Abstract
Phosphatidylserine (PtdSer) is an important anionic phospholipid found in eukaryotic cells and has been proven to serve as a beneficial factor in the treatment of neurodegenerative diseases. PtdSer resides in the inner leaflet of the plasma membrane, where it is involved in regulating the AKT and PKC signaling pathways; however, it becomes exposed to the extracellular leaflet during neurodevelopmental processes and neurodegenerative diseases, participating in microglia-mediated synaptic and neuronal phagocytosis. In this paper, we review several characteristics of PtdSer, including the synthesis and translocation of PtdSer, the functions of cytoplasmic and exposed PtdSer, and different PtdSer-detection materials used to further understand the role of PtdSer in the nervous system.




Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- AKT:
-
Protein kinase B
- AnxA5:
-
Annexin A5
- BAI1:
-
Brain-specific angiogenesis inhibitor 1
- BDNF:
-
Brain-derived neurotrophic factor
- C1q:
-
Complement component 1, q subcomponent
- CDC50A:
-
Cell division cycle protein 50A
- CNS:
-
Central nervous system
- CR3:
-
Complement receptor 3
- DHA:
-
Docosahexaenoic acid
- GABA:
-
Gamma-aminobutyric acid
- Caspase:
-
Cysteine-containing aspartate-specific proteases
- GAS6:
-
Growth arrest-specific 6
- GPR56:
-
Adhesion G protein-coupled receptor G1
- Lact-C2:
-
C2 domain in Lactadherin
- MFGE8:
-
Milk fat globule epidermal growth factor 8
- NBD:
-
Nitrobenzoxadiazole
- NBD-PS:
-
Nitrobenzoxadiazole-labeled Ptdser
- P4-ATPase:
-
Type IV P-type adenosine triphosphatase
- PET:
-
Positron emission tomography
- PH domain:
-
Pleckstrin homology domain
- PIP3 :
-
Phosphatidylinositol (3,4,5)-trisphosphate
- PKC:
-
Protein kinase C
- PtdCho:
-
Phosphatidylcholine
- PtdEtn:
-
Phosphatidylethanolamine
- PtdSer:
-
Phosphatidylserine
- pSIVA:
-
Polarity-sensitive annexin-based biosensor
- PSS:
-
Phosphatidylserine synthase
- RAGE:
-
Receptor for advanced glycation end products
- ROS:
-
Reactive oxygen species
- RTKs:
-
Receptor protein tyrosine kinase
- SAMP8:
-
Senescence-accelerated mouse-prone 8
- TAM:
-
Receptor tyrosine kinases TYRO3, AXL, and MER
- TIM:
-
T cell/transmembrane, immunoglobulin and mucin
- TMEM16F:
-
Transmembrane protein 16F
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
- Xkr8:
-
Xk-related protein 8
References
Svennerholm L (1968) Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res 9(5):570–579
Kimura AK, Kim HY (2013) Phosphatidylserine synthase 2: high efficiency for synthesizing phosphatidylserine containing docosahexaenoic acid. J Lipid Res 54(1):214–222
Kim HY, Huang BX, Spector AA (2014) Phosphatidylserine in the brain: metabolism and function. Prog Lipid Res 56:1–18
Scott-Hewitt N et al (2020) Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. Embo j 39(16):e105380
Park J et al (2021) Microglial MERTK eliminates phosphatidylserine-displaying inhibitory post-synapses. Embo j 40(15):e107121
Bader Lange ML et al (2008) Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol Dis 29(3):456–464
Brelstaff J et al (2018) Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep 24(8):1939-1948.e4
Brelstaff JH et al (2021) Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci Adv 7(43):eabg4980
Pampuscenko K et al (2020) Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. J Neurochem 154(3):316–329
Huang Y et al (2021) Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat Immunol 22(5):586–594
Vance JE (2008) Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 49(7):1377–1387
Arikketh D, Nelson R, Vance JE (2008) Defining the importance of phosphatidylserine synthase-1 (PSS1): unexpected viability of PSS1-deficient mice. J Biol Chem 283(19):12888–12897
Pettegrew JW et al (2001) Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res 26(7):771–782
Kim BK, Park SK (2020) Phosphatidylserine modulates response to oxidative stress through hormesis and increases lifespan via DAF-16 in Caenorhabditis elegans. Biogerontology 21(2):231–244
Akyol S et al (2021) Lipid profiling of Alzheimer’s disease brain highlights enrichment in glycerol(phospho)lipid, and sphingolipid metabolism. Cells 10(10):2591
Zhang W et al (2015) n-3 Polyunsaturated fatty acids reduce neonatal hypoxic/ischemic brain injury by promoting phosphatidylserine formation and Akt signaling. Stroke 46(10):2943–2950
Kingsley M (2006) Effects of phosphatidylserine supplementation on exercising humans. Sports Med 36(8):657–669
More MI, Freitas U, Rutenberg D (2014) Positive effects of soy lecithin-derived phosphatidylserine plus phosphatidic acid on memory, cognition, daily functioning, and mood in elderly patients with Alzheimer’s disease and dementia. Adv Ther 31(12):1247–1262
Chaung HC et al (2013) Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem 138(1):342–347
Nishizuka Y (1984) Turnover of inositol phospholipids and signal transduction. Science 225(4668):1365–1370
Suzuki S et al (2001) Oral administration of soybean lecithin transphosphatidylated phosphatidylserine improves memory impairment in aged rats. J Nutr 131(11):2951–2956
Kay JG, Fairn GD (2019) Distribution, dynamics and functional roles of phosphatidylserine within the cell. Cell Commun Signal 17(1):126
Park YJ et al (2021) Phosphatidylserine synthase plays an essential role in glia and affects development, as well as the maintenance of neuronal function. iScience 24(8):102899
von Schacky C (2021) Importance of EPA and DHA blood levels in brain structure and function. Nutrients 13(4):1074
Lauritzen L et al (2016) DHA effects in brain development and function. Nutrients 8(1):6
Kim HY, Akbar M, Kim YS (2010) Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids 82(4–6):165–172
Guo M et al (2007) Neuronal specific increase of phosphatidylserine by docosahexaenoic acid. J Mol Neurosci 33(1):67–73
Wu A, Ying Z, Gomez-Pinilla F (2008) Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 155(3):751–759
Wurtman RJ (2008) Synapse formation and cognitive brain development: effect of docosahexaenoic acid and other dietary constituents. Metabolism 57(Suppl 2):S6-10
Benfenati F et al (1989) Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J Cell Biol 108(5):1851–1862
Cheetham JJ et al (2001) Identification of synapsin I peptides that insert into lipid membranes. Biochem J 354(Pt 1):57–66
Zhou MM et al (2018) Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J Nutr Biochem 59:64–75
Xu ZJ et al (2021) A comparative study of the effects of phosphatidylserine rich in DHA and EPA on Aβ-induced Alzheimer’s disease using cell models. Food Funct 12(10):4411–4423
He Y et al (2021) Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther 6(1):425
Alkon DL, Sun MK, Nelson TJ (2007) PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol Sci 28(2):51–60
Vasudevan KM, Garraway LA (2010) AKT signaling in physiology and disease. Curr Top Microbiol Immunol 347:105–133
Huang BX et al (2011) Phosphatidylserine is a critical modulator for Akt activation. J Cell Biol 192(6):979–992
Zhang Z et al (2018) PI3K/Akt and HIF1 signaling pathway in hypoxiaischemia (Review). Mol Med Rep 18(4):3547–3554
Rai SN et al (2019) The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox Res 35(3):775–795
Duggan MR, Weaver M, Khalili K (2021) PAM (PIK3/AKT/mTOR) signaling in glia: potential contributions to brain tumors in aging. Aging (Albany NY) 13(1):1510–1527
Gabbouj S et al (2019) Altered insulin signaling in Alzheimer’s disease brain—special emphasis on PI3K-Akt pathway. Front Neurosci 13:629
Mellor H, Parker PJ (1998) The extended protein kinase C superfamily. Biochem J 332(Pt 2):281–292
Geribaldi-Doldan N et al (2019) Protein kinase C: targets to regenerate brain injuries? Front Cell Dev Biol 7:39
Newton AC (2010) Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab 298(3):E395-402
Verdaguer N et al (1999) Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. Embo j 18(22):6329–6338
Saito N, Shirai Y (2002) Protein kinase C gamma (PKC gamma): function of neuron specific isotype. J Biochem 132(5):683–687
Chopra R et al (2018) Protein kinase C activity is a protective modifier of Purkinje neuron degeneration in cerebellar ataxia. Hum Mol Genet 27(8):1396–1410
Schumacher D et al (2021) Phosphatidylserine supplementation as a novel strategy for reducing myocardial infarct size and preventing adverse left ventricular remodeling. Int J Mol Sci 22(9):4401
Kim PM, Kornberg MD (2022) Targeting PKC in microglia to promote remyelination and repair in the CNS. Curr Opin Pharmacol 62:103–108
Hornik TC, Neniskyte U, Brown GC (2014) inflammation induces multinucleation of microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J Neurochem 128(5):650–661
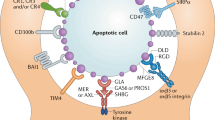
Poon IK et al (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14(3):166–180
Nagata S, Sakuragi T, Segawa K (2020) Flippase and scramblase for phosphatidylserine exposure. Curr Opin Immunol 62:31–38
Shin HW, Takatsu H (2020) Phosphatidylserine exposure in living cells. Crit Rev Biochem Mol Biol 55(2):166–178
Palmgren MG, Nissen P (2011) P-type ATPases. Annu Rev Biophys 40:243–266
Andersen JP et al (2016) P4-ATPases as phospholipid flippases-structure, function, and enigmas. Front Physiol 7:275
Coleman JA, Molday RS (2011) Critical role of the beta-subunit CDC50A in the stable expression, assembly, subcellular localization, and lipid transport activity of the P4-ATPase ATP8A2. J Biol Chem 286(19):17205–17216
Segawa K, Kurata S, Nagata S (2018) The CDC50A extracellular domain is required for forming a functional complex with and chaperoning phospholipid flippases to the plasma membrane. J Biol Chem 293(6):2172–2182
Timcenko M et al (2019) Structure and autoregulation of a P4-ATPase lipid flippase. Nature 571(7765):366–370
Timcenko M et al (2021) Structural basis of substrate-independent phosphorylation in a P4-ATPase lipid flippase. J Mol Biol 433(16):167062
Shin HW, Takatsu H (2019) Substrates of P4-ATPases: beyond aminophospholipids (phosphatidylserine and phosphatidylethanolamine). Faseb j 33(3):3087–3096
Wang J et al (2018) Proteomic analysis and functional characterization of P4-ATPase phospholipid flippases from murine tissues. Sci Rep 8(1):10795
Segawa K et al (2014) Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344(6188):1164–1168
Segawa K et al. (2021) A sublethal ATP11A mutation associated with neurological deterioration causes aberrant phosphatidylcholine flipping in plasma membranes. J Clin Invest 131(18):e148005. https://doi.org/10.1172/JCI148005
Nakanishi H et al (2020) Transport cycle of plasma membrane flippase ATP11C by Cryo-EM. Cell Rep 32(13):108208
Takatsu H et al (2011) ATP9B, a P4-ATPase (a putative aminophospholipid translocase), localizes to the trans-Golgi network in a CDC50 protein-independent manner. J Biol Chem 286(44):38159–38167
Levano K et al (2012) Atp8a1 deficiency is associated with phosphatidylserine externalization in hippocampus and delayed hippocampus-dependent learning. J Neurochem 120(2):302–313
Wang J et al (2019) ATP11B deficiency leads to impairment of hippocampal synaptic plasticity. J Mol Cell Biol 11(8):688–702
Xu Q et al (2012) P4-ATPase ATP8A2 acts in synergy with CDC50A to enhance neurite outgrowth. FEBS Lett 586(13):1803–1812
Sann S et al (2009) Roles of endosomal trafficking in neurite outgrowth and guidance. Trends Cell Biol 19(7):317–324
Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94(2):419–459
Suzuki J et al (2013) Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem 288(19):13305–13316
Fujii T et al (2015) TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc Natl Acad Sci U S A 112(41):12800–12805
Zhang Y et al (2020) TMEM16F aggravates neuronal loss by mediating microglial phagocytosis of neurons in a rat experimental cerebral ischemia and reperfusion model. Front Immunol 11:1144
Suzuki J et al (2013) Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341(6144):403–406
Suzuki J, Imanishi E, Nagata S (2016) Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure. Proc Natl Acad Sci U S A 113(34):9509–9514
Sakuragi T, Kosako H, Nagata S (2019) Phosphorylation-mediated activation of mouse Xkr8 scramblase for phosphatidylserine exposure. Proc Natl Acad Sci U S A 116(8):2907–2912
Kautzman AG et al (2018) Xkr8 modulates bipolar cell number in the mouse retina. Front Neurosci 12:876
Segawa K, Nagata S (2015) An Apoptotic “Eat Me” signal: phosphatidylserine exposure. Trends Cell Biol 25(11):639–650
Lemke G (2019) How macrophages deal with death. Nat Rev Immunol 19(9):539–549
Païdassi H et al (2008) C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol 180(4):2329–2338
Filipello F et al (2018) The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48(5):979-991e8
Li T et al (2020) A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. Embo j 39(16):e104136
O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34:185–204
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Weinhard L et al (2018) Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun 9(1):1228
Basilico B et al (2019) Microglia shape presynaptic properties at developing glutamatergic synapses. Glia 67(1):53–67
Filipello F et al (2018) The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48(5):979-991.e8
Werneburg S et al (2020) Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52(1):167-182.e7
Lemke G (2013) Biology of the TAM receptors. Cold Spring Harb Perspect Biol 5(11):a009076
Lemke G, Rothlin CV (2008) Immunobiology of the TAM receptors. Nat Rev Immunol 8(5):327–336
Lemke G (2017) Phosphatidylserine is the signal for TAM receptors and their ligands. Trends Biochem Sci 42(9):738–748
Huang M et al (2003) Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol 10(9):751–756
Lew ED et al (2014) Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife 3:e03385
Geng K et al (2017) Requirement of gamma-carboxyglutamic acid modification and phosphatidylserine binding for the activation of Tyro3, Axl, and Mertk receptors by growth arrest-specific 6. Front Immunol 8:1521
Fourgeaud L et al (2016) TAM receptors regulate multiple features of microglial physiology. Nature 532(7598):240–244
Chung WS et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504(7480):394–400
Ji R et al (2014) TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS ONE 9(12):e115140
Ji R et al (2015) TAM receptor deficiency affects adult hippocampal neurogenesis. Metab Brain Dis 30(3):633–644
Blades F et al (2018) The TAM receptor TYRO3 is a critical regulator of myelin thickness in the central nervous system. Glia 66(10):2209–2220
Tondo G, Perani D, Comi C (2019) TAM receptor pathways at the crossroads of neuroinflammation and neurodegeneration. Dis Markers 2019:2387614
Shafit-Zagardo B, Gruber RC, DuBois JC (2018) The role of TAM family receptors and ligands in the nervous system: From development to pathobiology. Pharmacol Ther 188:97–117
Prieto AL et al (2007) Localization and signaling of the receptor protein tyrosine kinase Tyro3 in cortical and hippocampal neurons. Neuroscience 150(2):319–334
Gautier EL et al (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13(11):1118–1128
Wu H et al (2021) Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation 18(1):2
Condello C et al (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun 6:6176
Owlett LD et al (2022) Gas6 induces inflammation and reduces plaque burden but worsens behavior in a sex-dependent manner in the APP/PS1 model of Alzheimer’s disease. J Neuroinflammation 19(1):38
Freeman GJ et al (2010) TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 235(1):172–189
De Maeyer RPH et al (2020) Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol 21(6):615–625
Liu W et al (2019) Tim-4 Inhibits NLRP3 Inflammasome via the LKB1/AMPKα Pathway in Macrophages. J Immunol 203(4):990–1000
Tan S et al (2020) Tim-3 hampers tumor surveillance of liver-resident and conventional NK cells by disrupting PI3K signaling. Cancer Res 80(5):1130–1142
Min C et al (2020) Tim-4 functions as a scavenger receptor for phagocytosis of exogenous particles. Cell Death Dis 11(7):561
Wang HW et al (2015) Microglia activity modulated by T cell Ig and mucin domain protein 3 (Tim-3). Cell Immunol 293(1):49–58
Koh HS et al (2015) The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun 6:6340
Mazaheri F et al (2014) Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat Commun 5:4046
Park D, Ravichandran KS (2010) Emerging roles of brain-specific angiogenesis inhibitor 1. Adv Exp Med Biol 706:167–178
Park D et al (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450(7168):430–434
Tu YK, Duman JG, Tolias KF (2018) The adhesion-GPCR BAI1 promotes excitatory synaptogenesis by coordinating bidirectional trans-synaptic signaling. J Neurosci 38(39):8388–8406
Sokolowski JD et al (2011) Brain-specific angiogenesis inhibitor-1 expression in astrocytes and neurons: implications for its dual function as an apoptotic engulfment receptor. Brain Behav Immun 25(5):915–921
Duman JG, Tu YK, Tolias KF (2016) Emerging roles of BAI adhesion-GPCRs in synapse development and plasticity. Neural Plast 2016:8301737
Zhu D et al (2015) BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. J Clin Invest 125(4):1497–1508
Duman JG et al (2013) The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J Neurosci 33(16):6964–6978
Nishi C et al (2014) Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol Cell Biol 34(8):1512–1520
Yanagihashi Y et al (2017) Mouse macrophages show different requirements for phosphatidylserine receptor Tim4 in efferocytosis. Proc Natl Acad Sci U S A 114(33):8800–8805
Park D, Hochreiter-Hufford A, Ravichandran KS (2009) The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr Biol 19(4):346–351
Lee J et al (2019) A scaffold for signaling of Tim-4-mediated efferocytosis is formed by fibronectin. Cell Death Differ 26(9):1646–1655
Salzman GS et al (2016) Structural basis for regulation of GPR56/ADGRG1 by its alternatively spliced extracellular domains. Neuron 91(6):1292–1304
Fricker M et al (2012) MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci 32(8):2657–2666
Elliott MR, Ravichandran KS (2010) Clearance of apoptotic cells: implications in health and disease. J Cell Biol 189(7):1059–1070
Akakura S et al (2004) The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res 292(2):403–416
Cserép C et al (2020) Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 367(6477):528–537
Butler CA et al (2021) Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem 158(3):621–639
Carpanini SM, Torvell M, Morgan BP (2019) Therapeutic inhibition of the complement system in diseases of the central nervous system. Front Immunol 10:362
van de Bovenkamp FS et al (2021) Circulating C1q levels in health and disease, more than just a biomarker. Mol Immunol 140:206–216
Dalakas MC, Alexopoulos H, Spaeth PJ (2020) Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol 16(11):601–617
Martin M, Leffler J, Blom AM (2012) Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells. J Biol Chem 287(40):33733–33744
Wang S. et al. (2020) Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer's disease model. J Exp Med 217(9):e20200785. https://doi.org/10.1084/jem.20200785
Ulland TK et al (2017) TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170(4):649-663.e13
Wang Y et al (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160(6):1061–1071
Konishi H, Kiyama H (2018) Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci 12:206
Cosker K et al (2021) Microglial signalling pathway deficits associated with the patient derived R47H TREM2 variants linked to AD indicate inability to activate inflammasome. Sci Rep 11(1):13316
Akkermann R et al (2017) The TAM receptor Tyro3 regulates myelination in the central nervous system. Glia 65(4):581–591
Lemke G, Burstyn-Cohen T (2010) TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci 1209:23–29
Schwanzel-Fukuda M, Pfaff DW (1989) Origin of luteinizing hormone-releasing hormone neurons. Nature 338(6211):161–164
Allen MP et al (2002) Adhesion-related kinase repression of gonadotropin-releasing hormone gene expression requires Rac activation of the extracellular signal-regulated kinase pathway. J Biol Chem 277(41):38133–38140
Bellan M, Pirisi M, Sainaghi PP (2016) The Gas6/TAM System and Multiple Sclerosis. Int J Mol Sci 17(11):1807
Weinger JG et al (2011) Loss of the receptor tyrosine kinase Axl leads to enhanced inflammation in the CNS and delayed removal of myelin debris during experimental autoimmune encephalomyelitis. J Neuroinflammation 8:49
Binder MD et al (2016) Common and low frequency variants in MERTK are independently associated with multiple sclerosis susceptibility with discordant association dependent upon HLA-DRB1*15:01 Status. PLoS Genet 12(3):e1005853
Zheng L et al (2020) Inhibition of TIM-4 protects against cerebral ischaemia-reperfusion injury. J Cell Mol Med 24(2):1276–1285
Chen ZQ et al (2019) Negative regulation of glial Tim-3 inhibits the secretion of inflammatory factors and modulates microglia to antiinflammatory phenotype after experimental intracerebral hemorrhage in rats. CNS Neurosci Ther 25(6):674–684
Kim HS et al (2020) Glial TIM-3 Modulates Immune Responses in the Brain Tumor Microenvironment. Cancer Res 80(9):1833–1845
Choi JS et al (2018) New targets for Parkinson’s disease: adhesion G protein-coupled receptor B1 is downregulated by AMP-activated protein kinase activation. Omics-a J Integr Biol 22(7):493–501
Cork SM, Van Meir EG (2011) Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J Mol Med (Berl) 89(8):743–752
Murayama AY et al (2020) The polymicrogyria-associated GPR56 promoter preferentially drives gene expression in developing GABAergic neurons in common marmosets. Sci Rep 10(1):21516
Chiou B et al (2021) Cell type-specific evaluation of ADGRG1/GPR56 function in developmental central nervous system myelination. Glia 69(2):413–423
Li QS, Sun Y, Wang T (2020) Epigenome-wide association study of Alzheimer’s disease replicates 22 differentially methylated positions and 30 differentially methylated regions. Clin Epigenetics 12(1):149
Alavi MS, Karimi G, Roohbakhsh A (2019) The role of orphan G protein-coupled receptors in the pathophysiology of multiple sclerosis: A review. Life Sci 224:33–40
Baek WY et al (2019) Polymorphisms of MFGE8 are associated with susceptibility and clinical manifestations through gene expression modulation in Koreans with systemic lupus erythematosus. Sci Rep 9(1):18565
Lagos-Cabré R et al (2019) Connexins in astrocyte migration. Front Pharmacol 10:1546
Hong S et al (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352(6286):712–716
Yuan T, Orock A, Greenwood-Van Meerveld B (2021) Amygdala microglia modify neuronal plasticity via complement C1q/C3-CR3 signaling and contribute to visceral pain in a rat model. Am J Physiol Gastrointest Liver Physiol 320(6):1092
Sudom A et al (2018) Molecular basis for the loss-of-function effects of the Alzheimer’s disease-associated R47H variant of the immune receptor TREM2. J Biol Chem 293(32):12634–12646
Bailey CC, DeVaux LB, Farzan M (2015) The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J Biol Chem 290(43):26033–26042
Poliani PL et al (2015) TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest 125(5):2161–2170
Piras S et al (2016) RAGE Expression and ROS generation in neurons: differentiation versus damage. Oxid Med Cell Longev 2016:9348651
Zhang S et al (2020) HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J Neuroinflammation 17(1):15
Teissier T, Boulanger É (2019) The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology 20(3):279–301
Sajithlal G et al (2002) Receptor for advanced glycation end products plays a more important role in cellular survival than in neurite outgrowth during retinoic acid-induced differentiation of neuroblastoma cells. J Biol Chem 277(9):6888–6897
Jones RS et al (2013) Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol 8(1):301–311
Cai Z et al (2016) Role of RAGE in Alzheimer’s disease. Cell Mol Neurobiol 36(4):483–495
Jiang X et al (2018) RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci Lett 672:65–69
Huang L et al (2020) The activation of phosphatidylserine/CD36/TGF-β1 pathway prior to surgical brain injury attenuates neuroinflammation in rats. Oxid Med Cell Longev 2020:4921562
Banesh S, Ramakrishnan V, Trivedi V (2018) Mapping of phosphatidylserine recognition region on CD36 ectodomain. Arch Biochem Biophys 660:1–10
Rawji KS et al (2020) Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol 139(5):893–909
Cusulin C et al (2012) Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells 30(12):2657–2671
Dobri AM et al (2021) CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 453:301–311
Grajchen E et al (2020) CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation. J Neuroinflammation 17(1):224
Kay JG, Grinstein S (2013) Phosphatidylserine-mediated cellular signaling. In: Capelluto DGS (ed) lipid-mediated protein signaling. Springer, New York, pp 177–193
Marinetti GV, Love R (1976) Differential reaction of cell membrane phospholipids and proteins with chemical probes. Chem Phys Lipid 16(4):239–254
Boon JM, Smith BD (2002) Chemical control of phospholipid distribution across bilayer membranes. Med Res Rev 22(3):251–281
Daleke DL (2003) Regulation of transbilayer plasma membrane phospholipid asymmetry (vol 44, pg 233, 2003). J Lipid Res 44(12):2429–2429
Martin OC, Pagano RE (1987) Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process(es). J Biol Chem 262(12):5890–8
Haldar S, Chattopadhyay A (2013) Application of NBD-labeled lipids in membrane and cell biology. In: Mély Y, Duportail G (eds) fluorescent methods to study biological membranes. Berlin Heidelberg, Springer, pp 37–50
Saijo S et al (2006) Discrimination of early and late apoptotic cells by NBD-phosphatidylserine-labelling and time-lapse observation of phagocytosis of apoptotic cells by macrophages. The Journal of Biochemistry 141(3):301–307
Chattopadhyay A, London E (1987) Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry 26(1):39–45
Reutelingsperger CP, van Heerde WL (1997) Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell Mol Life Sci 53(6):527–532
Kang TH et al (2020) Annexin A5 as an immune checkpoint inhibitor and tumor-homing molecule for cancer treatment. Nat Commun 11(1):1137
Peng B et al (2014) Annexin A5 as a potential marker in tumors. Clin Chim Acta 427:42–48
van Genderen HO et al (2008) Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim Biophys Acta 1783(6):953–963
Olofsson A, Mallouh V, Brisson A (1994) Two-dimensional structure of membrane-bound annexin V at 8 A resolution. J Struct Biol 113(3):199–205
Jacob T et al (2002) Modulation of cytosolic Ca(2+) concentration in airway epithelial cells by Pseudomonas aeruginosa. Infect Immun 70(11):6399–6408
Calderon F, Kim HY (2008) Detection of intracellular phosphatidylserine in living cells. J Neurochem 104(5):1271–1279
Liu T et al (2009) Detection of apoptosis based on the interaction between annexin V and phosphatidylserine. Anal Chem 81(6):2410–2413
van Engeland M et al (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31(1):1–9
Kim YE et al (2010) Engineering a polarity-sensitive biosensor for time-lapse imaging of apoptotic processes and degeneration. Nat Methods 7(1):67–73
Li T et al (2021) Phospholipid-flippase chaperone CDC50A is required for synapse maintenance by regulating phosphatidylserine exposure. EMBO J 40(21):15
Kolodgie FD et al (2003) Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V - A technique with potential for noninvasive imaging of vulnerable plaque. Circulation 108(25):3134–3139
Kim H et al (2020) A quenched Annexin V-fluorophore for the real-time fluorescence imaging of apoptotic processes in vitro and in vivo. Adv Sci 7(24):12
Smith BA et al (2011) In vivo targeting of cell death using a synthetic fluorescent molecular probe. Apoptosis 16(7):722–731
Smith BA et al (2012) Multicolor fluorescence imaging of traumatic brain injury in a cryolesion mouse model. ACS Chem Neurosci 3(7):530–537
Chan MM et al (2015) Non-invasive in vivo imaging of arthritis in a collagen-induced murine model with phosphatidylserine-binding near-infrared (NIR) dye. Arthritis Res Ther 17(1):50
Mazzoni F et al (2019) Non-invasive in vivo fluorescence imaging of apoptotic retinal photoreceptors. Sci Rep 9(1):1590
Yeung T et al (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319(5860):210–213
Krappa R et al (1999) Evectins: Vesicular proteins that carry a pleckstrin homology domain and localize to post-Golgi membranes. Proc Natl Acad Sci USA 96(8):4633–4638
Uchida Y et al (2011) Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes. Proc Natl Acad Sci USA 108(38):15846–15851
Hasegawa J et al (2021) A role of phosphatidylserine in the function of recycling endosomes. Front Cell Dev Biol 9:8
Chung J et al (2015) PI4P/phosphatidylserine countertransport at ORP5-and ORP8-mediated ER-plasma membrane contacts. Science 349(6246):428–432
Li YE et al (2021) TMEM41B and VMP1 are scramblases and regulate the distribution of cholesterol and phosphatidylserine. J Cell Biol 220(6):e202103105
Acknowledgements
We would apologize to authors whose relevant research was not cited due to the space limitation.
Funding
This work was sponsored by Basic Research Program of Shanghai (20JC1412200), The National Key Research and Development Program of China (2020YFA0113000, 2018YFA0109800), and National Natural Science Foundation of China (81971324).
Author information
Authors and Affiliations
Contributions
All authors reviewed the literature and wrote first drafts of the respective sections. J.W., J.Z., Y.Z., H.S., and N. W. integrated the sections to form the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Informed Consent
Not applicable.
Consent for Publication
Not applicable.
Institutional Review Board Statement
The authors declare no conflict of interest.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhuang, J., Zhang, Y., Shu, H. et al. Phosphatidylserine in the Nervous System: Cytoplasmic Regulator of the AKT and PKC Signaling Pathways and Extracellular “Eat-Me” Signal in Microglial Phagocytosis. Mol Neurobiol 60, 1050–1066 (2023). https://doi.org/10.1007/s12035-022-03133-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03133-6