Abstract
The aberrant Wnt/β-catenin signaling pathway facilitates cancer stem cell renewal, cell proliferation and differentiation, thus exerting crucial roles in tumorigenesis and therapy response. Accumulated investigations highlight the therapeutic potential of agents targeting Wnt/β-catenin signaling in cancer. Wnt ligand/ receptor interface, β-catenin destruction complex and TCF/β-catenin transcription complex are key components of the cascade and have been targeted with interventions in preclinical and clinical evaluations. This scoping review aims at outlining the latest progress on the current approaches and perspectives of Wnt/β-catenin signaling pathway targeted therapy in various cancer types. Better understanding of the updates on the inhibitors, antagonists and activators of Wnt/β-catenin pathway rationalizes innovative strategies for personalized cancer treatment. Further investigations are warranted to confirm precise and secure targeted agents and achieve optimal use with clinical benefits in malignant diseases.
Similar content being viewed by others
Introduction
The Wnt/β-catenin signaling pathway, also called the canonical Wnt signaling pathway, is a conserved signaling axis participating in diverse physiological processes such as proliferation, differentiation, apoptosis, migration, invasion and tissue homeostasis [1,2,3]. Increasing evidence indicates that dysregulation of the Wnt/β-catenin cascade contributed to the development and progression of some solid tumors and hematological malignancies [4,5,6,7,8].
In the Wnt/β-catenin pathway, abnormal regulation of the transcription factor β-catenin, which is the pivotal component of the Wnt signaling pathway, leads to early events in carcinogenesis [9,10,11,12]. Within the degradation complex, glycogen synthase kinase 3β (GSK3β) and casein kinase 1α (CK1α) mediate the phosphorylation of β-catenin, promoting its ubiquitination and subsequent proteasomal degradation [13, 14]. The β-catenin-dependent signaling pathway is triggered by the binding of secreted cysteine-rich glycoprotein ligands Wnts to the LRP-5/6 receptors and FZD receptors. In the presence of Wnt ligand, the binding of Wnt ligand and receptors on the cell surface induces disheveled (DVL), causing the aggregation of the complex (AXIN, GSK3β, CK1, APC) to the receptor [15]. Subsequently, the phosphorylation and inhibition of GSK3β ensure an elevation of cytosolic β-catenin concentration. Un-phosphorylated β-catenin in the cytosol migrates to the nucleus and accumulates, interacting with T cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) and co-activators, such as Pygopus and Bcl-9, to trigger the Wnt target genes like c-Myc, cyclin D1 and CDKN1A, resulting in the upregulation of TCF/LEF target gene.
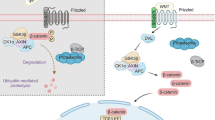
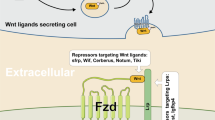
In addition, multiple regulatory mechanisms have been identified on the phosphorylation and ubiquitination of β-catenin by the degradation complex. Notum, which removes palmitoleate from Wnt proteins, blocks their extracellular secretion. Dickkopf (DKK) negatively regulates the initiation of Wnt protein-mediated signaling by competitively binding to LRP5/6 receptors. Besides, secreted FZD-related proteins (sFRPs), which bind to FZD receptors also blocking the initiation of Wnt protein-mediated signaling. Moreover, Wnt inhibitory factor (WIF) inhibits signaling by binding directly to Wnt proteins [16]. The transmembrane molecules ZNRF3 and RNF43 act on FZD molecules with E3 ubiquitin ligase activity [14, 17]. The 7-transmembrane receptor LGR4, LGR5 and LGR6 bind to R-spondins (RSPO) with high affinity to enhance the Wnt signal at a low dose of Wnt ligand [14, 18]. To elucidate the mechanism of Wnt/β-catenin signaling pathway activation and inhibition, a schematic diagram was depicted in Fig. 1.
Schematic representation of activated and inhibited Wnt/β-catenin pathway. “WNT ON state”: Upon ligation of Wnts to their receptors composed of frizzled proteins and LRP5/6, the cytoplasmic protein DVL is activated and induces the suppression of GSK3β. Subsequently, stabilized β-catenin translocates into the nucleus and binds to TCF/LEF transcription factors to lead to target gene transcription. “WNT OFF state”: In the absence of WNT ligand, the destruction complex of β-catenin, a tertiary complex formed by AXIN, CK1α, GSK3β and APC, phosphorylates β-catenin, which subsequently undergoes the ubiquitin-proteasomal degradation
Furthermore, Wnt/β-catenin signaling orchestrates multiple cell signaling cascades, such as epidermal growth factor receptor (EGFR), Hippo/YAP, nuclear factor kappa-B (NF-κB), Notch, Sonic Hedgehog and PI3K/Akt pathway, which contribute to pivotal molecular mechanism in cancer development [19,20,21,22,23,24]. EGFR could form a complex with β-catenin and promotes the invasion and metastasis of cancer cells [25, 26]. Moreover, the Hippo pathway has been shown to inhibit Dvl phosphorylation, nuclear accumulation of β-catenin and transcription of β-catenin/TCF-target genes in the Wnt/β-catenin signaling [21, 27]. Besides, the activation of Wnt/β-catenin pathway interacted with PI3K/AKT/GSK-3 cascade in glioblastoma cells and further provided mechanistic basis for the chemoresistance to temozolomide [22]. Additionally, AKT kinase could also activate β-catenin. Therefore, the cross talk between Wnt/β-catenin and PI3K-AKT pathway was confirmed to promote tumorigenesis and resistance to cancer therapy [23, 28].
Collectively, underscoring the physiological importance of Wnt/β-catenin signaling pathway in tumorigenesis, targeted agents are explored and presented promising therapeutic potential in preclinical studies and clinical trials of some cancer types. In the present review, we elaborated on the advances and challenges of Wnt/β-catenin signaling pathway targeted interventions in malignancies, aiming to provide rationales and insights on novel strategies in cancer therapy.
Wnt/β-catenin signaling pathway interventions for cancer
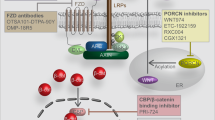
The deregulation of Wnt/β-catenin signaling pathway is closely related to the initiation and progression of various types of cancers [4, 5, 29]. Thus, inhibitors, antagonists and agonists were designed to target this cascade in solid tumors (Table 1) and hematological malignancies (Table 2). Formulas and structures of agents targeted Wnt/β-catenin signaling pathway are listed in Additional file 1. Hallmarks of diverse categories of Wnt/β-catenin targeted agents in malignancies are illustrated in Fig. 2. In addition, Fig. 3 is plotted to present a panoramic overview of Wnt/β-catenin signaling pathway targeted interventions in cancer therapy, which was deciphered in the following aspects.
Inhibitors targeting Wnt ligand/ receptor interface
Porcupine inhibitors
Porcupine (PORCN), a family member of membrane-bound O-acyltransferases (MBOAT), is key for the secretion of Wnt ligands [30, 31]. Several inhibitors that target PORCN prevent the palmitoylation of Wnt proteins in the endoplasmic reticulum, which subsequently prevents their secretion [13, 24]. Blocking the acylation of WNT with a PORCN inhibitor to abolish WNT secretion becomes an effective treatment strategy. WNT974 (LGK974) is an orally available small molecule inhibitor that decreases epithelial ovarian cancer (EOC) cell viability in vitro and inhibits tumor growth in vivo [24, 32]. In EOC preclinical mouse models, WNT974 presents enhanced anti-tumor effects with the combination of paclitaxel [33]. There is currently a phase I clinical trial investigating WNT974 monotherapy for patients with pancreatic cancer, triple-negative breast cancer and cervical squamous cell carcinoma (NCT01351103). CGX1321, another PORCN inhibitor, inhibits both canonical and non-canonical Wnt signaling pathways. The single-dose escalation of CGX1321 is invested in a phase 1 clinical trial (NCT02675946) in solid tumors. In an EOC mouse model, treatment with CGX1321 led to prolonged overall survival, decreased tumor burden and increased immune cell infiltration. Furthermore, effects of some other PORCN inhibitors were evaluated in preclinical studies [34, 35]. It was reported that the combination of the PORCN inhibitor ETC-159 and the PI3K inhibitor GDC-0941 decreased RNF43-mutant pancreatic cancer cell proliferation and xenograft growth in vivo [36]. Besides, IWP-O1 was observed with significantly improved metabolic stability and inhibit the phosphorylation of DVL in Hela cells [37]. Moreover, GNF-6231 demonstrated potent inhibition activities and induced robust anti-tumor efficacy in a breast cancer mouse model [38].
Wnt/FZD antagonists
With the antagonism of Wnt ligands and FZD receptors, canonical Wnt signaling pathway was suppressed and indicated potential strategy in cancer therapy. Ipafricept (OMP54F28; IPA) is a recombinant fusion protein, including the cysteine-rich domain of FZD8 fused to a human IgG1 Fc fragment [39]. This structure could bind directly to Wnt ligands, competing for the binding of Wnt ligands with FZD8 receptor, thereby inhibiting Wnt regulated processes [40]. In patient-derived ovarian cancer xenograft mice models, ipafricept displayed activity to decrease the population of stem cells, suppress tumor development and promote differentiation. In addition, in preclinical studies, ipafricept exhibits synergistic anti-tumor effects combined with taxanes when given prior to chemotherapy two to three days, with 82% of the patients achieved a partial or complete response [41]. Ipafricept was also investigated in a phase 1b dose-escalation study in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. The combination of these three agents produced similar response rates and survival outcomes compared with historical treatment regimens. Nevertheless, bone toxicities at efficacy doses prevented further testing of this treatment regimen. A phase 1b clinical trials suggested that ipafricept could also be administered with nab-paclitaxel and gemcitabine with reasonable tolerance in patients with previously untreated stage IV pancreatic cancer [42].
OMP-18R5 (vantictumab) is a monoclonal antibody targeting FZD1, FZD2, FZD5, FZD7 and FZD8 [43,44,45]. OMP-18R5 blocks tumor growth in xenograft mouse models of breast, pancreatic, colon, lung, and head and neck cancers and is being evaluated in a number of phase I trials for these tumor types [43, 46]. In a clinical trial, OTSA-101 was demonstrated that radioimmunotherapy targeting FZD10 is feasible in synovial sarcoma patients [47]. Besides, Pavlovic et al. utilized combinatorial antibody engineering by phage display to generate a variant antibody F2.A with specificity of FZD4 [44]. F2.A suppresses pancreatic cancer tumor growth in xenograft mouse models. Interestingly, carbamazepine, an antiepileptic drug, was recently reported to bind the cysteine-rich domain of FZD8, which suggests been explored as a promising therapy option in cancers [48]. Additionally, Fz7-21, a selective FZD7-binding peptide, disrupts intestinal stem cells and organoids, implicating the potential of therapeutic application in malignant diseases [49].
LRP5/6 inhibitors
As the co-receptor of Wnt, the phosphorylation of LRP5/6 promotes the activation of Wnt/β-catenin signaling pathway. The molecular complex Wnt-FZD-LRP5/6-DVL forms a structural region for AXIN interaction that disrupts degradation of β-catenin. BMD4503-2, a quinoxaline moiety, was identified as a new small-molecule inhibitor of the LRP5/6-sclerostin interaction through pharmacophore-based virtual screening and in vitro assays. The compound BMD4503-2 could revert the down-regulated activity of the Wnt/β-catenin signaling pathway through competitively binding to the LRP5/6-sclerostin complex [50].
DVL inhibitors
DVL is important for Wnt signal transduction by recruiting components of the β-catenin destruction complex to the cell membrane [51, 52]. DVL binds to the cytoplasmic carboxyl terminal end of FZD proteins through its PDZ domain [53]. NSC668036, FJ9, and 3289–8625 are some agents that block the DVL-PDZ interaction, resulting in subsequently inhibition of the signal transduction pathway [54, 55]. The non-electrophilic indole-2-carbinol-based chemical scaffold of FJ9 disrupted the interaction between FZD and the PDZ domain of DVL. NSC668036 and 3289–8625 were confirmed to down-regulate Wnt/β-catenin signaling and inhibit tumor cell growth in lung, colorectal and cervical cancer cell lines in vitro, as well as in a lung cancer xenografts [54].
Agents targeting the β-catenin-destruction complex
Tankyrase inhibitors
Scaffolding protein AXIN is the rate-limiting component of the β-catenin destruction complex, which are constantly surveyed and regulated by tankyrases [56,57,58]. Tankyrases belong to the Poly (ADP-ribose) polymerases (PARPs) family, regulating the stability of AXIN1 and AXIN2 through directing AXIN ubiquitylation by RNF146 and proteasomal degradation [59, 60]. There are two isoforms, Tankyrase 1 (PARP5a) and Tankyrase 2 (PARP5b) involved in the Wnt/β-catenin signaling, increasing the degradation of AXIN by the ubiquitin–proteasome pathway [61,62,63]. Tankyrase inhibitor, XAV939 and IWR-1 regulated AXIN by inhibiting Tankyrase 1 and Tankyrase 2 [64, 65]. Treatment with XAV939 decreased the viability of EOC cell lines and increased radio-sensitivity in cervical cancer cells [66]. Furthermore, the tankyrase-specific inhibitor, JW74 and JW55 affects cell cycle progression and induced apoptosis and differentiation in osteosarcoma and colon carcinoma cells, respectively [67, 68]. In addition, mice xenografts and patient-derived sphere cultures of colorectal cancer (CRC) were incubated with a Tankyrase inhibitor NVP-TNKS656 combination with AKT and PI3K inhibitors. A decreased nuclear β-catenin level predicted for apoptosis suggesting the tankyrase inhibitor could overcome resistance to AKT and PI3K inhibitors [61]. The same antineoplastic effect was observed in LZZ-02, a novel Tankyrase 1/2 inhibitor [69]. Concerns of gastrointestinal toxicity have been noted in analysis of these inhibitors, and further studies are needed [70].
CK1 agonists
Stabilizing the β-catenin destruction complex can block the nuclear localization of β-catenin, suggesting as an attractive therapeutic target. Feasible strategy for the repositioning of existing FDA approved drugs is explored for the treatment of malignancies with deregulated Wnt signaling. For example, pyrvinium, an existing FDA approved drug, can bind all CK1 family members in vitro, selectively potentiating CK1α kinase activity [71]. Colon cancer cells with APC mutations were sensitive to pyrvinium treatment with a decrease in both Wnt signaling and cell proliferation. Pyrvinium inhibits platinum-resistant tumor growth and induces apoptosis in vitro and in vivo, and these effects are enhanced when combined with paclitaxel. Pyrvinium blocks Wnt signal by decreasing β-catenin levels and suppressing the transcription of β-catenin targeted genes. However, cancer cells with increasing level of β-catenin are no longer impacted by pyrvinium [72, 73]. In addition, a novel small-molecule CK1α activator called SSTC3 has been proved to inhibit the growth of CRC xenografts in mice and also attenuate the growth of patient-derived metastatic CRC xenograft [74, 75].
Inhibitors targeting β-catenin/TCF transcription complex
Several compounds targeting the downstream effectors, like transcription complex and co-activators, were identified by high through-put ELISA screening, such as PFK115-584 and CGP049090, which can block the β-catenin/TCF complex in a dose-dependent manner [76]. LF3, a 4-thioureido-benzenesulfonamide derivative, robustly disrupts the critical interaction between β-catenin and the transcription factor TCF4. Besides, LF3 reduced tumor growth and induced differentiation in a mouse xenograft model of colon cancer [77]. KYA1797K/ KY1220 effectively suppressed the growth of colorectal cancer and breast cancer cells via the destabilization of both β-catenin and Ras [78,79,80]. Mantle cell lymphoma-initiating cells were particularly sensitive to Wnt pathway inhibitors. Targeting β-catenin-TCF4 interaction with CCT036477, iCRT3, iCRT5, iCRT14 or PKF118-310 preferentially eliminated the survival of malignant cells of acute lymphoblastic leukemia, gastric cancer, and breast cancer [81,82,83,84]. ZINC02092166 suppresses canonical Wnt signaling, downregulates the expression of Wnt target genes and inhibits the growth of colorectal cancer cells [85]. Based on the acylhydrazone component, the inhibitory activities were evaluated in cellular assays. NLS-StAx-h, a selective cell-penetrating peptide inhibitor of β-catenin-transcription factor interactions suppressed proliferation and migration of colorectal cancer cells. CWP232291 (CWP291), another small molecule inhibited Wnt-mediated transcriptional activity, was under evaluation on phase 1 clinical trial in patients with relapsed or refractory AML and myelodysplastic syndrome (MDS) [86]. Active form of CWP232204 binds to Src-associated substrate in mitosis of 68 kDa (SAM68), which regulates alternative splicing TCF, and promotes β-catenin degradation via apoptosis. Further investigations will explore CWP291, with a mechanism of aiming at eradication of earlier progenitors via Wnt pathway blockade, as combination therapy.
There are several co-activators of β-catenin-dependent transcription, including CREB binding protein (CBP). The CBPs are key transcriptional co-activators essential for a multitude of cellular processes and involved in human pathological conditions and cancer [87, 88]. Several CBP inhibitors have been developed in recent years and have shown promising antineoplastic effects in preclinical models with minimal off-target effects, such as PRI-724, ICG001, GNE-781, 1-(1H-indol-1-yl)ethenone, JW67, JW74, NLS-StAx-h, et al. [89,90,91]. PRI-724 is a first-in-class small molecule antagonist that inhibits the interaction between β-catenin and CBP [92]. It was phosphorylated-C-82 and was rapidly hydrolyzed to its active form C-82 in vivo [93]. In chemotherapy resistant EOC with hyperactivated CBP/β-catenin signaling, PRI-724 increased sensitization to platinum chemotherapy and preclinical studies had shown considerable toxicity profile [93, 94]. Monotherapy with ICG-001 led to the reduction of tumor-related characteristics [95, 96]. GNE-781 displayed anti-tumor activity in an acute myeloid leukemia (AML) model and was also shown to decrease Foxp3 transcript levels in a dose-dependent manner [90]. 1-(1H-indol-1-yl) ethenone markedly inhibited cell growth in several prostate cancer cell lines [89]. JW67 and JW74 were identified specifically inhibiting canonical Wnt pathway at the level of the destruction complex and inhibited the growth of colorectal cancer mouse xenograft model and multiple intestinal neoplasia mice [97]. Moreover, isoquercitrin showed anti-tumor effects on colon cancer cells (SW480, DLD-1 and HCT116), whereas exerting no significant effect on non-tumor colon cell (IEC-18), suggesting a specific effect in tumor cells in vitro [98].
Natural agents and new activity of old drugs
It is notable that some of the natural agents exert anti-tumor activities via regulating canonical Wnt signaling pathway [99, 100]. Curcumin, isolated from the rhizome of Curcuma longa, modulates Wnt signaling pathway and exerts anti-tumor activities in melanoma, lung cancer, breast cancer, colon cancer, endothelial carcinoma, gastric carcinoma and hepatocellular carcinoma [101]. 3,3′-diindolylmethane (DIM), a natural compound derived from cruciferous vegetables inhibited proliferation of colon and colorectal cancer cells via Wnt/β-catenin pathway, highlighting as a promising chemo-preventive agent or chemo-radio-sensitizer for the prevention of tumor recurrence in cancer therapy [102]. Formononetin, isolated from the red clover, displayed anti-tumor activities in breast cancer and glioma cells with high-level IC50 values. To achieve high potency, formononetin was modified with a coumarin unit to design a derivate 10 via the molecular hybridization strategy. The analog 10 presented anti-proliferative effects through Wnt/β-catenin pathway in gastric cancer [103]. Besides, Wogonin, a major flavonoid compound isolated from Scutellaria radix, decreased intracellular levels of Wnt proteins and activated degradation β-catenin for proteasomal degradation [104]. Gigantol, a bibenzyl compound from orchid species, was also reported to inhibit Wnt/β-catenin signaling through down-regulation of phosphorylated LRP6 and cytosolic β-catenin in breast cancer cells [105]. Additionally, treatment of echinacoside, a phenylethanoid glycoside from Tibetan herbs, significantly reduced tumor growth and regulation of Wnt/β-catenin signaling [106]. Besides, nimbolide, a limonoid present in leaves of the neem tree, concurrently abrogated canonical Wnt signaling and induced intrinsic apoptosis in hepatocarcinoma cells [107]. Moreover, isoquercitrin, a natural flavonol compound, exerted an inhibitory effect on Wnt/β-catenin, where the flavonoid regulated downstream of β-catenin translocation to the nucleus [108]. It was also noted that triptonide, a diterpenoid epoxide presented in Tripterygium wilfordii, could effectively inhibit canonical Wnt/β-catenin signaling by targeting the downstream C-terminal transcription domain of β-catenin or a nuclear component associated with β-catenin and induced apoptosis of Wnt-dependent cancer cells [109]. Moreover, the fungus Exobasidium vexans and its subcomponent atranorin were reported to inhibit lung cancer cell motility and tumorigenesis by affecting nuclear import of β-catenin and downregulating β-catenin/LEF downstream target genes [110].
In addition, researchers had found some old drugs performed new tricks, which play important roles in tumor growth, invasion and metastasis via regulating Wnt/β-catenin signaling pathway. Carbamazepine, an antiepileptic drug, was recently reported to bind the cysteine-rich domain of FZD8, which suggested to been explored as a promising therapy option in cancers [48]. It was also reported that psychiatric agent hexachlorophene attenuated Wnt/β-catenin signaling through suppressing β-catenin degradation in colon cancer cells [111]. Salinomycin, a type of antibiotics, was reported to trigger ionic changes to inhibit proximal Wnt signaling by interfering with LPR6 phosphorylation, and thus impairing the survival of cells that depend on Wnt signaling at the plasma membrane [112,113,114,115,116]. Besides, hematein was found to inhibit cancer cell growth and increased apoptosis through Wnt/TCF pathway [117]. Trifluoperazine (TFP), used as an antipsychotic and antiemetics, had been found to inhibit lung CSC spheroid formation ability and suppress expression of lung CSC markers (e.g., CD44/CD133) by inhibiting Wnt/β-catenin signal transduction [118]. The similar activities were also investigated in thioridazine, pimozide and diphenylbutylpiperidine class, other antiangiogenic agents [119,120,121]. It is notable that cyclooxygenases (COX1 and 2) inhibitors (e.g., aspirin, celecoxib, sulindac and ursolic acid) could inhibit Wnt/β-catenin pathway in cancer cells [122,123,124]. Aspirin increased expression of the Wnt antagonist Dickkopf-1, which suppressed activities of cancer stem cells in CRC cells [125].
Cancer stem cells -Wnt/β-catenin signaling pathway inhibitors
CSCs display many characteristics of embryonic or tissue stem cells and often show continuous activation of highly conserved signaling pathways related to development and tissue homeostasis [126, 127]. The Wnt/β-catenin signaling pathway is associated with regulating the pluripotency, self-renewal of stem cells and differentiation ability [1, 128].
Abnormal activation of the Wnt/β-catenin pathway promotes CSC progression and thus leads to the deterioration and metastasis of cancer [129]. For instance, abnormal activation of Wnt signaling disrupted the normal growth and differentiation of colonic crypt stem cells, resulting in a colorectal CSC phenotype by upregulating expression of target genes such as c-MYC and cyclin D [130]. Moreover, one study showed that experimental knockdown of CD146 could dedifferentiate colorectal cancer cells to acquire a stem cell phenotype through inhibiting GSK-3β which in turn promoted nuclear translocation of β-catenin for Wnt signaling activation [131]. Recent studies identified SAM68 as a novel transcriptional modulator selectively targeting CSCs over healthy stem cells via Wnt/β-catenin signaling [132]. Wnt/β-catenin signaling also exerts a crucial role in early hematopoiesis, notably in hematopoietic stem cells (HSCs). Loss- and gain-of-function studies demonstrated that Wnt signaling and β-catenin activity were necessary for proper function and cellularity control of hematopoietic cells including HSCs and MKs12-15 [133]. Overactive Wnt/β-catenin signaling led to exhaustion of HSCs, causing multilineage differentiation block and compromised hematopoietic stem cell maintenance [134].
Several compounds have been identified to target CSCs via Wnt/β-catenin signaling pathway (Table 3, Fig. 4). It has been reported that PORCN inhibitor WNT974 (LGK-974) inhibited the proliferation of breast CSCs [135, 136]. Niclosamide, an FDA approved anti-helminthic agent was identified as an inhibitor of the Wnt/β-catenin pathway and showed anti-tumor properties to selectively target ovarian CSCs [137]. In addition, niclosamide decreased the level of CSCs by reducing the expression of LRP6 and β-catenin in basal-like breast cancer [138, 139]. Notably, in a phase 2 trial, the safety and effectiveness of niclosamide was proved in the treatment of colorectal cancer [140]. Furthermore, niclosamide can reduce the expression of many components in the Wnt/β-catenin signaling pathway, the self-renewal ability and population of CSCs in CRC [141]. Additionally, ONC201, which is in a phase I/II study for patients with advanced cancer (NCT02038699), induced significant CSC-suppression and repress the expression of CSC-related genes in prostate and glioblastoma tumors through suppressing the Wnt signaling pathway [142,143,144].
Furthermore, many potential compounds targeting CSCs through inhibiting Wnt/β-catenin signaling pathway have been undertaken in preclinical evaluations. For example, XAV939 inhibited β-catenin signaling, thus attenuated CSC progression, thereby eliminating the CSC-mediated chemical resistance in head and neck squamous cell carcinoma (HNSCC) and colon cancer cells [145,146,147]. IWR-1, a tankyrase inhibitor, can hamper the expression of key stem markers in osteosarcoma, impair osteosarcoma CSC self-renewal and enhance doxorubicin sensitivity by affecting β-catenin translocation in vivo [148]. Trifluoperazine (TFP), used as an antipsychotic and antiemetics, has been found to inhibit lung CSC spheroid formation ability and suppress expression of lung CSC markers (e.g., CD44/ CD133) by inhibiting Wnt/β-catenin signal transduction [118]. Additionally, actinomycin D (AD) and telmisartan (TS) can also attenuate the number and activity of CSC and reduce CSC marker expression (such as ALDH1, SOX2 and NOS2) in lung cancer by blocking the Wnt/β-catenin signaling pathway. Besides, chelerythrine was identified to down-regulate the level of β-catenin and inhibited CSC invasion, spheroid formation and the expression of the stem marker SOX2 in non-small cell lung carcinoma (NSCLC) [149, 150]. Wnt-C59 (C59), an inhibitor of Wnt, decreased the sphere formation ability of CSCs dose-dependently in nasopharyngeal carcinoma (NPC) [151]. IC-2, a novel small-molecule Wnt inhibitor, reduced the population of CD44+ cells (liver CSCs) and the sphere-forming ability of hepatocellular carcinoma (HCC) cells, as well as in CRC and bladder cancer cells [152]. In addition, IC-2 increased the sensitivity of 5-FU in the DLD-1 cells, a CRC cell line. Moreover, JIB-04, a selective inhibitor of histone demethylase, significantly attenuated CSC tumor sphere formation, migration and invasion in vitro by regulating the recruitment of β-catenin [153]. A similar phenomenon was noted in FH535, which could suppress the expression of the liver CSC marker CD24 and CD44 [154, 155]. The combination of docetaxel (DTX) and sulforaphane (SFN) and pyrvinium pamoate (PP) can both inhibit the EMT (epithelial–mesenchymal transition), CSC self-renewal ability and drug resistance by decreasing β-catenin expression in BCSCs [156,157,158]. Additionally, SKL2001, an agonist of the Wnt/β-catenin pathway, stabilizes intracellular β-catenin via disruption of the AXIN/β-catenin interaction [159]. The treatment of mesenchymal stem cells with SKL2001 promoted osteoblastogenesis and suppressed adipocyte differentiation, providing a new strategy to regulate mesenchymal stem cell differentiation by modulation of the Wnt/β-catenin pathway. Besides, 5-FU was reported to promote stemness of colorectal cancer via p53-mediated WNT/β-catenin pathway activation [160]. Anti-progastrin humanized antibodies were investigated to decrease self-renewal of CSCs via Wnt signaling and represent potential novel strategies for K-RAS-mutated colorectal cancer [161].
Challenges of Wnt/β-catenin signaling targeted agents in cancer
Aberrant activation of Wnt/β-catenin signaling drives oncogenic transformation in a wide range of cancers, indicating the key pathway modulators as attractive therapeutic targets in malignancies. Despite that Wnt/β-catenin targeted therapies are varied and clinical experience nascent, with the development of the targeted agents and combination strategies under investigation, the risk for off-targeting effectivity, side effects and toxicities are not allowed to be neglected. Of note, the critical role of Wnt/β-catenin signaling in stem cell maintenance raised concerns regarding the dose-limiting toxicity of targeted agents in bone, hair and gastrointestinal tract as well as in hematopoiesis, which limited of its clinical application [162,163,164]. Besides, considerable cross talks between the Wnt/β-catenin signaling pathway with other pathways are critical to designing effective therapeutic approaches. The combination therapy with agents that have impacts on multiple pathways in solid and hematologic malignancies needs long-term follow-up observation. Therefore, further exploration and evaluation are warranted to identify precise and safe targeted agents and achieve optimal use with clinical benefits in cancer.
Conclusions
Novel strategies are imperative to improve the outcome of cancer patients. With great advances in the knowledge of molecular basis and the constant effort for improvement, preclinical investigations and clinical trials have been conducted on the Wnt/β-catenin signaling targeted interventions in malignancies. The Wnt/β-catenin signaling targeted regimens have been proved to represent promising candidates of individualized approaches in the treatment of cancer patients. Further investigations are expected on confirming the safety, efficacy, patient stratification and drug delivery of innovative Wnt/β-catenin targeted therapies in cancer.
Availability of data and materials
Not applicable.
Abbreviations
- PORCN:
-
Porcupine
- CBP:
-
CREB binding protein
- DVL:
-
Disheveled
- EOC:
-
Epithelial ovarian cancer
- CRC:
-
Colorectal cancer
- CSC:
-
Cancer stem cells
- FZD:
-
Frizzled
- LRP:
-
Low density lipoprotein receptor-related protein
- TCF/LEF:
-
Transcription factor/ Lymphoid Enhancer Binding Factor
- AXIN:
-
Anti-Neurexin
- GSK3β:
-
Glycogen synthase kinase-3β
- APC:
-
Adenomatous polyposis coli gene
- LGR:
-
Leucine-rich repeat containing G protein-coupled receptors
- RSPO:
-
R-spondin
- AML:
-
Acute myeloid leukemia
- APL:
-
Acute promyelocytic leukemia
- ALL:
-
Acute lymphocytic leukemia
- CML:
-
Chronic myeloid leukemia
- CLL:
-
Chronic lymphocytic leukemia
- MDS:
-
Myelodysplastic Syndromes
- BL:
-
Burkitt’s lymphoma
- HL:
-
Hodgkin lymphoma
- MCL:
-
Mantle cell lymphoma
References
Salik B, Yi H, Hassan N, Santiappillai N, Vick B, Connerty P, Duly A, Trahair T, Woo A, Beck D et al: Targeting RSPO3-LGR4 signaling for leukemia stem cell eradication in acute myeloid leukemia. Cancer Cell 2020.
Soleas J, D’Arcangelo E, Huang L, Karoubi G, Nostro M, McGuigan A, Waddell T. Assembly of lung progenitors into developmentally-inspired geometry drives differentiation via cellular tension. Biomaterials. 2020;254:120128.
Choi B, Cave C, Na C, Sockanathan S. GDE2-dependent activation of canonical wnt signaling in neurons regulates oligodendrocyte maturation. Cell Rep. 2020;31(5):107540.
Zhang M, Weng W, Zhang Q, Wu Y, Ni S, Tan C, Xu M, Sun H, Liu C, Wei P, et al. The lncRNA NEAT1 activates Wnt/β-catenin signaling and promotes colorectal cancer progression via interacting with DDX5. J Hematol Oncol. 2018;11(1):113.
Cao M-Q, You AB, Zhu X-D, Zhang W, Zhang Y-Y, Zhang S-Z, Zhang K-W, Cai H, Shi W-K, Li X-L, et al. miR-182-5p promotes hepatocellular carcinoma progression by repressing FOXO3a. J Hematol Oncol. 2018;11(1):12.
Ge X, Wang X. Role of Wnt canonical pathway in hematological malignancies. Journal of hematology & oncology. 2010;3:33.
He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851.
Gajos-Michniewicz A, Czyz M: WNT Signaling in melanoma. Int J Mol Sci 2020, 21(14).
Zhang X, Wang L, Qu Y. Targeting the β-catenin signaling for cancer therapy. Pharmacol Res 2020:104794
Wei C, Zhu M, Yang Y, Zhang P, Yang X, Peng R, Gao C, Lu J, Wang L, Deng X, et al. Downregulation of RNF128 activates Wnt/β-catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J Hematol Oncol. 2019;12(1):21.
Zhou J, Toh SHM, Chan Z-L, Quah JY, Chooi J-Y, Tan TZ, Chong PSY, Zeng Q, Chng W-J. A loss-of-function genetic screening reveals synergistic targeting of AKT/mTOR and WTN/β-catenin pathways for treatment of AML with high PRL-3 phosphatase. J Hematol Oncol. 2018;11(1):36.
Lim Z-F, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12(1):134.
Wiese KE, Nusse R, van Amerongen R: Wnt signalling: conquering complexity. Development 2018, 145(12).
Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99.
Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316(5831):1619–22.
Jaschke N, Hofbauer LC, Gobel A, Rachner TD. Evolving functions of Dickkopf-1 in cancer and immunity. Cancer Lett. 2020;482:1–7.
Ci Y, Li X, Chen M, Zhong J, North B, Inuzuka H, He X, Li Y, Guo J, Dai X. SCF E3 ubiquitin ligase targets the tumor suppressor ZNRF3 for ubiquitination and degradation. Protein Cell. 2018;9(10):879–89.
Zebisch M, Xu Y, Krastev C, MacDonald B, Chen M, Gilbert R, He X, Jones E. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun. 2013;4:2787.
Hu T, Li C. Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236.
Chen L, Qin F, Deng X, Avruch J, Zhou D. Hippo pathway in intestinal homeostasis and tumorigenesis. Protein Cell. 2012;3(4):305–10.
Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, Cheng C, Li L, Pi J, Si Y et al: The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res 2020.
Tomar VS, Patil V, Somasundaram K. Temozolomide induces activation of Wnt/β-catenin signaling in glioma cells via PI3K/Akt pathway: implications in glioma therapy. Cell Biol Toxicol. 2020;36(3):273–8.
Perry JM, Tao F, Roy A, Lin T, He XC, Chen S, Lu X, Nemechek J, Ruan L, Yu X, et al. Overcoming Wnt-β-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat Cell Biol. 2020;22(6):689–700.
Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60.
Suzuki M, Shigematsu H, Nakajima T, Kubo R, Motohashi S, Sekine Y, Shibuya K, Iizasa T, Hiroshima K, Nakatani Y, et al. Synchronous alterations of Wnt and epidermal growth factor receptor signaling pathways through aberrant methylation and mutation in non small cell lung cancer. Clin Cancer Res. 2007;13(20):6087–92.
Joosten SPJ, Spaargaren M, Clevers H, Pals ST. Hepatocyte growth factor/MET and CD44 in colorectal cancer: partners in tumorigenesis and therapy resistance. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188437.
Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012;31(5):1109–22.
Toh TB, Lim JJ, Hooi L, Rashid MBMA. Chow EK-H: Targeting Jak/Stat pathway as a therapeutic strategy against SP/CD44+ tumorigenic cells in Akt/β-catenin-driven hepatocellular carcinoma. J Hepatol. 2020;72(1):104–18.
Spaan I, Raymakers RA, van de Stolpe A, Peperzak V. Wnt signaling in multiple myeloma: a central player in disease with therapeutic potential. J Hematol Oncol. 2018;11(1):67.
Torres V, Godoy J, Inestrosa N. Modulating Wnt signaling at the root: porcupine and Wnt acylation. Pharmacol Ther. 2019;198:34–45.
Kabiri Z, Numata A, Kawasaki A. Edison, Tenen D, Virshup D: Wnts are dispensable for differentiation and self-renewal of adult murine hematopoietic stem cells. Blood. 2015;126(9):1086–94.
Agarwal P, Zhang B, Ho Y, Cook A, Li L, Mikhail F, Wang Y, McLaughlin M, Bhatia R. Enhanced targeting of CML stem and progenitor cells by inhibition of porcupine acyltransferase in combination with TKI. Blood. 2017;129(8):1008–20.
Doo DW, Meza-Perez S, Londono AI, Goldsberry WN, Katre AA, Boone JD, Moore DJ, Hudson CT, Betella I, McCaw TR, et al. Inhibition of the Wnt/beta-catenin pathway enhances antitumor immunity in ovarian cancer. Ther Adv Med Oncol. 2020;12:1758835920913798.
Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35(17):2197–207.
García-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, Henne-Bruns D, Pichlo C, Brunstein E, Baumann U, et al. Discovery of inhibitor of wnt production 2 (IWP-2) and related compounds as selective ATP-competitive inhibitors of casein Kinase 1 (CK1) δ/ε. J Med Chem. 2018;61(9):4087–102.
Zhong Z, Sepramaniam S, Chew XH, Wood K, Lee MA, Madan B, Virshup DM. PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers. Oncogene. 2019;38(40):6662–77.
You L, Zhang C, Yarravarapu N, Morlock L, Wang X, Zhang L, Williams NS, Lum L, Chen C. Development of a triazole class of highly potent Porcn inhibitors. Bioorg Med Chem Lett. 2016;26(24):5891–5.
Cheng D, Liu J, Han D, Zhang G, Gao W, Hsieh MH, Ng N, Kasibhatla S, Tompkins C, Li J, et al. Discovery of pyridinyl acetamide derivatives as potent, selective, and orally bioavailable porcupine inhibitors. ACS Med Chem Lett. 2016;7(7):676–80.
Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11.
Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA, Morgan MA, Kapoun AM, Brachmann RK, Stagg R, et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2019;154(2):294–301.
Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL, Cain J, Tam R, Cheng CY, Evans JW, et al. WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv. 2017;3(6):e1700090.
Dotan E, Cardin DB, Lenz H-J, Messersmith W, O'Neil B, Cohen SJ, Denlinger CS, Shahda S, Astsaturov I, Kapoun AM et al: Phase Ib study of Wnt inhibitor ipafricept with gemcitabine and nab-paclitaxel in patients with previously untreated stage IV pancreatic cancer. Clin Cancer Res 2020.
Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109(29):11717–22.
Pavlovic Z, Adams JJ, Blazer LL, Gakhal AK, Jarvik N, Steinhart Z, Robitaille M, Mascall K, Pan J, Angers S, et al. A synthetic anti-Frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties. MAbs. 2018;10(8):1157–67.
Flanagan D, Barker N, Costanzo N, Mason E, Gurney A, Meniel V, Koushyar S, Austin C, Ernst M, Pearson H, et al. Frizzled-7 is required for wnt signaling in gastric tumors with and without mutations. Can Res. 2019;79(5):970–81.
Le PN, Keysar SB, Miller B, Eagles JR, Chimed TS, Reisinger J, Gomez KE, Nieto C, Jackson BC, Somerset HL, et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol Carcinog. 2019;58(3):398–410.
Giraudet A-L, Cassier PA, Iwao-Fukukawa C, Garin G, Badel J-N, Kryza D, Chabaud S, Gilles-Afchain L, Clapisson G, Desuzinges C, et al. A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer. 2018;18(1):646.
Zhao Y, Ren J, Hillier J, Lu W, Jones EY. Antiepileptic drug carbamazepine binds to a novel pocket on the wnt receptor frizzled-8. J Med Chem. 2020;63(6):3252–60.
Nile AH, de Sousa EMF, Mukund S, Piskol R, Hansen S, Zhou L, Zhang Y, Fu Y, Gogol EB, Komuves LG, et al. A selective peptide inhibitor of Frizzled 7 receptors disrupts intestinal stem cells. Nat Chem Biol. 2018;14(6):582–90.
Choi J, Lee K, Kang M, Lim S-K, Tai No K. In silico discovery of quinoxaline derivatives as novel LRP5/6-sclerostin interaction inhibitors. Bioorg Med Chem Lett. 2018;28(6):1116–21.
Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T, et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9(7):743–54.
Jiang X, Charlat O, Zamponi R, Yang Y, Cong F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol Cell. 2015;58(3):522–33.
Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12(5):1251–60.
Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, et al. An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res. 2007;67(2):573–9.
Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem. 2009;284(24):16256–63.
Lee E, Salic A, Krüger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1(1):E10.
Bernkopf D, Brückner M, Hadjihannas M, Behrens J. An aggregon in conductin/axin2 regulates Wnt/β-catenin signaling and holds potential for cancer therapy. Nat Commun. 2019;10(1):4251.
Ji L, Lu B, Zamponi R, Charlat O, Aversa R, Yang Z, Sigoillot F, Zhu X, Hu T, Reece-Hoyes J, et al. USP7 inhibits Wnt/β-catenin signaling through promoting stabilization of Axin. Nat Commun. 2019;10(1):4184.
Xu D, Liu J, Fu T, Shan B, Qian L, Pan L, Yuan J. USP25 regulates Wnt signaling by controlling the stability of tankyrases. Genes Dev. 2017;31(10):1024–35.
Masuda M, Sawa M, Yamada T. Therapeutic targets in the Wnt signaling pathway: feasibility of targeting TNIK in colorectal cancer. Pharmacol Ther. 2015;156:1–9.
Arques O, Chicote I, Puig I, Tenbaum SP, Argiles G, Dienstmann R, Fernandez N, Caratu G, Matito J, Silberschmidt D, et al. Tankyrase inhibition blocks Wnt/beta-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22(3):644–56.
Schatoff E, Goswami S, Zafra M, Foronda M, Shusterman M, Leach B, Katti A, Diaz B, Dow L. Distinct colorectal cancer-associated APC mutations dictate response to tankyrase inhibition. Cancer Discov. 2019;9(10):1358–71.
Distler A, Deloch L, Huang J, Dees C, Lin N, Palumbo-Zerr K, Beyer C, Weidemann A, Distler O, Schett G, et al. Inactivation of tankyrases reduces experimental fibrosis by inhibiting canonical Wnt signalling. Ann Rheum Dis. 2013;72(9):1575–80.
Tian XH, Hou WJ, Fang Y, Fan J, Tong H, Bai SL, Chen Q, Xu H, Li Y. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/beta-catenin signaling pathway. J Exp Clin Cancer Res. 2013;32:100.
Riffell JL, Lord CJ, Ashworth A. Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov. 2012;11(12):923–36.
Zhang J, Si J, Gan L, Guo M, Yan J, Chen Y, Wang F, Xie Y, Wang Y, Zhang H. Inhibition of Wnt signalling pathway by XAV939 enhances radiosensitivity in human cervical cancer HeLa cells. Artif Cells Nanomed Biotechnol. 2020;48(1):479–87.
Stratford EW, Daffinrud J, Munthe E, Castro R, Waaler J, Krauss S, Myklebost O. The tankyrase-specific inhibitor JW74 affects cell cycle progression and induces apoptosis and differentiation in osteosarcoma cell lines. Cancer Med. 2014;3(1):36–46.
Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova O, et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Can Res. 2012;72(11):2822–32.
Li B, Liang J, Lu F, Zeng G, Zhang J, Ma Y, Liu P, Wang Q, Zhou Q, Chen L: Discovery of novel inhibitor for WNT/β-catenin pathway by tankyrase 1/2 structure-based virtual screening. Molecules 2020, 25(7).
Zhong Y, Katavolos P, Nguyen T, Lau T, Boggs J, Sambrone A, Kan D, Merchant M, Harstad E, Diaz D, et al. Tankyrase inhibition causes reversible intestinal toxicity in mice with a therapeutic index < 1. Toxicol Pathol. 2016;44(2):267–78.
Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol. 2010;6(11):829–36.
Zhang C, Zhang Z, Zhang S, Wang W, Hu P. Targeting of Wnt/beta-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med Sci Monit. 2017;23:266–75.
Wei W, Liu H, Yuan J, Yao Y: Targeting Wnt/beta-catenin by anthelmintic drug niclosamide overcomes paclitaxel resistance in esophageal cancer. Fundam Clin Pharmacol 2020.
Li B, Orton D, Neitzel LR, Astudillo L, Shen C, Long J, Chen X, Kirkbride KC, Doundoulakis T, Guerra ML et al: Differential abundance of CK1α provides selectivity for pharmacological CK1α activators to target WNT-dependent tumors. Sci Signal 2017, 10(485).
Rodriguez-Blanco J, Li B, Long J, Shen C, Yang F, Orton D, Collins S, Kasahara N, Ayad NG, McCrea HJ, et al. A CK1α activator penetrates the brain and shows efficacy against drug-resistant metastatic medulloblastoma. Clin Cancer Res. 2019;25(4):1379–88.
Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5(1):91–102.
Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI, von Kries JP, Birchmeier W. A Small-molecule antagonist of the β-Catenin/TCF4 interaction blocks the self-renewal of cancer stem cells and suppresses tumorigenesis. Can Res. 2016;76(4):891–901.
Cha P-H, Cho Y-H, Lee S-K, Lee J, Jeong W-J, Moon B-S, Yun J-H, Yang JS, Choi S, Yoon J, et al. Small-molecule binding of the axin RGS domain promotes β-catenin and Ras degradation. Nat Chem Biol. 2016;12(8):593–600.
Cha P-H, Choi K-Y. Simultaneous destabilization of β-catenin and Ras via targeting of the axin-RGS domain as a potential therapeutic strategy for colorectal cancer. BMB Rep. 2016;49(9):455–6.
Ryu W-J, Lee JD, Park J-C, Cha P-H, Cho Y-H, Kim JY, Sohn JH, Paik S, Choi K-Y. Destabilization of β-catenin and RAS by targeting the Wnt/β-catenin pathway as a potential treatment for triple-negative breast cancer. Exp Mol Med. 2020;52(5):832–42.
Mathur R, Sehgal L, Braun FK, Berkova Z, Romaguerra J, Wang M, Rodriguez MA, Fayad L, Neelapu SS, Samaniego F. Targeting Wnt pathway in mantle cell lymphoma-initiating cells. J Hematol Oncol. 2015;8:63.
Dandekar S, Romanos-Sirakis E, Pais F, Bhatla T, Jones C, Bourgeois W, Hunger SP, Raetz EA, Hermiston ML, Dasgupta R, et al. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2014;167(1):87–99.
Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J Transl Med. 2013;11:280.
Ji L, Qian W, Gui L, Ji Z, Yin P, Lin GN, Wang Y, Ma B, Gao W-Q. Blockade of β-Catenin-Induced CCL28 suppresses gastric cancer progression via inhibition of treg cell infiltration. Can Res. 2020;80(10):2004–16.
Catrow JL, Zhang Y, Zhang M, Ji H. Discovery of selective small-molecule inhibitors for the β-catenin/T-cell factor protein-protein interaction through the optimization of the acyl hydrazone moiety. J Med Chem. 2015;58(11):4678–92.
Lee JH, Faderl S, Pagel JM, Jung CW, Yoon SS, Pardanani AD, Becker PS, Lee H, Choi J, Lee K, et al. Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Adv. 2020;4(9):2032–43.
Kleszcz R, Szymanska A, Krajka-Kuzniak V, Baer-Dubowska W, Paluszczak J. Inhibition of CBP/beta-catenin and porcupine attenuates Wnt signaling and induces apoptosis in head and neck carcinoma cells. Cell Oncol (Dordr). 2019;42(4):505–20.
Che M, Kweon SM, Teo JL, Yuan YC, Melstrom LG, Waldron RT, Lugea A, Urrutia RA, Pandol SJ, Lai KKY: Targeting the CBP/beta-Catenin Interaction to Suppress Activation of Cancer-Promoting Pancreatic Stellate Cells. Cancers (Basel) 2020, 12(6).
Xiang Q, Wang C, Zhang Y, Xue X, Song M, Zhang C, Li C, Wu C, Li K, Hui X, et al. Discovery and optimization of 1-(1H-indol-1-yl)ethanone derivatives as CBP/EP300 bromodomain inhibitors for the treatment of castration-resistant prostate cancer. Eur J Med Chem. 2018;147:238–52.
Romero FA, Murray J, Lai KW, Tsui V, Albrecht BK, An L, Beresini MH, de Leon BG, Bronner SM, Chan EW, et al. GNE-781, A highly advanced potent and selective bromodomain inhibitor of cyclic adenosine monophosphate response element binding protein, binding protein (CBP). J Med Chem. 2017;60(22):9162–83.
Evangelisti C, Chiarini F, Cappellini A, Paganelli F, Fini M, Santi S, Martelli AM, Neri LM, Evangelisti C. Targeting Wnt/beta-catenin and PI3K/Akt/mTOR pathways in T-cell acute lymphoblastic leukemia. J Cell Physiol. 2020;235(6):5413–28.
Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105(9):1087–92.
Osawa Y, Oboki K, Imamura J, Kojika E, Hayashi Y, Hishima T, Saibara T, Shibasaki F, Kohara M, Kimura K. Inhibition of cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB)-binding protein (CBP)/β-catenin reduces liver fibrosis in mice. EBioMedicine. 2015;2(11):1751–8.
Wu G, Cao L, Zhu J, Tan Z, Tang M, Li Z, Hu Y, Yu R, Zhang S, Song L, et al. Loss of RBMS3 confers platinum resistance in epithelial ovarian cancer via activation of miR-126-5p/beta-catenin/CBP signaling. Clin Cancer Res. 2019;25(3):1022–35.
Fendler A, Bauer D, Busch J, Jung K, Wulf-Goldenberg A, Kunz S, Song K, Myszczyszyn A, Elezkurtaj S, Erguen B, et al. Inhibiting WNT and NOTCH in renal cancer stem cells and the implications for human patients. Nature Commun. 2020;11(1):929.
Wiese M, Hamdan FH, Kubiak K, Diederichs C, Gielen GH, Nussbaumer G, Carcaboso AM, Hulleman E, Johnsen SA, Kramm CM. Combined treatment with CBP and BET inhibitors reverses inadvertent activation of detrimental super enhancer programs in DIPG cells. Cell Death Dis. 2020;11(8):673.
Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D, Gradl D, Paulsen JE, Machonova O, Dembinski JL, et al. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71(1):197–205.
Amado NG, Predes D, Fonseca BF, Cerqueira DM, Reis AH, Dudenhoeffer AC, Borges HL, Mendes FA, Abreu JG. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J Biol Chem. 2014a;289(51):35456–67.
Bahrami A, Amerizadeh F, ShahidSales S, Khazaei M, Ghayour-Mobarhan M, Sadeghnia HR, Maftouh M, Hassanian SM, Avan A. Therapeutic potential of targeting wnt/beta-catenin pathway in treatment of colorectal cancer: rational and progress. J Cell Biochem. 2017;118(8):1979–83.
Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21(11):1541–7.
Ashrafizadeh M, Ahmadi Z, Mohamamdinejad R, Yaribeygi H, Serban MC, Orafai HM, Sahebkar A. Curcumin therapeutic modulation of the wnt signaling pathway. Curr Pharm Biotechnol. 2020;21(11):1006–15.
Banerjee S, Kong D, Wang Z, Bao B, Hillman GG, Sarkar FH. Attenuation of multi-targeted proliferation-linked signaling by 3,3’-diindolylmethane (DIM): from bench to clinic. Mutat Res. 2011;728(1–2):47–66.
Yao JN, Zhang XX, Zhang YZ, Li JH, Zhao DY, Gao B, Zhou HN, Gao SL, Zhang LF. Discovery and anticancer evaluation of a formononetin derivative against gastric cancer SGC7901 cells. Invest New Drugs. 2019;37(6):1300–8.
He L, Lu N, Dai Q, Zhao Y, Zhao L, Wang H, Li Z, You Q, Guo Q. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. Toxicology. 2013;312:36–47.
Yu S, Wang Z, Su Z, Song J, Zhou L, Sun Q, Liu S, Li S, Li Y, Wang M, et al. Gigantol inhibits Wnt/β-catenin signaling and exhibits anticancer activity in breast cancer cells. BMC complementary and alternative medicine. 2018;18(1):59.
Tang C, Gong L. Lvzi Xu, Qiu K, Zhang Z, Wan L: Echinacoside inhibits breast cancer cells by suppressing the Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun. 2020;526(1):170–5.
Kavitha K, Vidya Priyadarsini R, Anitha P, Ramalingam K, Sakthivel R, Purushothaman G, Singh A, Karunagaran D, Nagini S. Nimbolide, a neem limonoid abrogates canonical NF-κB and Wnt signaling to induce caspase-dependent apoptosis in human hepatocarcinoma (HepG2) cells. Eur J Pharmacol. 2012;681:6–14.
Amado N, Predes D, Fonseca B, Cerqueira D, Reis A, Dudenhoeffer A, Borges H, Mendes F, Abreu J. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J Biol Chem. 2014b;289(51):35456–67.
Chinison J, Aguilar JS, Avalos A, Huang Y, Wang Z, Cameron DJ, Hao J. Triptonide effectively inhibits Wnt/β-catenin signaling via c-terminal transactivation domain of β-catenin. Sci Rep. 2016;6:32779.
Zhou R, Yang Y, Park S-Y, Nguyen TT, Seo Y-W, Lee KH, Lee JH, Kim KK, Hur J-S, Kim H. The lichen secondary metabolite atranorin suppresses lung cancer cell motility and tumorigenesis. Sci Rep. 2017;7(1):8136.
Park S, Gwak J, Cho M, Song T, Won J, Kim D, Shin J, Oh S. Hexachlorophene inhibits Wnt/beta-catenin pathway by promoting Siah-mediated beta-catenin degradation. Mol Pharmacol. 2006;70(3):960–6.
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108(32):13253–7.
Tang Q-L, Zhao Z-Q, Li J-C, Liang Y, Yin J-Q, Zou C-Y, Xie X-B, Zeng Y-X, Shen J-N, Kang T, et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011;311(1):113–21.
Sánchez-Tilló E, Fanlo L, Siles L, Montes-Moreno S, Moros A, Chiva-Blanch G, Estruch R, Martinez A, Colomer D, Győrffy B, et al. The EMT activator ZEB1 promotes tumor growth and determines differential response to chemotherapy in mantle cell lymphoma. Cell Death Differ. 2014;21(2):247–57.
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039.
Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/β-catenin and mTORC1 signaling in breast and prostate cancer cells. J Cell Biochem. 2014;115(10):1799–807.
Hung M, Xu Z, Chen Y, Smith E, Mao J, Hsieh D, Lin Y, Yang C, Jablons D, You L. Hematein, a casein kinase II inhibitor, inhibits lung cancer tumor growth in a murine xenograft model. Int J Oncol. 2013;43(5):1517–22.
Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, Ho CC, Chen CC, Kuo YL, Lee PY, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186(11):1180–8.
Shaw V, Srivastava S, Srivastava SK: Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin Cancer Biol 2019.
Li B, Yan J, Phyu T, Fan S, Chung TH, Mustafa N, Lin B, Wang L, Eichhorn PJA, Goh BC, et al. MELK mediates the stability of EZH2 through site-specific phosphorylation in extranodal natural killer/T-cell lymphoma. Blood. 2019;134(23):2046–58.
Ren Y, Tao J, Jiang Z, Guo D, Tang J. Pimozide suppresses colorectal cancer via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 2018;209:267–73.
Zhang R, Li Y, Tian D, Liu Y, Nian W, Zou X, Chen Q, Zhou L, Deng Z, He B. Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int J Oncol. 2016;49(5):1973–82.
Li N, Xi Y, Tinsley H, Gurpinar E, Gary B, Zhu B, Li Y, Chen X, Keeton A, Abadi A, et al. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol Cancer Ther. 2013;12(9):1848–59.
Deng Y, Su Q, Mo J, Fu X, Zhang Y, Lin E. Celecoxib downregulates CD133 expression through inhibition of the Wnt signaling pathway in colon cancer cells. Cancer Invest. 2013;31(2):97–102.
Dunbar K, Valanciute A, Lima A, Vinuela P, Jamieson T, Rajasekaran V, Blackmur J, Ochocka-Fox A, Guazzelli A, Cammareri P et al: Aspirin rescues wnt-driven stem-like phenotype in human intestinal organoids and increases the wnt antagonist dickkopf-1. Cellular Mol Gastroenterol Hepatol 2020.
Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. 2020;13(1):60.
Aulicino F, Pedone E, Sottile F, Lluis F, Marucci L, Cosma MP: Canonical wnt pathway controls mESC self-renewal through inhibition of spontaneous differentiation via beta-catenin/TCF/LEF functions. Stem Cell Rep 2020.
Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106.
Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16(12):3153–62.
Koury J, Zhong L, Hao J. Targeting signaling pathways in cancer stem cells for cancer treatment. Stem Cells Int. 2017;2017:2925869.
Liu D, Du L, Chen D, Ye Z, Duan H, Tu T, Feng J, Yang Y, Chen Q, Yan X. Reduced CD146 expression promotes tumorigenesis and cancer stemness in colorectal cancer through activating Wnt/β-catenin signaling. Oncotarget. 2016;7(26):40704–18.
Benoit YD, Mitchell RR, Risueno RM, Orlando L, Tanasijevic B, Boyd AL, Aslostovar L, Salci KR, Shapovalova Z, Russell J, et al. Sam68 allows selective targeting of human cancer stem cells. Cell Chem Biol. 2017;24(7):833-844.e9.
Shooshtarizadeh P, Helness A, Vadnais C, Brouwer N, Beauchemin H, Chen R, Bagci H, Staal FJT, Coté J-F, Möröy T. Gfi1b regulates the level of Wnt/β-catenin signaling in hematopoietic stem cells and megakaryocytes. Nat Commun. 2019;10(1):1270.
Kirstetter P, Anderson K, Porse BT, Jacobsen SEW, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7(10):1048–56.
Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M, Liu C. Nestin positively regulates the Wnt/beta-catenin pathway and the proliferation, survival and invasiveness of breast cancer stem cells. Breast Cancer Res. 2014;16(4):408.
Solzak JP, Atale RV, Hancock BA, Sinn AL, Pollok KE, Jones DR, Radovich M. Dual PI3K and Wnt pathway inhibition is a synergistic combination against triple negative breast cancer. Npj Breast Cancer. 2017;3:17.
Lin CK, Bai MY, Hu TM, Wang YC, Chao TK, Weng SJ, Huang RL, Su PH, Lai HC. Preclinical evaluation of a nanoformulated antihelminthic, niclosamide, in ovarian cancer. Oncotarget. 2016;7(8):8993–9006.
Londono-Joshi AI, Arend RC, Aristizabal L, Lu W, Samant RS, Metge BJ, Hidalgo B, Grizzle WE, Conner M, Forero-Torres A, et al. Effect of niclosamide on basal-like breast cancers. Mol Cancer Ther. 2014;13(4):800–11.
Ye T, Xiong Y, Yan Y, Xia Y, Song X, Liu L, Li D, Wang N, Zhang L, Zhu Y, et al. The anthelmintic drug niclosamide induces apoptosis, impairs metastasis and reduces immunosuppressive cells in breast cancer model. PLoS ONE. 2014;9(1):e85887.
Burock S, Daum S, Keilholz U, Neumann K, Walther W, Stein U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: the NIKOLO trial. BMC Cancer. 2018;18(1):297.
Park SY, Kim JY, Choi JH, Kim JH, Lee CJ, Singh P, Sarkar S, Baek JH, Nam JS. Inhibition of LEF1-mediated DCLK1 by niclosamide attenuates colorectal cancer stemness. Clin Cancer Res. 2019;25(4):1415–29.
Prabhu VV, Lulla AR, Madhukar NS, Ralff MD, Zhao D, Kline CLB, Van den Heuvel APJ, Lev A, Garnett MJ, McDermott U, et al. Cancer stem cell-related gene expression as a potential biomarker of response for first-in-class imipridone ONC201 in solid tumors. PLoS ONE. 2017;12(8):e0180541.
Lev A, Lulla AR, Ross BC, Ralff MD, Makhov PB, Dicker DT, El-Deiry WS. ONC201 targets AR and AR-V7 signaling, reduces PSA, and synergizes with everolimus in prostate cancer. Mol Cancer Res. 2018;16(5):754–66.
Arrillaga-Romany I, Odia Y, Prabhu VV, Tarapore RS, Merdinger K, Stogniew M, Oster W, Allen JE, Mehta M, Batchelor TT, et al. Biological activity of weekly ONC201 in adult recurrent glioblastoma patients. Neuro Oncol. 2020;22(1):94–102.
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20.
Roy S, Roy S, Kar M, Chakraborty A, Kumar A, Delogu F, Asthana S, Hande MP, Banerjee B. Combined treatment with cisplatin and the tankyrase inhibitor XAV-939 increases cytotoxicity, abrogates cancer-stem-like cell phenotype and increases chemosensitivity of head-and-neck squamous-cell carcinoma cells. Mutat Res. 2019;846:503084.
Wu X, Luo F, Li J, Zhong X, Liu K. Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. Int J Oncol. 2016;48(4):1333–40.
Martins-Neves SR, Paiva-Oliveira DI, Fontes-Ribeiro C, Bovee J, Cleton-Jansen AM, Gomes CMF. IWR-1, a tankyrase inhibitor, attenuates Wnt/beta-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of a subcutaneous human osteosarcoma xenograft. Cancer Lett. 2018;414:1–15.
Heng WS, Cheah SC: Chelerythrine chloride downregulates beta-catenin and inhibits stem cell properties of non-small cell lung carcinoma. Molecules 2020, 25(1).
Medvetz D, Sun Y, Li C, Khabibullin D, Balan M, Parkhitko A, Priolo C, Asara JM, Pal S, Yu J, et al. High-throughput drug screen identifies chelerythrine as a selective inducer of death in a TSC2-null setting. Mol Cancer Res. 2015;13(1):50–62.
Cheng Y, Phoon YP, Jin X, Chong SY, Ip JC, Wong BW, Lung ML. Wnt-C59 arrests stemness and suppresses growth of nasopharyngeal carcinoma in mice by inhibiting the Wnt pathway in the tumor microenvironment. Oncotarget. 2015;6(16):14428–39.
Wu L, He S, He Y, Wang X, Lu L. IC-2 Suppresses proliferation and induces apoptosis of bladder cancer cells via the wnt/beta-catenin pathway. Med Sci Monit. 2018;24:8074–80.
Kim M, Cho H, Yoon H, Ahn Y, Park E, Jin Y, Jang Y. JIB-04, a small molecule histone demethylase inhibitor, selectively targets colorectal cancer stem cells by inhibiting the wnt/β-catenin signaling pathway. Sci Rep. 2018;8(1):6611.
Gedaly R, Galuppo R, Daily MF, Shah M, Maynard E, Chen C, Zhang X, Esser KA, Cohen DA, Evers BM, et al. Targeting the Wnt/beta-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS ONE. 2014;9(6):e99272.
Razak S, Afsar T, Almajwal A, Alam I, Jahan S. Growth inhibition and apoptosis in colorectal cancer cells induced by Vitamin D-Nanoemulsion (NVD): involvement of Wnt/β-catenin and other signal transduction pathways. Cell Biosci. 2019;9:15.
Mackenzie G, Ouyang N, Xie G, Vrankova K, Huang L, Sun Y, Komninou D, Kopelovich L, Rigas B. Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice. Cancer Prevent Res (Philadelphia, Pa). 2011;4(7):1052–60.
de Bessa Garcia SA, Pavanelli AC. Cruz E Melo N, Nagai MA: Prostate apoptosis response 4 (PAR4) expression modulates WNT signaling pathways in MCF7 breast cancer cells: A possible mechanism underlying PAR4-mediated docetaxel chemosensitivity. Int J Mol Med. 2017;39(4):809–18.
Xu L, Zhang L, Hu C, Liang S, Fei X, Yan N, Zhang Y, Zhang F. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. Int J Oncol. 2016;48(3):1175–86.
Gwak J, Hwang SG, Park H-S, Choi SR, Park S-H, Kim H, Ha N-C, Bae SJ, Han J-K, Kim D-E, et al. Small molecule-based disruption of the Axin/β-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res. 2012;22(1):237–47.
Cho YH, Ro EJ, Yoon JS, Mizutani T, Kang DW, Park JC, Il Kim T, Clevers H, Choi KY. 5-FU promotes stemness of colorectal cancer via p53-mediated WNT/beta-catenin pathway activation. Nat Commun. 2020;11(1):5321.
Prieur A, Cappellini M, Habif G, Lefranc MP, Mazard T, Morency E, Pascussi JM, Flaceliere M, Cahuzac N, Vire B, et al. Targeting the wnt pathway and cancer stem cells with anti-progastrin humanized antibodies as a potential treatment for K-Ras-mutated colorectal cancer. Clin Cancer Res. 2017;23(17):5267–80.
Cui C, Zhou X, Zhang W, Qu Y, Ke X. Is beta-catenin a druggable target for cancer therapy? Trends Biochem Sci. 2018;43(8):623–34.
Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13(7):513–32.
Lamture G, Crooks PA, Borrelli MJ. Actinomycin-D and dimethylamino-parthenolide synergism in treating human pancreatic cancer cells. Drug Dev Res. 2018;79(6):287–94.
Acknowledgments
Not applicable.
Funding
This study was funded by Translational Research Grant of National Clinical Research Center for Hematologic Diseases (NCRCH) (No.2020ZKMB01); National Natural Science Foundation (Nos. 82000195, 82070203, 81770210, 81473486 and 81270598); Key Research and Development Program of Shandong Province (No. 2018CXGC1213); Technology Development Projects of Shandong Province (No. 2017GSF18189); Taishan Scholars Program of Shandong Province; Shandong Provincial Natural Science Foundation (No.ZR201911140028); Shandong Provincial Engineering Research Center of Lymphoma; Academic Promotion Programme of Shandong First Medical University; Shandong Provincial Hospital Youth Talent Plan; Shandong Provincial Hospital Research Incubation Fund.
Author information
Authors and Affiliations
Contributions
Y.Z. drafted the manuscript. X.W. and Y.Z. revised the manuscript. Both authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Formulas and structures of agents targeted Wnt/β-catenin signaling pathway.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 13, 165 (2020). https://doi.org/10.1186/s13045-020-00990-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-020-00990-3