Abstract
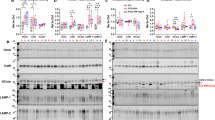
Frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) is characterized by progressive decline in behavior, executive function, and language. Progranulin (GRN) gene mutations are pathogenic for FTLD-TDP, and GRN transcript haploinsufficiency is the proposed disease mechanism. However, the evidence for this hypothesis comes mainly from blood-derived cells; we measured progranulin expression in brain. We characterized mRNA and protein levels of progranulin from four brain regions (frontal cortex, temporal cortex, occipital cortex, and cerebellum) in FTLD-TDP patients with and without GRN mutations, as well as neurologically normal individuals. Moreover, we performed immunohistochemistry to evaluate the degree of TDP-43 pathology and microglial infiltration present in these groups. In most brain regions, patients with GRN mutations showed mRNA levels comparable to normal controls and to FTLD-TDP without GRN mutations. However, GRN transcript levels in a brain region severely affected by disease (frontal cortex) were increased in mutation-bearing patients. When compared with normal individuals, GRN mutation-bearing cases had a significant reduction in the amount of progranulin protein in the cerebellum and occipital cortex, but not in the frontal and temporal cortices. In GRN mutant cases, GRN mRNA originated from the normal allele, and moderate microglial infiltration was observed. In conclusion, GRN mutation carriers have increased levels of mRNA transcript from the normal allele in brain, and proliferation of microglia likely increases progranulin levels in affected regions of the FTLD-TDP brain, and whether or not these findings underlie the accumulation of TDP-43 pathology in FTLD-TDP linked to GRN mutations remains to be determined.







Similar content being viewed by others
References
Akiyama H, McGeer PL (1990) Brain microglia constitutively express beta-2 integrins. J Neuroimmunol 30:81–93
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Asheuer M, Pflumio F, Benhamida S et al (2004) Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc Natl Acad Sci USA 101:3557–3562
Baker CA, Manuelidis L (2003) Unique inflammatory RNA profiles of microglia in Creutzfeldt–Jakob disease. Proc Natl Acad Sci USA 100:675–679
Baker M, Mackenzie IR, Pickering-Brown SM et al (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919
Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S (1990) Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun 173:1161–1168
Bateman A, Bennett HP (1998) Granulins: the structure and function of an emerging family of growth factors. J Endocrinol 158:145–151
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing (English summary). J Roy Statist Soc Ser B 57:289–300
Bhandari V, Palfree RG, Bateman A (1992) Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci USA 89:1715–1719
Bhandari V, Giaid A, Bateman A (1993) The complementary deoxyribonucleic acid sequence, tissue distribution, and cellular localization of the rat granulin precursor. Endocrinology 133:2682–2689
Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10(Suppl):S2–S9
Bronner IF, Rizzu P, Seelaar H et al (2007) Progranulin mutations in Dutch familial frontotemporal lobar degeneration. Eur J Hum Genet 15:369–374
Cairns NJ, Neumann M, Bigio EH et al (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171:227–240
Chen-Plotkin AS, Geser F, Plotkin JB et al (2008) Variations in the progranulin gene affect global gene expression in frontotemporal lobar degeneration. Hum Mol Genet 17:1349–1362
Coppola G, Karydas A, Rademakers R et al (2008) Gene expression study on peripheral blood identifies progranulin mutations. Ann Neurol 64:92–96
Cruts M, Gijselinck I, van der Zee J et al (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442:920–924
Cruts M, Van Broeckhoven C (2008) Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet 24:186–194
Daniel R, He Z, Carmichael KP, Halper J, Bateman A (2000) Cellular localization of gene expression for progranulin. J Histochem Cytochem 48:999–1009
Davoust N, Vuaillat C, Androdias G, Nataf S (2008) From bone marrow to microglia: barriers and avenues. Trends Immunol 29:227–234
Finch N, Baker M, Crook R et al (2009) Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132:583–591
Forman MS, Farmer J, Johnson JK et al (2006) Frontotemporal dementia: clinicopathological correlations. Ann Neurol 59:952–962
Gass J, Cannon A, Mackenzie IR et al (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15:2988–3001
Geser F, Brandmeir NJ, Kwong LK et al (2008) Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol 65:636–641
Ghidoni R, Benussi L, Glionna M, Franzoni M, Binetti G (2008) Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology 71:1235–1239
He Z, Bateman A (2003) Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 81:600–612
He Z, Ong CH, Halper J, Bateman A (2003) Progranulin is a mediator of the wound response. Nat Med 9:225–229
Huey ED, Grafman J, Wassermann EM et al (2006) Characteristics of frontotemporal dementia patients with a progranulin mutation. Ann Neurol 60:374–380
Le Ber I, van der Zee J, Hannequin D et al (2007) Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 28:846–855
Leverenz JB, Yu CE, Montine TJ et al (2007) A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain 130:1360–1374
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Mackenzie IR (2007) The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol 114:49–54
Mackenzie IR, Neumann M, Bigio EH et al (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117:15–18
McGeer EG, Klegeris A, McGeer PL (2005) Inflammation, the complement system and the diseases of aging. Neurobiol Aging 26(Suppl 1):94–97
McKhann GM, Albert MS, Grossman M et al (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58:1803–1809
Mukherjee O, Wang J, Gitcho M et al (2008) Molecular characterization of novel progranulin (GRN) mutations in frontotemporal dementia. Hum Mutat 29:512–521
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Plowman GD, Green JM, Neubauer MG et al (1992) The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem 267:13073–13078
Rademakers R, Eriksen JL, Baker M et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642
Ratnavalli E, Brayne C, Dawson K, Hodges JR (2002) The prevalence of frontotemporal dementia. Neurology 58:1615–1621
Rollinson S, Rohrer JD, van der Zee J et al (2009) No association of PGRN 3′UTR rs5848 in frontotemporal lobar degeneration. Neurobiol Aging 13 May 2009 [Epub ahead of print]
Rosso SM, Donker Kaat L, Baks T et al (2003) Frontotemporal dementia in the Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 126:2016–2022
Sampathu DM, Neumann M, Kwong LK et al (2006) Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 169:1343–1352
Schroeder A, Mueller O, Stocker S et al (2006) The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3
Shi L, Reid LH, Jones WD et al (2006) The microarray quality control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 24:1151–1161
Shoyab M, McDonald VL, Byles C, Todaro GJ, Plowman GD (1990) Epithelins 1 and 2: isolation and characterization of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci USA 87:7912–7916
Sleegers K, Brouwers N, Van Damme P et al (2009) Serum biomarker for progranulin-associated frontotemporal lobar degeneration. Ann Neurol 65:603–609
Van Damme P, Van Hoecke A, Lambrechts D et al (2008) Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol 181:37–41
Van Deerlin VM, Wood EM, Moore P et al (2007) Clinical, genetic, and pathologic characteristics of patients with frontotemporal dementia and progranulin mutations. Arch Neurol 64:1148–1153
Venneti S, Wang G, Nguyen J, Wiley CA (2008) The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol 67:1001–1010
Zhu J, Nathan C, Jin W et al (2002) Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111:867–878
Acknowledgments
This work was supported by the National Institutes of Health [K08AG033101-01; AG10124; AG17586; NS44266; AG15116; NS53488], the American Academy of Neurology-ALS Association [Clinician–Scientist Development Award to ACP], and the Burroughs Wellcome Fund [Career Award for Medical Scientists to ACP]. VMYL is the John H. Ware, 3rd, Professor of Alzheimer’s disease research. JQT is the William Maul Measey-Truman G. Schnabel, Jr., Professor of Geriatric Medicine and Gerontology. We thank Theresa Schuck for assistance in immunohistochemical studies. We thank Linda Kwong for helpful advice and discussions. Finally, we thank our patients and their families whose generosity made this work possible.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen-Plotkin, A.S., Xiao, J., Geser, F. et al. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol 119, 111–122 (2010). https://doi.org/10.1007/s00401-009-0576-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0576-2