Abstract
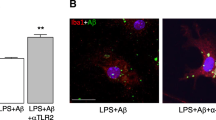
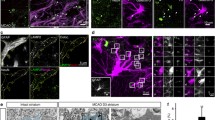
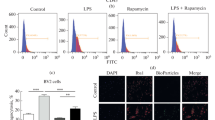
Astrocytes, the most numerous glial cell in the brain, have multiple functions and are key to maintenance of homeostasis in the central nervous system. Microglia are the resident immunocompetent cells in the brain and share several functions with macrophages, including their phagocytic ability. Indeed microglia are the resident phagocytes in the brain and express numerous cell surface proteins which act to enable receptor-mediated phagocytosis. However recent evidence suggests that astrocytes express some genes which permit phagocytosis of phosphatidylserine-decorated cells and this probably explains sporadic reports in the literature which suggest that astrocytes become phagocytic following brain trauma. Here we examined the potential of astrocytes to phagocytose fluorescently-labelled latex beads and amyloid-β (Aβ) and report that they competently engulf both in a manner that relies on actin polymerization since it was inhibited by cytochalasin D. The data indicate that incubation of cultured astrocytes or microglia with Aβ increased phagocytosis and markers of activation of both cell types. Aβ was found to markedly increase expression of the putative Aβ-binding receptors CD36 and CD47 in astrocytes, while it decreased expression of the receptor for advanced glycation endproducts (RAGE). It is demonstrated that blocking these receptors using a neutralizing antibody attenuated Aβ-induced phagocytosis of latex beads by astrocytes. Interestingly blocking these receptors also decreased uptake of beads even in the absence of Aβ. Here we demonstrate that astrocytes are competent phagocytes and are capable of engulfing Aβ.







Similar content being viewed by others
References
al-Ali SY, al-Hussain SM (1996) An ultrastructural study of the phagocytic activity of astrocytes in adult rat brain. J Anat 188(Pt 2):257–262
Alarcon R, Fuenzalida C, Santibanez M, von Bernhardi R (2005) Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem 280(34):30406–30415
Askarova S, Yang X, Sheng W, Sun GY, Lee JC (2011) Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience 199:375–385
Bao Y, Qin L, Kim E, Bhosle S, Guo H, Febbraio M, Haskew-Layton RE, Ratan R, Cho S (2012) CD36 is involved in astrocyte activation and astroglial scar formation. J Cereb Blood Flow Metab 32(8):1567–1577
Bechmann I, Nitsch R (1997) Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia 20(2):145–154
Benveniste EN, Nguyen VT, O’Keefe GM (2001) Immunological aspects of microglia: relevance to Alzheimer's disease. Neurochem Int 39(5–6):381–391
Berbel P, Innocenti GM (1988) The development of the corpus callosum in cats: a light- and electron-microscopic study. J Comp Neurol 276(1):132–156
Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M (2005) Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci 29(3):381–393
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci Official J Soc Neurosci 28(1):264–278
Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C, Zubair AC, Dickson D, Golde TE, Das P (2010) Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J Official Pub Fed Am Soc Experiment Biol 24(2):548–559
Downer EJ, Cowley TR, Lyons A, Mills KH, Berezin V, Bock E, Lynch MA (2010) A novel anti-inflammatory role of NCAM-derived mimetic peptide, FGL. Neurobiol Aging 31(1):118–128
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13(4):432–438
Floden AM, Combs CK (2006) Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. J Neurosci 26(17):4644–4648
Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, Abraham E (2011) Participation of the receptor for advanced glycation end products in efferocytosis. J Immunol 186(11):6191–6198
Gitik M, Liraz-Zaltsman S, Oldenborg PA, Reichert F, Rotshenker S (2011) Myelin down-regulates myelin phagocytosis by microglia and macrophages through interactions between CD47 on myelin and SIRPalpha (signal regulatory protein-alpha) on phagocytes. J Neuroinflammation 8:24
Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M (2009) Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci 12(11):1361–1363
Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL (2006) Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203(12):2613–2625
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci Official J Soc Neurosci 28(33):8354–8360
Hofer U, Lehmann AD, Waelti E, Amacker M, Gehr P, Rothen-Rutishauser B (2009) Virosomes can enter cells by non-phagocytic mechanisms. J Liposome Res 19(4):301–309
Kim S, Park SY, Kim SY, Bae DJ, Pyo JH, Hong M, Kim IS (2012) Cross talk between engulfment receptors stabilin-2 and integrin alphavbeta5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol Cell Biol 32(14):2698–2708
Koenigsknecht J, Landreth G (2004) Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci Official J Soc Neurosci 24(44):9838–9846
Koenigsknecht-Talboo J, Landreth GE (2005) Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci Official J Soc Neurosci 25(36):8240–8249
Lantos PL (1974) An electron microscope study of reacting astrocytes in gliomas induced by n-ethyl-n-nitrosourea in rats. Acta Neuropathol 30(2):175–181
Lee CY, Landreth GE (2010) The role of microglia in amyloid clearance from the AD brain. J Neural Transm 117(8):949–960
Lewandowska E, Wierzba-Bobrowicz T, Kosno-Kruszewska E, Lechowicz W, Schmidt-Sidor B, Szpak GM, Bertrand E, Pasennik E, Gwiazda E (2004) Ultrastructural evaluation of activated forms of microglia in human brain in selected neurological diseases (SSPE, Wilson’s disease and Alzheimer’s disease). Folia Neuropathol 42(2):81–91
Lindberg C, Selenica ML, Westlind-Danielsson A, Schultzberg M (2005) Beta-amyloid protein structure determines the nature of cytokine release from rat microglia. J Mol Neurosci 27(1):1–12
Loov C, Hillered L, Ebendal T, Erlandsson A (2012) Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS One 7(3):e33090
Lynch AM, Murphy KJ, Deighan BF, O’Reilly JA, Gun’ko YK, Cowley TR, Gonzalez-Reyes RE, Lynch MA (2010) The impact of glial activation in the aging brain. Aging Dis 1(3):262–278
Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE (2009) Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci Official J Soc Neurosci 29(13):4252–4262
Mandrekar-Colucci S, Karlo JC, Landreth GE (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a Murine model of Alzheimer’s disease. J Neurosci Official J Soc Neurosci 32(30):10117–10128
Mapes J, Chen YZ, Kim A, Mitani S, Kang BH, Xue D (2012) CED-1, CED-7, and TTR-52 regulate surface phosphatidylserine expression on apoptotic and phagocytic cells. Curr Biol CB 22(14):1267–1275
Matozaki T, Murata Y, Okazawa H, Ohnishi H (2009) Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 19(2):72–80
Mizwicki MT, Menegaz D, Zhang J, Barrientos-Duran A, Tse S, Cashman JR, Griffin PR, Fiala M (2012) Genomic and nongenomic signaling induced by 1alpha,25(OH)2-vitamin D3 promotes the recovery of amyloid-beta phagocytosis by Alzheimer’s disease macrophages. J Alzheimer’s Dis JAD 29(1):51–62
Nguyen JV, Soto I, Kim KY, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CH, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N (2011) Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A 108(3):1176–1181
Noda M, Suzumura A (2012) Sweepers in the CNS: microglial migration and phagocytosis in the Alzheimer disease pathogenesis. Int J Alzheimer’s Dis 2012:891087
Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M (2008) Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer’s disease. Glia 56(2):154–163
Seidel K, Vinet J, Dunnen WF, Brunt ER, Meister M, Boncoraglio A, Zijlstra MP, Boddeke HW, Rub U, Kampinga HH, Carra S (2012) The HSPB8-BAG3 chaperone complex is upregulated in astrocytes in the human brain affected by protein aggregation diseases. Neuropathol Appl Neurobiol 38(1):39–53
Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O’Banion MK (2007) Sustained hippocampal IL-1beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest 117(6):1595–1604
Sick E, Boukhari A, Deramaudt T, Ronde P, Bucher B, Andre P, Gies JP, Takeda K (2011) Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia 59(2):308–319
Simard AR, Soulet D, Gowing G, Julien JP, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49(4):489–502
Streit WJ, Braak H, Xue QS, Bechmann I (2009) Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 118(4):475–485
Van Amersfoort ES, Van Strijp JA (1994) Evaluation of a flow cytometric fluorescence quenching assay of phagocytosis of sensitized sheep erythrocytes by polymorphonuclear leukocytes. Cytometry 17(4):294–301
Veerhuis R, Boshuizen RS, Morbin M, Mazzoleni G, Hoozemans JJ, Langedijk JP, Tagliavini F, Langeveld JP, Eikelenboom P (2005) Activation of human microglia by fibrillar prion protein-related peptides is enhanced by amyloid-associated factors SAP and C1q. Neurobiol Dis 19(1–2):273–282
Wilkinson K, El Khoury J (2012) Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimer’s Dis 2012:489456
Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9(4):453–457
Zotova E, Holmes C, Johnston D, Neal JW, Nicoll JA, Boche D (2011) Microglial alterations in human Alzheimer’s disease following Abeta42 immunization. Neuropathol Appl Neurobiol 37(5):513–524
Acknowledgments
This work was funded by Science Foundation Ireland (07/IN.1/B949). RSJ was supported by a Health Research Board-funded structured PhD program in Neuroscience (PhD/2008/13). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, R.S., Minogue, A.M., Connor, T.J. et al. Amyloid-β-Induced Astrocytic Phagocytosis is Mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol 8, 301–311 (2013). https://doi.org/10.1007/s11481-012-9427-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-012-9427-3