Abstract
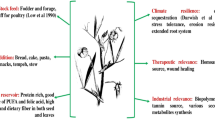
An efficient plantlet regeneration protocol using immature zygotic embryos (IZEs) via somatic embryogenesis has been developed in Pterocarpus marsupium Roxb. The regenerated plantlets were evaluated for their genetic stability. IZEs were incubated on Murashige and Skoog (MS) media augmented with 1.07–16.11 μM naphthalene acetic acid (NAA) or 0.90–13.97 μM 2,4-dichlorophenoxyacetic acid. The optimum callus induction (96.6%) was observed on MS medium augmented with 5.37 μM NAA. Induction of somatic embryos (SEs) was observed after sub-culture of calli on medium with decreased concentrations of NAA (0.54–5.37 μM), either alone or 2.69 μM NAA in combination with 2.22–8.90 μM benzyladenine (BA) or 2.32–9.30 μM Kinetin. Maximum number (33.4 ± 0.85) of SEs occurred on MS medium augmented with 2.69 μM NAA + 4.40 μM BA + 3% sucrose. Highest percentage (67.3 ± 0.37) of SEs matured and developed into cotyledonary stage by subsequent subculture on the same medium. SE formation and maturation decreased when sucrose concentrations were higher than 3%. Seventy percent of mature somatic embryos developed into plantlets on half strength MS medium augmented with 5.80 µM gibberellic acid. The various stages of development during somatic embryogenesis include globular, heart, torpedo and mature stages as revealed by the stereomicroscopic and histological studies of explants. Plantlets derived from SEs were successfully acclimatized in the greenhouse with a survival rate of 78%. Among the survived plantlets, 9 plantlets were randomly selected for inter-simple sequence repeat (ISSR) analysis. Of the 13 primers used, 8 produced reproducible and monomorphic bands. ISSR analysis revealed a homogenous amplification profile for all regenerated plantlets analyzed validating the genetic stability of somatic embryo derived plantlets.




Similar content being viewed by others
Abbreviations
- MS:
-
Murashige and Skoog
- PGR:
-
Plant growth regulators
- BA:
-
6-Benzyladenine
- GA3 :
-
Gibberellic acid
- Kn:
-
Kinetin
- NAA:
-
Naphthaleneacetic acid
- IZE:
-
Immature zygotic embryo
- SE:
-
Somatic embryo
- ISSR:
-
Inter-simple sequence repeats
References
Anis M, Husain MK, Shahzad A (2005) In vitro plantlet regeneration of Pterocarpus marsupium Roxb., an endangered leguminous tree. Curr Sci 88:861–863
Anjaneyulu C, Giri CC (2008) Factors influencing somatic embryo maturation, high frequency germination and plantlet formation in Terminalia chebula Retz. Plant Biotechnol Rep 2:153–161
Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Chakraborty A, Gupta N, Ghosh K, Roy P (2010) In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol In Vitro 24:1215–1228
Chand S, Singh AK (2004) In vitro shoot regeneration from cotyledonary node explants of a multipurpose leguminous tree, Pterocarpus marsupium Roxb. In Vitro Cell Dev Biol Plant 40:167–170
Chaturani GDG, Subasinghe S, Jayatilleke MP (2006) In vitro establishment, germination and growth performance of Red sandal wood (Pterocarpus santalinus L.). Trop Agric Res Ext 9:116–130
Corredoira E, Ballester A, Ibarra M, Vieitez AM (2015) Induction of somatic embryogenesis in explants of shoot cultures established from adult Eucalyptus globulus and E. saligna × E. maidenii trees. Tree Physiol 35:678–690
Das P (2011) In vitro somatic embryogenesis in some oil yielding tropical tree species. Am J Plant Sci 2:217–222
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Husain MK, Anis M, Shahzad A (2008) In vitro propagation of a multipurpose leguminous tree (Pterocarpus marsupium Roxb.) using nodal explants. Acta Physiol Plant 30:353–359
Husain MK, Anis M, Shahzad A (2010) Somatic embryogenesis and plant regeneration in Pterocarpus marsupium Roxb. Trees 24:781–787
Kim YW, Park SY, Park IS, Moon HK (2007) Somatic embryogenesis and plant regeneration from immature seeds of Magnolia obovata Thunberg. Plant Biotechnol Rep 1:237–242
Kirtikar KR, Basu BD (2012) Indian medicinal plants, vol 1, 2nd edn. International Book Distributors, New Delhi, p 828
Kong DM, Preece JE, Shen HL (2012) Somatic embryogenesis in immature cotyledons of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tissue Organ Cult 108:485–492
Kosaraju J, Madhunapantula SV, Chinni S, Khatwal RB, Dubala A, Nataraj SKM, Basavan D (2014) Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behav Brain Res 267:55–65
Lara-Chavez A, Flinn BS, Egertsdotter U (2011) Initiation of somatic embryogenesis from immature zygotic embryos of Oocarpa pine (Pinus oocarpa Schiede ex Schlectendal). Tree Physiol 31:539–554
Manickam M, Ramanathan M, Jahromi MA, Chansouria JP, Ray AB (1997) Anti-hyperglycemic activity of phenolics from Pterocarpus marsupium. J Nat Prod 60:609–610
Martin M, Sarmento D, Oliveira MM (2004) Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep 23:492–496
Mauri PV, Manzanera JA (2004) Effect of abscisic acid and stratification on somatic embryo maturation and germination of holm oak (Quercus ilex L.). In Vitro Cell Dev Biol Plant 40:495–498
Mikuła A, Pożoga M, Grzyb M, Rybczyński JJ (2015) An unique system of somatic embryogenesis in the tree fern Cyathea delgadii Sternb.: the importance of explant type, and physical and chemical factors. Plant Cell Tissue Organ Cult 123:467–478
Murashige T, Skoog FA (1962) Revised medium for growth and bioassay with tobacco tissue culture. Plant Physiol 15:473–479
Naz R, Anis M, Alatar AA (2016) ISSR marker-based detection of genomic stability in Cassia occidentalis L. plantlets derived from somatic embryogenesis. Eng Life Sci 16:17–24
Prakash MG, Gurumurthi K (2010) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult 100:13–20
Radhika T, Mahendar P, Venkatesham A, Reddy ARN, Narsimha RY, Krishna DR, Christopher T, Sadanandam A (2010) Antioxidant and analgesic activities of Pterocarpus marsupium Roxb. J Herbs Spices Med Plants 16:63–68
Radhika T, Laxmi Jaya Shankar P, Mahendar P, Sirisha K, Sadanandam A, Christopher T (2013a) Pterostilbene as a potential novel telomerase inhibitor: molecular docking studies and its in vitro evaluation. Curr Pharm Biotechnol 14:1027–1035
Radhika T, Rajesh Y, Mallesham B, Mahendar P, Sadanandam A, Christopher T (2013b) In vitro plantlet regeneration and Agrobacterium tumefaciens-mediated genetic transformation of Indian Kino tree (Pterocarpus marsupium Roxb.). Acta Physiol Plant 35:3437–3446
Rai MK, Phulwaria M, Harish Gupta AK, Shekhawat NS, Jaiswal U (2012) Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tissue Organ Cult 111:259–264
Rizvi SI, Mishra N (2013) Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res. https://doi.org/10.1155/2013/712092
Rutledge RG, Stewart D, Caron S, Overton C, Boyle B, MacKay J, Klimaszewska K (2013) Potential link between biotic defense activation and recalcitrance to induction of somatic embryogenesis in shoot primordia from adult trees of white spruce (Piceaglauca). BMC Plant Biol 13:1–17
Saeed T, Shahzad A (2015) High frequency plant regeneration in Indian Siris via cyclic somatic embryogenesis with biochemical, histological and SEM investigations. Ind Crop Prod 76:623–637
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449
Singh AK, Chand S (2003) Somatic embryogenesis and plant regeneration from cotyledon explants of timber yielding leguminous tree Dalbergia sissoo Roxb. J Plant Physiol 160:415–421
Siragusa M, Carra A, Salvia L, Puglia AM, De Pasquale F, Carimi F (2007) Genetic instability in calamondin (Citrus madurensis Lour.) plants derived from somatic embryogenesis induced by diphenylurea derivatives. Plant Cell Rep 26:1289–1296
Thengane SR, Deodhar SR, Bhosl SV, Rawal SK (2006) Direct somatic embryogenesis and plant regeneration in Garcinia indica Choiss. Curr Sci 91:1074–1078
Tiwari S, Pankaj S, Kanchan S (2004) In vitro propagation of Pterocarpus marsupium Roxb. An endangered medicinal tree. Ind J Biotechnol 3:422–425
Wang W, Zhao X, Zhuang G, Wang S, Chen F (2008) Simple hormonal regulation of somatic embryogenesis and/or shoot organogenesis in caryopsis cultures of Pogonatherum paniceum (Poaceae). Plant Cell Tissue Organ Cult 95:57–67
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interation. Ann Bot (Lond) 95:707–735
World Conservation Monitoring Centre (1998) Pterocarpus marsupium IUCN red list of threatened species. Version 2006. International Union for Conservation of Nature. Retrieved 11 May 2006. Listed as Vulnerable (VU A1 cd v2.3)
Yang L, Li Y, Shen H (2012) Somatic embryogenesis and plant regeneration from immature zygotic embryo cultures of mountain ash (Sorbus pohuashanensis). Plant Cell Tissue Organ Cult 109:547–556
Yang L, Bian L, Shen HL, Li YH (2013) Somatic embryogenesis and plantlet regeneration from mature zygotic embryos of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tissue Organ Cult 115:115–125
Acknowledgements
T. Radhika is thankful to Jawaharlal Nehru Memorial Fund, New Delhi, India for financial support (Ref: SU-A/180/2011-12/404). The authors gratefully acknowledge Prof. A. Sadanandam, Department of Biotechnology, Kakatiya University, Warangal, Telangana, India for providing ISSR primers.
Author information
Authors and Affiliations
Contributions
The authors have made the following declarations regarding their contributions. CT, RSN: Conceived and designed the experiments. RT: Performed the experiments. RT, PM: Analyzed the data. CT, RSN: Contributed reagents/materials. RT: Wrote the paper. CT, RSN, PM: Reviewed the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of the submitted manuscript has declared any conflict of interest.
Practical application
The protocol is useful for mass multiplication, genetic engineering, synthetic seed preparation and cryopreservation of P. marsupium plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig.
1 Effect of date of collection of explant on the induction of somatic embryogenesis in P. marsupium. The MS media was supplemented with 2.69 µM NAA +4.40 µM BA and 3% sucrose. Values are shown as mean ± SE of four independent experiments. At least 12 cultures were raised for each experiment. (TIFF 270 kb)
Rights and permissions
About this article
Cite this article
Tippani, R., Nanna, R.S., Mamidala, P. et al. Assessment of genetic stability in somatic embryo derived plantlets of Pterocarpus marsupium Roxb. using inter-simple sequence repeat analysis. Physiol Mol Biol Plants 25, 569–579 (2019). https://doi.org/10.1007/s12298-018-0602-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-018-0602-8