Abstract
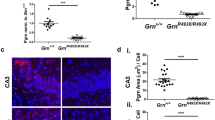
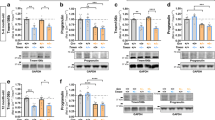
Heterozygous loss-of-function mutations in the progranulin gene (GRN) are a major cause of frontotemporal dementia due to progranulin haploinsufficiency; complete deficiency of progranulin causes neuronal ceroid lipofuscinosis. Several progranulin-deficient mouse models have been generated, including both knockout mice and knockin mice harboring a common patient mutation (R493X). However, the GrnR493X mouse model has not been characterized completely. Additionally, while homozygous GrnR493X and Grn knockout mice have been extensively studied, data from heterozygous mice is still limited. Here, we performed more in-depth characterization of heterozygous and homozygous GrnR493X knockin mice, which includes biochemical assessments, behavioral studies, and analysis of fluid biomarkers. In the brains of homozygous GrnR493X mice, we found increased phosphorylated TDP-43 along with increased expression of lysosomal genes, markers of microgliosis and astrogliosis, pro-inflammatory cytokines, and complement factors. Heterozygous GrnR493X mice did not have increased TDP-43 phosphorylation but did exhibit limited increases in lysosomal and inflammatory gene expression. Behavioral studies found social and emotional deficits in GrnR493X mice that mirror those observed in Grn knockout mouse models, as well as impairment in memory and executive function. Overall, the GrnR493X knockin mouse model closely phenocopies Grn knockout models. Lastly, in contrast to homozygous knockin mice, heterozygous GrnR493X mice do not have elevated levels of fluid biomarkers previously identified in humans, including neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) in both plasma and CSF. These results may help to inform pre-clinical studies that use this Grn knockin mouse model and other Grn knockout models.






Similar content being viewed by others
Data Availability
All relevant data are within the paper and its Supplementary Information files.
References
Petkau TL, Leavitt BR (2014) Progranulin in neurodegenerative disease. Trends Neurosci 37(7):388–398. https://doi.org/10.1016/j.tins.2014.04.003
Bateman A, Cheung ST, Bennett HPJ (2018) A brief overview of progranulin in health and disease. Methods Mol Biol 1806:3–15. https://doi.org/10.1007/978-1-4939-8559-3_1
Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G (2012) Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J Biol Chem 287(39):32298–32306. https://doi.org/10.1074/jbc.R112.399170
Kao AW, McKay A, Singh PP, Brunet A, Huang EJ (2017) Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci 18(6):325–333. https://doi.org/10.1038/nrn.2017.36
Nguyen AD, Nguyen TA, Martens LH, Mitic LL, Farese RV Jr (2013) Progranulin: at the interface of neurodegenerative and metabolic diseases. Trends Endocrinol Metab 24(12):597–606. https://doi.org/10.1016/j.tem.2013.08.003
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J et al (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442(7105):916–919. https://doi.org/10.1038/nature05016
Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R et al (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442(7105):920–924. https://doi.org/10.1038/nature05017
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S et al (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15(20):2988–3001. https://doi.org/10.1093/hmg/ddl241
Miller B, Llibre Guerra JJ (2019) Frontotemporal dementia. Handb Clin Neurol 165:33–45. https://doi.org/10.1016/b978-0-444-64012-3.00003-4
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133. https://doi.org/10.1126/science.1134108
Bevan-Jones WR, Cope TE, Jones PS, Kaalund SS, Passamonti L, Allinson K, Green O, Hong YT et al (2020) Neuroinflammation and protein aggregation co-localize across the frontotemporal dementia spectrum. Brain 143(3):1010–1026. https://doi.org/10.1093/brain/awaa033
Rohrer JD, Woollacott IOC, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R et al (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87(13):1329–1336
Meeter LH, Dopper EG, Jiskoot LC, Sanchez-Valle R, Graff C, Benussi L, Ghidoni R, Pijnenburg YA et al (2016) Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol 3(8):623–636. https://doi.org/10.1002/acn3.325
Zhu N, Santos-Santos M, Illán-Gala I, Montal V, Estellés T, Barroeta I, Altuna M, Arranz J et al (2021) Plasma glial fibrillary acidic protein and neurofilament light chain for the diagnostic and prognostic evaluation of frontotemporal dementia. Transl Neurodegener 10(1):50. https://doi.org/10.1186/s40035-021-00275-w
Suárez-Calvet M, Dols-Icardo O, Lladó A, Sánchez-Valle R, Hernández I, Amer G, Antón-Aguirre S, Alcolea D et al (2014) Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J Neurol Neurosurg Psychiatry 85(6):684–691. https://doi.org/10.1136/jnnp-2013-305972
Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, Snowden JS, Allsop D et al (2008) TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol 116(2):141–146. https://doi.org/10.1007/s00401-008-0389-8
Foulds PG, Davidson Y, Mishra M, Hobson DJ, Humphreys KM, Taylor M, Johnson N, Weintraub S et al (2009) Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta Neuropathol 118(5):647–658. https://doi.org/10.1007/s00401-009-0594-0
Heller C, Foiani MS, Moore K, Convery R, Bocchetta M, Neason M, Cash DM, Thomas D et al (2020) Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J Neurol Neurosurg Psychiatry 91(3):263–270. https://doi.org/10.1136/jnnp-2019-321954
Rojas JC, Wang P, Staffaroni AM, Heller C, Cobigo Y, Wolf A, Goh SM, Ljubenkov PA et al (2021) Plasma neurofilament light for prediction of disease progression in familial frontotemporal lobar degeneration. Neurology 96(18):e2296–e2312. https://doi.org/10.1212/wnl.0000000000011848
Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D et al (2012) Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet 90(6):1102–1107. https://doi.org/10.1016/j.ajhg.2012.04.021
Almeida M, Macario M, Ramos L, Baldeiras I, Ribeiro M, Santana I (2016) Portuguese family with the co-occurrence of frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis phenotypes due to progranulin gene mutation. Neurobiol Aging 41(200):e201–e205. https://doi.org/10.1016/j.neurobiolaging.2016.02.019
Huin V, Barbier M, Bottani A, Lobrinus JA, Clot F, Lamari F, Chat L, Rucheton B et al (2020) Homozygous GRN mutations: new phenotypes and new insights into pathological and molecular mechanisms. Brain 143(1):303–319. https://doi.org/10.1093/brain/awz377
Martens LH, Zhang J, Barmada SJ, Zhou P, Kamiya S, Sun B, Min SW, Gan L et al (2012) Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest 122(11):3955–3959. https://doi.org/10.1172/JCI63113
Petkau TL, Neal SJ, Milnerwood A, Mew A, Hill AM, Orban P, Gregg J, Lu G et al (2012) Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis 45(2):711–722. https://doi.org/10.1016/j.nbd.2011.10.016
Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y et al (2010) Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med 207(1):117–128. https://doi.org/10.1084/jem.20091568
Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R et al (2007) Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res 185(2):110–118. https://doi.org/10.1016/j.bbr.2007.07.020
Fujita K, Chen X, Homma H, Tagawa K, Amano M, Saito A, Imoto S, Akatsu H et al (2018) Targeting Tyro3 ameliorates a model of PGRN-mutant FTLD-TDP via tau-mediated synaptic pathology. Nat Commun 9(1):433. https://doi.org/10.1038/s41467-018-02821-z
Nguyen AD, Nguyen TA, Zhang J, Devireddy S, Zhou P, Xu X, Karydas AM, Miller BL, Rigo F et al (2018) Murine knockin model for progranulin-deficient frontotemporal dementia with nonsense-mediated mRNA decay. Proc Natl Acad Sci USA 115(12):E2849–E2858
Tanaka Y, Chambers JK, Matsuwaki T, Yamanouchi K, Nishihara M (2014) Possible involvement of lysosomal dysfunction in pathological changes of the brain in aged progranulin-deficient mice. Acta Neuropathol Commun 2:78
Götzl JK, Mori K, Damme M, Fellerer K, Tahirovic S, Kleinberger G, Janssens J, van der Zee J et al (2014) Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol 127(6):845–860. https://doi.org/10.1007/s00401-014-1262-6
Chang MC, Srinivasan K, Friedman BA, Suto E, Modrusan Z, Lee WP, Kaminker JS, Hansen DV et al (2017) Progranulin deficiency causes impairment of autophagy and TDP-43 accumulation. J Exp Med 214(9):2611–2628. https://doi.org/10.1084/jem.20160999
Lui H, Zhang J, Makinson S, Cahill M, Kelley K, Huang H, Shang Y, Oldham M et al (2016) Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165(4):921–935
Ward ME, Chen R, Huang HY, Ludwig C, Telpoukhovskaia M, Taubes A, Boudin H, Minami SS et al (2017) Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci Transl Med 9(385). https://doi.org/10.1126/scitranslmed.aah5642
Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA et al (2010) Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol 177(1):311–324. https://doi.org/10.2353/ajpath.2010.090915
Filiano AJ, Martens LH, Young AH, Warmus BA, Zhou P, Diaz-Ramirez G, Jiao J, Zhang Z et al (2013) Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J Neurosci 33(12):5352–5361. https://doi.org/10.1523/JNEUROSCI.6103-11.2013
Yin F, Dumont M, Banerjee R, Ma Y, Li H, Lin MT, Beal MF, Nathan C et al (2010) Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J 24(12):4639–4647. https://doi.org/10.1096/fj.10-161471
Frew J, Nygaard HB (2021) Neuropathological and behavioral characterization of aged Grn R493X progranulin-deficient frontotemporal dementia knockin mice. Acta Neuropathol Commun 9(1):57. https://doi.org/10.1186/s40478-021-01158-x
Arrant A, Filiano A, Warmus B, Hall A, Roberson E (2016) Progranulin haploinsufficiency causes biphasic social dominance abnormalities in the tube test. Genes Brain Behav 15(6):588–603
Krabbe G, Minami SS, Etchegaray JI, Taneja P, Djukic B, Davalos D, Le D, Lo I et al (2017) Microglial NFκB-TNFα hyperactivation induces obsessive-compulsive behavior in mouse models of progranulin-deficient frontotemporal dementia. Proc Natl Acad Sci USA 114(19):5029–5034
Ghoshal N, Dearborn JT, Wozniak DF, Cairns NJ (2012) Core features of frontotemporal dementia recapitulated in progranulin knockout mice. Neurobiol Dis 45(1):395–408. https://doi.org/10.1016/j.nbd.2011.08.029
Deacon R (2012) Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp 59:e2607
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3(5):287–302. https://doi.org/10.1111/j.1601-1848.2004.00076.x
Farr SA, Niehoff ML, Kumar VB, Roby DA, Morley JE (2019) Inhibition of glycogen synthase kinase 3beta as a treatment for the prevention of cognitive deficits after a traumatic brain injury. J Neurotrauma 36(11):1869–1875. https://doi.org/10.1089/neu.2018.5999
Farr SA, Banks WA, La Scola ME, Flood JF, Morley JE (2000) Permanent and temporary inactivation of the hippocampus impairs T-maze footshock avoidance acquisition and retention. Brain Res 872(1-2):242–249. https://doi.org/10.1016/s0006-8993(00)02495-1
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916:105–111. https://doi.org/10.1007/978-1-4939-8994-2_10
Hammond RS, Tull LE, Stackman RW (2004) On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82(1):26–34. https://doi.org/10.1016/j.nlm.2004.03.005
Ben Abdallah NM, Fuss J, Trusel M, Galsworthy MJ, Bobsin K, Colacicco G, Deacon RM, Riva MA et al (2011) The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol 227(1):42–52. https://doi.org/10.1016/j.expneurol.2010.09.008
O'Connor AM, Burton TJ, Leamey CA, Sawatari A (2014) The use of the puzzle box as a means of assessing the efficacy of environmental enrichment. J Vis Exp 94:52225. https://doi.org/10.3791/52225
Nicholson A, Finch N, Almeida M, Perkerson R, van Blitterswijk M, Wojtas A, Cenik B, Rotondo S et al (2016) Prosaposin is a regulator of progranulin levels and oligomerization. Nat Commun 7:11992
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351(3):602–611. https://doi.org/10.1016/j.bbrc.2006.10.093
Wils H, Kleinberger G, Pereson S, Janssens J, Capell A, Van Dam D, Cuijt I, Joris G et al (2012) Cellular ageing, increased mortality and FTLD-TDP-associated neuropathology in progranulin knockout mice. J Pathol 228(1):67–76. https://doi.org/10.1002/path.4043
Zhang J, Velmeshev D, Hashimoto K, Huang YH, Hofmann JW, Shi X, Chen J, Leidal AM et al (2020) Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency. Nature 588(7838):459–465. https://doi.org/10.1038/s41586-020-2709-7
Weskamp K, Tank EM, Miguez R, McBride JP, Gómez NB, White M, Lin Z, Gonzalez CM et al (2020) Shortened TDP43 isoforms upregulated by neuronal hyperactivity drive TDP43 pathology in ALS. J Clin Invest 130(3):1139–1155. https://doi.org/10.1172/jci130988
Ling JP, Pletnikova O, Troncoso JC, Wong PC (2015) TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science 349(6248):650–655. https://doi.org/10.1126/science.aab0983
Melamed Z, López-Erauskin J, Baughn MW, Zhang O, Drenner K, Sun Y, Freyermuth F, McMahon MA et al (2019) Premature polyadenylation-mediated loss of stathmin-2 is a hallmark of TDP-43-dependent neurodegeneration. Nat Neurosci 22(2):180–190. https://doi.org/10.1038/s41593-018-0293-z
Ma XR, Prudencio M, Koike Y, Vatsavayai SC, Kim G, Harbinski F, Briner A, Rodriguez CM et al (2022) TDP-43 represses cryptic exon inclusion in the FTD-ALS gene UNC13A. Nature 603(7899):124–130. https://doi.org/10.1038/s41586-022-04424-7
Brown AL, Wilkins OG, Keuss MJ, Hill SE, Zanovello M, Lee WC, Bampton A, Lee FCY et al (2022) TDP-43 loss and ALS-risk SNPs drive mis-splicing and depletion of UNC13A. Nature 603(7899):131–137. https://doi.org/10.1038/s41586-022-04436-3
Prudencio M, Humphrey J, Pickles S, Brown AL, Hill SE, Kachergus JM, Shi J, Heckman MG et al (2020) Truncated stathmin-2 is a marker of TDP-43 pathology in frontotemporal dementia. J Clin Invest 130(11):6080–6092. https://doi.org/10.1172/jci139741
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L et al (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14(10):577–589. https://doi.org/10.1038/s41582-018-0058-z
Arrant AE, Filiano AJ, Unger DE, Young AH, Roberson ED (2017) Restoring neuronal progranulin reverses deficits in a mouse model of frontotemporal dementia. Brain 140(5):1447–1465. https://doi.org/10.1093/brain/awx060
Chiba S, Matsuwaki T, Yamanouchi K, Nishihara M (2009) Alteration in anxiety with relation to the volume of the locus ceruleus in progranulin-deficient mice. J Reprod Dev 55(5):518–522. https://doi.org/10.1262/jrd.20239
Petkau TL, Hill A, Leavitt BR (2016) Core neuropathological abnormalities in progranulin-deficient mice are penetrant on multiple genetic backgrounds. Neuroscience 315:175–195. https://doi.org/10.1016/j.neuroscience.2015.12.006
Handley SL, McBlane JW (1993) An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods 29(3):129–138. https://doi.org/10.1016/1056-8719(93)90063-k
Sestakova N, Puzserova A, Kluknavsky M, Bernatova I (2013) Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol 6(3):126–135. https://doi.org/10.2478/intox-2013-0020
Benussi A, Premi E, Gazzina S, Brattini C, Bonomi E, Alberici A, Jiskoot L, van Swieten JC et al (2021) Progression of behavioral disturbances and neuropsychiatric symptoms in patients with genetic frontotemporal dementia. JAMA Netw Open 4(1):e2030194. https://doi.org/10.1001/jamanetworkopen.2020.30194
Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM (2004) Clinicopathological correlates in frontotemporal dementia. Ann Neurol 56(3):399–406. https://doi.org/10.1002/ana.20203
Possin KL, Feigenbaum D, Rankin KP, Smith GE, Boxer AL, Wood K, Hanna SM, Miller BL et al (2013) Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology 80(24):2180–2185. https://doi.org/10.1212/WNL.0b013e318296e940
Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL (2005) Neuroanatomical correlates of behavioural disorders in dementia. Brain 128(Pt 11):2612–2625. https://doi.org/10.1093/brain/awh628
van der Ende EL, Heller C, Sogorb-Esteve A, Swift IJ, McFall D, Peakman G, Bouzigues A, Poos JM et al (2022) Elevated CSF and plasma complement proteins in genetic frontotemporal dementia: results from the GENFI study. J Neuroinflammation 19(1):217. https://doi.org/10.1186/s12974-022-02573-0
Boland S, Swarup S, Ambaw YA, Malia PC, Richards RC, Fischer AW, Singh S, Aggarwal G et al (2022) Deficiency of the frontotemporal dementia gene GRN results in gangliosidosis. Nat Commun 13(1):5924. https://doi.org/10.1038/s41467-022-33500-9
Logan T, Simon MJ, Rana A, Cherf GM, Srivastava A, Davis SS, Low RLY, Chiu CL et al (2021) Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic. Cell 184(18):4651–4668.e4625. https://doi.org/10.1016/j.cell.2021.08.002
Roberson ED (2012) Mouse models of frontotemporal dementia. Ann Neurol 72(6):837–849. https://doi.org/10.1002/ana.23722
Petkau TL, Life B, Lu G, Yang J, Fornes O, Wasserman W, Simpson EM, Leavitt BR (2021) Human progranulin-expressing mice as a novel tool for the development of progranulin-modulating therapeutics. Neurobiol Dis 153:105314. https://doi.org/10.1016/j.nbd.2021.105314
Chien HF, Rodriguez RD, Bonifati V, Nitrini R, Pasqualucci CA, Gelpi E, Barbosa ER (2021) Neuropathologic findings in a patient with juvenile-onset levodopa-responsive parkinsonism due to ATP13A2 mutation. Neurology 97(16):763–766. https://doi.org/10.1212/wnl.0000000000012705
Takahashi K, Nelvagal HR, Lange J, Cooper JD (2022) Glial dysfunction and its contribution to the pathogenesis of the neuronal ceroid lipofuscinoses. Front Neurol 13:886567. https://doi.org/10.3389/fneur.2022.886567
Nakanishi H, Zhang J, Koike M, Nishioku T, Okamoto Y, Kominami E, von Figura K, Peters C et al (2001) Involvement of nitric oxide released from microglia-macrophages in pathological changes of cathepsin D-deficient mice. J Neurosci 21(19):7526–7533. https://doi.org/10.1523/jneurosci.21-19-07526.2001
Partanen S, Haapanen A, Kielar C, Pontikis C, Alexander N, Inkinen T, Saftig P, Gillingwater TH et al (2008) Synaptic changes in the thalamocortical system of cathepsin D-deficient mice: a model of human congenital neuronal ceroid-lipofuscinosis. J Neuropathol Exp Neurol 67(1):16–29. https://doi.org/10.1097/nen.0b013e31815f3899
Kashyap SN, Boyle NR, Roberson ED (2023) Preclinical interventions in mouse models of frontotemporal dementia due to progranulin mutations. Neurotherapeutics 20(1):140–153. https://doi.org/10.1007/s13311-023-01348-6
Acknowledgements
We thank Robert Farese, Jr., Tobias Walther, John Morley, and Thi Nguyen for helpful discussions, the Bluefield Project to Cure FTD for progranulin antibodies and Grn mouse plasma, Yuna Ayala for advice about TDP-43 analysis, Sami Barmada for qPCR primer sequences for sTDP-43 isoforms, the NeuroBehavior Laboratory at the Harvard NeuroDiscovery Center, the Quanterix Accelerator Lab, and Millipore Sigma for plasma NfL measurements using the SMC platform
Funding
This work was supported by grants from the National Institutes of Health (AG047339 and AG064069) and the Bluefield Project to Cure FTD to ADN. Research reported in this publication was also supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
D. M. Smith performed experiments, analyzed data, and prepared figures. G. Aggarwal performed experiments. M. L. Niehoff performed experiments. S. A. Jones performed experiments. S. Banerjee performed experiments. S. A. Farr performed experiments, analyzed data, and prepared figures. A. D. Nguyen designed the study, analyzed data, prepared figures, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All methods and animal procedures were approved by the Institutional Animal Care and Use Committee at Saint Louis University or the Harvard Medical Area Standing Committee on Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Smith, D.M., Aggarwal, G., Niehoff, M.L. et al. Biochemical, Biomarker, and Behavioral Characterization of the GrnR493X Mouse Model of Frontotemporal Dementia. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04190-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04190-9