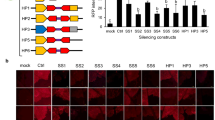
Abstract
Sugarcane, a complex polyploid, shows highly efficient and rapidly imposed silencing of diverse transgene constructs. Silencing is 5′-sequence-specific, copy-number independent, developmentally regulated and post-transcriptional in the plants first regenerated from transgenic callus. The results challenge some early generalizations from studies in model dicotyledonous plants, about causes of transgene silencing and approaches to avoid the problem. We reason that patchy and progressive transgene silencing, seen in most other plant species, may be associated with increasing endopolyploidy during maturation of differentiated tissues. The experimentally tractable sugarcane system allows features likely to trigger or protect from silencing to be tested, and ultimately controlled to improve the efficiency of plant improvement by genetic transformation.





Similar content being viewed by others
Abbreviations
- cDNA:
-
complementary DNA
- DNA:
-
deoxyribonucleic acid
- dsRNA:
-
double-stranded RNA
- GUS:
-
glucuronidase (encoded by the uidA gene)
- HDGS:
-
homology-dependent gene silencing
- LUC:
-
luciferase (encoded by the luc gene)
- NPTII:
-
neomycin phosphotransferase (encoded by the aphA gene)
- PTGS:
-
post-transcriptional gene silencing
- RNA:
-
ribonucleic acid
- RNAi:
-
RNA interference (RNA-mediated gene silencing)
- siRNA:
-
small interfering RNA (intermediate in RNAi)
- TGS:
-
transcriptional gene silencing
- Ubi:
-
ubiquitin
References
Baulcombe DC, English JJ (1996) Ectopic pairing of homologous DNA and post-transcriptional gene silencing in transgenic plants. Curr Opin Biotechnol 7:173–180
Birch RG (1997) Plant transformation: problems and strategies for practical application. Annu Rev Plant Physiol Plant Mol Biol 48:297–326
Birch RG, Bower R, Elliott AR, Potier BAM, Franks T, Cordeiro G (1996) Expression of foreign genes in sugarcane. In: Cock JH, Brekelbaum T (eds) Proc Int Soc Sugarcane Technol XXII Congress. Tecnicana, Cali, pp 368–373
Bird AP (1995) Gene number, noise reduction and biological complexity. Trends Genet 11:94–100
Bower R, Elliott AR, Potier BAM, Birch RG (1996) High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol Breed 2:239–249
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Brodersen P, Voinnet O (2006) The diversity of RNA silencing pathways in plants. Trends Genet 22:268–280
Cesarone CF, Bolognesi C, Santi L (1979) Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem 100:188–197
Chamberlain DA, Brettel RIS, Last DI, Witrzens B, McElroy D, Dolferus R, Dennis ES (1994) The use of the Emu promoter with antibiotic and herbicide resistance genes for the selection of transgenic wheat callus and rice plants. Aust J Plant Physiol 21:95–112
Chapman EJ, Carrington JC (2007) Specialization and evolution of endogenous small RNA pathways. Nat Rev Genet 8:884–896
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Cox KH, Goldberg RB (1988) Analysis of plant gene expression. In: Shaw CH (ed) Plant molecular biology: A practical approach. IRL Press, Oxford, pp 1–35
Dehio C, Schell J (1994) Identification of plant genetic loci involved in a posttranscriptional mechanism for meiotically reversible transgene silencing. Proc Natl Acad Sci USA 91:5538–5542
Eamens A, Wang MB, Smith NA, Waterhouse PM (2008) RNA silencing in plants: Yesterday, today, and tomorrow. Plant Physiol 147:456–468
Finnegan J, McElroy D (1994) Transgene inactivation: Plants fight back! Biotechnology 12:883–888
Fojtova M, Van Houdt H, Depicker A, Kovarik A (2003) Epigenetic switch from posttranscriptional to transcriptional silencing is correlated with promoter hypermethylation. Plant Physiol 133:1240–1250
Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR (1999) Ploidy regulation of gene expression. Science 285:251–254
Gallo-Meagher M, Irvine JE (1993) Effects of tissue type and promoter strength on transient GUS expression in sugarcane following particle bombardment. Plant Cell Rep 12:666–670
Graham MW, Mudge SR, Sternes PR, Birch RG (2009) Understanding and avoiding transgene silencing. In: Stewart CN, Touraev A, Citovsky V, Tzfira T (eds) Plant Transformation Technologies. Wiley-Blackwell, New York
Grivet L, D’Hont A, Roques D, Feldmannaud C, Glaszmann JC (1996) RFLP mapping in cultivated sugarcane (Saccharum spp.): Genome organization in a highly polyploid and aneuploid interspecific hybrid. Genetics 142:987–1000
Guo M, Davis D, Birchler JA (1996) Dosage effects on gene expression in a maize ploidy series. Genetics 142:1349–1355
Ha M, Lu J, Tian L, Ramachandran V, Kasschau KD, Chapman EJ, Carrington JC, Chen XM, Wang XJ, Chen ZJ (2009) Small RNAs serve as a genetic buffer against genomic shock in Arabidopsis interspecific hybrids and allopolyploids. Proc Natl Acad Sci USA 106:17835–17840
Hansom S, Bower R, Zhang L, Potier B, Elliott A, Basnayake S, Cordeiro G, Hogarth DM, Cox M, Berding N, Birch RG (1999) Regulation of transgene expression in sugarcane. In: Singh V (ed) Proceedings of the international society of sugarcane technologists XXIII congress. STAI, New Delhi, pp 278–290
Hobbs SLA, Kpodar P, DeLong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Ingelbrecht IL, Irvine JE, Mirkov TE (1999) Posttranscriptional gene silencing in transgenic sugarcane. Dissection of homology-dependent virus resistance in a monocot that has a complex polyploid genome. Plant Physiol 119:1187–1197
Jorgensen RA (1995) Cosuppression, flower color patterns, and metastable gene expression states. Science 268:686–691
Kanazawa A, O’Dell M, Hellens RP (2007) Epigenetic inactivation of chalcone synthase-A transgene transcription in petunia leads to a reversion of the post-transcriptional gene silencing phenotype. Plant Cell Physiol 48:638–647
Kovarik A, Van Houdt H, Holy A, Depicker A (2000) Drug-induced hypomethylation of a posttranscriptionally silenced transgene locus of tobacco leads to partial release of silencing. FEBS Lett 467:47–51
Koziel MG, Carozzi NB, Desai N (1996) Optimizing expression of transgenes with an emphasis on post-transcriptional events. Plant Mol Biol 32:393–405
Krizova K, Fojtova M, Depicker A, Kovarik A (2009) Cell culture-induced gradual and frequent epigenetic reprogramming of invertedly repeated tobacco transgene epialleles. Plant Physiol 149:1493–1504
Kumpatla SP, Hall TC (1998) Recurrent onset of epigenetic silencing in rice harboring a multi-copy transgene. Plant J 14:129–135
Kumpalta SP, Chandrasekharan MB, Iyer LM, Li G, Hall TC (1998) Genome intruder scanning and modulation systems and transgene silencing. Trends Plant Sci 3:97–104
Kunz C, Schob H, Stam M, Kooter JM, Meins F (1996) Developmentally regulated silencing and reactivation of tobacco chitinase transgene expression. Plant J 10:437–450
Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K (1993) Light-regulated and cell-specific expression of tomato rbcs-gusA and rice rbcs-gusA fusion genes in transgenic rice. Plant Physiol 102:991–1000
Lakshmanan P, Geijskes RJ, Aitken KS, Grof CLP, Bonnett GD, Smith GR (2005) Sugarcane biotechnology: The challenges and opportunities. In Vitro Cell Dev Biol Plant 41:345–363
Leitch IJ, Bennett MD (1997) Polyploidy in angiosperms. Trends Plant Sci 2:470–476
Marjanac G, Karimi M, Naudts M, Beeckman T, Depicker A, De Buck S (2009) Gene silencing induced by hairpin or inverted repeated sense transgenes varies among promoters and cell types. New Phytol 184:851–864
Matzke AJM, Matzke MA (1998a) Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol 1:142–148
Matzke M, Kanno T, Claxinger L, Huettel B, Matzke AJM (2009) RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol 21:367–376
Matzke MA, Matzke AJM (1995) How and why do plants inactivate homologous (trans)genes? Plant Physiol 107:679–685
Matzke MA, Matzke AJM (1998b) Epigenetic silencing of plant transgenes as a consequence of diverse cellular defence responses. Cell Mol Life Sci 54:94–103
Matzke MA, Matzke AJM (1998c) Gene silencing in plants: Relevance for genome evolution and the acquisition of genomic methylation patterns. Ciba Foundation Symposia 214:168–186
McElroy D, Blowers AD, Jenes B, Wu R (1991) Construction of expression vectors based on the rice actin 1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet 231:150–160
Millar AJ, Short SR, Chua N-H, Kay SA (1992) A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4:1075–1087
Mourrain P, van Blokland R, Kooter JM, Vaucheret H (2007) A single transgene locus triggers both transcriptional and post-transcriptional silencing through double-stranded RNA production. Planta 225:365–379
Mudge SR, Lewis-Henderson W, Birch RG (1996) Comparison of Vibrio and firefly luciferases as reporter gene systems for use in bacteria and plants. Aust J Plant Physiol 23:75–83
Mudge SR, Osabe K, Casu RE, Bonnett GD, Manners JM, Birch RG (2009) Efficient silencing of reporter transgenes coupled to known functional promoters in sugarcane, a highly polyploid crop species. Planta 229:549–558
Ossowski S, Schwab R, Weigel D (2008) Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53:674–690
Ow DW, Wood KV, DeLuca M, deWet JR, Helinski DR, Howell SH (1986) Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234:856–859
Palauqui J-C, Vaucheret H (1998) Transgenes are dispensible for the RNA degradation step of cosuppression. Proc Natl Acad Sci USA 95:9675–9680
Peach C, Velten J (1991) Transgene expression variability (position effect) of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Mol Biol 17:49–60
Rathus C, Bower R, Birch RG (1993) Effects of promoter intron and enhancer elements on transient gene expression in sugarcane and carrot protoplasts. Plant Mol Biol 23:613–618
Rogers SO, Bendich AJ (1988) Extraction of DNA from plant tissues. In: Gelvin SB, Schilperoort RA, Verma DPS (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp A6/1–10
Sambrook J, Russell DW (2001) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, New York
Scheid OM, Jakovleva L, Afsar K, Maluszynska J, Paszkowski J (1996) A change of ploidy can modify epigenetic silencing. Proc Natl Acad Sci USA 93:7114–7119
Schledzewski K, Mendel RR (1994) Quantitative transient gene expression: comparison of the promoters for maize polyubiquitin1, rice actin1, maize-derived Emu and 35 S in cells of barley, maize and tobacco. Transgenic Res 3:249–255
Soltis DE, Soltis PS (1993) Molecular data and the dynamic nature of polyploidy. Crit Rev Plant Sci 12:243–273
Traas J, Hulskamp M, Gendreau E, Hofte H (1998) Endoreduplication and development: Rule without dividing? Curr Opin Plant Biol 1:498–503
Vaucheret H (2006) Post-transcriptional small RNA pathways in plants: Mechanisms and regulations. Genes Dev 20:759–771
Vaucheret H, Beclin C, Elmayan T, Feuerbach F, Godon C, Morel J-B, Mourrain P, Palauqui J-C, Vernhettes S (1998) Transgene-induced gene silencing in plants. Plant J 16:651–659
Voinnet O (2008) Use, tolerance and avoidance of amplified RNA silencing by plants. Trends Plant Sci 13:317–328
Voinnet O, Vain P, Angell S, Baulcombe DC (1998) Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95:177–187
Wang ML, Goldstein C, Su W, Moore PH, Albert HH (2005) Production of biologically active GM-CSF in sugarcane: A secure biofactory. Transgenic Res 14:167–178
Wei HR, Wang ML, Moore PH, Albert HH (2003) Comparative expression analysis of two sugarcane polyubiquitin promoters and flanking sequences in transgenic plants. J Plant Physiol 160:1241–1251
Xu Y, Uchholz WG, DeRose RT, Hall TC (1995) Characterization of a rice gene family encoding root-specific proteins. Plant Mol Biol 27:237–248
Zhang L, Xu J, Birch RG (1999) Engineered detoxification confers resistance against a pathogenic bacterium. Nat Biotechnol 17:1021–1024
Zhong H, Zhang SB, Warkentin D, Sun BL, Wu TY, Wu R, Sticklen MB (1996) Analysis of the functional activity of the 1.4-kb 5′-region of the rice actin 1 gene in stable transgenic plants of maize (Zea mays L). Plant Sci 116:73–84
Acknowledgements
We thank Tony Cavallaro, Janette McDouall Stuart, and Simon Hansom for research assistance, and BSES Limited for assistance with sugarcane. This work was supported by the Australian Research Council through the industry collaborative grant scheme, and by the Sugar Research and Development Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Ray Ming
Rights and permissions
About this article
Cite this article
Birch, R.G., Bower, R.S. & Elliott, A.R. Highly Efficient, 5′-Sequence-Specific Transgene Silencing in a Complex Polyploid. Tropical Plant Biol. 3, 88–97 (2010). https://doi.org/10.1007/s12042-010-9047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12042-010-9047-0