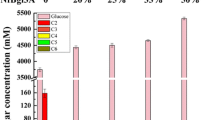
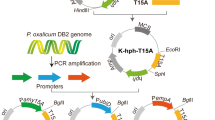
Abstract
The filamentous fungus Talaromyces cellulolyticus (formerly Acremonium cellulolyticus) is currently being intensively studied as a promising industrial producer of a number of secreted cellulolytic enzymes. In this study, the T. cellulolyticus gene lacA, which encodes a protein orthologous to the fungal extracellular β-galactosidases of family 35, was identified. The substitution of the lacA upstream region with a constitutive promoter demonstrated that the product of this gene is effectively secreted and possesses β-galactosidase activity. The optimal pH and temperature values for the hydrolysis of o-nitrophenyl-β-D-galactopyranoside by this enzyme were determined to be pH 4.5–5.5 and 50 °C, respectively. The negligible production of β-galactosidase activity by strains expressing lacA under native regulation raises the possibility of using lacA as a reporter gene. To test this hypothesis, the native promoter of lacA was replaced with the strong inducible promoter of the T. cellulolyticus cellobiohydrolase I gene. The cultivation of the resulting strain in various media showed that the β-galactosidase activity depends on cultivation conditions similar to the cellobiohydrolase activity. Thus, the suitability of lacA as a reporter for evaluating promoters with a wide range of expression profiles was demonstrated.







Similar content being viewed by others
References
Fujii, T., Hoshino, T., Inoue, H., & Yano, S. (2014). Taxonomic revision of the cellulose-degrading fungus Acremonium cellulolyticus nomen nudum to Talaromyces based on phylogenetic analysis. FEMS Microbiology Letters, 351, 32–41.
Yamanobe, T., Mitsiushi, Y., & Takasaki, Y. (1987). Isolation of a cellulolytic enzyme producing microorganism, culture conditions and some properties of the enzymes. Agricultural and Biological Chemistry, 51, 65–74.
Fang, X., Yano, S., Inoue, H., & Sawayama, S. (2009). Strain improvement of Acremonium cellulolyticus for cellulase production by mutation. Journal of Bioscience and Bioengineering, 107, 256–261.
Fujii, T., Iwata, K., Murakami, K., Yano, S., & Sawayama, S. (2012). Isolation of uracil auxotrophs of the fungus Acremonium cellulolyticus and the development of a transformation system with the pyrF gene. Bioscience, Biotechnology, and Biochemistry, 76, 245–249.
Inoue, H., Fujii, T., Yoshimi, M., Taylor, L. E., 2nd., Decker, S. R., Kishishita, S., Nakabayashi, M., & Ishikawa, K. (2013). Construction of a starch-inducible homologous expression system to produce cellulolytic enzymes from Acremonium cellulolyticus. Journal of Industrial Microbiology and Biotechnology, 40, 823–830.
Fujii, T., Koike, H., Sawayama, S., Yano, S., & Inoue, H. (2015). Draft genome sequence of Talaromyces cellulolyticus Strain Y-94, a source of lignocellulosic biomass-degrading enzymes. Genome Announcements, 3, e00014–e00015. https://doi.org/10.1128/genomeA.00014-15
Inoue, H., Decker, S. R., Taylor, L. E., 2nd., Yano, S., & Sawayama, S. (2014). Identification and characterization of core cellulolytic enzymes from Talaromyces cellulolyticus (formerly Acremonium cellulolyticus) critical for hydrolysis of lignocellulosic biomass. Biotechnology for Biofuels, 7, 151.
Ptitsyn, L. R., Yampolskaya, T. A., & Kutukova, E. A. (2017). Identification of core cellulolytic enzymes from Talaromyces cellulolyticus strain S6–25. FEBS J. 284, P.1.3–026.
van den Brink, J., & de Vries, R. P. (2011). Fungal enzyme sets for plant polysaccharide degradation. Applied Microbiology and Biotechnology, 91, 1477–1492.
Widmer, F., & Leuba, J. L. (1979). β-Galactosidase from Aspergillus niger. Separation and characterization of three multiple forms. European Journal of Biochemistry, 100, 559–567.
Kumar, V., Ramakrishnan, S., Teeri, T. T., Knowles, J. K., & Hartley, B. S. (1992). Saccharomyces cerevisiae cells secreting an Aspergillus niger beta-galactosidase grow on whey permeate. Biotechnology (N. Y), 10, 82–85.
Niu, D., Tian, X., Mchunu, N., Jia, C., Singh, S., Liu, X., Prior, B., & Lu, F. (2017). Biochemical characterization of three Aspergillus niger β-galactosidases. Electronic Journal of Biotechnology., 27, 37–43.
Rico-Díaz, A., Vizoso Vázquez, Á., Cerdán, M. E., Becerra, M., & Sanz-Aparicio, J. (2014). Crystallization and preliminary X-ray diffraction data of β-galactosidase from Aspergillus niger. Acta Crystallogr F Struct Biol Commun., 70, 1529–1531. https://doi.org/10.1107/S2053230X14019815
Rico-Díaz, A., Ramírez-Escudero, M., Vizoso-Vázquez, Á., Cerdán, M. E., Becerra, M., & Sanz-Aparicio, J. (2017). Structural features of Aspergillus niger β-galactosidase define its activity against glycoside linkages. FEBS Journal, 284, 1815–1829. https://doi.org/10.1111/febs.14083
Park, Y., Santi, M., & Pastore, G. (1979). Production and characterization of β-galactosidase from Aspergillus oryzae. Journal of Food Science, 44, 100–103.
Maksimainen, M. M., Lampio, A., Mertanen, M., Turunen, O., & Rouvinen, J. (2013). The crystal structure of acidic β-galactosidase from Aspergillus oryzae. International Journal of Biological Macromolecules, 60, 109–115. https://doi.org/10.1016/j.ijbiomac.2013.05.003
Nikolaev, I. V., Epishin, S. M., Zakharova, E. S., Kotenko, S. V., & Vinetskiĭ Iu. P. (1992). [Molecular cloning of the gene for secreted beta-galactosidase of the filamentous fungus Penicillium canescens]. [Article in Russian] Mol Biol (Mosk). 26, 869–875.
Rojas, A. L., Nagem, R. A., Neustroev, K. N., Arand, M., Adamska, M., Eneyskaya, E. V., Kulminskaya, A. A., Garratt, R. C., Golubev, A. M., & Polikarpov, I. (2004). Crystal structures of β-galactosidase from Penicillium sp. and its complex with galactose. Journal of Molecular Biology, 343, 1281–1292. https://doi.org/10.1016/j.jmb.2004.09.012
Adalberto, P. R., Massabni, A. C., Goulart, A. J., Contiero, J., Carmona, E. C., Cardello, L., & Monti, R. (2006). Production of β-galactosidase by Trichoderma reesei FTKO-39 in wheat bran: Partial purification of two isozymes. Applied Biochemistry and Biotechnology, 133, 163–170.
Maksimainen, M., Hakulinen, N., Kallio, J. M., Timoharju, T., Turunen, O., & Rouvinen, J. (2011). Crystal structures of Trichoderma reesei β-galactosidase reveal conformational changes in the active site. Journal of Structural Biology, 174, 156–163. https://doi.org/10.1016/j.jsb.2010.11.024
Tatusov, R. L., Koonin, E. V., & Lipman, D. J. (1997). A genomic perspective on protein families. Science, 278, 631–637. https://doi.org/10.1126/science.278.5338.631
Yilmaz, N., Visagie, C. M., Houbraken, J., Frisvad, J. C., & Samson, R. A. (2014). Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology, 78, 175–341. https://doi.org/10.1016/j.simyco.2014.08.001
Fairhead, C., Llorente, B., Denis, F., Soler, M., & Dujon, B. (1996). New vectors for combinatorial deletions in yeast chromosomes and for gap-repair cloning using ‘split-marker’ recombination. Yeast, 12, 1439–1457.
Catlett, N. L., Lee, B., Yoder, O. C., & Turgeon, B. G. (2003). Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genetics Reports. https://doi.org/10.4148/1941-4765.1150
Nielsen, M. L., Albertsen, L., Lettier, G., Nielsen, J. B., & Mortensen, U. H. (2006). Efficient PCR-based gene targeting with a recyclable marker for Aspergillus nidulans. Fungal Genetics and Biology, 43, 54–64. https://doi.org/10.1016/j.fgb.2005.09.005
Chernoglazov, V. M., Jafarova, A. N., & Klyosov, A. A. (1989). Continuous photometric determination of endo-1,4-beta-D-glucanase (cellulase) activity using 4-methylumbelliferyl-beta-D-cellobioside as a substrate. Analytical Biochemistry, 179, 186–189. https://doi.org/10.1016/0003-2697(89)90222-4
Collart, M. A., & Oliviero, S. (2001). Preparation of yeast RNA. Current Protocols in Molecular Biology. https://doi.org/10.1002/0471142727.mb1312s23
Smith, B. J. (1984). SDS polyacrylamide gel electrophoresis of proteins. Methods in Molecular Biology (Clifton, NJ), 1, 41–55. https://doi.org/10.1385/0-89603-062-8:41
Lum, G., & Min, X. J. (2011). FunSecKB: the Fungal Secretome KnowledgeBase. Database, 2011, bar001. https://doi.org/10.1093/database/bar001
von Heijne, G. (1985). Signal sequences. The limits of variation. Journal of Molecular Biology, 184, 99–105. https://doi.org/10.1016/0022-2836(85)90046-4
Nielsen, H., Engelbrecht, J., Brunak, S., & von Heijne, G. (1997). Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein engineering, 10, 1–6. https://doi.org/10.1093/protein/10.1.1
Baker, D., Shiau, A. K., & Agard, D. A. (1993). The role of pro regions in protein folding. Current Opinion in Cell Biology, 5, 966–970. https://doi.org/10.1016/0955-0674(93)90078-5
Eder, J., & Fersht, A. R. (1995). Pro-sequence-assisted protein folding. Molecular microbiology, 16, 609–614. https://doi.org/10.1111/j.1365-2958.1995.tb02423.x
Mizuno, K., Nakamura, T., Ohshima, T., Tanaka, S., & Matsuo, H. (1989). Characterization of KEX2-encoded endopeptidase from yeast Saccharomyces cerevisiae. Biochemical and Biophysical Research Communications, 159, 305–311.
Brenner, C., & Fuller, R. S. (1992). Structural and enzymatic characterization of a purified prohormone-processing enzyme: Secreted, soluble Kex2 protease. Proceedings of the National Academy of Sciences of the United States of America., 89, 922–926.
Bevan, A., Brenner, C., & Fuller, R. S. (1998). Quantitative assessment of enzyme specificity in vivo: P2 recognition by Kex2 protease defined in a genetic system. Proceedings of the National Academy of Sciences of the United States of America, 95, 10384–10389.
Jalving, R., van de Vondervoort, P. J., Visser, J., & Schaap, P. J. (2000). Characterization of the kexin-like maturase of Aspergillus niger. Applied and Environment Microbiology, 66, 363–368.
Fuglsang, C. C., Berka, R. M., Wahleithner, J. A., Kauppinen, S., Shuster, J. R., Rasmussen, G., Halkier, T., Dalboge, H., & Henrissat, B. (2000). Biochemical analysis of recombinant fungal mutanases. A new family of alpha1,3-glucanases with novel carbohydrate-binding domains. Journal of Biological Chemistry, 275, 2009–2018.
O’Connell, S., & Walsh, G. (2008). Application relevant studies of fungal beta-galactosidases with potential application in the alleviation of lactose intolerance. Applied Biochemistry and Biotechnology, 149, 129–138.
Cardoso, B. B., Silvério, S. C., Abrunhosa, L., Teixeira, J. A., & Rodrigues, L. R. (2017). β-galactosidase from Aspergillus lacticoffeatus: A promising biocatalyst for the synthesis of novel prebiotics. International Journal of Food Microbiology, 257, 67–74.
Cruz, R., Cruz, V., Belote, J. N., Khenayfes, M. D., Dorta, C., & Oliveira, L. H. (1999). Properties of a new fungal β-galactosidase with potential application in the dairy industry. Revista de Microbiologia, 30, 265–271.
v. d. Veen, P., Flipphi, M.J., Voragen, A.G. and Visser, J. (1993). Induction of extracellular arabinases on monomeric substrates in Aspergillus niger. Archives of Microbiology, 159, 66–71.
Fernández-Espinar, M., Piñaga, F., Graaff, L., Visser, J., Ramón, D., & Vallés, S. (2004). Purification, characterization and regulation of the synthesis of an Aspergillus nidulans acidic xylanase. Applied Microbiology and Biotechnology., 42, 555–562.
Kumar, S., & Ramón, D. (1996). Purification and regulation of the synthesis of a β-xylosidase from Aspergillus nidulans. FEMS Microbiology Letters., 135, 287–293.
de Vries, R. P., Visser, J., & de Graaff, L. H. (1999). CreA modulates the XlnR-induced expression on xylose of Aspergillus niger genes involved in xylan degradation. Research in Microbiology, 150, 281–285.
Rizzatti, A. C., Freitas, F., Bertolini, M., Peixoto-Nogueira, S. C., Terenzi, H., Jorge, J., & Polizeli, M. D. (2007). Regulation of xylanase in Aspergillus phoenicis: A physiological and molecular approach. Journal of Industrial Microbiology & Biotechnology., 35, 237–244.
Nikolaev, I. V., & Vinetski, Y. P. (1998). L-Arabinose induces synthesis of secreted beta-galactosidase in the filamentous fungus Penicillium canescens. Biochemistry (Moscow), 63, 1294–1298.
Vavilova, E. A., & Vinetskiĭ, Iu. P. (2003). Induction of endo-1,4-beta-xylanase and beta-galactosidase in the original and recombinant strains of the fungus Penicillium canescens]. [Article in Russian. Prikladnaia Biokhimiia i Mikrobiologiia, 39, 167–172.
Midoh, N., Sumida, N., Okakura, K., Murakami, T., & Yamanobe, T. (2009). New promoter for expressing protein products. Japanese patent JP4257759
Hideno, A., Inoue, H., Fujiim, T., Yano, S., Tsukahara, K., Murakami, K., Yunokawa, H., & Sawayama, S. (2013). High-coverage gene expression profiling analysis of the cellulase-producing fungus Acremonium cellulolyticus cultured using different carbon sources. Applied Microbiology and Biotechnology, 97, 5483–5492. https://doi.org/10.1007/s00253-013-4689-0
Fujii, T., Inoue, H., & Ishikawa, K. (2013). Enhancing cellulase and hemicellulase production by genetic modification of the carbon catabolite repressor gene, creA, in Acremonium cellulolyticus. AMB Express, 3, 73. https://doi.org/10.1186/2191-0855-3-73
Pardy, K. (1994). Reporter enzymes for the study of promoter activity. Molecular Biotechnology, 2, 23–27. https://doi.org/10.1007/BF02789287
Kain, S. R., & Ganguly, S. (2001). Overview of genetic reporter systems. Current Protocols in Molecular Biology, 9(Unit9), 6. https://doi.org/10.1002/0471142727.mb0906s36
Ghim, C. M., Lee, S. K., Takayama, S., & Mitchell, R. J. (2010). The art of reporter proteins in science: Past, present and future applications. BMB Reports, 43, 451–460. https://doi.org/10.5483/bmbrep.2010.43.7.451
Saloheimo, A., Aro, N., Ilmén, M., & Penttilä, M. (2000). Isolation of the ace1 gene encoding a Cys(2)-His(2) transcription factor involved in regulation of activity of the cellulase promoter cbh1 of Trichoderma reesei. The Journal of biological chemistry, 275, 5817–5825. https://doi.org/10.1074/jbc.275.8.5817
Chulkin, A. M., Vavilova, E. A., & Benevolenskiĭ, S. V. (2011). The mutational analysis of carbon catabolite repression in filamentous fungus Penicillium canescens. Molekuliarnaia Biologiia, 45, 871–878. https://doi.org/10.1134/S0026893311050049
Honda, Y., Tanigawa, E., Tsukihara, T., Nguyen, D. X., Kawabe, H., Sakatoku, N., Watari, J., Sato, H., Yano, S., Tachiki, T., Irie, T., Watanabe, T., & Watanabe, T. (2019). Stable and transient transformation, and a promoter assay in the selective lignin-degrading fungus Ceriporiopsis subvermispora. AMB Express, 9, 92. https://doi.org/10.1186/s13568-019-0818-1
Song, H. Y., Choi, D., Han, D. M., Kim, D. H., & Kim, J. M. (2018). A Novel Rapid Fungal Promoter Analysis System Using the Phosphopantetheinyl Transferase Gene, npgA, in Aspergillus nidulans. Mycobiology, 46, 429–439. https://doi.org/10.1080/12298093.2018.1548806
Miller, J. H. (1972). Experiments in molecular genetics. ColdSpring Harbor Laboratory Press.
Bitter, G. A., Chang, K. K., & Egan, K. M. (1991). A multi-component upstream activation sequence of the Saccharomyces cerevisiae glyceraldehyde-3-phosphate dehydrogenase gene promoter. Molecular & General Genetics: MGG, 231, 22–32. https://doi.org/10.1007/BF00293817
Flagfeldt, D. B., Siewers, V., Huang, L., & Nielsen, J. (2009). Characterization of chromosomal integration sites for heterologous gene expression in Saccharomyces cerevisiae. Yeast, 26, 545–551. https://doi.org/10.1002/yea.1705
Nevoigt, E., Kohnke, J., Fischer, C. R., Alper, H., Stahl, U., & Stephanopoulos, G. (2006). Engineering of promoter replacement cassettes for fine-tuning of gene expression in Saccharomyces cerevisiae. Applied and Environmental Microbiology, 72, 5266–5273. https://doi.org/10.1128/AEM.00530-06
Acknowledgements
We would like to thank Dr. Natalia P. Zakataeva (Ajinomoto-Genetika Research Institute, 1st Dorozhny proezd, 1-1, Moscow 117545, Russia) for critical reading of the manuscript and for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Orleneva, A.P., Serebrianyi, V.A., Kutukova, E.A. et al. Identification of the Talaromyces cellulolyticus Gene Encoding an Extracellular Enzyme with β-galactosidase Activity and Testing it as a Reporter for Gene Expression Assays. Mol Biotechnol 64, 637–649 (2022). https://doi.org/10.1007/s12033-022-00453-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00453-9