yu ZY, Huang Q, Qiao ZJ, Zong LS, Yan L, Li JX, Kang JL. Ultrahigh areal capacity of self-combusted nanoporous NiCuMn/Cu flexible anode for Li-ion battery. Chem Eng J. 2020;383:123097. https://doi.org/10.1016/j.cej.2019.123097.
Liu SD, Kang L, Zhang J, Jung E, Lee S, Jun SC. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020;32:167. https://doi.org/10.1016/j.ensm.2020.07.017.
Liu YF, Xiong LQ, Li PX, Fu HY, Hou ZC, Zhu L, Li WZ. Self-supported CuO nanoflake arrays on nanoporous Cu substrate as high-performance negative-electrodes for lithium-ion batteries. J Power Sour. 2019;428:20. https://doi.org/10.1016/j.jpowsour.2019.04.102.
Yang Y, Yuan W, Zhang XQ, Wang C, Yuan YH, Huang Y, Ye YT, Qiu ZQ, Tang Y. A review on FexOy-based materials for advanced lithium-ion batteries. Renew Sustain Energy Rev. 2020;127:109884. https://doi.org/10.1016/j.rser.2020.109884.
Hou CX, Wang B, Murugadoss V, Vupputuri S, Chao YF, Guo ZH, Wang CY, Du W. Recent advances in Co3O4 as anode materials for high-performance lithium-ion batteries. Eng Sci. 2020;11(5):19. https://doi.org/10.30919/es8d1128.
Yao LH, Cao WQ, Zhao JG, Zheng Q, Wang YC, Jiang S, Pan QL, Song J, Zhu YQ, Cao MS. Regulating bifunctional flower-like NiFe2O4/graphene for green EMI shielding and lithium ion storage. J Mater Sci Technol. 2022;127:48. https://doi.org/10.1016/j.jmst.2022.04.010.
Zong LS, Zhang ZJ, Yan L, Li P, Yu ZY, Qiao ZJ, Zhang SF, Kang JL. Multilayered interlaced SnO2 nanosheets@porous copper tube textile as thin and flexible electrode for lithium-ion batteries. Electrochim Acta. 2021;385:138436. https://doi.org/10.1016/j.electacta.2021.138436.
Langdon J, Manthiram A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2021;37:143. https://doi.org/10.1016/j.ensm.2021.02.003.
Cui ZH, Xie Q, Manthiram A. Zinc-doped high-nickel, low-cobalt layered oxide cathodes for high-energy-density lithium-ion batteries. ACS Appl Mater Interfaces. 2021;13(13):15324. https://doi.org/10.1021/acsami.1c01824.
Chen Y, Chen XY, Zhang YL. A comprehensive review on metal-oxide nanocomposites for high-performance lithium-ion battery anodes. Energy Fuels. 2021;35(8):6420. https://doi.org/10.1021/acs.energyfuels.1c00315.
Dyck O, Zhang LZ, Yoon M, Swett JL, Hensley D, Zhang C, Rack PD, Fowlkes JD, Lupini AR, Jesse S. Doping transition-metal atoms in graphene for atomic-scale tailoring of electronic, magnetic, and quantum topological properties. Carbon. 2021;173:205. https://doi.org/10.1016/j.carbon.2020.11.015.
An YL, Fei HF, Zeng GF, Ci LJ, Xiong SL, Feng JK, Qian YT. Green, scalable, and controllable fabrication of nanoporous silicon from commercial alloy precursors for high-energy lithium-ion batteries. ACS Nano. 2018;12(5):4993. https://doi.org/10.1021/acsnano.8b02219.
He W, Ye FJ, Lin J, Wang Q, Xie QS, Pei F, Zhang CY, Liu PF, Li XW, Wang LS, Qu BH, Peng DL. Boosting the electrochemical performance of Li-and Mn-rich cathodes by a three-in-one strategy. Nano-Micro Lett. 2021;13:205. https://doi.org/10.1007/s40820-021-00725-0.
Wang D, Jiang SD, Duan CQ, Mao J, Dong Y, Dong KZ, Wang ZY, Luo SH, Liu YG, Qi XW. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J Alloys Compd. 2020;844:156158. https://doi.org/10.1016/j.jallcom.2020.156158.
Vinnik DA, Trofimov EA, Zhivulin VE, Zaitseva OV, Gudkova SA, Starikov AY, Zherebtsov DA, Kirsanova AA, Habner M, Niewa R. High-entropy oxide phases with magnetoplumbite structure. Ceram Int. 2019;45(10):12942. https://doi.org/10.1016/j.ceramint.2019.03.221.
Zheng YN, Yi YK, Fan MH, Liu HY, Li X, Zhang R, Li MT, Qiao ZA. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries. Energy Stor Mater. 2019;23:678. https://doi.org/10.1016/j.ensm.2019.02.030.
Zhao J, Yang X, Huang Y, Du F, Zeng Y. Entropy stabilization effect and oxygen vacancies enabling spinel oxide highly reversible lithium-ion storage. ACS Appl Mater Interfaces. 2021;13(49):58674. https://doi.org/10.1021/acsami.1c18362.
Xiao B, Wu G, Wang TD, Wei ZG, Sui YW, Shen BL, Qi JQ, Wei FX, Zheng JC. High-entropy oxides as advanced anode materials for long-life lithium-ion batteries. Nano Energy. 2022;95:106962. https://doi.org/10.1016/j.nanoen.2022.106962.
Tian KH, Duan CQ, Ma Q, Li XL, Wang ZY, Sun HY, Luo SH, Wang D, Liu YG. High-entropy chemistry stabilizing spinel oxide (CoNiZnXMnLi)3O4 (X=Fe, Cr) for high-performance anode of Li-ion batteries. Rare Met. 2022;41(4):1265. https://doi.org/10.1007/s12598-021-01872-4.
Sarkar A, Velasco L, Wang D, Wang QS, Talasila G, Biasi L, Kubel C, Brezesinski T, Bhattacharya SS, Hahn H, Breitung B. High entropy oxides for reversible energy storage. Nat Commun. 2018;9:783. https://doi.org/10.1038/s41467-018-05774-5.
Qiu N, Chen H, Yang ZM, Sun S, Wang Y, Cui YH. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J Alloys Compd. 2019;777:767. https://doi.org/10.1016/j.jallcom.2018.11.049.
Nguyen TX, Patra J, Chang JK, Ting JM. High entropy spinel oxide nanoparticles for superior lithiation–delithiation performance. J Mater Chem A. 2020;8(36):18963. https://doi.org/10.1039/D0TA04844E.
Pan K, Zou F, Canova M, Zhu Y, Kim JH. Systematic electrochemical characterizations of Si and SiO anodes for high-capacity Li-ion batteries. J Power Sour. 2019;413:20. https://doi.org/10.1016/j.jpowsour.2018.12.010.
Liu H, Xi C, Xin JH, Zhang GL, Zhang SF, Zhang ZJ, Huang Q, Li JX, Liu H, Kang J. Free-standing nanoporous NiMnFeMo alloy: an efficient non-precious metal electrocatalyst for water splitting. Chem Eng J. 2021;404:126530. https://doi.org/10.1016/j.cej.2020.126530.
Shi YX, Pan XF, Li B, Zhao MM, Pang H. Co3O4 and its composites for high-performance Li-ion batteries. Chem Eng J. 2018;343:427. https://doi.org/10.1016/j.cej.2018.03.024.
Mujtaba J, Sun HY, Zhao YY, Xiang GL, Xu SM, Zhu J. High-performance lithium storage based on the synergy of atomic-thickness nanosheets of TiO2 (B) and ultrafine Co3O4 nanoparticles. J Power Sour. 2017;363:110. https://doi.org/10.1016/j.jpowsour.2017.07.076.
Kang L, Zhang MY, Zhang J, Liu SD, Zhang N, Yao WJ, Ye Y, Luo C, Gong ZW, Cl W, Zhou XF, Wu X, Jun SC. Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J Mater Chem A. 2020;8(47):25443. https://doi.org/10.1039/D0TA08979F.
Yang J, Hu SY, Fang YR, Hoang S, Li L, Yang WW, Liang ZF, Wu J, Hu JP, Xiao W, Pan CQ, Luo Z, Ding J, Zhang LZ, Guo YB. Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature CO oxidation. ACS Catal. 2019;9(11):9751. https://doi.org/10.1021/acscatal.9b02408.
Zhang ZY, Li ML, Gao Y, Wei ZX, Zhang MN, Wang CZ, Zeng Y, Zou B, Chen G, Du F. Fast potassium storage in hierarchical Ca0.5Ti2(PO4)3@C microspheres enabling high-performance potassium-ion capacitors. Adv Funct Mater. 2018;28(36):1802684. https://doi.org/10.1002/adfm.201802684.
Lokcu E, Toparli C, Anik M. Electrochemical performance of (MgCoNiZn)1–xLixO high-entropy oxides in lithium-ion batteries. ACS Appl Mater Interfaces. 2020;12(21):23860. https://doi.org/10.1021/acsami.0c03562.
Han ZY, Li XY, Li Q, Li HS, Xu J, Li N, Zhao GX, Wang X, Li HL, Li SD. Construction of the POMOF@polypyrrole composite with enhanced ion diffusion and capacitive contribution for high-performance lithium-ion batteries. ACS Appl Mater Interfaces. 2021;13(5):6265. https://doi.org/10.1021/acsami.0c20721.
Zhang SF, Zhang ZZ, Zhang XY, Kang JL. Carbon coated NixCoyMn1-x-yO/Mn3O4 with robust deficiencies grown on nanoporous alloy for enhanced Li-ion storage. Electrochim Acta. 2020;332:135468. https://doi.org/10.1016/j.electacta.2019.135468.
Liu CL, Luo SH, Huang HB, Wang ZY, Wang Q, Zhang YH, Liu YG, Zhai YC, Wang ZW. Potassium vanadate K0.23V2O5 as anode materials for lithium-ion and potassium-ion batteries. J Power Sour. 2018;389:77. https://doi.org/10.1016/j.jpowsour.2018.04.014.
Zhang M, He YX, Xu HJ, Ma C, Liang JF, Wang YY, Zhu J. Nb2O5 nanoparticles embedding in graphite hybrid as a high-rate and long-cycle anode for lithium-ion batteries. Rare Met. 2022;41(3):814. https://doi.org/10.1007/s12598-021-01863-5.
Huang CY, Huang CW, Wu MC, Patra J, Nguyen TX, Chang MT, Clemens O, Ting JM, Li J, Chang JK. Atomic-scale investigation of Lithiation/Delithiation mechanism in high-entropy spinel oxide with superior electrochemical performance. Chem Eng J. 2021;420:129838. https://doi.org/10.1016/j.cej.2021.129838.
Luo XF, Patra J, Chuang WT, Nguyen TX, Ting JM, Li J, Pao CW, Chang JK. Charge-discharge mechanism of high-entropy co-free spinel oxide toward Li+ storage examined using operando quick-scanning X-ray absorption spectroscopy. Adv Sci. 2022;9(21):2201219. https://doi.org/10.1002/advs.202201219.
Sarkar A, Wang QS, Schiele A, Chellali MR, Bhattacharya SS, Wang D, Brezesinski T, Hahn H, Velasco L, Breitung B. High-entropy oxides: high-entropy oxides: fundamental aspects and electrochemical properties. Adv Mater. 2019;31(26):1970189. https://doi.org/10.1002/adma.201970189.
Gao MJ, Le K, Wang GW, Wang Z, Wang FL, Liu W, Liu JR. Core-shell Cu2-xS@CoS2 heterogeneous nanowire array with superior electrochemical performance for supercapacitor application. Electrochim Acta. 2019;323:134839. https://doi.org/10.1016/j.electacta.2019.134839.
Zhou SH, Huang P, Xiong TZ, Yang F, Yang H, Huang YC, Li D, Deng JQ, Balogun MS. Sub-thick electrodes with enhanced transport kinetics via in situ epitaxial heterogeneous interfaces for high areal-capacity lithium ion batteries. Small. 2021;17(26):2100778. https://doi.org/10.1002/smll.202100778.
Liu SD, Kang L, Zhang J, Jun SC, Yamauchi Y. Carbonaceous anode materials for non-aqueous sodium-and potassium-ion hybrid capacitors. ACS Energy Lett. 2021;6(11):4127. https://doi.org/10.1021/acsenergylett.1c01855.
Yan YT, Lin JH, Liu T, Liu BS, Wang B, Qiao L, Tu JC, Cao J, Qi JL. Corrosion behavior of stainless steel-tungsten carbide joints brazed with AgCuX (X = In, Ti) alloys. Corros Sci. 2022;200:110231. https://doi.org/10.1016/j.corsci.2022.110231.
Liu DH, Li WH, Zheng YP, Cui Z, Yan X, Liu DS, Wang JW, Zhang Y, Lü HY, Bai FY, Guo JZ, Wu XL. In situ encapsulating α-MnS into N, S-codoped nanotube-like carbon as advanced anode material: α→β phase transition promoted cycling stability and superior Li/Na-storage performance in half/full cells. Adv Mater. 2018;30(21):1706317. https://doi.org/10.1002/adma.201706317.
Liu ZL, Wang XX, Wu ZY, Yang SJ, Yang SL, Chen SP, Wu XT, Chang XH, Yang PP, Zheng J, Liu XG. Ultrafine Sn4P3 nanocrystals from chloride reduction on mechanically activated Na surface for sodium/lithium ion batteries. Nano Res. 2020;13(11):3157. https://doi.org/10.1007/s12274-020-2987-2.
Ling JK, Karuppiah C, Reddy MV, Pal B, Yang CC, Jose R. Unraveling synergistic mixing of SnO2-TiO2 composite as anode for Li-ion battery and their electrochemical properties. J Mater Res. 2021;36(20):4120. https://doi.org/10.1557/s43578-021-00313-3.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 52271011, 52102291 and 51701142). We are also very grateful for the description of the Analytical Testing Center of Tiangong University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, ZY., Sun, Q., Li, H. et al. Tuning single-phase medium-entropy oxides derived from nanoporous NiCuCoMn alloy as a highly stable anode for Li-ion batteries. Rare Met. 42, 2982–2992 (2023). https://doi.org/10.1007/s12598-023-02293-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02293-1