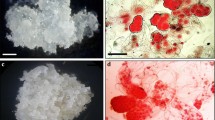
Abstract
Plant regeneration was achieved through direct and indirect somatic embryogenesis in Eucalyptus camaldulensis. Callus was induced from mature zygotic embryos and from cotyledon explants collected from 10, 15, 25, and 30-day-old seedlings cultured on Murashige and Skoog (MS) basal medium supplemented with different concentrations of naphthaleneacetic acid (NAA). Maximum callus induction from mature zygotic embryos was obtained on MS basal medium containing 1 mg l−1 NAA. The frequency of callus development varied based on the age of the cotyledon explants 10-day-old explants giving highest percentage on MS basal medium supplemented with 1 mg l−1 NAA. Callus obtained from mature zygotic embryos gave highest frequency of somatic embryogenesis on MS basal medium containing 0.5 mg l−1 benzyladenine (BA) and 0.1 mg l−1 NAA. Separate age wise culture of the calli, obtained from cotyledons of different ages cultured separately, revealed high somatic embryogenic potential on callus from 10-day-old cotyledons. Direct somatic embryogenesis too was obtained from hypocotyl explants without an intervening callus phase on MS basal medium containing 0.5 mg l−1 BA. The effects of abscisic acid (ABA), sucrose, and different strengths of MS medium on somatic embryo maturation and germination were also investigated. Number of mature somatic embryos increased with lower concentrations (0–1 mg l−1) of ABA while no significant differences were observed at higher concentrations (2–5 mg l−1) of ABA. Compared to basal medium containing lower concentrations of sucrose (1%), the MS medium supplemented with higher levels of sucrose (4%) showed significantly lower frequency of mature somatic embryos. Basal medium without any dilution gave the highest number of immature embryos. However, the number of mature embryos was high at higher medium dilutions.





Similar content being viewed by others
Abbreviations
- BA:
-
Benzyladenine
- 2,4-D :
-
2,4-Dichlorophenoxyacetic acid
- NAA:
-
α-Naphthaleneacetic acid
- IBA:
-
Indolebutyric acid
- ABA:
-
Abscisic acid
- MS:
-
Murashige and Skoog (1962) medium
References
Ammirato PV (1997) Hormonal control of somatic embryo development from cultured cells of caraway. Interaction of abscisic acid, zeatin and gibberellic acid. Plant Physiol 59:579–586
Ara H, Jaiswal U, Jaiswal VS (1999) Germination and plantlet regeneration from encapsulated somatic embryos of mango (Mangifera indica L.). Plant Cell Rep 19:166–170
Attree SM, Fowke LC (1993) Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult 35:1–35
Bandyopadhyay S, Cane K, Rasmussen G, Hamill JD (1999) Efficient plant regeneration from seedling explants of two commercially important temperate eucalypt species—Eucalyptus nitens and E. globulus. Plant Sci 140:189–198
Bennett IJ, McCombm JA, Tonkin CM, McDavid DAJ (1994) Alternating cytokinins in multiplication media stimulates in vitro shoot growth and rooting of Eucalyptus globulus Labill. Ann Bot 74:53–58
Choi YE, Soh WY (1996) Effect of plumule and radicle on somatic embryogenesis in the cultures of ginseng zygotic embryos. Plant Cell Tissue Organ Cult 45:137–143
Cotterill PP, Araújo C, Lencart P (2000) Project 3D: Celbi’s advanced-generation Eucalyptus globulus improvement. Celbi investigation report no. 75, vol 8, pp 67–78
Eldridge K, Davidson C, Harwood C, Van Wyk G (1994) Eucalypt domestication and breeding. Oxford University Press, New York
Gray DJ, Purohit A (1991) Somatic embryogenesis and development of synthetic seed technology. Crit Rev Plant Sci 10:33–61
Gutmann M, Aderkas PV, Label P, Lelu MA (1996) Effects of abscisic acid on somatic embryo maturation of hybrid larch. J Exp Bot 47:1905–1917
Harbard JL, Griffin AR, Espejo J (1999) Mass control pollination of Eucalyptus globulus: a practical reality. Can J For Res 29(10):1457–1463
Heirwegh KM, Banerjee GN, Nerum KV, Langhe ED (1985) Somatic embryogenesis and plantlet regeneration in Cichorium intybus L. Plant Cell Rep 4:108–111
Jain SM (2006) An update on overall recent progress on somatic embryogenesis in forest trees. In: Suzuki K, Ishii K, Sakurai S, Sasaki S (eds) Plantation technology in tropical forest science. Springer, Tokyo, pp 113–122
Komatsuda T, Lee W, Oka S (1992) Maturation and germination of somatic embryos as affected by sucrose and plant growth regulators in soybean Glycine gracilis Skvortz and Glycine max (L.) Merr. Plant Cell Tissue Organ Cult 28:103–113
Le Roux JJ, Van Steden J (1991) Micropropagation and tissue culture of Eucalyptus—a review. Tree Physiol 9:435–477
Leal AMC, Cotterill PP (1997) Mass control-pollination of Eucalyptus globulus. In: Proceedings of IUFRO symposium on silviculture and genetic improvement of Eucalyptus, Salvador, Brazil, 24–29 August 1997
MacRae S, van Staden J (1993) Agrobacterium rhizogenes-mediated transformation to improve rooting ability of eucalypts. Tree Physiol 12:411–418
McComb JA, Bennett IJ (1986) Eucalypts (Eucalyptus spp.). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 1: trees 1. Springer, Berlin, pp 340–362
Merkle SA (1995) Strategies for dealing with limitations of somatic embryogenesis in hardwood trees. Plant Tissue Cult Biotechnol 1:112–121
Merkle SA, Wiecko AT, Sotak RJ, Sommer HE (1990) Maturation and conversion of Liriodendron Tulipifera somatic embryos. In Vitro Cell Dev Biol 25:1086–1093
Muralidharan EM, Mascarenhas AF (1987) In vitro plantlet formation by organogenesis in Eucalyptus camaldulensis and by somatic embryogenesis in E. citriodora. Plant Cell Rep 6:256–259
Muralidharan EM, Mascarenhas AF (1995) Somatic embryogenesis in Eucalyptus. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 2-Angiosperms. Kluwer, Dordrecht, pp 23–40
Muralidharan EM, Gupta PK, Mascarenhas AF (1989) Plantlet production through high frequency somatic embryogenesis in long term cultures of Eucalyptus citriodora. Plant Cell Rep 8:41–43
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay of tobacco tissue cultures. Physiol Plant 15:473–497
Nadel BL, Altman A, Ziv M (1990) Regulation of somatic embryogenesis in celery cell suspension. 2. Early detection of embryogenic potential and induction of synchronized cell cultures. Plant Cell Tissue Organ Cult 20:119–124
Pinto G, Santos C, Neves L, Araújo C (2002) Somatic embryogenesis and plant regeneration in Eucalyptus globulus Labill. Plant Cell Rep 21:208–213
Pinto G, Silva S, Park YS, Neves L, Araújo C, Santos C (2008) Factors influencing somatic embryogenesis induction in Eucalyptus globulus Labill. basal medium and anti-browning agents. Plant Cell Tissue Organ Cult 27:1093–1101
Prakash MG, Gurumurthi K (2005) Somatic embryogenesis and plant regeneration in Eucalyptus tereticornis Sm. Current Sci 88:1311–1316
Redenbaugh K (1993) Synseeds: applications of synthetic seeds to crop improvement. CRC Press, Boca Raton
Smith DL, Krikorian AD (1989) Release of somatic embryogenic potential from excised zygotic embryos of carrot and maintenance of proembryogenic cultures in hormone-free medium. Am J Bot 76:1832–1843
Soh WY, Cho DY, Lee EK (1996) Multicotyledonary structure of somatic embryo formed from cell cultures of Daucus carota L. J Plant Biol 39:71–77
Stasolla C, Kong L, Yeung EC, Thorpe TA (2002) Maturation of somatic embryos in conifers: morphogenesis, physiology, biochemistry, and molecular biology. In vitro Cell Dev Biol Plant 38:93–105
Teulieres C, Marque C, Boutet AM (1994) Genetic transformation of Eucalyptus. In: Bajaj TPS (ed) Plant protoplasts and genetic engineering V. Springer, Berlin, pp 289–307
Tian L, Brown D (2000) Improvement of soybean somatic embryo development and maturation by abscisic acid treatment. Can J Plant Sci 80:271–276
Tibok A, Blackhall NW, Power JB, Davey MR (1995) Optimized plant regeneration from callus derived from seedling hypocotyls of Eucalyptus urophylla. Plant Sci 110:139–145
Trembly L, Trembly F (1995) Maturation of black spruce somatic embryos: sucrose hydrolysis and resulting osmotic pressure of the medium. Plant Cell Tissue Organ Cult 42:39–46
Tulecke W (1987) Somatic embryogenesis in woody perennials. In: Bonga JM, Durzan DJ (eds) Cell and tissue culture in forestry, vol 2. Martinus Nijhoff Publishers, Dordrecht, pp 61–69
Watt MP, Blakeway F, Cresswell CF, Herman B (1991) Somatic embryogenesis in Eucalyptus grandis. S Afr For J 157:59–64
Watt MP, Blakeway FC, Termignoni R, Jain SM (1999) Somatic embryogenesis in Eucalyptus grandis and E. dunnii. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 5. Kluwer, Dordrecht, pp 63–78
Wetzstein HY, Baker CM (1993) The relationship between somatic embryo morphology and conversion in peanut (Arachis hypogaea L.). Plant Sci 92:81–89
Acknowledgments
This work was performed as a part of the graduate thesis work by first author. The authors thank Director, Institute of Forest Genetics and Tree Breeding, Coimbatore, India for providing facilities to conduct the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prakash, M.G., Gurumurthi, K. Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis . Plant Cell Tiss Organ Cult 100, 13–20 (2010). https://doi.org/10.1007/s11240-009-9611-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9611-1