Abstract
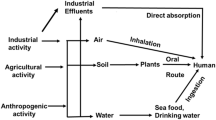
The environmental pollution of cadmium is worsening, and its significant carcinogenic effects on humans have been confirmed. Cadmium can induce cancer through various signaling pathways, including the ERK/JNK/p38MAPK, PI3K/AKT/mTOR, NF-κB, and Wnt. It can also cause cancer by directly damaging DNA and inhibiting DNA repair systems, or through epigenetic mechanisms such as abnormal DNA methylation, LncRNA, and microRNA. However, the detailed mechanisms of Cd-induced cancer are still not fully understood and require further investigation.
Graphical Abstract







Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Adams SV, Newcomb PA, White E (2012) Dietary cadmium and risk of invasive postmenopausal breast cancer in the VITAL cohort. Cancer Causes Control 23:845–854. https://doi.org/10.1007/s10552-012-9953-6
Adams SV, Quraishi SM, Shafer MM, Passarelli MN, Freney EP, Chlebowski RT, Luo J, Meliker JR, Mu L, Neuhouser ML, Newcomb PA (2014) Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women’s Health Initiative. Environ Health Perspect 122:594–600. https://doi.org/10.1289/ehp.1307054
Ahamed M, Akhtar MJ, Alhadlaq HA (2022) Combined effect of single-walled carbon nanotubes and cadmium on human lung cancer cells. Environ Sci Pollut Res Int 29:87844–87857. https://doi.org/10.1007/s11356-022-21933-0
Ali I, Damdimopoulou P, Stenius U, Halldin K (2015) Cadmium at nanomolar concentrations activates Raf-MEK-ERK1/2 MAPKs signaling via EGFR in human cancer cell lines. Chem Biol Interact 231:44–52. https://doi.org/10.1016/j.cbi.2015.02.014
Ali I, Hurmerinta T, Nurmi T, Berglund M, Rüegg J, Poutanen M, Halldin K, Mäkelä S, Damdimopoulou P (2016) From pure compounds to complex exposure: effects of dietary cadmium and lignans on estrogen, epidermal growth factor receptor, and mitogen activated protein kinase signaling in vivo. Toxicol Lett 253:27–35. https://doi.org/10.1016/j.toxlet.2016.04.020
Ali W, Ma Y, Zhu J, Zou H, Liu Z (2022) Mechanisms of cadmium-induced testicular injury: a risk to male fertility. Cells 11:3601. https://doi.org/10.3390/cells11223601
Amadou A, Praud D, Coudon T, Danjou AMN, Faure E, Leffondré K, Le Romancer M, Severi G, Salizzoni P, Mancini FR, Fervers B (2020) Chronic long-term exposure to cadmium air pollution and breast cancer risk in the French E3N cohort. Int J Cancer 146:341–351. https://doi.org/10.1002/ijc.32257
Amirkhah R, Schmitz U, Linnebacher M, Wolkenhauer O, Farazmand A (2015) MicroRNA–mRNA interactions in colorectal cancer and their role in tumor progression. Genes Chromosom Cancer 54:129–141. https://doi.org/10.1002/gcc.22231
Antoniali G, Marcuzzi F, Casarano E, Tell G (2015) Cadmium treatment suppresses DNA polymerase δ catalytic subunit gene expression by acting on the p53 and Sp1 regulatory axis. DNA Repair (Amst) 35:90–105. https://doi.org/10.1016/j.dnarep.2015.08.007
Apostoli P, Catalani S (2008) Mechanisms of action for metallic elements and their species classified carcinogen R 45 and R 49 by EU. G Ital Med Lav Ergon 30:382–391
Aria H, Ghaedrahmati F, Ganjalikhani-Hakemi M (2021) Cutting edge: metabolic immune reprogramming, reactive oxygen species, and cancer. J Cell Physiol 236:6168–6189. https://doi.org/10.1002/jcp.30303
Benbrahim-Tallaa L, Liu J, Webber MM, Waalkes MP (2007) Estrogen signaling and disruption of androgen metabolism in acquired androgen-independence during cadmium carcinogenesis in human prostate epithelial cells. Prostate 67:135–145. https://doi.org/10.1002/pros.20479
Benbrahim-Tallaa L, Tokar EJ, Diwan BA, Dill AL, Coppin J-F, Waalkes MP (2009) Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ Health Perspect 117:1847–1852. https://doi.org/10.1289/ehp.0900999
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88:1549–1559. https://doi.org/10.1016/j.biochi.2006.10.001
Bishak YK, Payahoo L, Osatdrahimi A, Nourazarian A (2015) Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac J Cancer Prev 16:9–21. https://doi.org/10.7314/apjcp.2015.16.1.9
Brama M, Gnessi L, Basciani S, Cerulli N, Politi L, Spera G, Mariani S, Cherubini S, Scotto d’Abusco A, Scandurra R, Migliaccio S (2007) Cadmium induces mitogenic signaling in breast cancer cell by an ERalpha-dependent mechanism. Mol Cell Endocrinol 264:102–108. https://doi.org/10.1016/j.mce.2006.10.013
Brocato J, Costa M (2013) Basic mechanics of DNA methylation and the unique landscape of the DNA methylome in metal-induced carcinogenesis. Crit Rev Toxicol 43:493–514. https://doi.org/10.3109/10408444.2013.794769
Cai J, Guan H, Jiao X, Yang J, Chen X, Zhang H, Zheng Y, Zhu Y, Liu Q, Zhang Z (2021) NLRP3 inflammasome mediated pyroptosis is involved in cadmium exposure-induced neuroinflammation through the IL-1β/IkB-α-NF-κB-NLRP3 feedback loop in swine. Toxicology 453:152720. https://doi.org/10.1016/j.tox.2021.152720
Cao X, Fu M, Bi R, Zheng X, Fu B, Tian S, Liu C, Li Q, Liu J (2021) Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 263:128346. https://doi.org/10.1016/j.chemosphere.2020.128346
Cao P, Nie G, Luo J, Hu R, Li G, Hu G, Zhang C (2022) Cadmium and molybdenum co-induce pyroptosis and apoptosis via the PTEN/PI3K/AKT axis in the livers of Shaoxing ducks (Anas platyrhynchos). Food Funct 13:2142–2154. https://doi.org/10.1039/d1fo02855c
Cartularo L, Laulicht F, Sun H, Kluz T, Freedman JH, Costa M (2015) Gene expression and pathway analysis of human hepatocellular carcinoma cells treated with cadmium. Toxicol Appl Pharmacol 288:399–408. https://doi.org/10.1016/j.taap.2015.08.011
Cartularo L, Kluz T, Cohen L, Shen SS, Costa M (2016) Molecular mechanisms of malignant transformation by low dose cadmium in normal human bronchial epithelial cells. PLoS ONE 11:e0155002. https://doi.org/10.1371/journal.pone.0155002
Chakraborty PK, Lee W-K, Molitor M, Wolff NA, Thévenod F (2010) Cadmium induces Wnt signaling to upregulate proliferation and survival genes in sub-confluent kidney proximal tubule cells. Mol Cancer 9:102. https://doi.org/10.1186/1476-4598-9-102
Chen GG, Liu ZM, Vlantis AC, Tse GMK, Leung BCH, van Hasselt CA (2004) Heme oxygenase-1 protects against apoptosis induced by tumor necrosis factor-alpha and cycloheximide in papillary thyroid carcinoma cells. J Cell Biochem 92:1246–1256. https://doi.org/10.1002/jcb.20157
Chen N, Su P, Wang M, Li Y-M (2018) Ascorbic acid inhibits cadmium-induced disruption of the blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Environ Sci Pollut Res 25:21713–21720. https://doi.org/10.1007/s11356-018-2138-4
Chen H, Yang X, Wang P, Wang Z, Li M, Zhao F-J (2018) Dietary cadmium intake from rice and vegetables and potential health risk: a case study in Xiangtan, southern China. Sci Total Environ 639:271–277. https://doi.org/10.1016/j.scitotenv.2018.05.050
Chen T-H, Huang J-J, Kung W-S, Lee S-S, Sun H-Y, Chuang H-Y (2019) The association of serum TNF-α levels and blood multi-elements modified by TNF-α gene polymorphisms in metal industrial workers. Int J Environ Res Public Health 16:4079. https://doi.org/10.3390/ijerph16214079
Chou X, Ma K, Shen Y, Min Z, Wu Q, Sun D (2021) Dual role of inositol-requiring enzyme 1α (IRE-1α) in Cd-induced apoptosis in human renal tubular epithelial cells: endoplasmic reticulum stress and STAT3 signaling activation. Toxicology 456:152769. https://doi.org/10.1016/j.tox.2021.152769
Chouchene L, Pellegrini E, Gueguen M-M, Hinfray N, Brion F, Piccini B, Kah O, Saïd K, Messaoudi I, Pakdel F (2016) Inhibitory effect of cadmium on estrogen signaling in zebrafish brain and protection by zinc. J Appl Toxicol 36:863–871. https://doi.org/10.1002/jat.3285
Chung C-J, Lee H-L, Chang C-H, Wu C-D, Liu C-S, Chung M-C, Hsu H-T (2023) Determination of potential sources of heavy metals in patients with urothelial carcinoma in central Taiwan: a biomonitoring case-control study. Environ Geochem Health. https://doi.org/10.1007/s10653-023-01481-3
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:92–99. https://doi.org/10.1016/j.tplants.2012.08.003
Correa JE, Ramírez R, Ruíz O, Leiva EI (2021) Effect of soil characteristics on cadmium absorption and plant growth of Theobroma cacao L. seedlings. J Sci Food Agric 101:5437–5445. https://doi.org/10.1002/jsfa.11192
Curcic M, Durgo K, Kopjar N, Ancic M, Vucinic S, Antonijevic B (2014) Cadmium and decabrominated diphenyl ether mixture: in vitro evaluation of cytotoxic, prooxidative and genotoxic effects. Environ Toxicol Pharmacol 38:663–671. https://doi.org/10.1016/j.etap.2014.07.021
Dewanjee S, Gangopadhyay M, Sahu R, Karmakar S (2013) Cadmium induced pathophysiology: prophylactic role of edible jute (Corchorus olitorius) leaves with special emphasis on oxidative stress and mitochondrial involvement. Food Chem Toxicol 60:188–198. https://doi.org/10.1016/j.fct.2013.07.043
Diaz D, Ujueta F, Mansur G, Lamas GA, Navas-Acien A, Arenas IA (2021) Low-level cadmium exposure and atherosclerosis. Curr Environ Health Rep 8:42–53. https://doi.org/10.1007/s40572-021-00304-w
DiDonato JA, Mercurio F, Karin M (2012) NF-κB and the link between inflammation and cancer. Immunol Rev 246:379–400. https://doi.org/10.1111/j.1600-065X.2012.01099.x
Divekar SD, Li H-H, Parodi DA, Ghafouri TB, Chen R, Cyrus K, Foxworth AE, Fornace AJ, Byrne C, Martin MB (2020) Arsenite and cadmium promote the development of mammary tumors. Carcinogenesis 41:1005–1014. https://doi.org/10.1093/carcin/bgz176
Djordjevic VR, Wallace DR, Schweitzer A, Boricic N, Knezevic D, Matic S, Grubor N, Kerkez M, Radenkovic D, Bulat Z, Antonijevic B, Matovic V, Buha A (2019) Environmental cadmium exposure and pancreatic cancer: evidence from case control, animal and in vitro studies. Environ Int 128:353–361. https://doi.org/10.1016/j.envint.2019.04.048
Eriksen KT, Halkjær J, Meliker JR, McElroy JA, Sørensen M, Tjønneland A, Raaschou-Nielsen O (2015) Dietary cadmium intake and risk of prostate cancer: a Danish prospective cohort study. BMC Cancer 15:177. https://doi.org/10.1186/s12885-015-1153-9
Filippini T, Torres D, Lopes C, Carvalho C, Moreira P, Naska A, Kasdagli M-I, Malavolti M, Orsini N, Vinceti M (2020) Cadmium exposure and risk of breast cancer: a dose-response meta-analysis of cohort studies. Environ Int 142:105879. https://doi.org/10.1016/j.envint.2020.105879
Florez-Garcia VA, Guevara-Romero EC, Hawkins MM, Bautista LE, Jenson TE, Yu J, Kalkbrenner AE (2023) Cadmium exposure and risk of breast cancer: a meta-analysis. Environ Res 219:115109. https://doi.org/10.1016/j.envres.2022.115109
Forcella M, Callegaro G, Melchioretto P, Gribaldo L, Frattini M, Stefanini FM, Fusi P, Urani C (2016) Cadmium-transformed cells in the in vitro cell transformation assay reveal different proliferative behaviours and activated pathways. Toxicol In Vitro 36:71–80. https://doi.org/10.1016/j.tiv.2016.07.006
Fu Z, Xi S (2020) The effects of heavy metals on human metabolism. Toxicol Mech Methods 30:167–176. https://doi.org/10.1080/15376516.2019.1701594
Fujiki K, Inamura H, Miyayama T, Matsuoka M (2017) Involvement of Notch1 signaling in malignant progression of A549 cells subjected to prolonged cadmium exposure. J Biol Chem 292:7942–7953. https://doi.org/10.1074/jbc.M116.759134
Fuse M, Tsunemi K (2013) Cross-border impacts of the restriction of hazardous substances: a perspective based on Japanese solders. Environ Sci Technol 47:9028–9034. https://doi.org/10.1021/es402581f
Gao X, Yu L, Moore AB, Kissling GE, Waalkes MP, Dixon D (2015) Cadmium and proliferation in human uterine leiomyoma cells: evidence of a role for EGFR/MAPK pathways but not classical estrogen receptor pathways. Environ Health Perspect 123:331–336. https://doi.org/10.1289/ehp.1408234
Gao Y, Xu Y, Wu D, Yu F, Yang L, Yao Y, Liang Z, Lau ATY (2017) Progressive silencing of the zinc transporter Zip8 (Slc39a8) in chronic cadmium-exposed lung epithelial cells. Acta Biochim Biophys Sin (Shanghai) 49:444–449. https://doi.org/10.1093/abbs/gmx022
Gaudet MM, Deubler EL, Kelly RS, Ryan Diver W, Teras LR, Hodge JM, Levine KE, Haines LG, Lundh T, Lenner P, Palli D, Vineis P, Bergdahl IA, Gapstur SM, Kyrtopoulos SA (2019) Blood levels of cadmium and lead in relation to breast cancer risk in three prospective cohorts. Int J Cancer 144:1010–1016. https://doi.org/10.1002/ijc.31805
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17:3782. https://doi.org/10.3390/ijerph17113782
Ghosh K, Chatterjee B, Behera P, Kanade SR (2020) The carcinogen cadmium elevates CpG-demethylation and enrichment of NFYA and E2F1 in the promoter of oncogenic PRMT5 and EZH2 methyltransferases resulting in their elevated expression in vitro. Chemosphere 242:125186. https://doi.org/10.1016/j.chemosphere.2019.125186
Gokey T, Hang B, Guliaev AB (2016) Cadmium(II) inhibition of human uracil-DNA glycosylase by catalytic water supplantation. Sci Rep 6:39137. https://doi.org/10.1038/srep39137
Goyer RA, Liu J, Waalkes MP (2004) Cadmium and cancer of prostate and testis. Biometals 17:555–558. https://doi.org/10.1023/b:biom.0000045738.59708.20
Gu J, Dai S, Liu Y, Liu H, Zhang Y, Ji X, Yu F, Zhou Y, Chen L, Tse WKF, Wong CKC, Chen B, Shi H (2018) Activation of Ca2+-sensing receptor as a protective pathway to reduce cadmium-induced cytotoxicity in renal proximal tubular cells. Sci Rep 8:1092. https://doi.org/10.1038/s41598-018-19327-9
Guo Y-S, Xu X-F, Li N, Sun N, Duan L-F (2019) Gene expression profiles in normal human prostate epithelial cells exposed to low-dose cadmium: a bioinformatics analysis. Zhonghua Nan Ke Xue 25:103–109
Gurel V, Sens DA, Somji S, Garrett SH, Weiland T, Sens MA (2005) Post-transcriptional regulation of metallothionein isoform 1 and 2 expression in the human breast and the MCF-10A cell line. Toxicol Sci 85:906–915. https://doi.org/10.1093/toxsci/kfi155
Haldsrud R, Krøkje A (2009) Induction of DNA double-strand breaks in the H4IIE cell line exposed to environmentally relevant concentrations of copper, cadmium, and zinc, singly and in combinations. J Toxicol Environ Health A 72:155–163. https://doi.org/10.1080/15287390802538964
Hankard PK, Bundy JG, Spurgeon DJ, Weeks JM, Wright J, Weinberg C, Svendsen C (2005) Establishing principal soil quality parameters influencing earthworms in urban soils using bioassays. Environ Pollut 133:199–211. https://doi.org/10.1016/j.envpol.2004.06.008
Hartwig A (1994) Role of DNA repair inhibition in lead- and cadmium-induced genotoxicity: a review. Environ Health Perspect 102(Suppl 3):45–50. https://doi.org/10.1289/ehp.94102s345
Hartwig A (2013) Cadmium and cancer. Met Ions Life Sci 11:491–507. https://doi.org/10.1007/978-94-007-5179-8_15
Hecht EM, Arheart K, Lee DJ, Hennekens CH, Hlaing WM (2016) A cross-sectional survey of cadmium biomarkers and cigarette smoking. Biomarkers 21:429–435. https://doi.org/10.3109/1354750X.2016.1153717
Hirao-Suzuki M, Takeda S, Kobayashi T, Kino K, Miyazawa H, Waalkes MP, Takiguchi M (2018) Cadmium down-regulates apolipoprotein E (ApoE) expression during malignant transformation of rat liver cells: direct evidence for DNA hypermethylation in the promoter region of ApoE. J Toxicol Sci 43:537–543. https://doi.org/10.2131/jts.43.537
Hirao-Suzuki M, Takeda S, Sakai G, Waalkes MP, Sugihara N, Takiguchi M (2021) Cadmium-stimulated invasion of rat liver cells during malignant transformation: evidence of the involvement of oxidative stress/TET1-sensitive machinery. Toxicology 447:152631. https://doi.org/10.1016/j.tox.2020.152631
Hoesel B, Schmid JA (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:86. https://doi.org/10.1186/1476-4598-12-86
Hossain Z, Huq F (2002) Studies on the interaction between Cd(2+) ions and DNA. J Inorg Biochem 90:85–96. https://doi.org/10.1016/s0162-0134(02)00412-9
House JS, Hall J, Park SS, Planchart A, Money E, Maguire RL, Huang Z, Mattingly CJ, Skaar D, Tzeng JY, Darrah TH, Vengosh A, Murphy SK, Jirtle RL, Hoyo C (2019) Cadmium exposure and MEG3 methylation differences between Whites and African Americans in the NEST Cohort. Environ Epigenet 5:dvz014. https://doi.org/10.1093/eep/dvz014
Hu K-H, Li W-X, Sun M-Y, Zhang S-B, Fan C-X, Wu Q, Zhu W, Xu X (2015) Cadmium induced apoptosis in MG63 cells by increasing ROS, activation of p38 MAPK and inhibition of ERK 1/2 pathways. Cell Physiol Biochem 36:642–654. https://doi.org/10.1159/000430127
Hu J, Wang H, Hu Y-F, Xu X-F, Chen Y-H, Xia M-Z, Zhang C, Xu D-X (2018) Cadmium induces inflammatory cytokines through activating Akt signaling in mouse placenta and human trophoblast cells. Placenta 65:7–14. https://doi.org/10.1016/j.placenta.2018.03.008
Huff MO, Todd SL, Smith AL, Elpers JT, Smith AP, Murphy RD, Bleser-Shartzer AS, Hoerter JE, Radde BN, Klinge CM (2016) Arsenite and cadmium activate MAPK/ERK via membrane estrogen receptors and G-protein coupled estrogen receptor signaling in human lung adenocarcinoma cells. Toxicol Sci 152:62–71. https://doi.org/10.1093/toxsci/kfw064
Iftode A, Drăghici GA, Macașoi I, Marcovici I, Coricovac DE, Dragoi R, Tischer A, Kovatsi L, Tsatsakis AM, Cretu O, Dehelean C (2021) Exposure to cadmium and copper triggers cytotoxic effects and epigenetic changes in human colorectal carcinoma HT-29 cells. Exp Ther Med 21:100. https://doi.org/10.3892/etm.2020.9532
Il’yasova D, Schwartz GG (2005) Cadmium and renal cancer. Toxicol Appl Pharmacol 207:179–186. https://doi.org/10.1016/j.taap.2004.12.005
Jeong E-M, Moon C-H, Kim C-S, Lee SH, Baik EJ, Moon CK, Jung Y-S (2004) Cadmium stimulates the expression of ICAM-1 via NF-kappaB activation in cerebrovascular endothelial cells. Biochem Biophys Res Commun 320:887–892. https://doi.org/10.1016/j.bbrc.2004.05.218
Jing Y, Liu L-Z, Jiang Y, Zhu Y, Guo NL, Barnett J, Rojanasakul Y, Agani F, Jiang B-H (2012) Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol Sci 125:10–19. https://doi.org/10.1093/toxsci/kfr256
Jing W, Lang L, Lin Z, Liu N, Wang L (2019) Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere 219:321–327. https://doi.org/10.1016/j.chemosphere.2018.12.033
Joseph P (2009) Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol 238:272–279. https://doi.org/10.1016/j.taap.2009.01.011
Ju-Kun S, Yuan D-B, Rao H-F, Chen T-F, Luan B-S, Xu X-M, Jiang F-N, Zhong W-D, Zhu J-G (2016) Association between Cd exposure and risk of prostate cancer: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 95:e2708. https://doi.org/10.1097/MD.0000000000002708
Junpradit C, Thooppeng P, Duangmal K, Prapagdee B (2021) Influence of cadmium-resistant Streptomycetes on plant growth and cadmium uptake by Chlorophytum comosum (Thunb.) Jacques. Environ Sci Pollut Res Int 28:39398–39408. https://doi.org/10.1007/s11356-021-13527-z
Kasemsuk T, Phuagkhaopong S, Yubolphan R, Rungreangplangkool N, Vivithanaporn P (2020) Cadmium induces CCL2 production in glioblastoma cells via activation of MAPK, PI3K, and PKC pathways. J Immunotoxicol 17:186–193. https://doi.org/10.1080/1547691X.2020.1829211
Katoh M (2005) WNT/PCP signaling pathway and human cancer (review). Oncol Rep 14:1583–1588
Kim S-Y, Kim G-Y, You H-J, Kang M-J (2022) Relationship between DNA mismatch repair and CRISPR/Cas9-mediated knock-in in the bovine β-casein gene locus. Anim Biosci 35:126–137. https://doi.org/10.5713/ab.21.0117
Kimura T, Hosaka T, Nakanishi T, Aozasa O (2019) Long-term cadmium exposure enhances metallothionein-1 induction after subsequent exposure to high concentrations of cadmium in P1798 mouse lymphosarcoma cells. J Toxicol Sci 44:309–316. https://doi.org/10.2131/jts.44.309
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238:215–220. https://doi.org/10.1016/j.taap.2009.03.026
Knani L, Bartolini D, Kechiche S, Tortoioli C, Murdolo G, Moretti M, Messaoudi I, Reiter RJ, Galli F (2019) Melatonin prevents cadmium-induced bone damage: first evidence on an improved osteogenic/adipogenic differentiation balance of mesenchymal stem cells as underlying mechanism. J Pineal Res 67:e12597. https://doi.org/10.1111/jpi.12597
Knani L, Venditti M, Kechiche S, Banni M, Messaoudi I, Minucci S (2020) Melatonin protects bone against cadmium-induced toxicity via activation of Wnt/β-catenin signaling pathway. Toxicol Mech Methods 30:237–245. https://doi.org/10.1080/15376516.2019.1701595
Kortenkamp A (2011) Are cadmium and other heavy metal compounds acting as endocrine disrupters? Met Ions Life Sci 8:305–317. https://doi.org/10.1039/9781849732116-00305
Kulkarni P, Dasgupta P, Bhat NS, Hashimoto Y, Saini S, Shahryari V, Yamamura S, Shiina M, Tanaka Y, Dahiya R, Majid S (2020) Role of the PI3K/Akt pathway in cadmium induced malignant transformation of normal prostate epithelial cells. Toxicol Appl Pharmacol 409:115308. https://doi.org/10.1016/j.taap.2020.115308
Li W, Gu X, Zhang X, Kong J, Ding N, Qi Y, Zhang Y, Wang J, Huang D (2015) Cadmium delays non-homologous end joining (NHEJ) repair via inhibition of DNA-PKcs phosphorylation and downregulation of XRCC4 and Ligase IV. Mutat Res 779:112–123. https://doi.org/10.1016/j.mrfmmm.2015.07.002
Li FJ, Surolia R, Li H, Wang Z, Liu G, Liu R-M, Mirov SB, Athar M, Thannickal VJ, Antony VB (2017) Low-dose cadmium exposure induces peribronchiolar fibrosis through site-specific phosphorylation of vimentin. Am J Physiol Lung Cell Mol Physiol 313:L80–L91. https://doi.org/10.1152/ajplung.00087.2017
Li K, Cao C, Ma Y, Su D, Li J (2019) Identification of cadmium bioaccumulation in rice (Oryza sativa L.) by the soil-plant transfer model and species sensitivity distribution. Sci Total Environ 692:1022–1028. https://doi.org/10.1016/j.scitotenv.2019.07.091
Li Z, Chi H, Zhu W, Yang G, Song J, Mo L, Zhang Y, Deng Y, Xu F, Yang J, He Z, Yang X (2021) Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch Toxicol 95:3497–3513. https://doi.org/10.1007/s00204-021-03157-2
Li FJ, Surolia R, Li H, Wang Z, Liu G, Kulkarni T, Massicano AVF, Mobley JA, Mondal S, de Andrade JA, Coonrod SA, Thompson PR, Wille K, Lapi SE, Athar M, Thannickal VJ, Carter AB, Antony VB (2021) Citrullinated vimentin mediates development and progression of lung fibrosis. Sci Transl Med 13:eaba2927. https://doi.org/10.1126/scitranslmed.aba2927
Li C-X, Talukder M, Xu Y-R, Zhu S-Y, Zhao Y-X, Li J-L (2023) Cadmium aggravates the blood-brain barrier disruption via inhibition of the Wnt7A/β-catenin signaling axis. Environ Pollut 324:121400. https://doi.org/10.1016/j.envpol.2023.121400
Lian S, Xia Y, Khoi PN, Ung TT, Yoon HJ, Kim NH, Kim KK, Jung YD (2015) Cadmium induces matrix metalloproteinase-9 expression via ROS-dependent EGFR, NF-кB, and AP-1 pathways in human endothelial cells. Toxicology 338:104–116. https://doi.org/10.1016/j.tox.2015.10.008
Lin X, Peng L, Xu X, Chen Y, Zhang Y, Huo X (2018) Connecting gastrointestinal cancer risk to cadmium and lead exposure in the Chaoshan population of Southeast China. Environ Sci Pollut Res Int 25:17611–17619. https://doi.org/10.1007/s11356-018-1914-5
Lin H-P, Wang Z, Yang C (2021) LncRNA DUXAP10 upregulation and the hedgehog pathway activation are critically involved in chronic cadmium exposure-induced cancer stem cell-like property. Toxicol Sci 184:33–45. https://doi.org/10.1093/toxsci/kfab099
Liu Z-M, Chen GG, Ng EKW, Leung W-K, Sung JJY, Chung SCS (2004) Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene 23:503–513. https://doi.org/10.1038/sj.onc.1207173
Liu Z-M, Chen GG, Vlantis AC, Tse GM, Shum CKY, van Hasselt CA (2007) Calcium-mediated activation of PI3K and p53 leads to apoptosis in thyroid carcinoma cells. Cell Mol Life Sci 64:1428–1436. https://doi.org/10.1007/s00018-007-7107-x
Liu X, Chen Y, Liu Y (2015) The inhibitory effect of CdCI, on DNA mismatch repair activity in ZR75—1 cells. J Wenzhou Med Univ 45:791–795
Liu J, Yu L, Castro L, Yan Y, Sifre MI, Bortner CD, Dixon D (2019) A nongenomic mechanism for “metalloestrogenic” effects of cadmium in human uterine leiomyoma cells through G protein-coupled estrogen receptor. Arch Toxicol 93:2773–2785. https://doi.org/10.1007/s00204-019-02544-0
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G (2022) Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 7:3. https://doi.org/10.1038/s41392-021-00762-6
Liu L, Zhao L, Liu Y, Yu X, Qiao X (2022) Rutin ameliorates cadmium-induced necroptosis in the chicken liver via inhibiting oxidative stress and MAPK/NF-κB pathway. Biol Trace Elem Res 200:1799–1810. https://doi.org/10.1007/s12011-021-02764-5
Lv Y-J, Song J, Xiong L-L, Huang R, Zhu P, Wang P, Liang X-X, Tan J-B, Wang J, Wu S-X, Wei Q-Z, Yang X-F (2021) Association of environmental cadmium exposure and bone remodeling in women over 50 years of age. Ecotoxicol Environ Saf 211:111897. https://doi.org/10.1016/j.ecoenv.2021.111897
Ma J, Bao X, Wang K, Wang C, Cui D (2021) Human health risk assessment of cadmium in soils: role of bioavailability and toxic effects. Asian J Ecotoxicol 16:120–132
Manning BD, Toker A (2017) AKT/PKB signaling: navigating the network. Cell 169:381–405. https://doi.org/10.1016/j.cell.2017.04.001
Matović V, Buha A, Bulat Z, Dukić-Ćosić D (2011) Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arh Hig Rada Toksikol 62:65–76. https://doi.org/10.2478/10004-1254-62-2011-2075
McElroy JA, Kruse RL, Guthrie J, Gangnon RE, Robertson JD (2017) Cadmium exposure and endometrial cancer risk: a large midwestern U.S. population-based case-control study. PLoS One 12:e0179360. https://doi.org/10.1371/journal.pone.0179360
McNeill DR, Narayana A, Wong H-K, Wilson DM (2004) Inhibition of Ape1 nuclease activity by lead, iron, and cadmium. Environ Health Perspect 112:799–804. https://doi.org/10.1289/ehp.7038
Méplan C, Mann K, Hainaut P (1999) Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells*. J Biol Chem 274:31663–31670. https://doi.org/10.1074/jbc.274.44.31663
Mérida-Ortega Á, López-Carrillo L, Rangel-Moreno K, Ramirez N, Rothenberg SJ (2021) Tobacco smoke exposure and urinary cadmium in women from Northern Mexico. Int J Environ Res Public Health 18:12581. https://doi.org/10.3390/ijerph182312581
Misra UK, Gawdi G, Pizzo SV (2003) Induction of mitogenic signalling in the 1LN prostate cell line on exposure to submicromolar concentrations of cadmium+. Cell Signal 15:1059–1070. https://doi.org/10.1016/s0898-6568(03)00117-7
Mitra S, Patra T, Saha D, Ghosh P, Mustafi SM, Varghese AC, Murmu N (2022) Sub-chronic cadmium and lead compound exposure induces reproductive toxicity and development of testicular germ cell neoplasia in situ in murine model: attenuative effects of resveratrol. J Biochem Mol Toxicol 36:e23058. https://doi.org/10.1002/jbt.23058
Mortoglou M, Buha Djordjevic A, Djordjevic V, Collins H, York L, Mani K, Valle E, Wallace D, Uysal-Onganer P (2022) Role of microRNAs in response to cadmium chloride in pancreatic ductal adenocarcinoma. Arch Toxicol 96:467–485. https://doi.org/10.1007/s00204-021-03196-9
Mustra DJ, Warren AJ, Wilcox DE, Hamilton JW (2007) Preferential binding of human XPA to the mitomycin C-DNA interstrand crosslink and modulation by arsenic and cadmium. Chem Biol Interact 168:159–168. https://doi.org/10.1016/j.cbi.2007.04.004
Naji, S., Issa, K., Eid, A., Iratni, R., Eid, A.H., 2019. Cadmium induces migration of colon cancer cells: roles of reactive oxygen species, P38 and cyclooxygenase-2. Cell Physiol Biochem 52, 1517–1534. https://doi.org/10.33594/000000106
Navarro Silvera SA, Rohan TE (2007) Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control 18:7–27. https://doi.org/10.1007/s10552-006-0057-z
Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J (2010) Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 23:769–782. https://doi.org/10.1007/s10534-010-9343-z
Neslund-Dudas CM, McBride RB, Kandegedara A, Rybicki BA, Kryvenko ON, Chitale D, Gupta N, Williamson SR, Rogers CG, Cordon-Cardo C, Rundle AG, Levin AM, Dou QP, Mitra B (2018) Association between cadmium and androgen receptor protein expression differs in prostate tumors of African American and European American men. J Trace Elem Med Biol 48:233–238. https://doi.org/10.1016/j.jtemb.2018.04.006
Nguyen HD, Kim M-S (2022) Cadmium, lead, and mercury mixtures interact with non-alcoholic fatty liver diseases. Environ Pollut 309:119780. https://doi.org/10.1016/j.envpol.2022.119780
Niture S, Gadi S, Lin M, Qi Q, Niture SS, Moore JT, Bodnar W, Fernando RA, Levine KE, Kumar D (2023) Cadmium modulates steatosis, fibrosis, and oncogenic signaling in liver cancer cells by activating notch and AKT/mTOR pathways. Environ Toxicol 38:783–797. https://doi.org/10.1002/tox.23731
Omidi M, Niknahad H, Noorafshan A, Fardid R, Nadimi E, Naderi S, Bakhtari A, Mohammadi-Bardbori A (2019) Co-exposure to an aryl hydrocarbon receptor endogenous ligand, 6-formylindolo[3,2-b]carbazole (FICZ), and cadmium induces cardiovascular developmental abnormalities in mice. Biol Trace Elem Res 187:442–451. https://doi.org/10.1007/s12011-018-1391-1
Ospondpant D, Phuagkhaopong S, Suknuntha K, Sangpairoj K, Kasemsuk T, Srimaroeng C, Vivithanaporn P (2019) Cadmium induces apoptotic program imbalance and cell cycle inhibitor expression in cultured human astrocytes. Environ Toxicol Pharmacol 65:53–59. https://doi.org/10.1016/j.etap.2018.12.001
Pal S, Kim JY, Park SH, Lim HB, Lee K-H, Song JM (2011) Quantitative classification of DNA damages induced by submicromolar cadmium using oligonucleotide chip coupled with lesion-specific endonuclease digestion. Environ Sci Technol 45:4460–4467. https://doi.org/10.1021/es200179j
Pal D, Suman S, Kolluru V, Sears S, Das TP, Alatassi H, Ankem MK, Freedman JH, Damodaran C (2017) Inhibition of autophagy prevents cadmium-induced prostate carcinogenesis. Br J Cancer 117:56–64. https://doi.org/10.1038/bjc.2017.143
Pan, S., Wang, Q., Zhang, Q., Zhou, M., Li, L., Zhou, X., 2021. A novel circular RNA, circPUS7 promotes cadmium-induced transformation of human bronchial epithelial cells by regulating Kirsten rat sarcoma viral oncogene homolog expression via sponging miR-770. Metallomics 13, mfab043. https://doi.org/10.1093/mtomcs/mfab043
Paniagua L, Diaz-Cueto L, Huerta-Reyes M, Arechavaleta-Velasco F (2019) Cadmium exposure induces interleukin-6 production via ROS-dependent activation of the ERK1/2 but independent of JNK signaling pathway in human placental JEG-3 trophoblast cells. Reprod Toxicol 89:28–34. https://doi.org/10.1016/j.reprotox.2019.06.008
Papa V, Bimonte VM, Wannenes F, D’Abusco AS, Fittipaldi S, Scandurra R, Politi L, Crescioli C, Lenzi A, Di Luigi L, Migliaccio S (2015) The endocrine disruptor cadmium alters human osteoblast-like Saos-2 cells homeostasis in vitro by alteration of Wnt/β-catenin pathway and activation of caspases. J Endocrinol Invest 38:1345–1356. https://doi.org/10.1007/s40618-015-0380-x
Pelch KE, Tokar EJ, Merrick BA, Waalkes MP (2015) Differential DNA methylation profile of key genes in malignant prostate epithelial cells transformed by inorganic arsenic or cadmium. Toxicol Appl Pharmacol 286:159–167. https://doi.org/10.1016/j.taap.2015.04.011
Peng L, Huang Y-T, Zhang F, Chen J-Y, Huo X (2019) Chronic cadmium exposure aggravates malignant phenotypes of nasopharyngeal carcinoma by activating the Wnt/β-catenin signaling pathway via hypermethylation of the casein kinase 1α promoter. Cancer Manag Res 11:81–93. https://doi.org/10.2147/CMAR.S171200
Peng C, Ouyang Y, Lu N, Li N (2020) The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front Immunol 11:1387. https://doi.org/10.3389/fimmu.2020.01387
Potts RJ, Watkin RD, Hart BA (2003) Cadmium exposure down-regulates 8-oxoguanine DNA glycosylase expression in rat lung and alveolar epithelial cells. Toxicology 184:189–202. https://doi.org/10.1016/s0300-483x(02)00579-6
Qu W, Fuquay R, Sakurai T, Waalkes MP (2006) Acquisition of apoptotic resistance in cadmium-induced malignant transformation: specific perturbation of JNK signal transduction pathway and associated metallothionein overexpression. Mol Carcinog 45:561–571. https://doi.org/10.1002/mc.20185
Qu W, Ke H, Pi J, Broderick D, French JE, Webber MM, Waalkes MP (2007) Acquisition of apoptotic resistance in cadmium-transformed human prostate epithelial cells: Bcl-2 overexpression blocks the activation of JNK signal transduction pathway. Environ Health Perspect 115:1094–1100. https://doi.org/10.1289/ehp.10075
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191:419. https://doi.org/10.1007/s10661-019-7528-7
Rasmi RR, Sakthivel KM, Guruvayoorappan C (2020) NF-κB inhibitors in treatment and prevention of lung cancer. Biomed Pharmacother 130:110569. https://doi.org/10.1016/j.biopha.2020.110569
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Rojas, E., Martínez-Pacheco, M., Rodríguez-Sastre, M.A., Valverde, M., 2019. As-Cd-Pb mixture induces cellular transformation via post-transcriptional regulation of Rad51c by miR-222. Cell Physiol Biochem 53, 910–920. https://doi.org/10.33594/000000181
Sawicka E, Saczko J, Kulbacka J, Szydełko M, Szymańska B, Piwowar A (2022) The influence of interaction between cadmium with 17β-estradiol, 2-methoxyestradiol and 16α-hydroxyestrone on viability and p-glycoprotein in ovarian cancer cell line. Int J Mol Sci 23:2628. https://doi.org/10.3390/ijms23052628
Schwerdtle T, Ebert F, Thuy C, Richter C, Mullenders LHF, Hartwig A (2010) Genotoxicity of soluble and particulate cadmium compounds: impact on oxidative DNA damage and nucleotide excision repair. Chem Res Toxicol 23:432–442. https://doi.org/10.1021/tx900444w
Seong JB, Bae YC, Lee H-S, Huh J-W, Lee S-R, Lee HJ, Lee D-S (2019) Increasing ERK phosphorylation by inhibition of p38 activity protects against cadmium-induced apoptotic cell death through ERK/Drp1/p38 signaling axis in spermatocyte-derived GC-2spd cells. Toxicol Appl Pharmacol 384:114797. https://doi.org/10.1016/j.taap.2019.114797
Sharma P, Caldwell TS, Rivera MN, Gullapalli RR (2020) Cadmium exposure activates Akt/ERK signaling and pro-inflammatory COX-2 expression in human gallbladder epithelial cells via a ROS dependent mechanism. Toxicol In Vitro 67:104912. https://doi.org/10.1016/j.tiv.2020.104912
Shati AA, El-Kott AF, Alkhateeb MA (2022) Resolvin D1 prevents cadmium chloride-induced memory loss and hippocampal damage in rats by activation/upregulation of PTEN-induced suppression of PI3K/Akt/mTOR signaling pathway. Clin Exp Pharmacol Physiol 49:275–290. https://doi.org/10.1111/1440-1681.13596
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48. https://doi.org/10.3322/caac.21763
So K-Y, Kim S-H, Jung K-T, Lee H-Y, Oh S-H (2017) MAPK/JNK1 activation protects cells against cadmium-induced autophagic cell death via differential regulation of catalase and heme oxygenase-1 in oral cancer cells. Toxicol Appl Pharmacol 332:81–91. https://doi.org/10.1016/j.taap.2017.07.022
Son Y-O, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee J-C, Shi X (2012) Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3β/β-catenin signaling. Toxicol Appl Pharmacol 264:153–160. https://doi.org/10.1016/j.taap.2012.07.028
Song X, Wei Z, Shaikh ZA (2015) Requirement of ERα and basal activities of EGFR and Src kinase in Cd-induced activation of MAPK/ERK pathway in human breast cancer MCF-7 cells. Toxicol Appl Pharmacol 287:26–34. https://doi.org/10.1016/j.taap.2015.05.010
Souza V, Escobar MDC, Bucio L, Hernández E, Gómez-Quiroz LE, Gutiérrez Ruiz MC (2009) NADPH oxidase and ERK1/2 are involved in cadmium induced-STAT3 activation in HepG2 cells. Toxicol Lett 187:180–186. https://doi.org/10.1016/j.toxlet.2009.02.021
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Suzuki M, Takeda S, Teraoka-Nishitani N, Yamagata A, Tanaka T, Sasaki M, Yasuda N, Oda M, Okano T, Yamahira K, Nakamura Y, Kobayashi T, Kino K, Miyazawa H, Waalkes MP, Takiguchi M (2017) Cadmium-induced malignant transformation of rat liver cells: potential key role and regulatory mechanism of altered apolipoprotein E expression in enhanced invasiveness. Toxicology 382:16–23. https://doi.org/10.1016/j.tox.2017.03.014
Tan HW, Liang Z-L, Yao Y, Wu D-D, Mo H-Y, Gu J, Chiu J-F, Xu Y-M, Lau ATY (2019) Lasting DNA damage and aberrant DNA repair gene expression profile are associated with post-chronic cadmium exposure in human bronchial epithelial cells. Cells 8:842. https://doi.org/10.3390/cells8080842
Tang J, Bei M, Zhu J, Xu G, Chen D, Jin X, Huang J, Dong J, Shi L, Xu L, Hu B (2021) Acute cadmium exposure induces GSDME-mediated pyroptosis in triple-negative breast cancer cells through ROS generation and NLRP3 inflammasome pathway activation. Environ Toxicol Pharmacol 87:103686. https://doi.org/10.1016/j.etap.2021.103686
Taş İ, Zhou R, Park S-Y, Yang Y, Gamage CDB, Son Y-J, Paik M-J, Kim H (2019) Inflammatory and tumorigenic effects of environmental pollutants found in particulate matter on lung epithelial cells. Toxicol In Vitro 59:300–311. https://doi.org/10.1016/j.tiv.2019.05.022
Thévenod F, Chakraborty PK (2010) The role of Wnt/beta-catenin signaling in renal carcinogenesis: lessons from cadmium toxicity studies. Curr Mol Med 10:387–404. https://doi.org/10.2174/156652410791316986
Trabelsi F, Khlifi R, Goux D, Guillamin M, Hamza-Chaffai A, Sichel F (2016) Genotoxic effects of cadmium in human head and neck cell line SQ20B. Environ Sci Pollut Res Int 23:16127–16136. https://doi.org/10.1007/s11356-016-6772-4
Vijayakumar V, Abern MR, Jagai JS, Kajdacsy-Balla A (2021) Observational study of the association between air cadmium exposure and prostate cancer aggressiveness at diagnosis among a nationwide retrospective cohort of 230,540 patients in the United States. Int J Environ Res Public Health 18:8333. https://doi.org/10.3390/ijerph18168333
Vincent-Hubert F, Châtel A, Gourlay-Francé C (2014) Metallothionein mRNA induction is correlated with the decrease of DNA strand breaks in cadmium exposed zebra mussels. Mutat Res Genet Toxicol Environ Mutagen 766:10–15. https://doi.org/10.1016/j.mrgentox.2014.03.006
Virani S, Rentschler KM, Nishijo M, Ruangyuttikarn W, Swaddiwudhipong W, Basu N, Rozek LS (2016) DNA methylation is differentially associated with environmental cadmium exposure based on sex and smoking status. Chemosphere 145:284–290. https://doi.org/10.1016/j.chemosphere.2015.10.123
Wang Y, Mandal AK, Son Y-O, Pratheeshkumar P, Wise JTF, Wang L, Zhang Z, Shi X, Chen Z (2018) Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol Appl Pharmacol 353:23–30. https://doi.org/10.1016/j.taap.2018.06.003
Wang D-H, Xu H, Zheng Y-H, Gu D-S, Zhu Y-J, Ren Y, Wang S-C, Yang L, Xu L-W (2020) Environmental exposure to lead and cadmium and hearing loss in Chinese adults: a case-control study. PLoS ONE 15:e0233165. https://doi.org/10.1371/journal.pone.0233165
Wang Q, Pan S, Jiang Q, Li L, Tu W, Zhang Q, Zhou X (2021) CircSPAG16 suppresses cadmium-induced transformation of human bronchial epithelial cells by decoying PIP5K1α to inactivate Akt. Mol Carcinog 60:582–594. https://doi.org/10.1002/mc.23325
Wang H, Wang A, Wang X, Zeng X, Xing H (2022) AMPK/PPAR-γ/NF-κB axis participates in ROS-mediated apoptosis and autophagy caused by cadmium in pig liver. Environ Pollut 294:118659. https://doi.org/10.1016/j.envpol.2021.118659
Wei Z, Shaikh ZA (2017) Cadmium stimulates metastasis-associated phenotype in triple-negative breast cancer cells through integrin and β-catenin signaling. Toxicol Appl Pharmacol 328:70–80. https://doi.org/10.1016/j.taap.2017.05.017
Wei, T., Jia, J., Wada, Y., Kapron, C.M., Liu, J., 2017. Dose dependent effects of cadmium on tumor angiogenesis. Oncotarget 8, 44944–44959. https://doi.org/10.18632/oncotarget.16572
Wei Z, Song X, Shaikh ZA (2015) Cadmium promotes the proliferation of triple-negative breast cancer cells through EGFR-mediated cell cycle regulation. Toxicol Appl Pharmacol 289:98–108. https://doi.org/10.1016/j.taap.2015.09.006
Wu L, Song J, Xue J, Xiao T, Wei Q, Zhang Z, Zhang Y, Li Z, Hu Y, Zhang G, Xia H, Li J, Yang X, Liu Q (2020) MircoRNA-143-3p regulating ARL6 is involved in the cadmium-induced inhibition of osteogenic differentiation in human bone marrow mesenchymal stem cells. Toxicol Lett 331:159–166. https://doi.org/10.1016/j.toxlet.2020.06.001
Xiao C, Liu Y, Xie C, Tu W, Xia Y, Costa M, Zhou X (2015) Cadmium induces histone H3 lysine methylation by inhibiting histone demethylase activity. Toxicol Sci 145:80–89. https://doi.org/10.1093/toxsci/kfv019
Xiao CL, Liu Y, Tu W, Xia YJ, Tian KM, Zhou X (2016) Research progress of the mechanisms underlying cadmium-induced carcinogenesis. Zhonghua Yu Fang Yi Xue Za Zhi 50:380–384. https://doi.org/10.3760/cma.j.issn.0253-9624.2016.04.021
Xiao T, Xue J, Shi M, Chen C, Luo F, Xu H, Chen X, Sun B, Sun Q, Yang Q, Dai X, Zhang A, Tang H, Liu Q (2018) Circ008913, via miR-889 regulation of DAB2IP/ZEB1, is involved in the arsenite-induced acquisition of CSC-like properties by human keratinocytes in carcinogenesis. Metallomics 10:1328–1338. https://doi.org/10.1039/c8mt00207j
Xu, J., Wise, J.T.F., Wang, L., Schumann, K., Zhang, Z., Shi, X., 2017. Dual roles of oxidative stress in metal carcinogenesis. JEP(T) 36. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2017025229
Xu J, Wang M, Zhang R, Wu L (2020) Toxicity of cadmium pollution in soil to organisms: a review. Asian Journal of Ecotoxicology 15:82–91
Xue, L., Zhou, J., Shi, X., 2005. Study on effects of CdCl2 on DNA repair in vitro and its mechanism. Ind Hlth & Occup Dis 404–408.
Yan L-J, Allen DC (2021) Cadmium-induced kidney injury: oxidative damage as a unifying mechanism. Biomolecules 11:1575. https://doi.org/10.3390/biom11111575
Yeh Y-H, Tsai C-C, Chen T-W, Lee C-H, Chang W-J, Hsieh M-Y, Li T-K (2022) Activation of multiple proteolysis systems contributes to acute cadmium cytotoxicity. Mol Cell Biochem 477:927–937. https://doi.org/10.1007/s11010-021-04298-9
Yu X, Filardo EJ, Shaikh ZA (2010) The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Toxicol Appl Pharmacol 245:83–90. https://doi.org/10.1016/j.taap.2010.02.005
Yu H, Lin L, Zhang Z, Zhang H, Hu H (2020) Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther 5:209. https://doi.org/10.1038/s41392-020-00312-6
Yu H-T, Zhen J, Leng J-Y, Cai L, Ji H-L, Keller BB (2021) Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol Sin 42:340–346. https://doi.org/10.1038/s41401-020-0396-4
Yuan D, Ye S, Pan Y, Bao Y, Chen H, Shao C (2013) Long-term cadmium exposure leads to the enhancement of lymphocyte proliferation via down-regulating p16 by DNA hypermethylation. Mutat Res 757:125–131. https://doi.org/10.1016/j.mrgentox.2013.07.007
Yue Y, Tan M, Luo Y, Deng P, Wang H, Li J, Hao R, Tian L, Xie J, Chen M, Yu Z, Zhou Z, Pi H (2022) miR-3614-5p downregulation promotes cadmium-induced breast cancer cell proliferation and metastasis by targeting TXNRD1. Ecotoxicol Environ Saf 247:114270. https://doi.org/10.1016/j.ecoenv.2022.114270
Yue Y, Deng P, Xiao H, Tan M, Wang H, Tian L, Xie J, Chen M, Luo Y, Wang L, Liang Y, Pi H, Zhou Z, Yu Z (2022) N6-methyladenosine-mediated downregulation of miR-374c-5p promotes cadmium-induced cell proliferation and metastasis by targeting GRM3 in breast cancer cells. Ecotoxicol Environ Saf 229:113085. https://doi.org/10.1016/j.ecoenv.2021.113085
Zeng L, Zhou J, Wang X, Zhang Y, Wang M, Su P (2021) Cadmium attenuates testosterone synthesis by promoting ferroptosis and blocking autophagosome-lysosome fusion. Free Radic Biol Med 176:176–188. https://doi.org/10.1016/j.freeradbiomed.2021.09.028
Zhang Y, Wang X (2020) Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 13:165. https://doi.org/10.1186/s13045-020-00990-3
Zhang, Hui, Zhang C, Zhang, Huizhu, Niu H (2022) Pollution status and health risk assessment of lead and cadmium in market vegetables in Heze City, 2017–2021. Chin J Health Lab Te 32:3058–3062+3066
Zhang C, Liang Y, Lei L, Zhu G, Chen X, Jin T, Wu Q (2013) Hypermethylations of RASAL1 and KLOTHO is associated with renal dysfunction in a Chinese population environmentally exposed to cadmium. Toxicol Appl Pharmacol 271:78–85. https://doi.org/10.1016/j.taap.2013.04.025
Zhang R, Zhu Y, Dong X, Liu B, Zhang N, Wang X, Liu L, Xu C, Huang S, Chen L (2017) Celastrol attenuates cadmium-induced neuronal apoptosis via inhibiting Ca2+ -CaMKII-dependent Akt/mTOR pathway. J Cell Physiol 232:2145–2157. https://doi.org/10.1002/jcp.25703
Zhang X, Ma L, Tang Y, Han J, Qi Y, Huang D (2021) Low-dose cadmium exposure facilitates cell proliferation by promoter hypermethylation of RASSF1A and DAPK1 genes. Environ Toxicol 36:2313–2321. https://doi.org/10.1002/tox.23345
Zhang Y, Guo S, Wang S, Li X, Hou D, Li H, Wang L, Xu Y, Ma B, Wang H, Jiang X (2021) LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf 220:112376. https://doi.org/10.1016/j.ecoenv.2021.112376
Zhang C, Lin T, Nie G, Hu R, Pi S, Wei Z, Wang C, Xing C, Hu G (2021) Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/AKT axis in duck renal tubular epithelial cells. Environ Pollut 272:116403. https://doi.org/10.1016/j.envpol.2020.116403
Zhang S-N, Xie W-Y, Zhai Z-Q, Chen C, Zhao F-J, Wang P (2023) Dietary intake of household cadmium-contaminated rice caused genome-wide DNA methylation changes on gene/hubs related to metabolic disorders and cancers. Environ Pollut 327:121553. https://doi.org/10.1016/j.envpol.2023.121553
Zhao X-J, Lu S-J, Xu R-J, Li B-L, Wu G-P, Wei F-S (2014) Soil heavy metal cadmium standard limit and range of background value research. Huan Jing Ke Xue 35:1491–1497
Zhao Y, Li Q, Yang Z, Shao Y, Xue P, Qu W, Jia X, Cheng L, He M, He R, Zhou Z, Zhang Y (2018) Cadmium activates noncanonical Wnt signaling to impair hematopoietic stem cell function in mice. Toxicol Sci 165:254–266. https://doi.org/10.1093/toxsci/kfy166
Zhou Z, Liu H, Wang C, Lu Q, Huang Q, Zheng C, Lei Y (2015) Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology. Sci Rep 5:15293. https://doi.org/10.1038/srep15293
Zhou M, Li L, Chen B, Pan S, Tu W, Hou Y, Chen P, Hernández RR, Zhou X (2021) Circ-SHPRH suppresses cadmium-induced transformation of human bronchial epithelial cells by regulating QKI expression via miR-224-5p. Ecotoxicol Environ Saf 220:112378. https://doi.org/10.1016/j.ecoenv.2021.112378
Zhu P, Liao L-Y, Zhao T-T, Mo X-M, Chen GG, Liu Z-M (2017) GPER/ERK&AKT/NF-κB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells. Mol Cell Endocrinol 442:68–80. https://doi.org/10.1016/j.mce.2016.12.007
Zhu L, Li B, Xue J (2022) Current research on the biological role and mechanism of circRNA through Wnt /β-catenin signal pathway. J Med Postgra 35:651–655. https://doi.org/10.16571/j.cnki.1008-8199.2022.06.015
Zimta A-A, Schitcu V, Gurzau E, Stavaru C, Manda G, Szedlacsek S, Berindan-Neagoe I (2019) Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ Res 178:108700. https://doi.org/10.1016/j.envres.2019.108700
Funding
This work was supported by grants from the Sichuan Natural Science Foundation (No. 2022NSFSC0741), Southwest Medical University Research Project (No. 2021ZKZD001) and Southwest Medical University Applied basic Project (No. 2022QN043).
Author information
Authors and Affiliations
Contributions
All authors have reviewed the manuscript, and they contributed equally.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
There is a close relationship between cadmium and neoplasms.
The Cd carcinogenesis is mainly due to the ERK/JNK/p38 MAPK, the PI3K/AKT/mTOR, the NF-κB, and the Wnt signaling pathways.
The DNA repair and epigenetic mechanisms are receiving wide attention.
The researches of the Cd carcinogenesis molecular mechanisms are beneficial to prevent tumors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Gan, X. & Tang, Y. Mechanisms of Heavy Metal Cadmium (Cd)-Induced Malignancy. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04189-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04189-2