Abstract
The identification of causative mutations in the (pro)granulin gene (GRN) has been a major breakthrough in the research on frontotemporal dementia (FTD). So far, all FTD-associated GRN mutations are leading to neurodegeneration through a “loss-of-function” mechanism, encouraging researchers to develop a growing number of cellular and animal models for GRN deficiency. GRN is a multifunctional secreted growth factor, and loss of its function can affect different cellular processes. Besides loss-of-function (i.e., mostly premature termination codons) mutations, which cause GRN haploinsufficiency through reduction of GRN expression, FTD-associated GRN missense mutations have also been identified. Several of these missense mutations are predicted to increase the risk of developing neurodegenerative diseases through altering various key biological properties of GRN-like protein secretion, proteolytic processing, and neurite outgrowth. With the use of cellular and animal models for GRN deficiency, the portfolio of GRN functions has recently been extended to include functions in important biological processes like energy and protein homeostasis, inflammation as well as neuronal survival, neurite outgrowth, and branching. Furthermore, GRN-deficient animal models have been established and they are believed to be promising disease models as they show accelerated aging and recapitulate at least some neuropathological features of FTD. In this review, we summarize the current knowledge on the molecular mechanisms leading to GRN deficiency and the lessons we learned from the established cellular and animal models. Furthermore, we discuss how these insights might help in developing therapeutic strategies for GRN-associated FTD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frontotemporal dementia (FTD) predominantly affects people below the age of 65 years. The average age-at-onset varies between 45 and 65 years with a mean onset age in the 1950s [1]. There is no apparent gender preference and depending on the study, the prevalence varies from 10 to 20/100,000 inhabitants [2]. Therefore, FTD is nowadays recognized as the second most common form of presenile dementia after Alzheimer disease (AD). Clinically, patients with FTD show progressive behavioral changes, language impairment, and/or executive dysfunction caused by an atrophy of the prefrontal and anterior temporal neocortex [3]. Pathologically, most of the patients are characterized by the presence of cellular inclusions of either the microtubule-associated protein tau or TAR DNA-binding protein-43 (FTD-TDP) in affected brain regions, leaving 10–15 % of patients with a different underlying pathological inclusion protein [4]. The number of FTD patients with a positive family history is high (40–50 %) with just over 10 % of these familial patients belonging to families with an autosomal dominant inheritance of disease [5]. Although recent genetic breakthroughs have increased the number of identified genetic causes for FTD, still >60 % of patients with familial FTD cannot be explained by a mutation in one of the currently known causative genes [6].
In this review, we focus on FTD-TDP caused by mutations in the (pro)granulin gene (GRN) and the recent progress realized through the generation and characterization of GRN-deficient cellular and animal models. We discuss the insights in the molecular mechanisms underlying GRN deficiency generated by these models and how current knowledge might be used to design potential therapeutic strategies for GRN-related FTD-TDP.
Genetics
In the mid-1990s, segregation studies in autosomal dominant families suffering from FTD with parkinsonism linked to chromosome 17 provided the first evidence of a genetic cause located in the chromosomal region 17q21 [7, 8]. A few years later, extensive mutation analyses resulted in the identification of mutations in MAPT, a gene located on chromosome 17q21 and encoding the microtubule associated protein tau [9–11]. Today, 44 MAPT mutations have been reported in 134 families including deletions, intronic splice site, and missense mutations [12, 13]. Nevertheless, in a large number of autosomal-dominant FTD patients linked to 17q21, no MAPT mutations could be identified. Systematic genetic studies in the 17q21 region ultimately led to the identification of mutations in another gene, the (pro)granulin gene (GRN) located proximate to MAPT and coding for a multifunctional growth factor [14, 15], Today, nearly half of the familial FTD patients can be explained by mutations in MAPT and GRN [16].
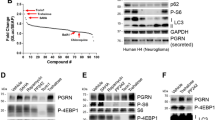
A total of 69 pathogenic GRN mutations have been reported in patients with FTD and related disorders [12, 17], the majority of which are loss-of-function mutations, unambiguously suggesting that GRN haploinsufficiency is at the basis of the disease pathogenesis. Loss of GRN can be achieved at several levels, either affecting the gene itself, the expression of its transcript or protein [18], the transport, stability, or processing of the mature protein (Fig. 1).
Overview of GRN loss-of-function mechanisms and their functional consequences. Loss of GRN can occur on the transcriptional (a, c), genomic (b), and translational level (d, e). Posttranscriptional mechanisms include cytosolic missorting (e), inefficient secretion (f), altered proteolytic processing into individual GRN peptides (i), and potentially also regulation of transcription via binding to cyclin T1 (g). GRN missense mutations can affect its neurotrophic properties (h) and eventually affect the pro-inflammatory response initiated by TNF-α through alteration of the binding affinity of GRN to the TNF receptor (j). Mutations in GRN might also affect binding of GRN to sortilin (k) thereby influencing the levels of extracellular GRN or inflammatory signaling cascades stimulated by CpG-DNA (l). SP signal peptide, NMD nonsense-mediated decay, PTC premature termination codon
Most mutations are nonsense or frameshift mutations introducing a premature termination codon (PTC) followed by degradation of the mutant transcript by nonsense-mediated mRNA decay (NMD; Fig. 1a; reviewed in [19]). Other mutations lead to genomic deletion of one whole copy of the gene (Fig. 1b) [20, 21], affect a splice donor site (Fig. 1c) resulting in the inclusion of the nuclear retention signal and degradation of the mutant transcript in the nucleus [15, 22, 23] or destroy the translation initiation codon preventing translation (Fig. 1d) [14, 15, 24, 25].
Together, all loss-of-function mutations in GRN explain 5–10 % of all FTD patients and near 25 % if the FTD patients have a positive family history of disease [17]. Besides classical loss-of-function mutations, 52 missense mutations have also been described. Twenty-six of those were only observed in patients suggesting their association with FTD and indicate a potential pathogenic effect [12]. The identified missense mutations are scattered over the entire GRN protein (Fig. 2) indicating that they either affect the function of the GRN precursor protein [17] or its proteolysis into functional granulin peptides.
Schematic representation of GRN showing the distribution of GRN missense variants within the GRN precursor protein. Black, missense mutations detected in patients; gray, missense mutations detected in patients and/or controls; red, missense mutations with functional evidence of their pathogenicity; blue asterisk, predicted glycosylation sites. SP signal peptide, aa amino acid
In addition to the chromosome 17q21 locus, another locus on chromosome 9 has been linked in autosomal dominant families to FTD often associated with motor neuron disease [26], and was confirmed by many research groups [25, 27–30]. Nevertheless, it took more than 10 years of collaborative efforts to identify a pathological hexanucleotide repeat expansion in the regulatory region of the C9orf72 gene explaining the linkage to chromosome 9 [6, 31, 32]. In some FTD cohorts, a mutation frequency of 6 % with expansions in C9orf72 was observed, similar to the GRN mutation frequency (7 %) [6], identifying C9orf72 as another major genetic cause of FTD. Functional analyses showed that the repeat expansion reduced mRNA expression in mutation carriers suggesting C9orf72 haploinsufficiency [6, 31]. Conversely, nuclear RNA foci positive for C9orf72 were also detected [31] pointing to multiple biological mechanisms that might contribute to disease in C9orf72 repeat expansion carriers. Rare mutations in the genes coding for the TAR DNA-binding protein 43 (TDP-43 or TARDBP), valosin-containing protein (VCP) and charged multivesicular body protein 2B (CHMP2B) also contribute to the FTD genetic etiology (reviewed in [33]).
To explore if potential genetic susceptibility factors might contribute to FTD, genome-wide association (GWA) studies were used [34]. The first GWA study was performed on FTD patients with confirmed TDP-43 neuropathology or clinical diagnosed FTD patients carrying mutations in GRN that predict TDP-43 pathology. This resulted in the identification of the first FTD risk gene, TMEM106B, coding for a type 2 transmembrane protein [35, 36]. Carriers of pathogenic GRN mutations showed the strongest association with TMEM106B pointing to a possible disease modifying effect of TMEM106B in GRN-associated FTD carriers (reviewed in [37]). Initial expression studies suggested a positive correlation with TMEM106B mRNA expression [36]; however, this could not be observed in clinically diagnosed FTD patients [38]. Until now, the functional biological link between GRN, TMEM106B and TDP-43 remains unclear. So far, one study showed a significant correlation of TMEM106B and GRN levels in plasma [39], and altered expression of microRNAs (miRNA) from the miRNA-132 cluster has been suggested to influence TMEM106B expression levels in FTD patients [40]. However, until now cell culture experiments using TMEM106B overexpression or knockdown showed no consistent effects on GRN levels [35, 40, 41]. Another GWA study indicated an association of FTD with the chromosomal region 1p13 near the sortilin gene as a regulator of plasma GRN levels [42]. Furthermore, on-going efforts using next-generation sequencing technologies might discover additional genetic causal and risk factors contributing to the FTD etiology. Increasing knowledge of the genetic etiology of FTD will likely increase our understanding of the underlying disease mechanisms and might help directing future functional biological studies to unravel disease pathways.
Clinical and Neuropathological Characteristics
FTD patients carrying a GRN mutation clinically present with a large phenotypic variability even within one family segregating the same mutation [43–45]. Despite this variability, patients generally present with behavioral changes, including apathy and social withdrawal as the most prominent clinical symptoms (reviewed in [46]). Some GRN mutation carriers also show clinical symptoms characteristic for AD, Parkinson disease (PD) [22], progressive nonfluent aphasia [47] and corticobasal syndrome [48]. Even some clinical overlap with psychiatric disorders was recently described [49]. Due to this clinical heterogeneity and because symptoms can change over time, it can be a challenging task for neurologists to provide patients with the precise clinical diagnosis. The age at onset in GRN mutation carriers is also highly variable and ranges from 35 to 89 years with a mean onset of around 60 years [46]. The penetrance of GRN mutations is incomplete with approximately 50 % carriers affected at age 60 and 90 % at 70 years [24]. Patients with GRN-associated FTD have considerable frontal atrophy but also temporoparietal atrophy is associated with GRN mutations [50]. Mostly, asymmetric distributed hemispheric atrophy can be observed in GRN mutation carriers and based on the asymmetric pattern, neuroimaging can differentiate GRN and MAPT mutation carriers [51]. Further studies also showed that FTD patients with a GRN mutation have a faster rate of whole brain atrophy than patients with a MAPT mutation resulting in smaller brain volumes in the GRN carrier group [51, 52]. Interestingly, a study reported potential compensatory mechanisms of brain plasticity in both presymptomatic GRN mutation carriers with normal cognitive and behavioral performances as in FTD patients with GRN mutations [53].
Neuropathologically, GRN-associated FTD is predominantly characterized by neuronal and glial cytoplasmic and/or lentiform intranuclear inclusions (NCI or NII, respectively) in the affected cortical regions, which are immunoreactive to ubiquitin and TDP-43 but not to tau and alpha-synuclein [54]. Other consistent neuropathological features caused by GRN deficiency are extensive astrogliosis, loss of myelin in the underlying white matter, hippocampal sclerosis and irregular dystrophic neurites [46]. Biochemically, GRN-associated FTD is characterized by the accumulation of abnormally phosphorylated TDP-43 and both TDP-43 full-length and C-terminal fragments (CTFs) are recovered in detergent insoluble urea fractions from affected brain regions [55–57]. The exact mechanism how GRN haploinsufficiency is linked to the generation of pathological TDP-43 is still not completely understood, but evidence is accumulating that GRN deficiency reduces the efficiency of cellular degradation processes and in turn increases the general susceptibility towards cellular stressors [58].
Progranulin: A Multifunctional Protein
Gene and Protein
In humans, the gene coding for GRN is located on chromosome 17 at cytogenetic band 17q21 and comprises 12 exons [59] (Fig. 3). GRN is coding for a 593 amino acid long secreted protein with a predicted molecular weight of 68.5 kDa. GRN contains a signal sequence and seven and a half tandem repeats of a unique 10–12 cysteine-containing motif called granulin domains, a modular organization resembling the precursor of the epidermal growth factor [60]. During maturation of GRN the signal peptide gets cleaved off and mature GRN is secreted as a glycosylated full-length protein with an apparent molecular weight of 90 kDa, which can undergo proteolysis resulting in the generation of individual granulin peptides [60].
The mouse homolog to human GRN (Grn) is located on chromosome 11 [61], and both mouse and rat Grn code for a 589-amino acid protein displaying 75 % overall identity and a similar modular structure [62]. Each granulin domain is encoded by two neighboring exons suggesting the formation of hybrid granulin-like proteins by alternative splicing [59]. Possible alternative spliced transcripts have been observed in some cell lines [63], however, their expression in vivo and thus biological relevance are still unknown. Several independent research groups have purified and characterized individual granulin peptides from different cells and organisms and named them granulins [64] or epithelins [65] due to their association with granulocytes or epithelial cells. The diverse functions of GRN are already reflected in the different designations used in the literature, i.e., proepithelin, GRN, granulin-epithelin precursor, acrogranin, 88 kDa glycoprotein, epithelial transforming growth factor, or PC cell-derived growth factor [66–69]. In this review paper, we use the official designated nomenclature for the human (GRN and GRN) and murine (Grn and Grn) gene and protein, respectively (http://www.genenames.org/).
Gene and Protein Expression
Gene expression can be detected throughout the whole body but GRN is predominantly expressed in epithelial and hematopoietic cells [60, 62]. Among the various analyzed tissues, protein expression was particularly high in spleen, placenta, and kidney [60, 70]. In the central nervous system (CNS), GRN is expressed both in neurons and microglia but not in astrocytes as observed in transgenic knock-in mice expressing a reporter gene from the Grn locus [71]. Two additional studies evaluating GRN mRNA and protein distribution, showed GRN expression in various brain areas including the cingulate and piriform cortices, the pyramidal cell layer and dentate gyrus of the hippocampus, the amygdala, the ventromedial, and arcuate nuclei of the hypothalamus and the Purkinje cell layer in the cerebellum [72, 73]. Expression of Grn mRNA in the hypothalamus is inducible by androgens suggesting an involvement of Grn in the masculinization of the brain [74–76]. GRN expression can also be regulated by miRNAs [77–80], which are posttranscriptional regulators of gene expression, and brains of GRN associated FTD patients show significant alterations in miRNA expression [40, 81]. Furthermore, increased methylation of the GRN promoter has recently been suggested to correlate with GRN expression in peripheral blood mononuclear cells [82] and further studies are awaited evaluating whether GRN promoter methylation can also be correlated with GRN expression in brains of FTD patients. In microglia, GRN expression is largely increased upon activation whereas in neurons it increases during maturation [71]. Although baseline levels of GRN expression in astrocytes were below the detection limit in most studies, stimulation with Toll-like receptor (TLR) ligands and Th1 cytokines led to significant upregulation of GRN expression in human primary astrocytes [83].
The reports on the expression of GRN in the brain during normal aging are contradictory. One study, analyzing mice up till 12 months of age, reported decreased Grn mRNA levels in an age-dependent manner in the hippocampus and hypothalamus as well as in the cerebral cortex [73], suggesting important functional consequences for the early pathogenesis of GRN-associated FTD. In contrast, a meta-analysis of a large set of expression arrays reported increased GRN mRNA levels in association with aging in a large variety of tissues including cortex and hippocampus [84]. It is possible though that region-specific reduction of GRN expression contributes to disease pathogenesis and the observed increases of GRN mRNA expression with age might reflect increased microglia reactivity due to chronic low-level neuroinflammation, a feature observed also in the normal aged brain [85].
GRN Protein Structure
Dissecting the protein structure of human GRN by high-resolution NMR shows that three of the granulin peptides (granulin A, C, and F) contain relatively well-defined three-dimensional structures in solution with a stable stack of two β-hairpins in their N-terminal subdomains [86]. This is in accordance with the previously reported more rigid stacked β-hairpin granulin fold of crap granulin A [87]. In contrast, the C-terminal subdomain of the granulins seems to be more flexible [86]. While granulin A, C and F represent well-folded peptides, the residual granulin peptides (granulins B, D, E, and G) exist as poorly structured disulfide isomers [86]. Whether and how these structural differences are responsible for the biological activity of the individual granulins or even the GRN protein, requests systematic analysis in the future.
GRN as a Mitogen and Neurotrophic Protein
Since the initial reports on the identification of GRN, a large body of literature has accumulated describing the mitogenic or inhibitory effects of GRN and its proteolytically cleaved granulins on various cell types (for a recent review, see [88]). Further evidence for the activity of GRN as an important growth factor came from the numerous oncological studies reporting increased GRN expression as a negative prognostic factor in many different cancers [89]. The effect of GRN on neuronal cells is less well described. Recently, extracellular administration of GRN was reported to stimulate neurite outgrowth in cultured motor and cortical neurons [90], demonstrating for the first time the neurotrophic properties of GRN. In similar experiments, the putative loss-of-function outcome of some FTD-associated GRN missense mutations could be demonstrated [91–93]. Moreover, GRN was able to increase neuronal survival [90] and to protect neurons from neuronal apoptosis caused by Grn deficiency [94] or by toxic insults [95], suggesting that GRN is a neuronal survival factor. Although granulin E showed similar neurotrophic and neuroprotective properties, it would not be surprising if some of the other granulins would have opposing effects, as it was observed on other cell types [86]. Further studies are awaited to determine the actual contribution of the individual granulins on neuronal homeostasis.
GRN Signaling
GRN is thought to exert its mitogenic effect through the stimulation of both the mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase pathways [96–99]. In neurons, GRN was shown to stimulate the phosphorylation of Akt [91, 94, 95] and glycogen synthase kinase (GSK)-3β [91, 95], while the effect on the MAPK pathway was not consistently observed [91, 94, 95]. Furthermore, GRN also affects insulin signaling in adipocytes downstream of the insulin receptor [100], is the only growth factor that can stimulate cells deficient for the insulin-like growth factor 1 (IGF-1) receptor [101] and can substitute for IGF-1 signaling in the regulation of muscle growth [102]. Initial attempts to find the GRN cell surface receptor by chemical crosslinking techniques resulted in the identification of two classes of binding sites on epithelial cells and fibroblasts: a high-affinity site with a relatively low number of receptors per cell and a low-affinity site with a larger number of receptors per cell [103, 104]. One study estimated a size of ≈120 kDa for the receptor [104], while another study reported the interaction of GRN with a receptor of ≈170–175 kDa [105].
Sortilin, a 100-kDa type-1 membrane receptor involved in lysosomal targeting [106] was identified as a high-affinity neuronal receptor of GRN [107]. Sortilin facilitates rapid endocytosis and delivery of GRN to the lysosomes through binding of the GRN C terminus to the beta-propeller region of sortilin [108] and thereby regulating extracellular levels of GRN [42, 107]. Sortilin not only regulates the release of pro-neurotrophins (proNT), but is also implicated, together with p75NTR, in regulating signal transduction by proNTs [109]. A study using hippocampal neurons from sortilin knockout mice showed that the neurotrophic effect of GRN is independent of sortilin since GRN stimulated neurite outgrowth was not affected in these neurons [92]. Hence, the identification of additional cell surface receptors for GRN will be a major step forward in understanding GRN-linked signaling events.
GRN and Inflammation
Both the human and murine promoter contain several regulatory cis-elements that are possibly involved in cytokine and growth factor regulated transcriptional gene expression [110], and the involvement of GRN in inflammatory processes has been well described [111–115]. GRN has a function during wound healing, where it increases the accumulation of neutrophils, macrophages, blood vessels, and fibroblasts in the wound [111]. Mediation of the inflammatory response involves proteolytical processing of anti-inflammatory GRN into pro-inflammatory granulins by the serine proteases neutrophil elastase and proteinase 3 [112, 115]. Some metalloproteinases (MMP-9, MMP-12, MMP-14, and ADAMTS-7) also show substrate specificity for GRN [83, 116–118] and can act as GRN convertases. Secretory leukocyte protease inhibitor (SLPI) is known as a natural regulator of the proteolytic process through binding to GRN and inhibiting the elastase-mediated GRN proteolysis [115]. Accordingly, SLPI-deficient mice show impaired wound healing, most likely due to a reduction of Grn caused by increased elastase activity [119]. Subcutaneous administration of GRN completely restored the proper wound healing process in SLPI-deficient mice [115]. Furthermore, GRN was described as a potent inhibitor of the inflammatory cytokine tumor necrosis factor alpha (TNF-α) signaling [115, 120], whereas individual granulins A and B are thought to be pro-inflammatory through the induction of the pro-inflammatory cytokines interleukin-8, TNF-α, and interleukin-1b expression [115, 121]. A further role for GRN as a soluble co-factor for the delivery of CpG-oligonucleotides to TLR 9 was described [122], but how exactly CpG-oligonucleotide-bound GRN meets TLR9 in the endolysosomal compartment is not known. One could speculate that binding of GRN to sortilin and subsequent endocytosis might be involved in this process [123].
In the CNS, GRN can act as a chemoattractant to recruit and/or activate microglia followed by increased endocytosis of extracellular peptides such as amyloid beta [124]. Increased GRN expression, especially in activated microglia, is a consistent feature of human neurodegenerative conditions [125–127] including FTD caused by GRN haploinsufficiency [14, 15, 128]. Grn was also among the top upregulated molecules around amyloid plaques in AD mouse models [129], and in activated microglia of models of motor neuron degeneration [130–132].
Inflammatory mediators like TNF-α and TGF-β are increased in the cerebrospinal fluid (CSF) but not in serum of patients with FTD [133], and FTD patients with GRN mutations have significantly higher circulating levels of IL-6 compared with FTD patients without a GRN mutation or control individuals [134]. Similarly, Grn knockout mice (Grn −/−) responded with an exaggerated production of pro-inflammatory cytokines upon LPS stimulation and delayed recovery of bacterial infections [114], suggesting that the GRN-mediated neurodegeneration could be a result of cumulative damage through deregulation of inflammation. This is in line with the sustained neuroinflammatory processes that contribute to neurodegenerative diseases such as AD [135] and PD [136].
GRN in Energy Homeostasis
Obesity and aging of the human population are two key concerns worldwide with a large social, medical, and economic impact. A link between neurodegenerative diseases and obesity has been suggested and a number of studies associate obesity with cognitive decline and enhanced vulnerability to brain injury [137]. Insulin resistance and type 2 diabetes are associated with the pathogenesis and pathophysiology of some human neurodegenerative diseases [138], and deficits in insulin signaling lead to hyperphosphorylation of tau [139]. In addition, increased GRN serum levels were linked to type 2 diabetes and physical training could significantly reduce GRN levels by about 20 % in these patients [140]. Furthermore, renal function has also been suggested to significantly affect GRN serum levels [141]. Increased GRN levels were also associated with insulin resistance in obese individuals, which had also a tendency for higher IL-6 and MCP-1 serum concentrations [142].
Mouse models of obesity also showed increased Grn levels in blood and adipose tissues, which could be normalized by the insulin-sensitizing agent pioglitazone [100]. High fat diet leads to insulin resistance through the induction of IL-6 and Grn was shown to be a key mediator of this process [100]. Extracellular administration of GRN results in impaired insulin signaling downstream of the insulin receptor and leads to insulin resistance, whereas Grn deficiency enhances insulin sensitivity resulting in reduced deposition of peritoneal fat in GRN-deficient mice [100]. In the hypothalamus, Grn is involved in glucose sensing and GRN levels have been inversely correlated with appetite and food intake [143] and behavioral changes in FTD patients, including GRN mutation carriers, include overeating as well as a preference for sweet food [16]. These studies suggest important functions of GRN as an adipokine and one might hypothesize that disturbances in energy homeostasis could contribute to precipitate neurodegeneration in GRN-associated FTD.
GRN Loss-of-Function Mechanisms
Besides classical loss-of-function mutations, mutations leading to non-synonymous amino acid substitutions have also been described and some of them are predicted in silico to affect protein function [17]. Due to the multifunctional character of GRN, the functional consequences of these missense mutations might be widespread and subtler. Some GRN missense mutations are potentially causing a partial haploinsufficiency by affecting protein translation, sorting and GRN secretion (Fig. 1e, f). The first identified missense mutation (p.A9D) was located in the GRN signal peptide [144], and functional analyses showed that the mutant protein was not secreted due to cytoplasmic missorting [145, 146]. Two other missense mutations (p.P248L and p.R432C) affected protein secretion and stability and potentially reduce the amount of available GRN [146]. Intracellular GRN has been shown to bind to cyclin T1 in the cytoplasm and blocking its translocation to the nucleus (Fig. 1g). This interference with the assembly of functional pTEFb complexes leads to inhibition of transcription from cellular promoters like the cad and c-myc promoter [147, 148]. It would therefore be interesting to investigate if GRN missense mutations affect gene transcription by altering cyclin T1 binding, ultimately resulting in a distinct molecular phenotype similar to that observed in brains of GRN-associated FTD patients [149]. Furthermore, missense mutations can affect the neurotrophic properties of GRN (Fig. 1h) as indicated by the reduced ability of mutant GRN to stimulate neurite outgrowth and neuronal survival [91, 93]. The exact mechanism is unknown but it was suggested that missense mutations might affect proper receptor-ligand interaction due to conformational changes in the protein [93]. Serine proteases like neutrophil elastase or proteinase 3 are involved in converting GRN into granulins (Fig. 1i) [112, 115], a process that is important during inflammatory conditions. A set of missense mutations, especially those affecting highly conserved cysteine residues (p.C139R and p.C521Y), were shown to interfere with proper proteolytic GRN processing [93]. This eventually affects the course of an inflammatory response and thus might contribute to neurodegenerative disease. It is also intriguing to consider that missense mutations in GRN might affect the regulation of inflammatory processes by affecting the ability of GRN to compete with TNF-α for its cognate receptors [120, 150] and thus attenuating the inflammatory reaction (Fig. 1j). A recombinant GRN peptide called antagonist of TNF/TNFR signaling via targeting to TNF receptors (ATSTTRIN) that includes parts of granulins F, A, and C, proved even more potent than GRN in attenuating the response to TNF-α [120]. Of note is that eight GRN missense mutations affect amino acids inside this region [12]. Moreover, it would also not be surprising if some missense mutations would affect binding of GRN to sortilin (Fig. 1k) and by this either alter the levels of extracellular GRN or influence the innate immune response by affecting the, until now hypothetical, model of CpG-oligonucleotide delivery to TLR9 in the endosomes (Fig. 1l) [122, 123]. Detailed information on how GRN missense variants affect GRN function and/or processing is still scarce and therefore further functional studies will be tremendously important to group the missense mutations into functional clusters depending on the biological process they are affecting.
Modeling Progranulin Loss in Cells
Keeping the balance of bioavailable GRN seems to be crucial for maintaining cellular homeostasis as both GRN overexpression and deficiency are linked to the development and progression of cancers and neurodegeneration, respectively. Increased GRN levels are correlated with significantly increased tumorigenicity in several types of cancer and reducing the levels by RNA interference or neutralizing antibodies generally reduces cell proliferation and tumorigenicity [151]. Downregulation of GRN expression causes alterations in cell cycle progression due to reduction of cyclin D1, CDK4, and alpha-tubulin [152–154] and leads to caspase-mediated apoptosis or increased susceptibility to it, depending on the cell type [91, 94, 155–158].
Following the identification of GRN loss-of-function mutations in FTD patients [14, 15], and the proposed role of GRN as a modifying factor in other neurodegenerative diseases [159, 160], the neuroscience community has put increasing efforts in understanding and characterizing the role of GRN in the CNS. One of the key questions to answer is how GRN deficiency can lead to neurodegeneration. GRN is widely expressed in the CNS [72, 73] and its expression and secretion was confirmed in neuronal progenitor cells (NPCs), cultured primary neurons, motor neuron cell lines, and neurons derived from induced pluripotent stem (iPS) cells [94, 161–164], making those valuable tools to further study GRN function. The addition of exogenous GRN proved beneficial for neurite outgrowth and neuronal survival [90] and supported neuronal survival of motor neuron cell lines even under serum deprivation [164]. Both GRN and granulin E showed neurotrophic properties in vitro [90, 92] and three studies using mutant GRN proteins showed that the missense mutations interfere with the neurotrophic functions of GRN [91–93]. However, the exact mechanism how GRN promotes neurite outgrowth and neuronal survival is still not completely understood. The first study using neurons derived from iPS cells, generated from an FTD patient with a GRN nonsense mutation, suggests defects of GRN-deficient neurons in the PI3K/Akt and MAPK signaling pathways [161]. Furthermore, GRN preferentially activates the PI3K/Akt signaling pathway in cortical neurons derived from Grn −/− mice and Grn deficiency in these cells leads to a subtle reduction in phosphorylated Akt [94]. In NPCs [162] and primary neurons [91] GRN stimulated GSK3-Beta (GSK3β) phosphorylation, which could be abolished by PI3K inhibitors [162], supporting the involvement of the PI3K/Akt pathway in GRN-mediated survival signaling. While GRN stimulation results in significantly increased neurite outgrowth, siRNA-mediated GRN knockdown has the opposite effect [165] and causes impaired retinoic acid-induced neuronal differentiation of neuroblastoma cells possibly through reduced phosphorylation of GSK3β [91]. Together these data suggest an essential role of PI3K/Akt signaling and regulation of GSK3β in GRN-mediated neuronal integrity. GSK3β is known to be critically involved in the canonical Wnt-signaling pathway [166], but a recent study using inducible GRN knockdown in human NPCs also identified a major adaptive role for the noncanonical Wnt signaling pathway in GRN-associated FTD [163]. The amount of data and their functional impact further strengthen the validity of using such cellular models to find key molecular mechanisms that are affected by GRN deficiency.
Proper neuronal connectivity is crucial for maintaining brain homeostasis and Grn knockdown [165] or knockout [167] in primary hippocampal cultures has been associated with reduced neural connectivity. In these experiments, Grn loss led to decreased neuronal arborization and length as well as spine and synapse density [165, 167], which could be responsible for alterations in the synaptic output. Although Grn knockdown resulted in a significant reduction in synapse density, the number of synaptic vesicles per synapse was increased, a phenomenon also observed in postmortem brain sections of GRN-associated FTD patients [165]. In this study, the authors also reported an increased frequency of spontaneous glutamatergic transmission upon Grn knockdown in hippocampal neurons [165], thereby supporting the theory that GRN deficiency increases the probability of release at remaining synapses due to increased vesicle density. This might be directly caused by GRN deficiency or could be reflecting a compensation of the reduced number of synapses [165]. However, in hippocampal slice cultures the release probability was comparable between slices from Grn −/− and wild-type (wt) mice, supporting the theory that the decreased synaptic output might rather be caused by the reduced number of functional synapses [167]. Further studies are highly encouraged as the observed synaptic dysfunction significantly precedes most indications of neuropathological changes in this model [167].
Loss of GRN is ultimately associated with degeneration of cortical and hippocampal neurons [168] and the first cellular studies are highlighting the important role of GRN expression on neuronal survival. Complete loss of Grn [94] or persistent GRN knockdown in mouse primary cortical neurons [155, 169] and human NPCs [163] or GRN haploinsufficiency in patient derived iPS cells [161] led to significantly increased caspase activation and reduced neuronal survival, however, no effect on neuronal survival of rat hippocampal neurons was observed upon Grn knockdown in another study [165]. Furthermore, GRN deficiency was priming neuroblastoma cells for staurosporine-induced apoptosis [91] and increased the susceptibility of cultured neurons or hippocampal slice cultures to cellular stressors such as inhibiting proteasomal proteolysis [94], NMDA-mediated excitotoxicity, oxidative stress [155] glucose, and oxygen starvation [114] or kinase inhibitors [161]. Overexpression of GRN or addition of extracellular GRN rescued these effects [95, 155, 161] due to the activation of cell survival signaling pathways [95], suggesting that the increased susceptibility of neurons is specific for GRN deficiency.
Inadequate responses to inflammatory insults are also likely contributing to FTD pathogenesis and cellular studies using macrophages or microglia from Grn-deficient mice indicated a critical involvement of Grn in regulating TLR9 signaling [122]. Furthermore, Grn deficiency resulted in an exaggerated inflammatory response defined by an increased production of pro-inflammatory cytokines and reduced production of anti-inflammatory IL-10. Grn-deficient microglia were also more cytotoxic compared with wt controls [114, 163].
Lastly, the major biochemical feature of GRN-associated FTD is the redistribution of TDP-43 from the nucleus to the cytoplasm, increased TDP-43 phosphorylation and generation of aggregation-prone TDP-43 CTFs that accumulate in neuronal intranuclear or cytoplasmic inclusions [55, 57]. The first study investigating the cellular link between GRN and TDP-43 reported increased accumulation of TDP-43 CTFs, with no obvious redistribution of TDP-43 from its predominant nuclear localization in non-neuronal cells [158]. However, subsequent attempts to reproduce this effect in similar cell lines failed to detect increased caspase activation and TDP-43 fragmentation upon GRN knockdown in non-neuronal cells [94, 146, 170]. Moreover, N-terminal sequencing of the TDP-43 CTFs isolated from brains of FTD patients indicated that these CTFs are different from those generated upon caspase cleavage [171]. Interestingly, increased redistribution of TDP-43 from the nucleus to the cytoplasm was observed in cortical neurons upon persistent knockdown of Grn [155] and neurons derived from GRN-deficient iPS cells [161]. Additional stress by disturbing the proteasomal machinery also resulted in increased accumulation of phosphorylated full-length TDP-43 in primary cells derived from Grn −/− mice [94].
The established cellular models of GRN deficiency have already improved our understanding on the multiple cell biological functions of GRN. However, so far, no GRN-deficient cell-culture model could recapitulate all pathological hallmarks of FTD including the ubiquitinated and TDP-43 positive nuclear and/or cytoplasmic aggregates. As some of the FTD characteristic features might be difficult to completely recapitulate in vitro, animal models of GRN deficiency have been developed to further deepen our understanding of the GRN biology on the level of a whole organism.
Progranulin-Deficient Animal Models
The granulin/epithelin motif defines a family of structurally unique proteins, of great evolutionary antiquity [60, 172]. Granulin motif encoding genes are present in most commonly used laboratory animals including Caenorhabditis elegans [173], Danio rerio [172], Xenopus leavis [174], and Mus musculus [61], making them valuable tools to study the effect of GRN deficiency in an in vivo setting.
Nonrodent Models of GRN Deficiency
In the nematode C. elegans, the GRN gene encodes for a secreted protein with three predicted granulin domains, which is expressed in intestinal cells and selected neurons, but not in muscle cells [173]. GRN deletion mutants appeared grossly normal with a normal lifespan but they produced approximately 20 % less progeny. GRN-deficient C. elegans showed significantly fewer apoptotic bodies, a phenomenon that was attributed to an increased clearing of apoptotic cells [173]. Based on these results, Kao et al. proposed a speculative model suggesting that cells of GRN-deficient organisms do not have enough time to recover from sub-lethal stress ultimately leading to cumulative cellular loss over time [173]. Table 1 gives an overview of the currently described nonrodent GRN-deficient animal models.
In zebrafish (D. rerio), a useful model of vertebrate development and disease, four GRN paralogues were identified that are coding for two precursor proteins (zfGRN-A and B) and two shorter forms of GRN (zfGRN-1 and zfGRN-2) [172]. A noncoding RNA gene with antisense complementarity to both zfGRN1 and zfGRN2 has also been identified, with a possible function in regulating gene dosage [172]. Both zfGRN-A and zfGRN-B transcripts are expressed in a wide variety of tissues including the gills, heart, multiple visceral organs and at modest expression levels in the brain [172]. Knockdown of zfGRN-A using antisense morpholinos led to reduced proliferation and increased apoptosis in hepatocytes and zfGRN-A-deficient zebrafish had a decreased liver size. Impaired liver morphogenesis was linked to reduced expression of hepatic MET, a receptor tyrosine kinase known to have functions controlling liver size [175]. zfGRN-A expression can also be found within the peripheral and CNS and knockdown by antisense morpholinos resulted in truncated motor neurons (MNs) and inappropriate early branching [176]. In contrast, overexpression of zfGRN-A or human GRN caused increased MN branching and rescued the truncation defects caused by zfGRN-A deficiency, survival of motor neuron 1 (smn1) deficiency [176] or overexpression of mutant TDP-43 [177]. The effect of zfGRN knockdown on MN axonal growth was confirmed by another study where knockdown of zfGRN-A produced a greater decrease in axonal length than zfGRN-B knockdown [177]. Additional to the observed MN defects, and most likely as a consequence, zfGRN-A morphants showed a marked progressive swimming defect although the touch response was unaltered [176]. Cytoplasmic redistribution of TDP-43 or proteolytic processing into aggregation-prone CTFs are characteristic disease features of GRN-associated FTD, but downregulation of zfGRN did not cause any of these alterations [146, 177].
Rodent Models of Grn Deficiency
Rodent models are the most frequently used animal models in biomedical research for several reasons including their anatomical similarities to humans as well as the possibility to generate disease models through targeted gene manipulation such as gene knockout.
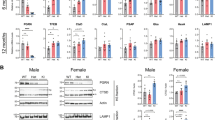
Five independent Grn −/− mouse models have been established to date [75, 114, 167, 173, 178], of which a general overview of their characteristics is included in Table 2. So far, most studies used all-tissue knockout of Grn, yet some of the models also allow tissue-specific knockout [114, 167, 173, 178]. Utilizing one of these conditional Grn knockout lines [173], Martens et al. report deregulated microglial activation in microglia-specific Grn −/− mice leading to increased neuron loss in a model of neuronal injury [179]. All described Grn −/− mice are viable, fertile and reproduce with a normal Mendelian pattern of inheritance. However, one follow up-study on the mice established by Kayasuga et al. [75] suggested a decreased generation frequency of Grn −/− mice and postnatal sensitivity to handling [180]. This was not observed in another follow-up study by Ghoshal et al. on the same Grn −/− mice [181]. Two further studies observed increased age related mortality with differences appearing from 10 months onwards [178, 181]. In contrast, no decreased survival was reported in two other independent mouse models [114, 167]. Although in humans, GRN haploinsufficiency is sufficient to lead to neurodegeneration, none of the Grn −/− mice studies identified any obvious defects yet, mimicking the condition in human disease. Therefore, all studies focused on characterizing the behavioral (Table 3) and neurohistological (Table 4) consequences of Grn loss in Grn −/− mice.
Initial studies, using siRNA-mediated knockdown or administration of neutralizing Grn antibodies, have suggested that hypothalamic Grn exerts anorexigenic effects affecting weight gain and loss, indicating a potential role of Grn in hypothalamic glucose sensing [143]. However, consistent with another report [182], no effect on body weight was yet described in Grn −/− mice [75, 114, 167, 178].
In rodents, Grn has been shown to be an androgen-inducible gene in the neonatal hypothalamus that is expressed at high levels in males, throughout the critical period for the sexual differentiation of the brain, while in females the Grn expression levels drop during this critical period [183, 184]. Accordingly, infusion of Grn antisense oligonucleotides into the third ventricle of neonatal male rats significantly suppressed male sexual behaviors, like frequency of mount, intromission and ejaculation in the adulthood [182]. Alterations in male sexual behavior, aggression, and anxiety were also observed in the first Grn −/− mouse model [75]. In the open-field test, wt females generally show higher levels of anxiety than males and Grn loss raises the anxiety level of males significantly to similar levels of females [75]. A follow up-study on the same mice linked increased anxiety in Grn −/− males to an increase in the volume and number of cells in the locus ceruleus [185], a nucleus involved in physiological responses to stress and anxiety. Furthermore, Grn −/− males exhibited enhanced aggressiveness towards females and increased frequency of biting attacks in the resident-intruder test, which was ascribed to alterations in the brain serotonergic system of Grn −/− mice [75]. However, diminished social interaction and passive disinterested behavior, rather than increased aggression was observed in two other studies [167, 181]. Yin et al. reported increased signs of depression in Grn −/− mice using a tail suspension and forced swimming test [186], while Petkau et al. did not observe any abnormalities in their Grn −/− model [167].
Perhaps the most consistent behavioral phenotype in Grn−/− mice is a reduction in social interactions [167, 181, 186], a feature observed already at very young age [186]. Assessment of spatial memory functions by Morris water maze was less consistent between the individual publications. While Petkau et al. did not observe any deficits of Grn −/− mice in the Morris water maze [167] other studies report subtle impairments in the oldest mice using the same test parameters [178, 181, 186]. Generally, motor functions seem not to be affected largely by Grn deficiency, however, Grn −/− mice tended to swim somewhat slower [178, 181], took longer to learn the rotarod task [167] and showed reduced performance in the inverted screen test at old ages [181]. Such subtle locomotor deficits might be explained by increased inflammation in joints as Grn −/− mice were recently shown to have increased susceptibility to collagen-induced arthritis [113]. Although all Grn −/− mouse models have been maintained in similar backgrounds (Table 2), subtle differences in background strain or in the applied test protocols, two important variables in behavioural studies [187, 188], might account for some of the observed phenotypic differences in Grn −/− mice.
Reports on the neuropathology of Grn −/− mice are more consistent (Table 4). All studies report a pronounced microgliosis and astrogliosis in brain areas including the cortex, hippocampus, thalamus and brainstem [114, 167, 178, 180, 181, 186]. Microgliosis tends to be detectable at earlier time points, with significant differences emerging from 12 months onwards, and both microgliosis and astrogliosis show a progressive worsening [178, 180, 186]. Accumulation of ubiquitinated proteins in the same brain areas is another consistent feature of all Grn −/− models [114, 167, 178, 180, 181, 186], suggesting either an overproduction of a ubiquitin target protein or more likely a perturbation in the functioning of the ubiquitin-proteasomal and/or autophagy-lysosomal degradation machineries. Furthermore, a robust increase in the accumulation of the aging pigment lipofuscin in the brains of Grn −/− mice [167, 178, 180], associated with vacuolation in the habenula and hippocampus [180], was observed. Interestingly, a homozygous GRN mutation has recently been identified in two siblings with neuronal ceroid lipofuscinosis (NCL) [189], a lysosomal storage disorder with prominent accumulation of lipofuscin [190], calling for future studies to determine whether the same pathways could be affected in FTD and GRN-related NCL.
Together with increased accumulation of the autophagy-related receptor p62 as well as lysosomal proteases such as cathepsin D [178], this points towards an involvement of the autophagy-lysosome degradation system in Grn-mediated neuropathology.
Although cell culture experiments suggested a link between GRN deficiency and caspase activation [94, 155, 158], no obvious signs of apoptosis were observed in the brains of Grn −/− mice [178, 180]. Neuronal loss is not a prominent feature of Grn −/− mice [178, 180], although some focal neuronal loss in the CA2-3 region of the hippocampus and small nonsignificant reduction in neuron density in the dorsal thalamus were observed in very old mice [180, 181]. While such changes might be very subtle and only detectable in old mice, impaired neuronal function is predicted to manifest much earlier. Accordingly, reduced hippocampal synaptic connectivity and impaired synaptic plasticity (e.g., reduced long-term potentiation) were reported in 10- to 12-month-old Grn −/− mice [167].
Another disease feature of GRN-associated FTD is the cytoplasmic mislocalisation of TDP-43 with concomitant nuclear clearing, abnormal phosphorylation and formation of nuclear and cytoplasmic TDP-43 positive aggregates [55, 57]. Histological studies were so far unsuccessful in detecting TDP-43 positive aggregates in brains of Grn −/− mice. While Yin et al. observed increased cytoplasmic reactivity to phosphorylated TDP-43 in hippocampal and thalamic areas [114, 186], other studies did not [167, 178, 180, 181]. Biochemically, Grn −/− brains did not show increased generation of TDP-43 CTFs [170, 178], but full-length phosphorylated TDP-43 was significantly increased from 12 months onwards in the insoluble fraction prepared from Grn −/− brains [178]. Absence of major pathological TDP-43 alterations like fragmentation and aggregation in Grn −/− mice suggests that these events might merely be a late event in the pathogenesis of FTD.
Besides consistent neuropathological changes in the brains of Grn −/− mice, other vital organs and processes are also negatively affected by Grn deficiency. For example, Grn −/− mice react with an exaggerated immune response to foreign pathogens and are less efficient in clearing bacterial infections resulting in prolonged inflammation [114]. While young Grn −/− mice did not show any morphological, hematological or biochemical abnormalities [114], the liver of aged Grn −/− mice showed increased signs of cellular ageing with abnormal hepatic and ductal morphology and significant upregulation of lysosomal proteases, like cathepsin D, in lysosomes within sinusoidal foamy histiocytes [178]. These findings, together with the recently reported biological role of Grn in energy homeostasis [100], call for the investment into more holistic approaches especially when studying deficiencies of multifunctional proteins such as GRN.
Restoring GRN Function: A Way to a Successful Therapy
Recent advances in functional genomics have brought us a few steps closer to understanding the biological mechanisms involved in the pathogenesis of FTD. Cellular and animal models for GRN-associated FTD, have produced a tremendous amount of information, nourishing our hope that, if effectively translated into treatment opportunities, we would be able at some stage to delay or cure this devastating disease. However, despite recent advances, treatments for FTD are still lacking and only limited symptomatic treatment options are available [191].
Targeting GRN Expression
Reduced GRN levels in biofluids like serum or CSF can be used as a reliable biomarker for the diagnosis and early detection of FTD caused by GRN mutations [192–194]. Because of the underlying haploinsufficiency mechanism, targeting or modifying GRN expression is assumed to be beneficial in preventing neurodegenerative diseases. Increasing Grn expression has already been shown to be advantageous in several animal models [173, 175–177, 195]. Modulating GRN expression by boosting the expression from the mutant or the wt allele might proof beneficial in delaying disease pathogenesis and could be a valid future therapeutic strategy. Stimulation of the mutant GRN allele by ribosomal read-through has been suggested as a worthwhile approach to be pursued in future FTD clinical trials [196]. Several compounds including ataluren (PTC124), aminoglycosides (e.g., gentamicin) and non-aminoglycosides have proven premature termination codon read-through activity in vitro and in vivo [197–199]. PTC124 has been shown to be safe and tolerable [198] and clinical trials for other diseases with genetic deficiency, like Duchenne muscular dystrophy and cystic fibrosis [200, 201] have been started.
An alternative strategy to normalize the levels of GRN could be to increase the expression and production from the wt allele. Increased transcription might be achieved by androgens as shown in the hypothalamus of neonatal rats [184], and administration of estrogen or selective stimulation of estrogen receptors in the brain could be considered a potential strategy for increasing GRN levels in the brain. A high-throughput screen of 1200 FDA approved drugs identified suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor, as potent activator of GRN expression [202]. Other pan-HDAC inhibitors showed similar GRN stimulating effects and administration of SAHA was able to normalize the expression of GRN in cells derived from GRN mutation carriers [202]. Posttranscriptional mechanisms might also be involved in regulating GRN expression, and inhibitors of vacuolar ATPase and the FDA-approved alkalizing drugs chloroquin, bepridile, and amiodarone were shown to increase intracellular and secreted GRN protein levels via a translational mechanism independent of lysosomal degradation, autophagy or endocytosis [203]. Identification of protein disulfide isomerase family members as GRN interactors and modulators of GRN secretion [204], together with the observed inefficient posttranslational processing and secretion of GRN in neurons and microglia, suggested that modulation of the endoplasmatic reticulum chaperone network might be another potential therapeutic target to increase GRN expression [204].
Rediscovery of already approved drugs with the potential to modify GRN expression and production are considered valuable approaches to accelerate the process of establishing first generation drugs for FTD and related disorders. However, because of the role of GRN in promoting tumor growth, future studies will need to delineate the tolerated GRN levels and the potential adverse effects of increasing or administering GRN over a longer period of time [196].
Targeting GRN Receptors
GRN is a secreted growth factor stimulating both growth and survival signals in various cell types. Recently, sortilin has been identified as the first neuronal receptor for GRN [107] and is thought to be involved in controlling the extracellular levels of GRN via receptor-mediated endocytosis [42, 107]. Molecules or compounds that could selectively interfere with the GRN/sortilin interaction and thus raise the levels of extracellular GRN could therefore be considered as potential candidates for future clinical and preclinical trials for FTD. Although the idea of raising GRN levels through compounds that modify GRN/sortilin interaction is intriguing, first the question needs to be addressed whether sortilin is only involved in GRN clearance or also in survival signaling.
In addition, GRN can bind with high affinity to the TNF receptors (TNFR1 and TNFR2) [113] and hence acquired a therapeutic potential in inflammatory conditions such as rheumatoid arthritis [113] or acute respiratory distress syndrome [150]. A modified hybrid granulin peptide (named ATSTTRIN) was even more effective than full-length GRN in diminishing the pro-inflammatory signaling cascade elicited by TNF-α. Neurodegenerative diseases, including FTD, have a strong neuroinflammatory component and it will therefore be important to test if administration of GRN or hybrid granulin peptides are beneficial in delaying the disease pathology. It is likely that additional receptors or combination of receptors are involved in GRN-mediated prosurvival signaling and their identification will hopefully provide additional points of entry for possible therapeutic interventions.
GRN and the Serotonergic System
The serotonergic system is important for behavioral modulation [205] and decreased serotonin receptor binding has been reported in affected brain areas in autopsied FTD patients [3]. Alterations in the serotonergic system were also recapitulated in Grn −/− mice, which have reduced expression of the serotonergic receptor 5-HT1A in the hippocampus after an aggressive encounter [75]. The exact role that GRN plays in modulating the serotonergic system is not well characterized, but it has been suggested to relate to the organization and/or activation of the serotonergic system in the brain [75]. In line with this, some studies using selective serotonin-reuptake inhibitors or 5-HT receptor agonists to treat behavioral deficits in FTD patients showed efficacy of the treatment [206, 207].
GSK3β and Wnt Signaling
GSK3β is an enzyme regulating many cellular functions including cellular structure and survival [166]. Deregulation of GSK3β is linked to several common pathological conditions, including diabetes and AD [166]. GSK3β is known to be involved in phosphorylating tau and clinical studies are evaluating the effects of GSK3β inhibitors like lithium chloride and valproic acid on AD pathogenesis [208]. Data obtained from the cellular models of GRN deficiency suggest that GRN is, at least to some extent, exerting its neurotrophic and neuroprotective function via regulating GSK3β phosphorylation [91, 162]. Interestingly, hnRNPs have also been shown to belong to the many substrates of GSK3β [166], but whether GSK3β also contributes to pathological TDP-43 phosphorylation is not known. While further data on this subject are awaited, Grn −/− mice showed a general increase in the phosphorylation state of proteins [178], and one could hypothesize that this could be caused by alterations in the activity of GSK3β.
Additionally, GRN loss has recently been implicated in altering the expression of FZD2, a receptor involved in the noncanonical Wnt signaling pathway [163]. This upregulation of FZD2 is thought to be neuroprotective and thus compensating for the lost GRN function. Manipulation of the expression of the FZD2 receptor or one of its downstream effectors through compounds with agonistic functions could therefore be considered as a potential future therapeutic strategy for GRN-associated FTD [163].
Modulating Conversion of Grn to Granulins
Many neurodegenerative diseases, including FTD, are characterized by considerable neuroinflammation [209]. GRN and its granulin peptides are critically involved in regulating inflammatory reactions through the conversion of anti-inflammatory GRN to pro-inflammatory granulins. Several proteinases such as neutrophil elastase, proteinase-3 and some metalloproteinases are involved in this process, which can be inhibited for example by the binding of SLPI to GRN [115]. Grn −/− mice show a predominant proinflammatory response [114] and micro- and astrogliosis are one of the most consistent pathological features of Grn −/− mice (see Table 4), most likely caused by the loss of full-length GRN. Pathogenic cysteine mutations also affect the processing of GRN into granulins [93] thereby potentially shifting the balance towards a proinflammatory response. Modulating the activity of GRN converting enzymes or increasing the expression of inhibitory proteins might therefore be considered as potential strategy to increase the GRN/granulin ratio. While topically applied SLPI peptides have already been tested to treat impaired wound healing in elderly people, systemic administration of proteinase inhibitors like SLPI might cause severe side effects as overexpression of SLPI has been associated with several forms of cancers [210]. The few existing studies evaluating the effect of proteinase inhibitors on neuronal survival report beneficial effects of a neutrophil elastase inhibitor in attenuating MN death or hippocampal neuronal damage after ischemic insults [211–213]. Whether these neuroprotective effects could be driven by stabilization of GRN was however not investigated. These initial results clearly encourage further studies investigating the neuroprotective effects of proteinase inhibitors and their potential for attenuating the neuropathology associated with GRN deficiency.
Conclusions
Deregulation of GRN is critically involved in cancer and neurodegeneration, two of the major pathological conditions our aging population has to face today. GRN deficiency is associated with FTD, a neurodegenerative condition for which currently no pharmacological treatment to cure or delay its progression is available. The generation and characterization of GRN cellular and animal models, as highlighted in this review, has been essential in increasing our knowledge about the diverse biological functions of GRN over the last couple of years. However, many biological functions of GRN are still poorly understood and the described models will help us to gain further insight into the GRN biology. Generating novel and ongoing characterization of the existing GRN models will hopefully provide us soon with enough mechanistic information to enable us to translate our findings into novel therapeutic strategies for neurodegenerative diseases related to GRN deficiency.
References
Cerami C, Scarpini E, Cappa SF, Galimberti D (2012) Frontotemporal lobar degeneration: current knowledge and future challenges. J Neurol 259:2278–2286
Van Langenhove T, van der Zee J, Van Broeckhoven C (2012) The molecular basis of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum. Ann Med (in press)
Neary D, Snowden J, Mann D (2005) Frontotemporal dementia. Lancet Neurol 4:771–780
Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM (2009) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, Lomen-Hoerth C, Wilhelmsen KC, Lee VM, Grossman M, Miller BL (2005) Comparison of family histories in FTLD subtypes and related tauopathies. Neurology 65:1817–1819
Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, Janssens J, Bettens K, Van Cauwenberghe C, Pereson S, Engelborghs S, Sieben A, De Jonghe P, Vandenberghe R, Santens P, De Bleecker J, Maes G, Baumer V, Dillen L, Joris G, Cuijt I, Corsmit E, Elinck E, Van Dongen J, Vermeulen S, Van den Broeck M, Vaerenberg C, Mattheijssens M, Peeters K, Robberecht W, Cras P, Martin JJ, De Deyn PP, Cruts M, Van Broeckhoven C (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol 11:54–65
Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D’Amato CJ, Gilman S (1997) Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference Participants. Ann Neurol 41:706–715
Lynch T, Sano M, Marder KS, Bell KL, Foster NL, Defendini RF, Sima AA, Keohane C, Nygaard TG, Fahn S (1994) Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology 44:1878–1884
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Heutink P (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393:702–705
Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 43:815–825
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B (1998) Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 95:7737–7741
Cruts M, Theuns J, Van Broeckhoven C (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat. doi:10.1002/humu.22117
Rademakers R, Cruts M, Van Broeckhoven C (2004) The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 24:277–295
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924
Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC (2011) Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry 82:476–486
Gijselinck I, Van Broeckhoven C, Cruts M (2008) Granulin mutations associated with frontotemporal lobar degeneration and related disorders: An update. Hum Mutat 29:1373–1386
Sleegers K, Brouwers N, Van Broeckhoven C (2010) Role of progranulin as a biomarker for Alzheimer’s disease. Biomark Med 4:37–50
Cruts M, Van Broeckhoven C (2008) Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet 24:186–194
Gijselinck I, van der Zee J, Engelborghs S, Goossens D, Peeters K, Mattheijssens M, Corsmit E, Del-Favero J, De Deyn PP, Van Broeckhoven C, Cruts M (2008) Progranulin locus deletion in frontotemporal dementia. Hum Mutat 29:53–58
Rovelet-Lecrux A, Deramecourt V, Legallic S, Maurage CA, Le BI, Brice A, Lambert JC, Frebourg T, Hannequin D, Pasquier F, Campion D (2008) Deletion of the progranulin gene in patients with frontotemporal lobar degeneration or Parkinson disease. Neurobiol Dis 31:41–45
Brouwers N, Nuytemans K, van der Zee J, Gijselinck I, Engelborghs S, Theuns J, Kumar-Singh S, Pickut BA, Pals P, Dermaut B, Bogaerts V, De Pooter T, Serneels S, Van den Broeck M, Cuijt I, Mattheijssens M, Peeters K, Sciot R, Martin JJ, Cras P, Santens P, Vandenberghe R, De Deyn PP, Cruts M, Van Broeckhoven C, Sleegers K (2007) Alzheimer and Parkinson diagnoses in progranulin null mutation carriers in an extended founder family. Arch Neurol 64:1436–1446
Le Ber I, van der Zee J, Hannequin D, Gijselinck I, Campion D, Puel M, Laquerriere A, De Pooter T, Camuzat A, Van den Broeck M, Dubois B, Sellal F, Lacomblez L, Vercelletto M, Thomas-Anterion C, Michel BF, Golfier V, Didic M, Salachas F, Duyckaerts C, Cruts M, Verpillat P, Van Broeckhoven C, Brice A (2007) Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 28:846–855
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL III, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15:2988–3001
Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, Puel M, Ghanim M, Lacomblez L, Mikol J, Deramecourt V, Lejeune P, de la Sayette V, Belliard S, Vercelletto M, Meyrignac C, Van Broeckhoven C, Lambert JC, Verpillat P, Campion D, Habert MO, Dubois B, Brice A (2008) Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 131:732–746
Hosler BA, Siddique T, Sapp PC, Sailor W, Huang MC, Hossain A, Daube JR, Nance M, Fan C, Kaplan J, Hung WY, McKenna-Yasek D, Haines JL, Pericak-Vance MA, Horvitz HR, Brown RH Jr (2000) Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21–q22. JAMA 284:1664–1669
Gijselinck I, Engelborghs S, Maes G, Cuijt I, Peeters K, Mattheijssens M, Joris G, Cras P, Martin JJ, De Deyn PP, Kumar-Singh S, Van Broeckhoven C, Cruts M (2010) Identification of 2 loci at chromosomes 9 and 14 in a multiplex family with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol 67:606–616
Morita M, Al Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, Mitchell JE, Habgood JJ, de Belleroche J, Xi J, Jongjaroenprasert W, Horvitz HR, Gunnarsson LG, Brown RH Jr (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66:839–844
Rollinson S, Mead S, Snowden J, Richardson A, Rohrer J, Halliwell N, Usher S, Neary D, Mann D, Hardy J, Pickering-Brown S (2011) Frontotemporal lobar degeneration genome wide association study replication confirms a risk locus shared with amyotrophic lateral sclerosis. Neurobiol Aging 32:758–7
Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, Baas F, de J, V, Shaw CE (2006) Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3. Brain 129:868–876
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, Kouri N, Wojtas A, Sengdy P, Hsiung GY, Karydas A, Seeley WW, Josephs KA, Coppola G, Geschwind DH, Wszolek ZK, Feldman H, Knopman DS, Petersen RC, Miller BL, Dickson DW, Boylan KB, Graff-Radford NR, Rademakers R (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256
Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta-Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering-Brown S, Morris HR, Tienari PJ, Traynor BJ (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268
Galimberti D, Scarpini E (2012) Genetics of frontotemporal lobar degeneration. Front Neurol 3:52
Hardy J, Singleton A (2009) Genomewide association studies and human disease. N Engl J Med 360:1759–1768
Lang CM, Fellerer K, Schwenk BM, Kuhn PH, Kremmer E, Edbauer D, Capell A (2012) Haass C (2012) Membrane orientation and subcellular localization of transmembrane protein 106B (TMEM106B), a major risk factor for frontotemporal lobar degeneration. J Biol Chem 287(23):19355–19365
Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, Arnold SE, Mann DM, Pickering-Brown SM, Seelaar H, Heutink P, van Swieten JC, Murrell JR, Ghetti B, Spina S, Grafman J, Hodges J, Spillantini MG, Gilman S, Lieberman AP, Kaye JA, Woltjer RL, Bigio EH, Mesulam M, Al-Sarraj S, Troakes C, Rosenberg RN, White CL III, Ferrer I, Llado A, Neumann M, Kretzschmar HA, Hulette CM, Welsh-Bohmer KA, Miller BL, Alzualde A, de Lopez MA, McKee AC, Gearing M, Levey AI, Lah JJ, Hardy J, Rohrer JD, Lashley T, Mackenzie IR, Feldman HH, Hamilton RL, DeKosky ST, van der Zee J, Kumar-Singh S, Van Broeckhoven C, Mayeux R, Vonsattel JP, Troncoso JC, Kril JJ, Kwok JB, Halliday GM, Bird TD, Ince PG, Shaw PJ, Cairns NJ, Morris JC, McLean CA, DeCarli C, Ellis WG, Freeman SH, Frosch MP, Growdon JH, Perl DP, Sano M, Bennett DA, Schneider JA, Beach TG, Reiman EM, Woodruff BK, Cummings J, Vinters HV, Miller CA, Chui HC, Alafuzoff I, Hartikainen P, Seilhean D, Galasko D, Masliah E, Cotman CW, Tunon MT, Martinez MC, Munoz DG, Carroll SL, Marson D, Riederer PF, Bogdanovic N, Schellenberg GD, Hakonarson H, Trojanowski JQ, Lee VM (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42:234–239
van der Zee J, Van Broeckhoven C (2011) TMEM106B a novel risk factor for frontotemporal lobar degeneration. J Mol Neurosci 45:516–521
van der Zee J, Van Langenhove T, Kleinberger G, Sleegers K, Engelborghs S, Vandenberghe R, Santens P, Van den Broeck M, Joris G, Brys J, Mattheijssens M, Peeters K, Cras P, De Deyn PP, Cruts M, Van Broeckhoven C (2011) TMEM106B is associated with frontotemporal lobar degeneration in a clinically diagnosed patient cohort. Brain 134:808–815
Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, Bertelsen S, Mayo K, Norton JB, Morris JC, Goate A (2011) Association of TMEM106B gene polymorphism with age at onset in granulin mutation carriers and plasma granulin protein levels. Arch Neurol 68:581–586
Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L, Busch JI, Akle S, Grossman M, Van DV, Trojanowski JQ, Lee VM (2012) TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. J Neurosci 32:11213–11227
Brady OA, Zheng Y, Murphy K, Huang M, Hu F (2012) The Frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum Mol Genet (in press)
Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, Rutherford NJ, Hunter TA, DeJesus-Hernandez M, Bisceglio GD, Mackenzie IR, Singleton A, Cookson MR, Crook JE, Dillman A, Hernandez D, Petersen RC, Graff-Radford NR, Younkin SG, Rademakers R (2010) Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet 87:890–897
Kelley BJ, Haidar W, Boeve BF, Baker M, Graff-Radford NR, Krefft T, Frank AR, Jack CR Jr, Shiung M, Knopman DS, Josephs KA, Parashos SA, Rademakers R, Hutton M, Pickering-Brown S, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, Ivnik RJ, Petersen RC (2009) Prominent phenotypic variability associated with mutations in progranulin. Neurobiol Aging 30:739–751
Larner AJ (2012) Intrafamilial clinical phenotypic heterogeneity with progranulin gene p.Glu498fs mutation. J Neurol Sci 316:189–190
Rademakers R, Baker M, Gass J, Adamson J, Huey ED, Momeni P, Spina S, Coppola G, Karydas AM, Stewart H, Johnson N, Hsiung GY, Kelley B, Kuntz K, Steinbart E, Wood EM, Yu CE, Josephs K, Sorenson E, Womack KB, Weintraub S, Pickering-Brown SM, Schofield PR, Brooks WS, Van Deerlin VM, Snowden J, Clark CM, Kertesz A, Boylan K, Ghetti B, Neary D, Schellenberg GD, Beach TG, Mesulam M, Mann D, Grafman J, Mackenzie IR, Feldman H, Bird T, Petersen R, Knopman D, Boeve B, Geschwind DH, Miller B, Wszolek Z, Lippa C, Bigio EH, Dickson D, Graff-Radford N, Hutton M (2007) Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C–>T (Arg493X) mutation: an international initiative. Lancet Neurol 6:857–868
van Swieten JC, Heutink P (2008) Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol 7:965–974
Cruchaga C, Fernandez-Seara MA, Seijo-Martinez M, Samaranch L, Lorenzo E, Hinrichs A, Irigoyen J, Maestro C, Prieto E, Marti-Climent JM, Arbizu J, Pastor MA, Pastor P (2009) Cortical atrophy and language network reorganization associated with a novel progranulin mutation. Cereb Cortex 19:1751–1760
Guerreiro RJ, Santana I, Bras JM, Revesz T, Rebelo O, Ribeiro MH, Santiago B, Oliveira CR, Singleton A, Hardy J (2008) Novel progranulin mutation: screening for PGRN mutations in a Portuguese series of FTD/CBS cases. Mov Disord 23:1269–1273
Rainero I, Rubino E, Negro E, Gallone S, Galimberti D, Gentile S, Scarpini E, Pinessi L (2011) Heterosexual pedophilia in a frontotemporal dementia patient with a mutation in the progranulin gene. Biol Psychiatry 70:e43–e44
Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, Rutherford NJ, Baker M, Knopman DS, Wszolek ZK, Parisi JE, Dickson DW, Petersen RC, Rademakers R, Jack CR Jr, Josephs KA (2012) Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain 135:794–806
Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, Rossor MN, Warren JD (2010) Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage 53:1070–1076
Whitwell JL, Weigand SD, Gunter JL, Boeve BF, Rademakers R, Baker M, Knopman DS, Wszolek ZK, Petersen RC, Jack CR Jr, Josephs KA (2011) Trajectories of brain and hippocampal atrophy in FTD with mutations in MAPT or GRN. Neurology 77:393–398
Borroni B, Alberici A, Cercignani M, Premi E, Serra L, Cerini C, Cosseddu M, Pettenati C, Turla M, Archetti S, Gasparotti R, Caltagirone C, Padovani A, Bozzali M (2012) Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol Aging 33(10):2506–2520
Mackenzie IR (2007) The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 114:49–54
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Kumar-Singh S (2011) Progranulin and TDP-43: mechanistic links and future directions. J Mol Neurosci 45:561–573
Bhandari V, Bateman A (1992) Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun 188:57–63
Bateman A, Bennett HP (1998) Granulins: the structure and function of an emerging family of growth factors. J Endocrinol 158:145–151
Bucan M, Gatalica B, Baba T, Gerton GL (1996) Mapping of Grn, the gene encoding the granulin/epithelin precursor (acrogranin), to mouse chromosome 11. Mamm Genome 7:704–705
Bhandari V, Giaid A, Bateman A (1993) The complementary deoxyribonucleic acid sequence, tissue distribution, and cellular localization of the rat granulin precursor. Endocrinology 133:2682–2689
Bhandari V, Palfree RG, Bateman A (1992) Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A 89:1715–1719
Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S (1990) Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun 173:1161–1168
Shoyab M, McDonald VL, Byles C, Todaro GJ, Plowman GD (1990) Epithelins 1 and 2: isolation and characterization of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci U S A 87:7912–7916
Baba T, Hoff HB III, Nemoto H, Lee H, Orth J, Arai Y, Gerton GL (1993) Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev 34:233–243
Parnell PG, Wunderlich J, Carter B, Halper J (1992) Transforming growth factor e: amino acid analysis and partial amino acid sequence. Growth Factors 7:65–72
Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, Shoyab M (1992) The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem 267:13073–13078
Zhou J, Gao G, Crabb JW, Serrero G (1993) Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. J Biol Chem 268:10863–10869
Bhandari V, Palfree RG, Bateman A (1992) Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A 89:1715–1719
Petkau TL, Neal SJ, Orban PC, MacDonald JL, Hill AM, Lu G, Feldman HH, Mackenzie IR, Leavitt BR (2010) Progranulin expression in the developing and adult murine brain. J Comp Neurol 518:3931–3947
Daniel R, He Z, Carmichael KP, Halper J, Bateman A (2000) Cellular localization of gene expression for progranulin. J Histochem Cytochem 48:999–1009
Matsuwaki T, Asakura R, Suzuki M, Yamanouchi K, Nishihara M (2011) Age-dependent changes in progranulin expression in the mouse brain. J Reprod Dev 57:113–119
Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M (2007) Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res 185:110–118
Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M (2007) Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res 185:110–118
Suzuki M, Yoshida S, Nishihara M, Takahashi M (1998) Identification of a sex steroid-inducible gene in the neonatal rat hypothalamus. Neurosci Lett 242:127–130
Jiao J, Herl LD, Farese RV, Gao FB (2010) MicroRNA-29b regulates the expression level of human progranulin, a secreted glycoprotein implicated in frontotemporal dementia. PLoS One 5:e10551
Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ, Finch N, Rutherford NJ, Crook RJ, Josephs KA, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Caselli RJ, Wszolek ZK, Uitti RJ, Feldman H, Hutton ML, Mackenzie IR, Graff-Radford NR, Dickson DW (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642
Wang WX, Kyprianou N, Wang X, Nelson PT (2010) Dysregulation of the mitogen granulin in human cancer through the miR-15/107 microRNA gene group. Cancer Res 70:9137–9142
Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, Dimayuga J, Stromberg AJ, Huang Q, Saatman KE, Nelson PT (2010) miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol 177:334–345
Kocerha J, Kouri N, Baker M, Finch N, DeJesus-Hernandez M, Gonzalez J, Chidamparam K, Josephs KA, Boeve BF, Graff-Radford NR, Crook J, Dickson DW, Rademakers R (2011) Altered microRNA expression in frontotemporal lobar degeneration with TDP-43 pathology caused by progranulin mutations. BMC Genomics 12:527
Galimberti D, D’Addario C, Dell’osso B, Fenoglio C, Marcone A, Cerami C, Cappa SF, Palazzo MC, Arosio B, Mari D, Maccarrone M, Bresolin N, Altamura AC, Scarpini E (2012) Progranulin gene (GRN) promoter methylation is increased in patients with sporadic frontotemporal lobar degeneration. Neurol Sci (in press)
Suh HS, Choi N, Tarassishin L, Lee SC (2012) Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12). PLoS One 7:e35115
Swindell WR (2009) Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics 10:585
Jurgens HA, Johnson RW (2012) Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol 233:40–48
Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, Bennett HP, Bateman A, Ni F (2008) Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci 17:711–724
Hrabal R, Chen Z, James S, Bennett HP, Ni F (1996) The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat Struct Biol 3:747–752
De Muynck L, Van Damme P (2011) Cellular effects of progranulin in health and disease. J Mol Neurosci 45:549–560
Toh H, Chitramuthu BP, Bennett HP, Bateman A (2011) Structure, function, and mechanism of progranulin; the brain and beyond. J Mol Neurosci 45:538–548
Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, Carmeliet P, Van Den Bosch L, Robberecht W (2008) Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol 181:37–41
Gao X, Joselin AP, Wang L, Kar A, Ray P, Bateman A, Goate AM, Wu JY (2010) Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3beta. Protein Cell 1:552–562
Gass J, Lee WC, Cook C, Finch N, Stetler C, Jansen-West K, Lewis J, Link CD, Rademakers R, Nykjaer A, Petrucelli L (2012) Progranulin regulates neuronal outgrowth independent of sortilin. Mol Neurodegener 7:33
Wang J, Van Damme P, Cruchaga C, Gitcho MA, Vidal JM, Seijo-Martinez M, Wang L, Wu JY, Robberecht W, Goate A (2010) Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J Neurochem 112:1305–1315
Kleinberger G, Wils H, Joris G, Ponsaerts P, Timmermans J-P, Van Broeckhoven C, Kumar-Singh S (2010) Increased caspase activation and decreased TDP-43 solubility in progranulin knockout cortical cultures. J Neurochem 115:735–747
Xu J, Xilouri M, Bruban J, Shioi J, Shao Z, Papazoglou I, Vekrellis K, Robakis NK (2011) Extracellular progranulin protects cortical neurons from toxic insults by activating survival signaling. Neurobiol Aging 32:2326.e5–2326.e16
Feng JQ, Guo FJ, Jiang BC, Zhang Y, Frenkel S, Wang DW, Tang W, Xie Y, Liu CJ (2010) Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J 24:1879–1892
Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A (2006) Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res 66:7103–7110
Ong CH, Bateman A (2003) Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histol Histopathol 18:1275–1288
Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R (1999) Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res 59:5331–5340
Matsubara T, Mita A, Minami K, Hosooka T, Kitazawa S, Takahashi K, Tamori Y, Yokoi N, Watanabe M, Matsuo E, Nishimura O, Seino S (2012) PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab 15:38–50
Xu SQ, Tang D, Chamberlain S, Pronk G, Masiarz FR, Kaur S, Prisco M, Zanocco-Marani T, Baserga R (1998) The granulin/epithelin precursor abrogates the requirement for the insulin-like growth factor 1 receptor for growth in vitro. J Biol Chem 273:20078–20083
Hu SY, Tai CC, Li YH, Wu JL (2012) Progranulin compensates for blocked IGF-1 signaling to promote myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR pathway. FEBS Lett 586:3485–3492
Culouscou JM, Carlton GW, Shoyab M (1993) Biochemical analysis of the epithelin receptor. J Biol Chem 268:10458–10462
Xia X, Serrero G (1998) Identification of cell surface binding sites for PC-cell-derived growth factor, PCDGF, (epithelin/granulin precursor) on epithelial cells and fibroblasts. Biochem Biophys Res Commun 245:539–543
Parnell PG, Carter BJ, Halper J (1995) Identification of a membrane-associated receptor for transforming growth factor type E. J Recept Signal Transduct Res 15:747–756
Braulke T, Bonifacino JS (2009) Sorting of lysosomal proteins. Biochim Biophys Acta 1793:605–614
Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM (2010) Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68:654–667
Zheng Y, Brady OA, Meng PS, Mao Y, Hu F (2011) C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS One 6:e21023
Nykjaer A, Willnow TE (2012) Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci 35:261–270
Bhandari V, Daniel R, Lim PS, Bateman A (1996) Structural and functional analysis of a promoter of the human granulin/epithelin gene. Biochem J 319(Pt 2):441–447
He Z, Ong CH, Halper J, Bateman A (2003) Progranulin is a mediator of the wound response. Nat Med 9:225–229
Kessenbrock K, Frohlich L, Sixt M, Lammermann T, Pfister H, Bateman A, Belaaouaj A, Ring J, Ollert M, Fassler R, Jenne DE (2008) Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest 118:2438–2447
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, Syed NM, Lai Y, Lin EA, Kong L, Su J, Yin F, Ding AH, Zanin-Zhorov A, Dustin ML, Tao J, Craft J, Yin Z, Feng JQ, Abramson SB, Yu XP, Liu CJ (2011) The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332:478–484
Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, Nathan C, Ding A (2009) Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med 207:117–128
Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A (2002) Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111:867–878
Bai XH, Wang DW, Kong L, Zhang Y, Luan Y, Kobayashi T, Kronenberg HM, Yu XP, Liu CJ (2009) ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol 29:4201–4219
Butler GS, Dean RA, Tam EM, Overall CM (2008) Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol 28:4896–4914
Xu D, Suenaga N, Edelmann MJ, Fridman R, Muschel RJ, Kessler BM (2008) Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics 7:2215–2228
Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM (2000) Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med 6:1147–1153
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, Syed NM, Lai Y, Lin EA, Kong L, Su J, Yin F, Ding AH, Zanin-Zhorov A, Dustin ML, Tao J, Craft J, Yin Z, Feng JQ, Abramson SB, Yu XP, Liu CJ (2011) The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 32:478–484
Okura H, Yamashita S, Ohama T, Saga A, Yamamoto-Kakuta A, Hamada Y, Sougawa N, Ohyama R, Sawa Y, Matsuyama A (2010) HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. J Atheroscler Thromb 17:568–577
Park B, Buti L, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, Nishihara M, Ploegh HL (2011) Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity 34:505–513
Moresco EM, Beutler B (2011) Special delivery: granulin brings CpG DNA to Toll-like receptor 9. Immunity 34:453–455
Pickford F, Marcus J, Camargo LM, Xiao Q, Graham D, Mo JR, Burkhardt M, Kulkarni V, Crispino J, Hering H, Hutton M (2011) Progranulin is a chemoattractant for microglia and stimulates their endocytic activity. Am J Pathol 178:284–295
Baker CA, Manuelidis L (2003) Unique inflammatory RNA profiles of microglia in Creutzfeldt–Jakob disease. Proc Natl Acad Sci U S A 100:675–679
Gliebus G, Rosso A, Lippa CF (2009) Progranulin and beta-amyloid distribution: a case report of the brain from preclinical PS-1 mutation carrier. Am J Alzheimers Dis Other Demen 24:456–460
Malaspina A, Kaushik N, de BJ (2001) Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J Neurochem 77:132–145
Chen-Plotkin AS, Xiao J, Geser F, Martinez-Lage M, Grossman M, Unger T, Wood EM, Van Deerlin VM, Trojanowski JQ, Lee VM (2010) Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol 119:111–122
Pereson S, Wils H, Kleinberger G, McGowan E, Vandewoestyne M, Van Broeck B, Joris G, Cuijt I, Deforce D, Hutton M, Van Broeckhoven C, Kumar-Singh S (2009) Progranulin expression correlates with dense-core amyloid plaque burden in Alzheimer disease mouse models. J Pathol 219:173–181
Ferraiuolo L, Heath PR, Holden H, Kasher P, Kirby J, Shaw PJ (2007) Microarray analysis of the cellular pathways involved in the adaptation to and progression of motor neuron injury in the SOD1 G93A mouse model of familial ALS. J Neurosci 27:9201–9219
Lobsiger CS, Boillee S, Cleveland DW (2007) Toxicity from different SOD1 mutants dysregulates the complement system and the neuronal regenerative response in ALS motor neurons. Proc Natl Acad Sci U S A 104:7319–7326
Philips T, De Muynck L, Thu HN, Weynants B, Vanacker P, Dhondt J, Sleegers K, Schelhaas HJ, Verbeek M, Vandenberghe R, Sciot R, Van Broeckhoven C, Lambrechts D, Van Leuven F, Van Den Bosch L, Robberecht W, Van Damme P (2010) Microglial upregulation of progranulin as a marker of motor neuron degeneration. J Neuropathol Exp Neurol 69:1191–1200
Sjogren M, Folkesson S, Blennow K, Tarkowski E (2004) Increased intrathecal inflammatory activity in frontotemporal dementia: pathophysiological implications. J Neurol Neurosurg Psychiatry 75:1107–1111
Bossu P, Salani F, Alberici A, Archetti S, Bellelli G, Galimberti D, Scarpini E, Spalletta G, Caltagirone C, Padovani A, Borroni B (2011) Loss of function mutations in the progranulin gene are related to pro-inflammatory cytokine dysregulation in frontotemporal lobar degeneration patients. J Neuroinflammation 8:65
McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27:741–749
Hirsch EC, Vyas S, Hunot S (2012) Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S210–S212
Bruce-Keller AJ, Keller JN, Morrison CD (2009) Obesity and vulnerability of the CNS. Biochim Biophys Acta 1792:395–400
Luchsinger JA, Gustafson DR (2009) Adiposity, type 2 diabetes, and Alzheimer’s disease. J Alzheimers Dis 16:693–704
Kim B, Feldman EL (2012) Insulin resistance in the nervous system. Trends Endocrinol Metab 23:133–141
Youn BS, Bang SI, Kloting N, Park JW, Lee N, Oh JE, Pi KB, Lee TH, Ruschke K, Fasshauer M, Stumvoll M, Bluher M (2009) Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes 58:627–636
Richter J, Focke D, Ebert T, Kovacs P, Bachmann A, Lossner U, Kralisch S, Kratzsch J, Beige J, Anders M, Bast I, Bluher M, Stumvoll M, Fasshauer M (2012) Serum levels of the adipokine progranulin depend on renal function. Diabetes Care (in press)
Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M (2010) Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 299:E506–E515
Kim HK, Shin MS, Youn BS, Namkoong C, Gil SY, Kang GM, Yu JH, Kim MS (2011) Involvement of progranulin in hypothalamic glucose sensing and feeding regulation. Endocrinology 152:4672–4682
Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JS, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Tenenholz GL, Liscic RM, Armendariz J, Morris JC, Goate AM (2006) HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol 60:314–322
Mukherjee O, Wang J, Gitcho M, Chakraverty S, Taylor-Reinwald L, Shears S, Kauwe JS, Norton J, Levitch D, Bigio EH, Hatanpaa KJ, White CL, Morris JC, Cairns NJ, Goate A (2008) Molecular characterization of novel progranulin (GRN) mutations in frontotemporal dementia. Hum Mutat 29:512–521
Shankaran SS, Capell A, Hruscha AT, Fellerer K, Neumann M, Schmid B, Haass C (2008) Missense mutations in the progranulin gene linked to frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions reduce progranulin production and secretion. J Biol Chem 283:1744–1753
Hoque M, Mathews MB, Pe’ery T (2010) Progranulin (granulin/epithelin precursor) and its constituent granulin repeats repress transcription from cellular promoters. J Cell Physiol 223:224–233
Hoque M, Young TM, Lee CG, Serrero G, Mathews MB, Pe’ery T (2003) The growth factor granulin interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol Cell Biol 23:1688–1702
Chen-Plotkin AS, Geser F, Plotkin JB, Clark CM, Kwong LK, Yuan W, Grossman M, Van Deerlin VM, Trojanowski JQ, Lee VM (2008) Variations in the progranulin gene affect global gene expression in frontotemporal lobar degeneration. Hum Mol Genet 17:1349–1362
Guo Z, Li Q, Han Y, Liang Y, Xu Z, Ren T (2012) Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators Inflamm 2012:540794
Bateman A, Bennett HP (2009) The granulin gene family: from cancer to dementia. Bioessays 31:1245–1254
Liu Y, Xi L, Liao G, Wang W, Tian X, Wang B, Chen G, Han Z, Wu M, Wang S, Zhou J, Xu G, Lu Y, Ma D (2007) Inhibition of PC cell-derived growth factor (PCDGF)/granulin-epithelin precursor (GEP) decreased cell proliferation and invasion through downregulation of cyclin D and CDK4 and inactivation of MMP-2. BMC Cancer 7:22
Lu R, Serrero G (2001) Mediation of estrogen mitogenic effect in human breast cancer MCF-7 cells by PC-cell-derived growth factor (PCDGF/granulin precursor). Proc Natl Acad Sci U S A 98:142–147
Park MY, Park YS, Nam JH (2011) RNA interference against granulin-epithelin precursor prevents hepatocellular carcinoma growth: its application as a therapeutic agent. Int J Oncol 39:853–861
Guo A, Tapia L, Bamji SX, Cynader MS, Jia W (2010) Progranulin deficiency leads to enhanced cell vulnerability and TDP-43 translocation in primary neuronal cultures. Brain Res 1366:1–8.
Kamrava M, Simpkins F, Alejandro E, Michener C, Meltzer E, Kohn EC (2005) Lysophosphatidic acid and endothelin-induced proliferation of ovarian cancer cell lines is mitigated by neutralization of granulin-epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene 24:7084–7093
Kong WJ, Zhang SL, Chen X, Zhang S, Wang YJ, Zhang D, Sun Y (2007) PC cell-derived growth factor overexpression promotes proliferation and survival of laryngeal carcinoma. Anticancer Drugs 18:29–40
Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson D, Petrucelli L (2007) Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci 27:10530–10534
Brouwers N, Sleegers K, Engelborghs S, Maurer-Stroh S, Gijselinck I, van der Zee J, Pickut BA, Van den Broeck M, Mattheijssens M, Peeters K, Schymkowitz J, Rousseau F, Martin JJ, Cruts M, De Deyn PP, Van Broeckhoven C (2008) Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology 71:656–664
Sleegers K, Brouwers N, Maurer-Stroh S, van Es MA, Van Damme P, van Vught PW, van der Zee J, Serneels S, De Pooter T, Van den Broeck M, Cruts M, Schymkowitz J, De Jonghe P, Rousseau F, van den Berg LH, Robberecht W, Van Broeckhoven C (2008) Progranulin genetic variability contributes to amyotrophic lateral sclerosis. Neurology 71:253–259
Almeida S, Zhang Z, Coppola G, Mao W, Futai K, Karydas A, Geschwind MD, Tartaglia MC, Gao F, Gianni D, Sena-Esteves M, Geschwind DH, Miller BL, Farese RV Jr, Gao FB (2012) Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell Rep 2:789–798
Nedachi T, Kawai T, Matsuwaki T, Yamanouchi K, Nishihara M (2011) Progranulin enhances neural progenitor cell proliferation through glycogen synthase kinase 3beta phosphorylation. Neuroscience 185:106–115
Rosen EY, Wexler EM, Versano R, Coppola G, Gao F, Winden KD, Oldham MC, Martens LH, Zhou P, Farese RV Jr, Geschwind DH (2011) Functional genomic analyses identify pathways dysregulated by progranulin deficiency, implicating wnt signaling. Neuron 71:1030–1042
Ryan CL, Baranowski DC, Chitramuthu BP, Malik S, Li Z, Cao M, Minotti S, Durham HD, Kay DG, Shaw CA, Bennett HP, Bateman A (2009) Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci 10:130
Tapia L, Milnerwood A, Guo A, Mills F, Yoshida E, Vasuta C, Mackenzie IR, Raymond L, Cynader M, Jia W, Bamji SX (2011) Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J Neurosci 31:11126–11132
Jope RS, Johnson GV (2004) The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29:95–102
Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, Gregg J, Lu G, Feldman HH, Mackenzie IR, Raymond LA, Leavitt BR (2012) Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis 45:711–722
Sleegers K, Cruts M, Van BC (2010) Molecular pathways of frontotemporal lobar degeneration. Annu Rev Neurosci 33:71–88
Lim HY, Albuquerque B, Haussler A, Myrczek T, Ding A, Tegeder I (2012) Progranulin contributes to endogenous mechanisms of pain defense after nerve injury in mice. J Cell Mol Med 16:708–721
Dormann D, Capell A, Carlson AM, Shankaran SS, Rodde R, Neumann M, Kremmer E, Matsuwaki T, Yamanouchi K, Nishihara M, Haass C (2009) Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem 110:1082–1094
Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, Neumann M, Trojanowski JQ, Lee VM (2009) Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J Biol Chem 284:8516–8524
Cadieux B, Chitramuthu BP, Baranowski D, Bennett HP (2005) The zebrafish progranulin gene family and antisense transcripts. BMC Genomics 6:156
Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, Zhou P, De LA, Neukomm LJ, Cabello J, Farese RV Jr, Kenyon C (2011) A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A 108:4441–4446
Pera EM, Hou S, Strate I, Wessely O, De Robertis EM (2005) Exploration of the extracellular space by a large-scale secretion screen in the early Xenopus embryo. Int J Dev Biol 49:781–796
Li YH, Chen MH, Gong HY, Hu SY, Li YW, Lin GH, Lin CC, Liu W, Wu JL (2010) Progranulin A-mediated MET signaling is essential for liver morphogenesis in zebrafish. J Biol Chem 285:41001–41009
Chitramuthu BP, Baranowski DC, Kay DG, Bateman A, Bennett HP (2010) Progranulin modulates zebrafish motoneuron development in vivo and rescues truncation defects associated with knockdown of survival motor neuron 1. Mol Neurodegener 5:41
Laird AS, Van Hoecke A, De Muynck L, Timmers M, Van Den Bosch L, Van Damme P, Robberecht W (2010) Progranulin is neurotrophic in vivo and protects against a mutant TDP-43 induced axonopathy. PLoS One 5:e13368
Wils H, Kleinberger G, Pereson S, Janssens J, Capell A, Van Dam D, Cuijt I, Joris G, De Deyn PP, Haass C, Van Broeckhoven C, Kumar-Singh S (2012) Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol 228(1):67–76
Martens LH, Zhang J, Barmada SJ, Zhou P, Kamiya S, Sun B, Min SW, Gan L, Finkbeiner S, Huang EJ, Farese RV Jr (2012) Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest 122:3955–3959
Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J (2010) Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggests a role for progranulin in successful aging. Am J Pathol 177:311–324
Ghoshal N, Dearborn JT, Wozniak DF, Cairns NJ (2012) Core features of frontotemporal dementia recapitulated in progranulin knockout mice. Neurobiol Dis 45:395–408
Suzuki M, Bannai M, Matsumuro M, Furuhata Y, Ikemura R, Kuranaga E, Kaneda Y, Nishihara M, Takahashi M (2000) Suppression of copulatory behavior by intracerebroventricular infusion of antisense oligodeoxynucleotide of granulin in neonatal male rats. Physiol Behav 68:707–713
Suzuki M, Lee HC, Kayasuga Y, Chiba S, Nedachi T, Matsuwaki T, Yamanouchi K, Nishihara M (2009) Roles of progranulin in sexual differentiation of the developing brain and adult neurogenesis. J Reprod Dev 55:351–355
Suzuki M, Nishiahara M (2002) Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab 75:31–37
Chiba S, Matsuwaki T, Yamanouchi K, Nishihara M (2009) Alteration in anxiety with relation to the volume of the locus ceruleus in progranulin-deficient mice. J Reprod Dev 55:518–522
Yin F, Dumont M, Banerjee R, Ma Y, Li H, Lin MT, Beal MF, Nathan C, Thomas B, Ding A (2010) Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J 24:4639–4647
Bailey KR, Rustay NR, Crawley JN (2006) Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J 47:124–131
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124
Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, Sims KB, Lewis J, Lin WL, Dickson DW, Dahl HH, Bahlo M, Berkovic SF (2012) Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet 90:1102–1107
Kohlschutter A, Schulz A (2009) Towards understanding the neuronal ceroid lipofuscinoses. Brain Dev 31:499–502
Kaye ED, Petrovic-Poljak A, Verhoeff NP, Freedman M (2010) Frontotemporal dementia and pharmacologic interventions. J Neuropsychiatry Clin Neurosci 22:19–29
Finch N, Baker M, Crook R, Swanson K, Kuntz K, Surtees R, Bisceglio G, Rovelet-Lecrux A, Boeve B, Petersen RC, Dickson DW, Younkin SG, Deramecourt V, Crook J, Graff-Radford NR, Rademakers R (2009) Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132:583–591
Ghidoni R, Stoppani E, Rossi G, Piccoli E, Albertini V, Paterlini A, Glionna M, Pegoiani E, Agnati LF, Fenoglio C, Scarpini E, Galimberti D, Morbin M, Tagliavini F, Binetti G, Benussi L (2012) Optimal plasma progranulin cutoff value for predicting null progranulin mutations in neurodegenerative diseases: a multicenter Italian study. Neurodegener Dis 9:121–127
Sleegers K, Brouwers N, Van Damme P, Engelborghs S, Gijselinck I, van der Zee J, Peeters K, Mattheijssens M, Cruts M, Vandenberghe R, De Deyn PP, Robberecht W, Van Broeckhoven C (2009) A serum biomarker for progranulin-associated frontotemporal lobar degeneration. Ann Neurol 65:603–609
Tao J, Ji F, Wang F, Liu B, Zhu Y (2012) Neuroprotective effects of progranulin in ischemic mice. Brain Res 1436:130–136
Vossel KA, Miller BL (2008) New approaches to the treatment of frontotemporal lobar degeneration. Curr Opin Neurol 21:708–716
Du L, Damoiseaux R, Nahas S, Gao K, Hu H, Pollard JM, Goldstine J, Jung ME, Henning SM, Bertoni C, Gatti RA (2009) Nonaminoglycoside compounds induce readthrough of nonsense mutations. J Exp Med 206: 2285–2297. Doi: 10.1084/jem.20081940
Manuvakhova M, Keeling K, Bedwell DM (2000) Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA 6:1044–1055
Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL (2007) PTC124 targets genetic disorders caused by nonsense mutations. Nature 447:87–91
Finkel RS (2010) Read-through strategies for suppression of nonsense mutations in Duchenne/Becker muscular dystrophy: aminoglycosides and ataluren (PTC124). J Child Neurol 25:1158–1164
Hirawat S, Welch EM, Elfring GL, Northcutt VJ, Paushkin S, Hwang S, Leonard EM, Almstead NG, Ju W, Peltz SW, Miller LL (2007) Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol 47:430–444
Cenik B, Sephton CF, Dewey CM, Xian X, Wei S, Yu K, Niu W, Coppola G, Coughlin SE, Lee SE, Dries DR, Almeida S, Geschwind DH, Gao FB, Miller BL, Farese RV Jr, Posner BA, Yu G, Herz J (2011) Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J Biol Chem 286:16101–16108
Capell A, Liebscher S, Fellerer K, Brouwers N, Willem M, Lammich S, Gijselinck I, Bittner T, Carlson AM, Sasse F, Kunze B, Steinmetz H, Jansen R, Dormann D, Sleegers K, Cruts M, Herms J, Van Broeckhoven C, Haass C (2011) Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J Neurosci 31:1885–1894
Almeida S, Zhou L, Gao FB (2011) Progranulin, a glycoprotein deficient in frontotemporal dementia, is a novel substrate of several protein disulfide isomerase family proteins. PLoS One 6:e26454
Kiser D, Steemers B, Branchi I, Homberg JR (2012) The reciprocal interaction between serotonin and social behaviour. Neurosci Biobehav Rev 36:786–798
Huey ED, Putnam KT, Grafman J (2006) A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 66:17–22
Lebert F, Stekke W, Hasenbroekx C, Pasquier F (2004) Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord 17:355–359
Trojanowski JQ, Duff K, Fillit H, Koroshetz W, Kuret J, Murphy D, Refolo L (2008) New directions for frontotemporal dementia drug discovery. Alzheimers Dement 4:89–93
Galimberti D, Fenoglio C, Scarpini E (2008) Inflammation in neurodegenerative disorders: friend or foe? Curr Aging Sci 1:30–41
Nukiwa T, Suzuki T, Fukuhara T, Kikuchi T (2008) Secretory leukocyte peptidase inhibitor and lung cancer. Cancer Sci 99:849–855
Matayoshi H, Hirata T, Yamashita S, Ishida K, Mizukami Y, Gondo T, Matsumoto M, Sakabe T (2009) Neutrophil elastase inhibitor attenuates hippocampal neuronal damage after transient forebrain ischemia in rats. Brain Res 1259:98–106
Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umemura K (2000) Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res 858:55–60
Yamauchi T, Sawa Y, Sakurai M, Hiroshi T, Matsumiya G, Abe K, Matsuda H (2006) ONO-5046 attenuation of delayed motor neuron death and effect on the induction of brain-derived neurotrophic factor, phosphorylated extracellular signal-regulated kinase, and caspase3 after spinal cord ischemia in rabbits. J Thorac Cardiovasc Surg 131:644–650
Acknowledgments
The research in the author’s group has been in part supported by the Interuniversity Attraction Poles program of the Belgian Science Policy Office, the Medical Foundation Queen Elisabeth, the Foundation for Alzheimer Research (SAO-FRMA), the Methusalem Excellence program of the Flemish Government, the Research Foundation Flanders, the Agency for Innovation by Science and Technology, and the Special Research Fund of the University of Antwerp, Belgium. The research was performed in the frame of the international consortium of Centers of Excellence in Neurodegenerative Brain Diseases (http://www.coen.org/) supported by the Flemish Government through the VIB (C.V.B.) and DZNE (C.H.). G.K. received a Ph.D. fellowship of the Research Foundation Flanders.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kleinberger, G., Capell, A., Haass, C. et al. Mechanisms of Granulin Deficiency: Lessons from Cellular and Animal Models. Mol Neurobiol 47, 337–360 (2013). https://doi.org/10.1007/s12035-012-8380-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8380-8