Abstract
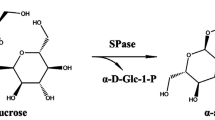
2-O-α-D-Glucosyl glycerol (2-αGG) can be used as a multipurpose anti-aging, cell-stimulating, and skin moisturizing agent in the cosmetic industry. Sucrose phosphorylase (SPase) has been widely used in the production of 2-αGG. In this paper, the gene encoding sucrose phosphorylase from Bifidobacterium longum (BlSP) was inserted into pRSF-Duet-1 to construct the recombinant plasmid pRSF-BlSP and was functionally expressed in E. coli BL21(DE3) to be used as a biocatalyst for the synthesis of 2-αGG firstly. The mutations of BlSP were carried out based on alanine scanning, and a positive mutant G293A with a 50% increase in activity for 2-αGG production was identified. Mutant G293A has less Km and bigger kcat/Km towards glycerol than the parental BlSP. Subsequently, the production of 177.6 g/L 2-αGG was attained from 1 M sucrose and 1.2 M glycerol catalyzed by 17 mg/mL G293A mutant. This study indicated that BlSP has good potential in the production of 2-αGG.












Similar content being viewed by others
Data Availability
All proteins in the present study are available from UniProtKB via the accession codes. The original data are provided with the paper.
References
Bolivar, J. M., Luley-Goedl, C., Leitner, E., Sawangwan, T., & Nidetzky, B. (2017). Production of glucosyl glycerol by immobilized sucrose phosphorylase: Options for enzyme fixation on a solid support and application in microscale flow format. Journal of Biotechnology, 257, 131–138.
Takenaka, F., Uchiyama, H., & Imamura, T. (2000). Identification of alpha-D-glucosylglycerol in sake. Bioscience, Biotechnology, and Biochemistry, 64, 378–385.
Schagen, S. K., Overhagen, S. & Bilstein, A. (2017). New data confirm skin revitalizing and stress protection by Glycoin® natural. Euro Cosmetic, 1/2, 24–27.
Weber, T. M., Kausch, M., Rippke, F., Schoelermann, A. M., & Filbry, A. W. (2012). Treatment of xerosis with a topical formulation containing glyceryl glucoside, natural moisturizing factors, and ceramide. The Journal of Clinical and Aesthetic Dermatology, 5, 29–39.
Schrader, A., Siefken, W., Kueper, T., Breitenbach, U., Gatermann, C., Sperling, G., Biernoth, T., Scherner, C., Stäb, F., Wenck, H., Wittern, K. P., & Blatt, T. (2012). Effects of glyceryl glucoside on AQP3 expression, barrier function and hydration of human skin. Skin Pharmacology and Physiology, 25, 192–199.
Harada, N., Zhao, J., Kurihara, H., Nakagata, N., & Okajima, K. (2010). Effects of topical application of alpha-D-glucosylglycerol on dermal levels of insulin-like growth factor-i in mice and on facial skin elasticity in humans. Bioscience, Biotechnology, and Biochemistry, 74, 759–765.
Su, C., Allum, A. J., Aizawa, Y., & Kato, T. A. (2016). Novel glyceryl glucoside is a low toxic alternative for cryopreservation agent. Biochemical and Biophysical Research Communications, 476, 359–364.
Tan, X., Luo, Q., & Lu, X. (2016). Biosynthesis, biotechnological production, and applications of glucosylglycerols. Applied Microbiology and Biotechnology, 100, 6131–6139.
Takenaka, F., & Uchiyama, H. (2000). Synthesis of alpha-D-glucosylglycerol by alpha-glucosidase and some of its characteristics. Bioscience, Biotechnology, and Biochemistry, 64, 1821–1826.
Franceus, J., & Desmet, T. (2020). Sucrose phosphorylase and related enzymes in glycoside hydrolase family 13: Discovery, application and engineering. International Journal of Molecular Sciences, 21, 2526.
Jeong, J. W., Seo, D. H., Jung, J. H., Park, J. H., Baek, N. I., Kim, M. J., & Park, C. S. (2014). Biosynthesis of glucosyl glycerol, a compatible solute, using intermolecular transglycosylation activity of amylosucrase from Methylobacillus flagellatus KT. Applied Biochemistry and Biotechnology, 173, 904–917.
Goedl, C., Sawangwan, T., Mueller, M., Schwarz, A., & Nidetzky, B. (2008). A high-yielding biocatalytic process for the production of 2-O-(alpha-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angewandte Chemie International Edition, 47, 10086–10089.
Franceus, J., Ubiparip, Z., Beerens, K., & Desmet, T. (2021). Engineering of a thermostable biocatalyst for the synthesis of 2-O-glucosylglycerol. ChemBioChem, 22, 2777–2782.
Luley-Goedl, C., Sawangwan, T., Mueller, M., Schwarz, A., & Nidetzky, B. (2010). Biocatalytic process for production of α-glucosylglycerol using sucrose phosphorylase. Food Technology and Biotechnology, 48, 276–283.
Xia, Y. Y., Li, X. Y., Yang, L. L., Luo, X. Z., Shen, W., Cao, Y., Peplowski, L., & Chen, X. Z. (2021). Development of thermostable sucrose phosphorylase by semi-rational design for efficient biosynthesis of alpha-D-glucosylglycerol. Applied Microbiology and Biotechnology, 105, 7309–7319.
Zhang, T., Yang, J., Tian, C., Ren, C., Chen, P., Men, Y., & Sun, Y. (2020). High-yield biosynthesis of glucosylglycerol through coupling phosphorolysis and transglycosylation reactions. Journal of Agriculture and Food Chemistry, 68, 15249–15256.
Kim, M., Kwon, T., Lee, H. J., Kim, K. H., Chung, D. K., Ji, G. E., Byeon, E. S., & Lee, J. H. (2003). Cloning and expression of sucrose phosphorylase gene from Bifidobacterium longum in E. coli and characterization of the recombinant enzyme. Biotechnology Letters, 25, 1211–1217.
Zhang, H., Sun, X., Li, W., Li, T., Li, S., & Kitaoka, M. (2018). Expression and characterization of recombinant sucrose phosphorylase. Protein Journal, 37, 93–100.
Shin, M. H., Cheong, N. Y., Lee, J. H., & Kim, K. H. (2009). Transglucosylation of caffeic acid by a recombinant sucrose phosphorylase in aqueous buffer and aqueous-supercritical CO2 media. Food Chemistry, 115, 1028–1033.
van den Broek, L. A., van Boxtel, E. L., Kievit, R. P., Verhoef, R., Beldman, G., & Voragen, A. G. (2004). Physico-chemical and transglucosylation properties of recombinant sucrose phosphorylase from Bifidobacterium adolescentis DSM20083. Applied Microbiology and Biotechnology, 65, 219–227.
Schägger, H., & Jagow, G. V. (1987). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Analytical Biochemistry, 166, 368–379.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–428.
Kim, D. E., Chivian, D., & Baker, D. (2004). Protein structure prediction and analysis using the Robetta server. Nucleic Acids Research, 32, W526–W531.
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30, 2785–2791.
Lemmon, G., & Meiler, J. (2012). Rosetta Ligand docking with flexible XML protocols. Methods in Molecular Biology, 819, 143–155.
Kartchner, B. K., Kazan, I. C., Ozkan, S. B., & Mills, J. H. (2020). A method for the incorporation of protein dynamics into computational enzyme design using the Rosetta software suite. Biophysical Journal, 118, 320–321.
Gordon, J. C., Myers, J. B., Timothy, F., Valia, S., Heath, L. S., & Alexey, O. (2017). H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Research, 33, 368–371.
Yang, B., Wang, H. J., Song, W., Chen, X. L., Liu, J., Luo, Q. L., & Liu, L. M. (2017). Engineering of the conformational dynamics of lipase to increase enantioselectivity. ACS Catalysis, 7, 7593–7599.
Weiner, P. K., & Kollman, P. A. (2010). Amber: Assisted model building with energy refinement. A general program for modeling molecules and their interactions. Journal of Computational Chemistry, 2, 287–303.
Maier, J. A., Martinez, C., Kasavajhala, K., Wickstrom, L., Hauser, K. E., & Simmerling, C. (2015). Ff14sb: Improving the accuracy of protein side chain and backbone parameters from ff99sb. Journal of Chemical Theory and Computation, 11, 3696–3713.
Humphrey, W., Dalke, A., & Schulten, K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics, 14, 33–38.
Sprogøe, D., van den Broek, L. A. M., Mirza, O., Kastrup, J. S., Voragen, A. G. J., Gajhede, M., & Skov, L. K. (2004). Crystal structure of sucrose phosphorylase from Bifidobacterium adolescentis. Biochemistry, 43, 1156–1162.
Schwarz, A., & Nidetzky, B. (2006). Asp-196→Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glucosyl transfer to and from phosphate. FEBS Letters, 580, 3905–3910.
Mueller, M., & Nidetzky, B. (2007). The role of Asp-295 in the catalytic mechanism of Leuconostoc mesenteroides sucrose phosphorylase probed with site-directed mutagenesis. FEBS Letters, 581, 1403–1408.
Wiesbauer, J., Goedl, C., Schwarz, A., Brecker, L., & Nidetzky, B. (2010). Substitution of the catalytic acid–base Glu237 by Gln suppresses hydrolysis during glucosylation of phenolic acceptors catalyzed by Leuconostoc mesenteroides sucrose phosphorylase. Journal of Molecular Catalysis. B, Enzymatic, 65, 24–29.
Mirza, O., Skov, L. K., Sprogøe, D., van den Broek, L. A. M., Beldman, G., Kastrup, J. S., & Gajhede, M. (2006). Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. Journal of Biological Chemistry, 281, 35576–35584.
Li, Y., Li, Z., He, X. Y., Chen, L. L., Cheng, Y. C., Jia, H. H., Yan, M., & Chen, K. Q. (2019). Characterisation of a Thermobacillus sucrose phosphorylase and its utility in enzymatic synthesis of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid. Journal of Biotechnology, 305, 27–34.
Kullin, B., Abratt, V. R., & Reid, S. J. (2006). A functional analysis of the Bifidobacterium longum cscA and scrP genes in sucrose utilization. Applied Microbiology and Biotechnology, 72, 975–981.
Fujii, K., Iiboshi, M., Yanase, M., Takaha, T., & Kuriki, T. (2006). Enhancing the thermal stability of sucrose phosphorylase from Streptococcus mutans by random mutagenesis. Journal of Applied Glycoscience, 53, 91–97.
He, X. Y., Li, Y., Tao, Y. H., Qi, X. L., Ma, R. Q., Jia, H. H., Yan, M., Chen, K. Q., & Hao, N. (2021). Discovering and efficiently promoting the extracellular secretory expression of Thermobacillus sp. ZCTH02-B1 sucrose phosphorylase in Escherichia coli. International Journal of Biological Macromolecules, 173, 532–540.
Goedl, C., Schwarz, A., Minani, A., & Nidetzky, B. (2007). Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: Characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of alpha-D-glucose 1-phosphate. Journal of Biotechnology, 129, 77–86.
Wang, M., Wu, J., & Wu, D. (2018). Cloning and expression of the sucrose phosphorylase gene in bacillus subtilis and synthesis of kojibiose using the recombinant enzyme. Microbial Cell Factories, 17, 23.
Kasperowicz, A., Stan-Glasek, K., Guczynska, W., Piknova, M., & Michalowski, T. (2009). Sucrose phosphorylase of the rumen bacterium Pseudobutyrivibrio ruminis strain A. Journal of Applied Microbiology, 107, 812–820.
Stan-Glasek, K., Kasperowicz, A., Guczyńska, W., Piknová, M., Pristaš, P., Nigutová, K., Javorský, P., & Michałowski, T. (2010). Phosphorolytic cleavage of sucrose by sucrose-grown ruminal bacterium Pseudobutyrivibrio ruminis strain k3. Folia Microbiologica, 55, 383–385.
Kawasaki, H., Nakamura, N., Ohmori, M., & Sakai, T. (1996). Cloning and expression in Escherichia coli of sucrose phosphorylase gene from Leuconostoc mesenteroides No. 165. Bioscience, Biotechnology, and Biochemistry, 60, 322–324.
Verhaeghe, T., Diricks, M., Aerts, D., Soetaert, W., & Desmet, T. (2013). Mapping the acceptor site of sucrose phosphorylase from Bifidobacterium adolescentis by alanine scanning. Journal of Molecular Catalysis. B, Enzymatic, 96, 81–88.
Guibert, A., & Monsan, P. (1988). Production and purification of sucrose phosphorylase from Leuconostoc mesenteroides application to the production of glucose-1-phosphate. Annals of the New York Academy of Sciences, 542, 307–311.
Schwaiger, K. N., Cserjan-Puschmann, M., Striedner, G., & Nidetzky, B. (2021). Whole cell-based catalyst for enzymatic production of the osmolyte 2-O-α-glucosylglycerol. Microbial Cell Factories, 20, 79.
Funding
This work was financed by the National Key R & D Program of China (2021YFC2101500), NSFC (21878155), the Jiangsu Synergetic Innovation Center, or Advanced Bio-manufacture, and PAPD.
Author information
Authors and Affiliations
Contributions
J.P.L. conducted most of the experiments. Y.L. and H.H.J. designed and supervised the project. Z.G. provided experimental reagents and equipment. K.X.T. conducted kinetic simulation analysis of enzymes. T.Z. performed activity assay and tested the related reactivity of substrates. All the authors discussed the design and results, commented on the manuscript, and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, J., Tang, K., Zhang, T. et al. Efficient Production of 2-O-α-D-Glucosyl Glycerol Catalyzed by an Engineered Sucrose Phosphorylase from Bifidobacterium longum. Appl Biochem Biotechnol 194, 5274–5291 (2022). https://doi.org/10.1007/s12010-022-03939-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03939-z