Abstract
This study examined the effects of pulsed electric field (PEF) treatment on enzymes, non-starch polysaccharides, and bread-making potential of oat and barley flour. Enzyme activity, microstructure, β-glucan extractability, molecular weight (Mw) and structure of non-starch polysaccharides, dough rheology, and flat bread properties were determined. An exponential decay model explained better the residual activity of oat β-glucanase across electric field intensity than barley β-glucanase. PEF treatment of flour at 12 kV/cm for 162 ms significantly reduced β-glucanase activity (40.2–76.5%) while increasing the concentration of total β-glucans (33.5%) and water-extractable arabinoxylans (36–41%). Mw of linear β-d-glucans decreased (9%) while Mw of branched arabinoxylans increased (6–33%). Scanning electron microscopy showed changes in microstructure of barley proteins. Blending wheat flour (70%) with oat or barley flour (30% weight) after PEF treatment enhanced gluten aggregation energy (29–19%) and breakdown viscosity (18–43%) of dough, as well as increased β-glucan content (21–32%) but reduced specific volume (11–24%). The findings of this study provide a comprehensive insight into the PEF’s potential for retarding enzymatic reactions and preserving integrity of cereal non-starch polysaccharides.
Similar content being viewed by others
Introduction
Bread, a global staple, continues to evolve to meet the demands of modern life. Flat bread, one of the oldest types of bread, possesses consumers’ interest due to its versatility and convenience. Increasing consumers’ demand necessitates the development of nutritionally enriched flat breads (Garzon et al., 2022). Oat and barley flour, known for their richness in fiber, minerals, vitamins, and antioxidants, are underutilized ingredients in flat bread. Globally, in 2021, 1.46 billion tons of oat and 22.6 million tons of barley were produced, as reported by the Food and Agriculture Organization (FAOSTAT, 2023). Hence, oat and barley flour are interesting ingredients for fiber-enrichment of bread (Mansoor et al., 2019; Rieder et al., 2012), but certain challenges need to be overcome.
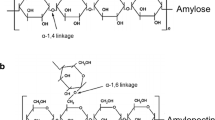
Barley and oat are characterized by their non-starch polysaccharides, β-glucans, and arabinoxylans (AX) in particular (Lazaridou et al., 2007; Zambrano et al., 2023). The β-glucan content in barley and oats ranges from 2.5 to 11.3% and 2.2 to 7.8%, respectively (Lazaridou et al., 2007). The proportion of AX in the endosperm is similar between barley and oats (1.2–1.3%), while their proportion in the bran is higher in barley (10.3%) than in oats (5.2%) (Zannini et al., 2022). Physiological and technological properties of β-glucans depend not only on their amount but also on their molecular weight (Mw), extractability, viscosity, and solubility (Lazaridou et al., 2007). The fine structure of cereal (1 \(\to\) 3) and (1 \(\to\) 4) β-D-glucans consists mainly of β-D-glucopyranosyl (β-d-Glcp) units that are 30% linked via 1,3-glycosidic bonds and 70% via 1,4-glycosidic bonds (Lazaridou et al., 2007; Zhang et al., 2019). β-Glucans of high Mw show high viscosity in the aqueous solution and pronounced gelation properties, which ultimately increases the physiological benefits (Goudar et al., 2020; Zhang et al., 2019). The AX is a heteroglycan whose structure consists of 1,4-linked β-d-xylopyranosyl residues (β-d-Xylp) with α-l-arabinofuranosyl units (α-l-Araf) (Zambrano et al., 2023). The AX is primarily consisting of insoluble dietary fiber, where the higher degree of Araf substitution to the Xylp backbone plays a crucial role in its solubility (Zambrano et al., 2023). β-Glucans and AX can lead to a weakening of the gluten network during bread production depending on their solubility and Mw (Cao et al., 2023; Courtin & Delcour, 2002).
The endogenous cereal β-glucanase depolymerizes β-D-glucans, i.e., it degrades the Mw of the β-glucans increasing its solubility but lowering the viscosity and reducing their physiological effect (Pérez-Quirce et al., 2017). Approaches used so far for inactivating β-glucanase in flour include high-intensity ultrasound, autoclaving, ethanol refluxing, scalding, and microwave processing (Grgić et al., 2023; Lazaridou et al., 2014; Pérez-Quirce et al., 2017; Pérez-Quirce et al., 2016). A harsh thermal or chemical processing can cause severe structural changes, e.g., disorder of the protein network, deformation of starch granules, and reorganization of amylose and amylopectin chains (Duque et al., 2020). Such modifications constraint the application of treated flour as a food ingredient. Therefore, innovative processing of flour to inactivate endogenous enzymes, i.e., β-glucanase, while preserving functional structure of its biopolymers, is needed.
The pulsed electric field (PEF) is emerging as a non-thermal technology for food processing. A food material located between two electrodes is subjected to a high-voltage electric field in short pulses (µs-ms) (Duque et al., 2019). The added energy is absorbed by the carbon backbone of biomacromolecules and is changing their configuration (Martín-Belloso & Elez-Martínez, 2005). Thus, PEF treatment can enhance the extraction of important compounds (Kumari et al., 2018, 2019) and alter the physicochemical properties of biomacromolecules (Duque et al., 2019, 2020; Jaeger, et al., 2010; Maniglia et al., 2021). Due to no chemical residues, low energy, and time consumption, PEF has already been successfully used to modulate the activity and stability of several enzymes (Jaeger et al., 2010; Li et al., 2022; Ohshima et al., 2007; Zhang et al., 2017). Moreover, the effects of PEF technology on polysaccharides such as corn, wheat and cassava starch, sugar beet pectin, and chitosan have been investigated (Han et al., 2012; Ma et al., 2012; Maniglia et al., 2021; Rivero-Ramos et al., 2023). To our knowledge, no study has yet investigated the influence of PEF processing on the activity of β-glucanase or non-starch polysaccharide structure of oat or barley flour.
The aim of this study was to investigate the influence of PEF treatment on the β-glucanase activity, the extractability of β-glucans, and the Mw of non-starch polysaccharides of oat and barley flour, but also to validate its functionality in further processing, i.e., making of composite bread. Therefore, we first investigated the kinetics of β-glucanase inactivation depending on the electric field intensities (EFI), the treatment time at selected EFI, and the specific energy input of the PEF treatment. Then, the changes in molecular structure and properties of its non-starch polysaccharides (β-glucans and arabinoxylans) after selected PEF treatment were determined. Finally, the application of PEF-treated flour on dough rheology and flat bread properties was investigated.
Materials and Methods
Materials
Oat flour was provided by Granolio Plc. (Zagreb, Croatia), barley flour by the family-run farm Ivan Varga (Orehovica, Croatia), and semi-refined wheat flour by Čakovečki mlinovi Plc. (Čakovec, Croatia) and stored at − 20 °C beforehand usage. Samples were not thermally treated beforehand. The proximate composition of flour samples was determined according to the AOAC Official Methods (2012). Oat, barley, and semi-refined wheat flour contained 11.0, 9.8, and 11.0% of protein (AOAC Method 920.87); 1.8, 2.3, and 0.8% of ash (AOAC Method 942.05); and 7.0, 1.6, and 2.3% of fat (AOAC Method 922.06), respectively. The particle geometric mean diameter, determined according to the ICC standard 207 (1998) by sieving at the mesh apertures of 670, 355, 282, 225, 180, and 125 µm and calculated as reported previously by Grgić et al. (2023), was 231 µm for oat flour and 295 µm for barley flour.
Methods
Pulsed Electric Field Treatment
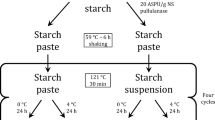
The oat and barley flour suspended in water (in a ratio of 2:1, w/w) was treated in a high-intensity PEF system (HVG60/1, Impel Ltd., Zagreb, Croatia). The batch treatment chamber was equipped with two parallel stainless-steel electrodes covering an area of 201 cm2 and a distance of 25 mm. The system administered bipolar square wave pulses with a uniform pulse width of 2 μs and a pulse frequency of 150 Hz.
First, the suspensions of both flour types were PEF-treated for 18 ms at four levels of EFI: 4.8, 8.4, 12, and 16 kV/cm. After defining the EFI which inactivated the most of β-glucanase, the influence of treatment time was investigated with the same aim at five levels: 18, 54, 108, and 162 ms.
Specific energy input (WS, kJ/kg) was calculated according to Lohani and Muthukumarappan (2016) following the equation (Eq. 1):
where C is the capacitance (F), V is the voltage (V), n is the number of pulses, and m is the sample weight (kg). The capacitance was measured using LCR meter (DE-5000, Deree Electrical Instrument Co., Ltd., New Taipei City, Taiwan). The WS of the oat and barley flour samples varied from 0.1 to 11.48 kJ/kg and 0.1 to 13.11 kJ/kg, respectively throughout the PEF treatments. Samples were treated at the initial temperature of 20 ± 0.1 °C which minimally increased during the treatment (Table 1). The temperature and conductivity of flour-water suspension were measured before and after PEF treatment using a handheld conductivity meter (S230, Mettler-Toledo, Greifensee, Switzerland).
The PEF-treated samples were freeze-dried (Alpha 1–4 LSCplus; Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) and stored at −20 °C for subsequent analyses or used directly for bread making.
Enzymatic Activity
The activity of β-glucanase was measured using the malt β-glucanase/lichenase assay kit (K-MBG4 08/18 method) (Megazyme, Bray, Ireland) according to the previously described protocol (Grgić et al., 2023). Extraction of β-glucanase from the samples was performed using the malt β-glucanase/lichenase extraction buffer with an MS3 shaker (IKA, Germany) for 15 min. After centrifugation at 6.200 rpm for 5 min (Rotina, Hettich, Germany), the extracted β-glucanase was incubated with the MBG4 substrate at 30 °C for 20 min, and the absorbance was measured at 400 nm.
According to Giner et al. (2000), the inhibition of enzyme activity by PEF was described with an exponential, first-order kinetics model (Eqs. 2 and 3):
where RA is the residual β-glucanase activity (%); RA0 is the intercept of the curve, k (ms−1) is the first-order kinetic constant, and Ws is specific energy input (kJ kg−1) or EFI (kV cm−1).
The α-amylase activity was determined according to AOAC Method 2002.01 and ICC Standard No. 303 using α-amylase SD assay kit (K-AMYLSD 04/19 method) (Megazyme, Bray, Ireland) according to the manufacturer’s instructions. The sample (0.5 g, particles < 0.5 mm) was extracted with 8 mL of extraction buffer (pH = 5.4) at 40 °C for 10 min with occasional mixing. The extract was then centrifuged at 10.696 rpm for 3 min (Rotina, Hettich, Kirchlengern, Germany), and 0.4 mL of the extract was used in a reaction with 0.1mL of amylase SD reagent solution at 40 °C for 10 min. After completion of the reaction, the absorbance was measured at 400 nm.
Polyphenol oxidase (PPO) activity was assessed in accordance with the AACC 22-85.01 method (AACC, 2000). To 50 mg of flour, 1.5 mL of a 10 mM solution of L-DOPA in a 50 mM MOPS buffer at pH 6.5 was added. After vigorous vortexing (MS 3 shaker, IKA, Germany) at 1.000 rpm for 15 min, the samples were subsequently centrifuged at 14.800 rpm for 5 min. The resulting supernatant was utilized for spectrophotometric analysis at 475 nm. PPO activity is reported as the average absorbance value obtained from three replicates.
The spectrophotometer PerkinElmer Lambda 35 UV/Vis (Waltham, MA, USA) was used for all spectrophotometric measurements done in duplicates.
Scanning Electron Microscopy
A scanning electron microscope (SEM, Vega 3 LMH, Tescan, Brno, Czech Republic) was used to observe microstructure of untreated and PEF-treated barley and oat flour samples. The samples were coated with a thin layer of a gold-palladium alloy prior to imaging with SEM operating at an electron acceleration voltage of 2.5 kV.
β-D-Glucan Content and Characterization of Polysaccharidic Fractions
Total β-D-glucan contents of control and PEF-treated flour as well as bread samples were determined in duplicate according to AOAC Method 995.16 using the enzymatic assay kit (K-BGLU 07/23, Megazyme, Bray, Ireland).
Prior to the extraction and characterization of polysaccharides, the homogenized material underwent a series of washing steps to remove lipids, pigments, and other ballast molecules. To remove water residues, samples were successively washed with hexane, acidified ethanol (0.2 mol L−1 HCl in 80% aqueous ethanol), aqueous ethanol (80%, v/v) until reaching a neutral pH, and finally with ethanol (96%, v/v) and acetone. After washing, the re-homogenized solids were first subjected to cold water extraction at 20 °C, followed by hot water reflux extraction at 100 °C.
Fourier Transform Infrared (FTIR) Measurement
The FTIR spectra of spectra and all obtained fractions were recorded using a Nicolet 6700 FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, the sample was mixed with 10 times (v/v) potassium bromide (KBr for IR spectroscopy, Supelco Sigma-Aldrich, MA, USA) and ground thoroughly into fine powder in a mortar. The mixture was then pelleted with a hand press (Pike Technologies, Madison, WI, USA). The FTIR spectra were collected in the transmission mode at wavenumber region from 400 to 4000 cm−1 with a spectral resolution of 2 cm−1 and in 64 scans on average. FTIR spectra were recorded and subsequently processed (smoothed, baseline corrected, and averaged) using Omnic 8.0 software (Thermo Fisher Scientific, Waltham, USA) and then exported in ASCII format to the Origin 8.0 software (OriginLab, Northampton, MA, USA) for the preparation of output graphs. The spectra are represented as an average value of two repeated measurements for each sample.
Determination of β-Glucan Molecular Weight
A Mw of samples was analyzed using GPC/SEC chromatography system Omnisec Reveal coupled with a multi-angle light scattering (low-angle light scattering (LALS) and right-angle light scattering (RALS)), viscosity (DP), and refractive index (RI) detectors (Malvern Panalytical, Westborough, USA). Two GPC/SEC columns YMC-Pack Diol-200/S-5 μm/8 × 30 mm and YMC-Pack Diol-120/S-5 μm/8 × 30 mm (YMC, Japan) and a guard column (DL12S05-0308WTG, YMC, Japan) were eluted by 0.1 M sodium nitrate at a flow rate of 0.7 mL/min. The detectors and the columns were tempered at 35 °C. Calibration was performed with a Polycal pullulan/dextran standards (Malvern Pananalytical, Westborough, USA). The samples and standards were injected twice at volume 100 μL. The chromatographic system, as well as the acquisition and data analysis, was controlled by OMNISEC software v11.35 (Malvern Pananalytical, Westborough, USA).
Monosaccharide Composition and Linkages (Methylation)
The monosaccharide composition was determined after hydrolysis of sample (1–2 mg) with 72% H2SO4, followed by reduction and acetylation (Passos and Coimbra, 2013). The resulting compounds were analyzed as alditol acetates by GC-FID (Shimadzu GC2010, Kyoto, Japan) on a capillary column DB-225 (30 m length, 0.25 mm internal diameter, and 0.15 µm film thickness).
The AX concentration was estimated according to Zambrano et al. (2023) following Eq. 4:
The yield calculation for AX also considered galactose, as small quantities of galactose have been suggested to be present in AX by Paz-Samaniego et al. (2019).
Polysaccharides were methylation in dry DMSO (1 mL) with CH3I, concentrated to dryness, hydrolyzed with 2 M TFA (120 °C for 1 h), reduced with NaBD4, and subsequently acetylated. Partially methylated alditol acetates were analyzed with GC–MS (Shimadzu 2010 SE, Kyoto, Japan) on a capillary column HP-5 (30 m length, 0.25 mm internal diameter, 0.15 µm film thickness) according to Passos and Coimbra (2013). Duplicate samples were prepared for both analyses.
NMR Spectroscopy
The purified water-soluble fractions (F1 and F2) were selected and analyzed on a Bruker Avance III™ 500 MHz NMR spectrometer (Bruker, Billerica, MA, USA). Proton NMR and 13C APT NMR spectra were recorded for D2O solutions at 20 °C and 80 °C and processed using MestReNova 10.0 software (Mestrelab Research, Santiago de Compostela, Spain). Correlation 2D NMR experiments 1H, 1H COSY, 1H, 13C HMQC and 1H, 13C HBMC were used for the proton and carbon signal assignment. The 1H NMR spectra of the chosen purified fractions were exported as the ASCII data files to the Origin 6.0 (Microcal Origin, Northampton, MA, USA) software and normalized using least-squares curve-fitting (PeakFit module of Origin 6.0) and multiple Voigt (Gaussian–Lorentzian mix) curves in the region of 4.40–4.85 ppm. The Voigt components centered at 4.53–4.56 and 4.74–4.76 ppm were used to calculate the ratio between the 1,4- and 1,3-linked β-d-glucopyranosyl units in the mixed-linkage β-d-glucan.
Determination of Gluten Aggregation and Pasting Properties of Wheat Flour and Oat/Barley Flour Blend
The pasting characteristics of the flour blend were evaluated following the AACC (2000) method 76–21.02 using a MicroViscoAmylograph (Brabender GmbH & Co. KG, Duisburg, Germany) in a measuring range of 235 cmg. Suspensions of flour blend (10 g of flour 14% moisture basis suspended in 105 mL of distilled water) were equilibrated at 30 °C, heated to 93 °C at a rate of 7.5 °C/min, upheld at 93 °C for 5 min, cooled to 50 °C at a rate of 7.5 °C/min, and held at 50 °C for 1 min. The parameters derived from the recorded curve included maximum viscosity (MV), cold paste viscosity at the end of the test (CPV), breakdown viscosity (BV), and setback viscosity (SV), all measured in Brabender Units (BU).
The wheat flour (70%, w/w) was blended with oat or barley flour (30%, w/w) both corrected to the 14% moisture. Gluten aggregation was evaluated using the GlutoPeak instrument (Brabender GmbH & Co. KG, Duisburg, Germany), following the method by Wang et al. (2018). The flour blend (8 g) was introduced into a 0.5 mol/L CaCl2 solution (10 g). The test was conducted at a stirring speed of 3.000 rpm, at a temperature of 20 °C, lasting for 3 min. The obtained parameters were encompassed Peak Maximum Time (PMT, in minutes) and Maximum Torque (BEM, in Brabender Units, BU), representing the time prior to maximum torque decline and the peak resistance during mixing, torque before maximum (AM), and gluten aggregation energy (AGGEN), respectively.
Experimental Bread Making
A control wheat single layer flat bread (typical for Croatia), as well as four types of composite breads in which 30% of wheat flour was replaced with oat or barley flour, untreated or PEF-treated was prepared. The addition of water was adjusted until a farinograph consistency of 200 BU was achieved in each formulation (75% for wheat control, 82% for barley, and 86% for oat composite bread) considering the amount of water already contained in PEF-treated samples. Instant yeast (Lesaffre Adriatic Ltd., Croatia, 0.4%), table salt (2%), and oil (4%) were added on flour weight basis.
Dough was kneaded in a spiral mixer (Diosna SP12, Osnabrück, Germany) in several stages. Flour was mixed with water for 2 min at 90 rpm; then, yeast and salt were added, and fast mixing was continued at 120 rpm for 5 min. After this time, oil (4% at flour weight) was added, and mixing continued for 2 more minutes. The resulting mixture was subjected to bulk fermentation (28 °C, relative humidity 75%, 1.5 h), divided into round balls (350 g), placed in metal pans (20 cm diameter), and subjected to proofing at 28 °C, RH 75%, 1 h (proofing cabinet, Wiesheu, Affalterbach, Germany). The flat bread was baked in a deck oven (Wiesheu, Affalterbach, Germany) at a temperature of 240 °C for 30 min with 0.21 mL cm−2 of steam. Breads were baked in triplicate and cooled for 1.5 h at ambient conditions for subsequent measurements.
Oscillatory Rheology Measurement
To assess the viscoelastic properties of dough prepared as previously described, oscillatory measurements were performed in duplicate with parallel plate geometry of 25 mm diameter and 2 mm gap (MCR 92 rheometer, Anton Paar, Graz, Austria). A constant frequency of 6.28 rad/s, a shear strain of 0.05%, a pressure of 0.1 to 100 Pa, and 0.1% of the shear strain, within the linear viscoelastic region, were set for the stress sweep test. The Peltier temperature control device P-PTD200/AIR (Anton Paar, Graz, Austria) was used to maintain a constant temperature during the test. The loss factor (tan δ) was calculated as the ratio between the energy lost and the energy stored in the test (G’’/G’).
Determination of Bread Physical Properties
The volume of flat breads was determined according to AACC 10–05.01 method (AACC, 2000) in duplicate. The specific volume of bread was calculated after dividing volume with weight (Eq. 5):
The height (4 edges of the bread and the center) and width (2 places) of the bread were measured with a caliper to calculate the spread ratio as width/height.
The texture profile (TPA) of the crumb was measured using a texture analyzer (TA1 Texture Analyzer, Ametek Lloyd Instruments Ltd., West Sussex, UK) with a 55 mm diameter aluminum probe. Immediately before the analysis, the bread slices with 12.5 mm thickness were cut into 36 mm diameter pieces. Two pieces stacked together were compressed to 50% of their original height at test speed 2 mm/s and 30 s pause between the first and second compression. The hardness, chewiness, and resilience were evaluated in six replicates using a NexygenPLUS Software (Ametek Lloyd Instruments Ltd., West Sussex, UK).
The L a b system was used for determining upper (top) crust color at five points of three bread pieces with a colorimeter (Konica Minolta CM-700d, Osaka, Japan).
Determination of Dietary Fiber Content in Bread
Preparation of flat bread samples for dry matter determination (AACC 44-15A method) was carried out according to AACC 62-05 method. Total dietary fiber (TDF) was determined according to AACC Method 32-05.0 and AOAC Method 985.29 using Total Dietary Fiber Assay Kit (Megazyme, Bray, Ireland). Analyses were done in duplicate.
Statistical Analyses
To assess significant differences between all samples, one-way analysis of variance (ANOVA) with Tukey post hoc test was performed. After excluding control wheat sample, the interactions between flour type and treatment were assessed with two-way ANOVA. ANOVA and Pearson correlation test were performed at a significance level of p < 0.05 using Statistica 14 (TIBCO Software Inc., CA, USA).
Results and Discussion
Effect of PEF Treatment on Flour β-Glucanase Activity
The initial activity of β-glucanase was 24% higher in oat than in barley flour. Oat β-glucanase appeared to be consistently inactivated across all tested EFI levels (Fig. 1a). The lowest residual activity was observed after treatment at 12 kV/cm, with only 29.8% of the initial activity retained. In contrast, barley β-glucanase exhibited an activity increase of 22.4% already after treatment at 4.5 kV/cm, while the lowest residual activity of 52.6% was recorded after treatment at 16 kV/cm. These findings are consistent with several previous studies. A similar pattern of increased enzyme activity at lower EFI, followed by a decrease at higher EFI, was reported by Li et al. (2022) and Zhang et al. (2017). In addition, Ohshima et al. (2007) found that horseradish peroxidase at low PEF intensities (< 12 kV/cm) shows a gradual increase in activity, while higher intensities (≥ 12 kV/cm) lead to a reduced activity.
The residual activity (RA) of oat and barley flour β-glucanase exposed to a different EFI at constant treatment time (18 ms), b different treatment time (ms) at constant EFI (12 kV/cm), and c residual β-glucanase activity in oat and barley flour submitted at different specific energy inputs supplied by high-intensity pulsed electric fields from 4.8 to 16 kV/cm for 18 ms. The values are shown as mean ± standard deviation (n = 2)
Further on, oat β-glucanase exhibited a substantial decline in activity (76.5%) already after 18 ms of PEF treatment at 12 kV/cm which remained constant even at prolonged treatment time (Fig. 1b). In contrast, barley β-glucanase showed a continuous activity decrease along the treatment time, reaching its maximum reduction of 40.2% after 162 ms at 12 kV/cm (Fig. 1a). Similary, Ohshima et al. (2007) proved that the activity of peroxidase and β-galactosidase decreases with longer treatment time.
Consistently with EFI, the effects of different specific energy intakes on the RA of β-glucanase differed between oat and barley flour. With increasing specific energy input, β-glucanase activity of oats was decreasing, whereas an initial activity increase followed by a decrease was observed in barley. The dependence of the RA of oat β-glucanase was well explained by EFI-based exponential decay model (Table 1). Nevertheless, this model did not significantly explain the RA of barley β-glucanase due to the recorded activity increase at low EFI. The Ws-based model significantly explained the dependence of the RA of barley β-glucanase with coefficient of determination of 68%. Despite the relatively low coefficient of determination, the model shows faster kinetics of the β-glucanase inactivation by PEF in oats compared to barley flour. PEF treatment has some mechanism to activate and inactivate enzymes, and the effective PEF strength differs by the type of enzymes and matrix (Ohshima et al., 2007). The enzyme inactivation by PEF treatment is explained by the impact of high electric field pulses on the three-dimensional structure of the globular protein (Duque et al., 2019). The PEF induces partial unfolding of protein molecules, enhances ionization of sulfhydryl (SH) groups within proteins, and triggers conformational changes and subsequent loss of activity due to challenges in substrate binding at the active site (Fernandez-Diaz et al., 2000). Our study shows that the inactivation of oat β-glucanase is easier than for barley β-glucanase which requires higher EFI, longer treatment time, and Ws. Such an extent and difference in the inactivation between barley and oat β-glucanase were obtained after treatment with high-intensity ultrasound (Grgić et al., 2023). Unlike with ultrasound, the sample heating during PEF treatment was avoided (Table 1).
Considering that after EFI treatment at 16 kV/cm, the activity of oat β-glucanase increased, and the highest inactivation of barley β-glucanase occurred after 162 ms of treatment; further investigation was continued with PEF treatment with EFI of 12 kV/cm and 162 ms (Table 1) of both samples under the same conditions.
Morphological Properties of PEF-Treated Oat and Barley Flour
The SEM images of the untreated and PEF-treated oat and barley flour samples are shown in Fig. 2. Untreated barley flour consisted of irregularly shaped particles with a size of 100–500 µm, which were mainly aggregates of starch, fiber, and proteins (Fig. 2a, b). A relatively large proportion of smaller particles with a size of 10–30 μm, mostly corresponding to individual starch granules, was also observed in barley flour (Fig. 2a). Untreated oat flour consisted predominantly of individual or clustered starch granules, typically 5–100 µm in size, with protein fragments closely adhering to their surface (Fig. 2d, e). In both the untreated oat and barley flour samples, the surface of the individual starch granules appeared uniformly smooth, without cracks or pits. The protein matrix in the untreated barley flour had a slightly wrinkled surface, while the protein fragments in the untreated oat flour exhibited a more granular appearance, but in both samples again without visible cracks or pits on the surface (Fig. 2c, f). The SEM images of the PEF-treated samples showed that the PEF treatment generally had no effect on the size and shape of the flour particles (Fig. 2g, i). In the PEF-treated barley flour samples, morphological changes in the form of small (approx. 100 nm) holes and circular or elliptical pits (100–1000 nm in size) were observed on the surface of the protein matrix (Fig. 2h), most likely caused by a localized high-energy discharge of the electric field. PEF treatment induces unfolding of protein molecules, affecting their secondary and tertiary configuration, which can subsequently lead to interactions and aggregation (Shams et al., 2024). In this study, these changes after the PEF treatment of oat flour were much more discrete and were manifested in the sporadic appearance of shallow pits on the surface of the protein fragments (Fig. 2j). In both PEF-treated samples, no morphological changes were observed on the starch granules even after a detailed SEM examination of the samples.
Total β-Glucan Content in Control and PEF-Treated Oat and Barley Flour
Cereal β-glucan is predominantly a soluble dietary fiber found mainly in the endosperm cell wall and aleurone layer of oats and barley (Shoukat & Sorrentino, 2021). Total β-glucan content was 10.5% higher in untreated oat flour than in barley flour (Table 2). In agreement, Zhang et al. (2019) found 2.65–4.73% of β-d-glucan in oat flour. Similarly, β-glucan content in barley grain is reported between 2 and 11% but most often ranges between 4 and 6% (Goudar et al., 2020). After PEF treatment, total β-d-glucan content significantly increased (33.5%) compared to untreated samples (Table 2). These results were a consequence of PEF-assisted extraction which increases the rate of mass transfer by electroporation of cell membranes (Kumari et al., 2019). An increased electrical conductivity of oat (20%) and barley (11%) flour (Table 1) after PEF treatment indicated the increase in cell membrane permeability resulting in glucans’ better extraction (Nowacka et al., 2019). In addition, the results may be attributed to the inactivation of endogenous enzymes, including β-glucanase by PEF processing, since β-d-glucan content correlated negatively with β-glucanase activity (r = −0.828, p = 0.011). In agreement, Duque et al. (2019) found an increase in β-d-glucan content in oat flour (12–20%) after PEF treatment at 4.1–4.3 kV/cm, which they assumed to be related with a possible decrease in β-glucanase activity.
Molecular Weight of Water-Soluble Fractions of Control and PEF-Treated Oat and Barley Flour
The results of GPC analysis of the purified water-soluble polysaccharide fractions F1 (cold water soluble) and F2 (hot water soluble) obtained from control and PEF-treated oat and barley flours are summarized in Table 3. As a rule, the Mw values of F1 were higher than those of F2 fractions. For the control flours, the Mw values of both fractions obtained from oat flour (371,000 and 318,000 g/mol) were higher than Mw of the corresponding fractions from barley flour (339,000 and 233,000 g/mol). The results are consistent with previous studies describing oat β-d-glucans as having a higher molecular weight than barley β-d-glucans. Liu and White (2011) reported the Mw of oat β-d-glucan ranging from 6.81 × 105 to 7.85 × 105 g/mol, and Karimi et al. (2019) found that the Mw of barley β-d-glucan varies between 0.8 × 104 and 3.77 × 105 g/mol. On the other hand, Zhang et al. (2019) found the average Mw 1.30 × 105 g/mol of oat β-d-glucan and a wide range from 4.05 × 104 to 5.22 × 105 g/mol. This distinction can be attributed to the environment and genotype differences in oats. Higher Mw parts of the fractions are associated with the contribution of β-d-glucan and AX, while the starch and protein residues retained after enzymatic treatments contribute to lower Mw parts. For F1 obtained from oat flour, the main polysaccharide was found to be β-d-glucan (see results of composition and linkage analyses and NMR assignments). Thus, in this case, the PEF treatment leading to a decrease of Mw from 3.71 × 105 to 3.39 × 105 g/mol relates to the partial degradation of this polysaccharide. Previously, the effect of PEF processing on the Mw of various polysaccharides has been studied (Han et al., 2012; Luo et al., 2010; Ma et al., 2012; Maniglia et al., 2021; Rivero-Ramos et al., 2023). For example, Han et al. (2012) demonstrated that PEF treatment causes depolymerization of corn starch and reduces its Mw. Ma et al. (2012) demonstrated a 9–31% decrease in Mw of sugar beet pectin after the PEF treatment (18–30 kV/cm, 806–2418 µs, 1 kHz). Luo et al. (2010) attributed the Mw decrease of PEF-treated chitosan to the breaks in the glycosidic linkages caused by the hydroxyl radicals ·OH. Yet, no study investigated the Mw of β-glucans. In this study, the opposite effect of PEF treatment was observed for F1 fractions from barley flour, i.e., an increase of Mw from 3.30 × 105 to 3.76 × 105 g/mol that could be due to the higher contribution of AX, while the polydispersity Mw/Mn changed slightly and was a little higher for the barley flour products (1.09–1.10) than for the oat products (1.02–1.06) because of more complex nature of the former as a mixture of linear β-d-glucan and branched AX macromolecules. In both samples, the PEF treatment led to significant decrease of intrinsic viscosity [η] from 2.8–4.0 to 2.0–2.35 dL/g. For the F2 fractions, the PEF treatment caused an increase of Mw by 0.20 × 105 g/mol for oat flour and 1.14 × 105 g/mol for barley flour. The effects of PEF on the composition and other GPC parameters of F2 were different for oat and barley flour. The oat product containing maximal contribution of AX (− 55 mol %) demonstrated the highest values of polydispersity (2.40) and [η] (3.24 dL/g), while the barley product having a minimal amount of AX (− 15 mol %) showed the lowest value of [η] (0.86 dL/g).
Monosaccharide Composition and Linkage
The composition of neutral monosaccharides in each fraction is summarized in Table 4. In both fractions of oat and barley flours, glucose prevailed, followed by xylose and arabinose, implying the presence of β-glucans and AX. In addition, the F2 fraction contained a substantial proportion of mannose and galactose, indicating the possible presence of some galactomannans. Glucose was predominant in all fractions, being higher in F1 in oat (94 mol %) than in barley flour (71 mol % in F1), but in F2, it was equally present in both samples.
The influence of PEF processing on glucose content among fractions was adverse; it had no significant effect in the F1 while 18% decreasing effect in F2 of oat flour, whereas 18% decrease in F1 and 52% increase in F2 were found in barley flour (Table 4). This can be related to changes in total β-glucan content found after PEF processing (Table 2). Barley and oat flour are a rich source of AX in addition to β-glucans (Zambrano et al., 2023). Compared to oat flour, the lower glucose content in barley flour was complemented with the higher content of arabinose, xylose, and hence AX in F1, while vice versa was in F2 (Table 4). After PEF treatment of barley flour, the content of arabinose and xylose substantially increased in F1 while it decreased in F2. The PEF treatment of oats led to a 40.6% increase in the AX proportion in the F1 fraction, while their increase in F2 was 10%.
PEF treatment did not significantly affect the Ara/Xyl ratio, but it significantly increased AX content in F1 of both flours, which might be explained by the greater extraction of this polysaccharide. The Ara/Xyl ratio as well as the positively correlated AX content further increased (13% and 10%, respectively) in F2 of oat flour, while Ara/Xyl ratio increased and the proportion of AX decreased (7% and 36%, respectively) in F2 of barley flour. This was confirmed by the negative correlation between these two parameters (r = − 0.987, p = 0.013). These changes in monosaccharides in fractions F1 and F2 after PEF treatment can be attributed to changes in the structure of the polysaccharides, which contribute to greater or lesser water solubility, and to their ability to bind with other polymers (such as hemicellulose) found in plant tissues (Zannini et al., 2022). In general, the higher the Ara/Xyl ratio, the higher the solubility of AX derived from the endosperm (Lazaridou et al., 2008; Zambrano et al., 2023). In contrast, Izydorczyk et al. (2003) demonstrated that insoluble AX from endosperm have higher A/X ratio than soluble AX. However, there is always some variation, usually due to the variety, the germination condition, or the nature of the other polymers in the grain (Zannini et al., 2022).
Table 5 demonstrates the results of methylation analysis for each fraction. Glucose predominating in F1 fractions obtained from control and PEF-treated oat flour is represented mainly by the 1,4-substituted glucosyls (~ 72—88%) and by fewer amounts of 1,3-linked glucosyls (~ 7%), which indicates mixed-linkage β-d-glucan as expected. A few terminal, 1,4,6- and 1,6-linked glucosyl fragments originated from starch, which were more pronounced for the PEF-treated oat flour, remained in F1 after the enzymatic hydrolysis. Additionally, in this fraction, xylose and galactose fragments were absent in the control. Therefore, the PEF treatment of oat flour supports the co-extraction of some AX and, probably, arabinogalactan together with β-d-glucan by cold water.
The corresponding F1 fraction from barley flour contained significantly fewer glucosyls (~ 45–55%) due to the presence of significant amount of the AX carbohydrates (~ 42–47%), represented mainly by the non-branched 1,4-linked xylopyranosyls (~ 20–28%). The mono- and disubstituted xylopyranosyls comprised ~ 8–11%, while 1,3,4-linked xylosyl fragments were found only in the PEF-modified sample (~ 4%). The terminal and 1,3-linked anabinosyl fragments represent this sugar (~ 10–11%), and the latter were found only in the control sample. Therefore, β-d-glucan and AX are extracted from barley flour at the comparable amounts, and the PEF treatment of barley flour caused partial degradation of the AX side chains.
In the F2 fraction obtained from control and PEF-treated oat flour, the AX fragments (~ 46–49.5%) prevailed significantly among those of glucans, and 1,4-linked glycosyl comprised only ~ 14–15%. The terminal, 1,4,6- and 1,6-linked glycosyls (~ 15%) confirmed the presence of starch residues, while the 1,3-linked glucosyls from β-d-glucan were much less pronounced (~ 2%). AX is represented mainly by terminal arabinofuranosyls (~ 12–13%) and 1,4- and 1,2,3,4-linked xylosyls (13–14 and 9–10%). Terminal xylosyls and 1,2- and 1,3-linked arabinosyls comprise disaccharide side chains of oat AX (Pastell et al., 2009). These fractions also contained galactosyl and mannosyl fragments, which could be associated with branched arabinogalactan (AG) and glucomannan (GM). Barley cell wall contains about 3–4% of GM (Bader Ui Ain, 2018), but in this polysaccharide, like in other homo- and heteromannans, mannose is represented by the 1,4-linked units (Voiniciuc, 2022). Commonly, AG is part of the structure of pectins and proteins (Su & Higashiyama, 2018), and AG proteins were found in barley cell walls (Makowska et al., 2017). Cereal AG consist of the (1 → 3)-β-d-galactan backbone with the β-d-galactosyl side chains bound at the O-6 position of some backbone galactoses, which in turn may carry α-l-arabinosyls as well as residues of other sugars (Bader UI Ain, 2018). The PEF treatment on the oat flour slightly influenced the composition and structure of AX and other polysaccharides in F2.
Finally, the F2 fraction from control and PEF-treated barley flour contained significantly more glucosyl fragments (~ 49–80%) and less AX fragments (~ 9–16%) than the corresponding fractions obtained from oat flour. However, the F2 fraction from PEF-treated barley flour contains much more glucosyl fragments (~ 80%) and less all other sugar fragments (~ 12%) than the corresponding control, which had about equal amounts of glucosyls and the other sugars. Therefore, the PEF treatment of barley flour promotes complete extraction of β-d-glucan with hot water, which agrees with the NMR data (see Table 4).
FTIR Spectra of Flours and isolated Polysaccharides
The FTIR spectra of the purified polysaccharide fractions F1 and F2 isolated from oat and barley flour, control and PEF-treated samples, are shown in Fig. 3. The spectra of the standard flour components are shown in Fig. S1 for comparison. In all these spectra, the strong and broad band at 3370–3400 cm−1 corresponded to the O–H stretching vibrations in water and hydroxyl groups involved in the intra- and intermolecular hydrogen bonding in the polysaccharide network. The narrow region at 2800–3000 cm−1 arose from the C–H stretching vibrations. In cold water extracts F1, two bands with almost equal intensity at 2922–2924 and 2889–2891 cm−1 were observed in this region, while in hot water extracts F2, only one band with a low-frequency shoulder at 2927–2931 cm−1 was observed. The scissor vibration of water molecules and the amide I vibration in protein residues contributed to the band at 1645–1653 cm−1 (Kong & Yu, 2007). Two shoulders observed for some F2 around 1725 and 1549 cm−1 were assigned to the C = O stretching and amide II vibrations in uronic acids (Bichara et al., 2016) and remaining proteins (Kong & Yu, 2007), respectively. Intense overlapping bands at 950–1200 cm−1 were attributed to C–O–C, C–O, and C–C stretching vibrations in polysaccharides. The broad absorbance at 400–800 cm−1 arose from the twisting vibration of water molecules participating in intermolecular hydrogen bonds. The band observed at 895–897 cm−1 for F1 and at 899–901 cm−1 for F2 was assigned to the C1β–H bending vibration characteristic for β-anomeric configuration in polysaccharides, i.e., β-d-glucans (Zhang et al., 2018) and β-d-xylans (Kačuráková et al., 1998, 2000), which are found in cereal grains and flour. Weak bands at 850–852 and ~ 762 cm−1 observed for F2 originate from C1α–H bending, CH2 deformation, and C–C stretching vibrations in the remaining starch (Fan et al., 2012; Kačuráková et al., 1998, 2000). For oat flour cold water extracts F1, the bands at 895–897, 1072–1074, 1159, 1201–1203, 1238, 1265, 1377, and 1417–1421 cm−1 are characteristic of “mixed linkage” β-d-glucan (Bai et al., 2021; Climova et al., 2021; Fusté et al., 2019; Sourki et al., 2017; Zhao et al., 2020). In the cold water extracts F1 from barley flour, most of the corresponding bands were observed at similar positions. However, the band of β-d-glucan at 1038 cm−1 was not pronounced in F1, and the band at 1026–1028 cm−1 was observed for oat flour and at 1041 cm−1 for barley flour instead due to the contribution of starch residues and AX, respectively (Kačuráková et al., 1999; Mikkelsen et al., 2010; Robert et al., 2005). For PEF-treated barley flour, the band at 1041 cm−1 was more pronounced than that of control barley flour, and the control band at 1261 cm−1 was shifted to 1257 cm−1. These features confirmed higher amount of AX, having corresponding bands at 1044 and 1253 cm−1. For oat flour hot water extracts F2, the band envelope at 900–1200 cm−1 is typical for AX, and the loss of peak multiplicity in this region is characteristic of highly substituted AX (Hromádková et al., 2013). The strong and sharp band at 1043 cm−1 and band at 1381–1383 and 899–901 cm−1 were attributive for β-d-xylan (Hromádková et al., 2013; Kačuráková et al., 1999; Robert et al., 2005). The weak band at 808–811 cm−1 originated from Araf furanoid ring vibration in AX (Kačuráková et al., 1998, 1999). The band observed for all F2 at 1076–1078 cm−1 indicates a contribution of β-d-galactan (Hromádková et al., 2013; Kačuráková et al., 1999), while starch residues contribute to this band and several weak bands at 532–534, 579, 704, 762, and 850 cm−1 (Mikkelsen et al., 2010). Finally, the spectrum obtained for fraction F2 from PEF-treated barley flour looks like a spectrum of β-d-glucan with α-d-glucan (starch) as a concomitant having characteristic bands of both these polysaccharides (Šandula et al., 1999).
The Composition and Structure of Isolated Polysaccharides by NMR
The 1D and correlation 2D NMR spectroscopy was applied to evaluate the composition and structure of polysaccharides in the purified polysaccharide fractions F1 and F2 obtained from control and PEF-treated oat and barley flours. Figure 4a, b demonstrates the 1H and 13C HMQC spectra observed for these products. Peak decomposition analysis of the 1H NMR spectrum of purified polysaccharide fraction F1 obtained from PEF-treated oat flour is shown as an example in Fig. S2. Table 6 summarizes the assignment of the proton and carbon resonance signals to the monosaccharide units, which are labeled as a–m as in the figures. The assignment was based on the relevant literature (Colleoni-Sirghie et al., 2003; Cui et al., 2000; Guo et al., 2019; Johansson et al., 2000; Pastell et al., 2009; Petersen et al., 2015; Roubroeks et al., 2000; Zhang et al., 2018). Unfortunately, not all signals were well expressed enough to identify all these units.
The signals of β-d-glucan (units a, b, and c) predominate in the HMQC spectra of the F1 fractions obtained from the control and PEF-treated oat flour. The spectrum of the control product also had weak signals from starch residues (unit d). In addition to the β-d-glucan signals mentioned above, the HMQC spectra of the corresponding fractions obtained from barley flour also contained weak signals of AX (units g–k), which were more pronounced for the control sample. In contrast to oat flour, the barley flour used in this study contained a cold water soluble AX fraction, which was found in the F1 extracts. The HMQC spectra of the F2 fractions obtained from the control flour and PEF-treated oat flour showed pronounced signals of AX structures (units g–k) and remaining starch fragments (units d, d′, e, and f), while the signals of β-d-glucan were not observed. The insert shows CH3 signals in O-acetyl groups, indicating that some AX units are esterified with acetic acid. The signals assigned to terminal β-d-xylopyranosyls and 1,2-linked α-l-arabinofuranosyls (units g′ and k) confirmed the presence of 2-O-β-d-xylopyranosyl-α-l-arabinofuranosyl side chains in the structure of oat AX (Pastell et al., 2009). The HMQC spectra of the F2 fractions obtained from the control flour and PEF-treated barley flour showed significant differences in the composition of these products. Firstly, the α-l-arabinofuranosyl signals (units i and j) predominated for the control product, while the β-d-xylopyranosyl and β-d-glucosyl signals were not pronounced. Secondly, the β-d-glucosyl signals of MLG (units a, b, and c) were the most intense for the PEF-treated product. Thirdly, the α-d-glucosyl signals of starch residue (units d, d′, e, and f) units were pronounced in both cases. These findings indicate that the PEF treatment of barley flour led to significant changes in the composition of F2 obtained by the hot water extraction.
The relative ratio between the integral areas of the anomeric H1 proton signals at 4.75 ppm (unit a) and 4.53–4.55 ppm (units b and c) represent the ratio between 1,3- and 1,4-linked β-d-glucopyranosyl units in cereal β-d-glucans (Colleoni-Sirghie et al., 2003; Mikkelsen et al., 2013; Roubroeks et al., 2000;). The 1H NMR spectra of the F2 fractions did not have the mentioned signals except that of PEF-treated barley flour, which contained a significant amount of β-d-glucan. In addition, other H1 signals, including those of β-d-xylosyls from AX, complicated the integration in the current spectral region. To avoid this, we used a peak-fitting procedure in the region of H1β signals (4.40–4.85 ppm) and obtained the Voigt components centered at 4.53–4.56 and 4.74–4.76 ppm which corresponded to the 1,4- and 1,3-linked β-d-glucopyranosyl units, respectively (Fig. 4). The ratio of 1,4- to 1,3-linkages in β-d-glucan were 2.46 (F1, control oat flour), 2.59 (F1, PEF-treated oat flour), 2.53 (F1, control barley flour), 2.44 (F1, PEF-treated barley flour), and 2.33 (F2, PEF-treated barley flour). The corresponding ratio in oat β-d-glucans obtained from the resonance signals of the anomeric protons ranged from 2.37 to 2.45 for oat varieties and changed slightly after partial hydrolysis (Colleoni-Sirghie et al., 2003). Mikkelsen et al. (2013) reported the β-d-glucan linkage patterns (ratio of β-1,4 to β-1,3 linkages) calculated from the 1H NMR spectra for barley mutant (2.28), barley control (2.42), and oat (2.48). The values determined for oat and barley glucans were quite similar, while the barley mutant showed a significant difference. In the current study, a slight increase in this value by 0.13 for the PEF-treated oat flour and a simultaneous decrease in Mw could be due to the preferable rupture of the 1,3-glycosidic bonds in oat β-d-glucan. Oppositely, for barley flour, the ratio of 1,4- to 1,3-linkages decreased after the PEF treatment by 0.09 for F1 and by 0.20 for F2, so the cellulose-like fragments could be more sensitive in this case due to the specificity of barley β-d-glucan structure or the influence of other flour components. Even though the changes in this ratio observed for the PEF-treated flours were relatively small, they could indicate a certain sensitivity of cereal β-d-glucans to the PEF treatment. The final effect of this treatment depends on the flour composition and the structural specificity of these polysaccharides, possibly on the distribution of DP3 and DP4 fragments in their chain.
Pasting Properties and Gluten Aggregation of Wheat Flour and Oat/Barley Flour Blend
The pasting profile mirrors real food production processes, aiding in the prediction of the potential industrial (bread making) application of flour since it reflects the starch quality and amylolytic activity of flour. The activity of α-amylase of untreated oat flour (322.04 U/kg) was threefold higher than its activity in barley flour (101.64 U/kg). After PEF treatment, the α-amylase activity of oat flour was reduced by 78%, whereas the amylase activity of barley flour remained unchanged.
Both the flour type and the treatment significantly (p < 0.05) affected the pasting properties. Compared to the control wheat sample, the pasting profile of the blend with untreated oat flour was more viscous, whereas the paste containing untreated barley flour was less viscous (Table 7). After adding PEF-treated flours, the MV, CPV, and SV of wheat-oat or wheat-barley paste slightly decreased, but the BV significantly increased compared to samples with untreated flours. The viscosity reduction was unexpected since the α-amylase activity of oat flour was reduced after PEF treatment. The reason could be the depolymerization of starch chains caused by PEF which yields more amylose leaching from the amylopectin (Duque et al., 2019). Jokinen et al. (2023) stated that higher BV with lower peak time, final, and setback viscosity is associated with starch damage and higher amylose content in oat flour. According to Duque et al. (2019), lower values of SV explained the reduced tendency for amylopectin retrogradation in PEF-treated oat samples. Such modification of starch induced by PEF is desirable since retrogradation is the main cause of bread staling (crumb hardening during storage). In this study, the SV values did not decrease significantly, which can be explained by the fact that the MVA analysis was performed on a mixture of wheat and oat/barley flour (for bread dough). We assume that the impact of PEF treatment would be more visible at a higher proportion of treated flour in the blend.
Flour quality can be categorized by the GlutoPeak parameters, where a short PMT and a high BEM indicate high-quality flours, while a longer PMT and lower BEM values indicate weaker flours (Amoriello et al., 2016). The PMT decreased with the addition of untreated oat flour, while it remained unchanged after adding barley flour compared to the control wheat sample (Table 7). Unlike flours with a high gluten content, flours with a high content of dietary fiber (including β-glucans), which have high water absorption capacity, require longer time for the formation of the gluten network (Cao et al., 2023). On the other hand, the unchanged PMT can happen even in the presence of fiber which affects analytical results (Amoriello et al., 2020). An additional factor could be the different protein composition, since oats are known for low gluten content, which is lower than in wheat and even lower than in barley (Schalk et al., 2017). Flours with a high protein content and high dough strength build up the gluten network faster and require more energy for effective gluten aggregation compared to lower quality flours (Amoriello et al., 2016). In our study, BEM and AGGEN were also reduced after adding either oat or barley untreated flours (Table 7). Partial replacement of wheat flour with oat or barley flour resulted in a lower gluten concentration and aggregation which indicates a weaker development of gluten network (Wang et al., 2018).
After adding PEF-treated flours, most GlutoPeak parameters changed compared to values obtained after adding untreated oat or barley flour (Table 7). The interaction between flour type and PEF treatment had a significant influence at AGGEN (p = 0.003) and somewhat at PMT (p = 0.053) and BEM (p = 0.057). The PMT of both blends was even shorter after adding PEF-treated flour compared to untreated flours, but the effect was more visible in oat blend. Unlike barley, BEM increased after PEF treatment of oat flour, reaching the value of the control wheat sample. Similary, AM and AGGEN increased after both oat and barley flour were treated in PEF (Table 7). The AGGEN increment was more visible for barley blend than for oat blend. Higher AM and AGGEN values indicate an improved bread making potential of flour (Amoriello et al., 2016; Karaduman et al., 2020). PMT values were inversely correlated with AM (r = − 0.875, p = 0.001). The AM value provides insight into gluten strength before the completion of gluten formation, i.e., higher AM indicates stronger gluten (Karaduman et al., 2020). Our results imply that replacing wheat flour with PEF-treated oat or barley flour has better potential in maintaining the strength of the gluten network than if untreated flours are used. A possible explanation for this is the lower Mw of β-glucans (oat flour) during PEF treatment but also increased proportion of water-extractable AX (Table 7). This was confirmed by the negative correlation between β-glucan Mw and AM and AGGEN of oat flour (r = 0.990 or r = − 0.997, p ≤ 0.01, respectively) as well as the positive correlation between the content of AX-F1 and AM and AGGEN of barley flour (r = 0.999, p = ≤ 0.001, for both). According to Courtin and Delcour (2002), water-extractable AX due to their high molecular weight form a secondary weaker network, which reenforces the gluten network. It can be concluded that PEF treatment of oat flour in particular had a positive effect on the formation of the gluten network, since a shorter PMT and higher BEM (only for oat), AM, and AGGEN are characteristics of rapid formation of a strong gluten network.
Dough Viscoelastic Properties
The complex viscosity and τmax were dependent on the interaction of flour type and PEF treatment (p ≤ 0.005). The complex viscosity of the bread dough significantly increased after partial replacement of wheat flour with oat or barley flour (Table 7). The reason for increased viscosity is the high dietary fiber content (including soluble β-d-glucan and arabinoxylan) of these alternative raw materials (Rieder et al., 2012). Unlike barley, the complex viscosity of dough containing oat flour after PEF treatment further increased by 28%. The τmax value increased significantly after adding oat flour untreated or even more if PEF-pretreated. The opposite, τmax drastically reduced after adding barley flour, equally with untreated or treated sample (Table 7). This means that barley samples were softer and consequently could withstand less force without structural deformation, while the oat samples became harder and consequently could withstand more force compared to the control. During PEF treatment, due to extraction, the proportion of soluble AX-F1 of oat flour increased by 40%, while the content of AX-F2 remained unchanged, resulting in a slight increase in the complex viscosity and τmax of the WOF-PEF sample. In barley flour, on the other hand, the proportion of AX-F1 increased by 36%, but at the same time, the proportion of AX-F2 (56%) decreased (Table 4); hence, the complex viscosity and τmax remained unchanged after PEF treatment. The Mw of β-d-glucan is also thought to affect the rheology results, but the reduction in Mw that occurred because of PEF treatment was masked due to the lower β-glucanase activity. Our study shows that PEF technology affects biopolymers and rheological properties of the dough depending on the flour type.
Physical and Nutritional Properties of Flat Bread Made with Oat or Barley Flour
Table 8 shows the nutritional and physical properties of flat breads. Replacing semi-refined wheat flour (30%, w/w) with oat or barley flour in the formulation resulted in flat breads with considerably higher β-d-glucans and total dietary fiber content. Flat breads made from PEF-treated oat and barley flour had higher β-d-glucan content (21–31%) compared to breads with untreated flours due to the inactivation of β-glucanase and improved β-d-glucan extraction during PEF treatment. Flat breads enriched with oat flour could be labeled as a “source of fiber,” while those containing barley flour were a “high fiber.”
The partial replacement of wheat flour with alternative (untreated) flours led to a significantly (p < 0.05) lower (19–24%) specific volume while a bigger spread ratio of flat bread (32%) compared to control wheat bread (Table 8). A similar reduction in specific volume after adding oat flour to wheat bread was previously demonstrated by Krochmal-Marczak et al. (2010). The reason for this was gluten dilution and the presence of fiber in barley and oat flour. Soluble fiber fractions in interaction with the gluten network make the retention of gasses difficult and, at the same time, make the dough too stiff to incorporate gasses during mixing and fermentation (Andrzej et al., 2019). Further, the water-unextractable AX represent a physical barrier for the formation of gluten network during dough development resulting in a weaker dough structure and lower bread volume (Courtin & Delcour, 2002). In this study, the specific volume of the flat bread was reduced even more, and the spread ratio increased even more if wheat-replacing flours were PEF-treated. Specific volume was influenced by the interaction between flour type and PEF treatment (p = 0.011); the reduction was only 11% in oat-containing bread while it decreased by 24% in barley-containing bread. This could be related to changes on barley proteins observed with SEM. Considering the results obtained with GlutoPeak which indicated shortening of PMT after PEF treatment, possibly a shortening of mixing time would be beneficial in preserving a higher bread volume. Another possible explanation is the reduced activity of α-amylase (78%) after oat flour PEF processing. Since this enzyme is necessary for assuring enough sugar for yeast fermentation and carbon dioxide production, amylase or sugar addition might have improved gas production and bread volume.
The crumb hardness, cohesiveness, resilience, and chewiness of barley breads were significantly lower compared to control wheat bread (Table 8). This is consistent with the study of Skendi et al. (2010), who demonstrated that incorporation of β-d-glucan isolated from barley leads to a decrease in the hardness of the final product. Such change in texture could be related to lower BEM after partial replacement of wheat flour with oat or barley flour in this study. One possible reason is that β-d-glucan can form a gel that has a soft structure, which consequently softens the bread crumb (Andrzej et al., 2019). The PEF treatment of barley flour resulted in a further reduction of cohesiveness and resilience of its bread which could be related to observed changes in protein matrix with SEM.
The interaction between the flour type and PEF treatment significantly (p < 0.05) influenced the crust lightness L*, redness a*, and yellowness b*. After the PEF treatment of flour, L* and b* were reduced in the case of oat bread. The darkening of oat bread could be related to the increased PPO activity (18%) of oat flour after PEF treatment, which was confirmed by its negative correlation with the L* parameter (r = − 0.875, p = 0.022). Overall, a small difference in color (dE < 1.5) from the control wheat bread was observed in all samples (Table 8).
Conclusions
This study demonstrated how the effectiveness of pulsed electric field technology in inactivating endogenous β-glucanase enzymes differs between oat and barley flour. Mixed linkage β-d-glucans from our oat and barley flour belonged to the MMW group, even after PEF treatment, after which their extractability was improved (33.5%). Therefore, it can be assumed that they have preserved physiological potential. In addition, PEF treatment was found to increase water-extractable arabinoxylans, which, in combination with β-glucans, positively contributed to the reinforcement of the gluten network in bread dough. Consequently, the use of PEF-treated raw materials in bread making is promising for improving the nutritional and technological properties of bread. However, it is important to note that the bread-making process should be adjusted since PEF technology affects many enzymes and biopolymers of flour. Although PEF technology offers the potential for processing flour with the aim of improving bread quality, further optimization of this treatment approach depending on the flour type is essential to achieve better physical properties of the final product. Future studies should investigate the consumers’ acceptability of food containing PEF-pretreated ingredients.
Data Availability
All relevant data are shown in the manuscript. Additional data is available upon request.
Code Availability
Not applicable.
References
AACC – American Association of Cereal Chemists. (2000). Method 22-85.01. Measurement of polyphenol oxi- dase in wheat kernels (L-DOPA substrate). Method 76-21.02 General pasting method for wheat or rye flour of starch using the rapid visco analyser. Method 10-05.01 Guidelines for measurement of volume by rapeseed displacement. Method 44-15A Moisture – air oven methods. Method 62-05 Preparation of sample: Bread. Method 32-05.0 Total dietary fiber. Approved Methods of the American Association of Cereal Chemists International, 10th ed. American Association of Cereal Chemists, Saint Paul, Minnesota, USA.
Amoriello, T., Mellara, F., Galli, V., Amoriello, M., & Ciccoritti, R. (2020). Technological properties and consumer acceptability of bakery products enriched with brewers’ spent grains. Foods. https://doi.org/10.3390/foods9101492
Amoriello, T., Turfani, V., Galli, V., Mellara, F., & Carcea, M. (2016). Evaluation of a new viscometer performance in predicting the technological quality of soft wheat flour. Cereal Chemistry, 93(4), 364–368. https://doi.org/10.1094/CCHEM-09-15-0185-R
Andrzej, K. M., Małgorzata, M., Sabina, K., Olaf, H., & Rodak, E. (2019). Application of rich in β-glucan flours and preparations in bread baked from frozen dough. Food Science and Technology International, 26(1), 53–64. https://doi.org/10.1177/1082013219865379
AOAC – Association of Official Analytical Chemists. (2012). Official Method 985.29 Total dietary fiber in foods. Enzymatic-gravimetric method. Official Method 920.87 Protein (total) in flour. Official Method 942.05 Determination of ash in animal feed. Official Method 922.06 Fat in flour. Acid hydrolysis method. Official Method 995.16 β-D-glucan in brley and oats. Official Method 2002.01 Measurement of alpha.gif (53 bytes)-Amylase Activity in White Wheat Flour, Milled Malt, and Microbial Enzyme Preparations Ceralpha Assay. Official Methods of Analysis of AOAC International, 19th ed. Washington, DC.
Bader UI Ain, H., Saeed, F., Ahmad, N., Imran, A., Niaz, B., Afzaal, M., Imran, M., Tufail, T., & Javed, A. (2018). Functional and health-endorsing properties of wheat and barley cell wall’s non-starch polysaccharides. International Journal of Food Properties, 21(1), 1463–1480. https://doi.org/10.1080/10942912.2018.1489837
Bai, Y. P., Zhou, H. M., Zhu, K. R., & Li, Q. (2021). Effect of thermal processing on the molecular, structural, and antioxidant characteristics of highland barley β-glucan. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2021.118416
Bichara, L. C., Alvarez, P. E., Bimbi, M. V. F., Vaca, H., Gervasi, C., & Brandán, S. A. (2016). Structural and spectroscopic study of a pectin isolated from citrus peel by using FTIR and FT-Raman spectra and DFT calculations. Infrared Physics and Technology, 76, 315–327. https://doi.org/10.1016/j.infrared.2016.03.009
Cao, H., Gao, F., Shen, H., Su, Q., Guan, X., Sun, Z., & Yu, Z. (2023). Influence of partial substitution of wheat flour with sprouted oat flours on physicochemical and textural characteristics of wheat bread. Journal of Cereal Science. https://doi.org/10.1016/j.jcs.2023.103649
Climova, A., Ibrahim, M. N., Salamahina, A., Savin, A. M., Dukhinova, M. S., Barakova, N. V., & Krivoshapkina, E. F. (2021). Application of extracted β-glucan from oat for β-carotene encapsulation. Journal of Food Science and Technology, 58, 2641–2650. https://doi.org/10.1007/s13197-020-04770-2
Colleoni-Sirghie, M., Fulton, D. B., & White, P. J. (2003). Structural features of water soluble (1,3) (1,4)-β-D-glucans from high-β-glucan and traditional oat lines. Carbohydrate Polymers, 54(2), 237–249. https://doi.org/10.1016/S0144-8617(03)00168-1
Courtin, C. M., & Delcour, J. A. (2002). Arabinoxylans and endoxylanases in wheat flour bread-making. Journal of Cereal Science, 35, 225–243. https://doi.org/10.1006/jcrs.2001.0433
Cui, W., Wood, P. J., Blackwell, B., & Nikiforuk, J. J. C. P. (2000). Physicochemical properties and structural characterization by two-dimensional NMR spectroscopy of wheat β-D-glucan—comparison with other cereal β-D-glucans. Carbohydrate Polymers, 41(3), 249–258. https://doi.org/10.1016/S0144-8617(99)00143-5
Duque, S. M. M., Leong, S. Y., Agyei, D., Singh, J., Larsen, N., & Oey, I. (2019). Understanding the impact of pulsed electric fields treatment on the thermal and pasting properties of raw and thermally processed oat flours. Food Research International. https://doi.org/10.1016/j.foodres.2019.108839
Duque, S. M. M., Leong, S. Y., Agyei, D., Singh, J., Larsen, N., & Oey, I. (2020). Modifications in the physicochemical properties of flour “fractions” after pulsed electric fields treatment of thermally processed oat. Innovative Food Science & Emerging Technologies. https://doi.org/10.1016/j.ifset.2020.102406
Fan, D., Ma, W., Wang, L., Huang, J., Zhao, J., Zhang, H., & Chen, W. (2012). Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Stärke, 64(8), 598–606. https://doi.org/10.1002/star.201100200
FAOSTAT. (2023). Food and Agriculture Organization. Retrieved November 3, 2023, from https://www.fao.org/faostat/en/#data/QCL
Fernandez-Diaz, M. D., Barsotti, L., Dumay, E., & Chedtel, J. C. (2000). Effects of pulsed electric fields on ovalbumin solutions and dialyzed egg white. Journal of Agricultural and Food Chemistry, 48, 2332–2339. https://doi.org/10.1021/jf9908796
Fusté, N. P., Guasch, M., Guillen, P., Anerillas, C., Cemeli, T., Pedraza, N., Ferrezuelo, F., Encinas, M., Moralejo, M., & Garí, E. (2019). Barley β-glucan accelerates wound healing by favoring migration versus proliferation of human dermal fibroblasts. Carbohydrate Polymers, 210, 389–398. https://doi.org/10.1016/j.carbpol.2019.01.090
Garzon, R., Gasparre, N., Pasqualone, A., Papageorgiou, M., Grgić, T., Le-Bail, P., Pablos, I. M., El Tomb, C., Magro, C., & Rosell, C. M. (2022). Flatbreads on the rise, what about their nutritional quality? The current state of the Mediterranean market. Medical Research Archives. https://doi.org/10.18103/mra.v10i7.2920
Giner, J., Gimeno, V., Espachs, A., Elez, P., Barbosa-Cánovas, G. V., & Martín, O. (2000). Inhibition of tomato (Licopersicon esculentum Mill.) pectin methylesterase by pulsed electric fields. Innovative Food Science & Emerging Technologies, 1, 57–67. https://doi.org/10.1016/S1466-8564(00)00003-5
Goudar, G., Sharma, P., Janghu, S., & Longvah, T. (2020). Effect of processing on barley β-glucan content, its molecular weight and extractability. International Journal of Biological Macromolecules, 162, 1204–1216. https://doi.org/10.1016/j.ijbiomac.2020.06.208
Grgić, T., Pavišić, Z., Maltar-Strmečki, N., Voučko, B., Čukelj Mustač, N., Ćurić, D., Le-Bail, A., & Novotni, D. (2023). Ultrasound-assisted modification of enzymatic and antioxidant activities, functional and rheological properties of oat and barley bran. Food and Bioprocess Technology, 16, 2416–2429. https://doi.org/10.1007/s11947-023-03074-5
Guo, R., Xu, Z., Wu, S., Li, X., Li, J., Hu, H., Wu, Y., & Ai, L. (2019). Molecular properties and structural characterization of an alkaline extractable arabinoxylan from hull-less barley bran. Carbohydrate Polymers, 218, 250–260. https://doi.org/10.1016/j.carbpol.2019.04.093
Han, Z., Yu, Q., Zeng, X. A., Luo, D. H., Yu, S. J., Zhang, B. S., & Chen, X. D. (2012). Studies on the microstructure and thermal properties of pulsed electric fields (PEF)-treated maize starch. International Journal of Biological Macromolecules, 8(1), 1–17. https://doi.org/10.1515/1556-3758.2375
Hromádková, Z., Paulsen, B. S., Polovka, M., Košťálová, Z., & Ebringerová, A. (2013). Structural features of two heteroxylan polysaccharide fractions from wheat bran with anti-complementary and antioxidant activities. Carbohydrate Polymers, 93(1), 22–30. https://doi.org/10.1016/j.carbpol.2012.05.021
ICC. (1998). Standard Methods of the ICC. Vienna, Austria: International Association of Cereal Science and Technology.
Izydorczyk, M. S., Jacobs, M., & Dexter, J. E. (2003). Distribution and structural variation of nonstarch polysaccharides in milling fractions of hull-less barley with variable amylose content. Cereal Chemistry, 80(6), 645–653. https://doi.org/10.1094/CCHEM.2003.80.6.645
Jaeger, H., Meneses, N., Moritz, J., & Knorr, D. (2010). Model for the differentiation of temperature and electric field effects during thermal assisted PEF processing. Journal of Food Engineering, 100, 109–118. https://doi.org/10.1016/j.jfoodeng.2010.03.034
Johansson, L., Virkki, L., Maunu, S., Lehto, M., Ekholm, P., & Varo, P. (2000). Structural characterization of water soluble β-glucan of oat bran. Carbohydrate Polymers, 42(2), 143–148. https://doi.org/10.1016/S0144-8617(99)00157-5
Jokinen, I., Veijalainen, P. S., Lille, M., Nordlund, E., & Mantila, U. H. (2023). Variability of carbohydrate composition and pasting properties of oat flakes and oat flours produced by industrial oat milling process – Comparison to non-heat-treated oat flours. Food Chemistry. https://doi.org/10.1016/j.foodchem.2022.134902
Kačuráková, M., Belton, P. S., Wilson, R. H., Hirsch, J., & Ebringerová, A. (1998). Hydration properties of xylan-type structures: An FTIR study of xylooligosaccharides. Journal of the Science of Food and Agriculture, 77(1), 38–44.
Kačuráková, M., Capek, P., Sasinková, V., Wellner, N., & Ebringerová, A. (2000). FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydrate Polymers, 43(2), 195–203. https://doi.org/10.1016/S0144-8617(00)00151-X
Kačuráková, M., Wellner, N., Ebringerová, A., Hromádková, Z., Wilson, R. H., & Belton, P. S. (1999). Characterisation of xylan-type polysaccharides and associated cell wall components by FT-IR and FT-Raman spectroscopies. Food Hydrocolloids, 13(1), 35–41. https://doi.org/10.1016/S0268-005X(98)00067-8
Karaduman, Y., Sayaslan, A., & Akın, A. (2020). GlutoPeak parameters of whole wheat flours for gluten quality evaluation in soft wheat breeding programs. Journal of Cereal Science. https://doi.org/10.1016/j.jcs.2020.103031
Karimi, R., Azizi, M. H., & Xu, Q. (2019). Effect of different enzymatic extractions on molecular weight distribution, rheological and microstructural properties of barley bran β-glucan. International Journal of Biological Macromolecules, 126, 298–309. https://doi.org/10.1016/j.ijbiomac.2018.12.165
Kong, J., & Yu, S. (2007). Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochimica Et Biophysica Sinica, 39(8), 549–559. https://doi.org/10.1111/j.1745-7270.2007.00320.x
Krochmal-Marczak, B., Tobiasz-Salach, R., & Kaszuba, J. (2010). The effect of adding oat flour on the nutritional and sensory quality of wheat bread. British Food Journal, 122(7), 2329–2339. https://doi.org/10.1108/BFJ-07-2019-0493
Kumari, B., Tiwari, B. K., Walsh, D., Griffin, T. P., Islam, N., Lyng, J. G., Brunton, N. P., & Rai, D. K. (2019). Impact of pulsed electric field pre-treatment on nutritional and polyphenolic contents and bioactivities of light and dark brewer’s spent grains. Innovative Food Science & Emerging Technologies, 54, 200–210. https://doi.org/10.1016/j.ifset.2019.04.012
Kumari, B., Tiwari, B. K., Hossain, M. B., & Brunton, N. P. (2018). Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food and Bioprocess Technology, 11, 223–241. https://doi.org/10.1007/s11947-017-1961-9
Lazaridou, A., Biliaderis, C. G., & Izydorczyk, M. S. (2007). Cereal β-glucans: Structures, physical properties, and physiological functions. In C. G. Biliaderis & M. S. Izydorczyk (Eds.), Functional Food Carbohydrates (pp. 2–58). CRC Press.
Lazaridou, A., Chornick, T., Biliaderis, C. G., & Izydorczyk, M. S. (2008). Sequential solvent extraction and structural characterization of polysaccharides from the endosperm cell walls of barley grown in different environments. Carbohydrate Polymers, 76, 621–639. https://doi.org/10.1016/j.carbpol.2008.01.001
Lazaridou, A., Marinopoulou, A., Matsoukas, N. P., & Biliaderis, C. G. (2014). Impact of flour particle size and autoclaving on β-glucan physicochemical properties and starch digestibility of barley rusks as assessed by in vitro assays. Bioactive Carbohydrates and Dietary Fibre, 4(1), 58–73. https://doi.org/10.1016/j.bcdf.2014.06.009
Li, Y., Zhang, S., Bao, Z., Sun, N., & Lin, S. (2022). Exploring the activation mechanism of alcalase activity with pulsed electric field treatment: Effects on enzyme activity, spatial conformation, molecular dynamics simulation and molecular docking parameters. Innovative Food Science & Emerging Technologies. https://doi.org/10.1016/j.ifset.2022.102918
Liu, Y., & White, P. J. (2011). Molecular weight and structure of water soluble (1→3), (1→4)-β-glucans affect pasting properties of oat flours. Journal of Food Science, 76(1), 68–74. https://doi.org/10.1111/j.1750-3841.2010.01912.x
Lohani, U. C., & Muthukumarappan, K. (2016). Application of pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innovative Food Science & Emerging Technologies, 35, 29–35. https://doi.org/10.1016/j.ifset.2016.03.012
Luo, W., Han, Z., Zeng, X., Yu, S., & Kennedy, J. F. (2010). Study on the degradation of chitosan by pulsed electric fields treatment. Innovative Food Science & Emerging Technologies, 11, 587–591. https://doi.org/10.1016/j.ifset.2010.04.002
Ma, S., Yu, S. J., Zhang, B., & Wang, Z. H. (2012). Physicochemical properties of sugar beet pulp pectin by pulsed electric field treatment. International Journal of Food Science and Technology, 47(12), 2538–2544. https://doi.org/10.1111/j.1365-2621.2012.03132.x
Makowska, K., Kałużniak, M., Oleszczuk, S., Zimny, J., Czaplicki, A., & Konieczny, R. (2017). Arabinogalactan proteins improve plant regeneration in barley (Hordeum vulgare L.) anther culture. Plant Cell, Tissue and Organ Culture, 131, 247–257. https://doi.org/10.1007/s11240-017-1280-x
Maniglia, B. C., Pataro, G., Ferrari, G., Esteves, P., Augusto, D., Le-Bail, P., & Le-Bail, A. (2021). Pulsed electric fields (PEF) treatment to enhance starch 3D printing application: Effect on structure, properties, and functionality of wheat and cassava starches. Innovative Food Science & Emerging Technologies. https://doi.org/10.1016/j.ifset.2021.102602
Mansoor, R., Ali, T., Arif, M., & Moin, A. (2019). Effects of barley flour on dough rheology, texture, sensory and glycemic index of traditional unleavened flat bread (Roti). Cereal Chemistry, 96(6), 1170–1179. https://doi.org/10.1002/cche.10228
Martín-Belloso, O., & Elez-Martínez, P. (2005). Enzymatic inactivation by pulsed electric fields. In D. W. Sun (Ed.), Emerging technologies for food processing (pp. 155–181). Academic Press. https://doi.org/10.1016/B978-012676757-5/50009-8
Mikkelsen, M. S., Jespersen, B. M., Larsen, F. H., Blennow, A., & Engelsen, S. B. (2013). Molecular structure of large-scale extracted β-glucan from barley and oat: Identification of a significantly changed block structure in a high β-glucan barley mutant. Food Chemistry, 136(1), 130–138. https://doi.org/10.1016/j.foodchem.2012.07.097
Mikkelsen, M. S., Jespersen, B. M., Møller, B. L., Lærke, H. N., Larsen, F. H., & Engelsen, S. B. (2010). Comparative spectroscopic and rheological studies on crude and purified soluble barley and oat β-glucan preparations. Food Research International, 43(10), 2417–2424. https://doi.org/10.1016/j.foodres.2010.09.016
Nowacka, M., Tappi, S., Wiktor, A., Rybak, K., Miszczykowska, A., Czyzewski, J., Drozdzal, K., Witrowa-Rajchert, D., & Tylewicz, U. (2019). The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods, 8(7), 244. https://doi.org/10.3390/foods8070244
Ohshima, T., Tamura, T., & Sato, M. (2007). Influence of pulsed electric field on various enzyme activities. Journal of Electrostatics, 65, 156–161. https://doi.org/10.1016/j.elstat.2006.07.005
Passos, C. P., & Coimbra, M. A. (2013). Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydrate Polymers, 94(1), 626–633. https://doi.org/10.1016/j.carbpol.2013.01.088
Pastell, H., Virkki, L., Harju, E., Tuomainen, P., & Tenkanen, M. (2009). Presence of 1→ 3-linked 2-O-β-d-xylopyranosyl-α-l-arabinofuranosyl side chains in cereal arabinoxylans. Carbohydrate Research, 344(18), 2480–2488. https://doi.org/10.1016/j.carres.2009.09.035
Paz-Samaniego, R., Sotelo-Cruz, N., Marquez-Escalante, J., Rascon-Chu, A., Campa-Mada, A. C., & Carvajal-Millan, E. (2019). Nixtamalized maize flour by-product as a source of health-promoting ferulated arabinoxylans (AX). In V. R. Preedy & R. R. Watson (Eds.), Flour and breads and their fortification in health and disease prevention (pp. 225–235). Academic Press.
Pérez-Quirce, S., Ronda, F., Lazaridou, A., & Biliaderis, C. G. (2017). Effect of microwave radiation pretreatment of rice flour on gluten-free breadmaking and molecular size of β-glucans in the fortified breads. Food and Bioprocess Technology, 10, 1412–1421. https://doi.org/10.1007/s11947-017-1910-7
Pérez-Quirce, S., Ronda, F., Melendre, C., Lazaridou, A., & Biliaderis, C. G. (2016). Inactivation of endogenous rice flour β-glucanase by microwave radiation and impact on physico-chemical properties of the treated flour. Food and Bioprocess Technology, 9, 1562–1573. https://doi.org/10.1007/s11947-016-1741-y
Petersen, B. O., Motawie, M. S., Møller, B. L., Hindsgaul, O., & Meier, S. (2015). NMR characterization of chemically synthesized branched α-dextrin model compounds. Carbohydrate Research, 403, 149–156. https://doi.org/10.1016/j.carres.2014.05.011
Rieder, A., Holtekjølen, A. K., Sahlstrøm, S., & Moldestad, A. (2012). Effect of barley and oat flour types and sourdoughs on dough rheology and bread quality of composite wheat bread. Journal of Cereal Science, 55, 44–52. https://doi.org/10.1016/j.jcs.2011.10.003
Rivero-Ramos, P., Unthank, M. G., Sanz, T., Rodrigo, M. D., & Benlloch-Tinoco, M. (2023). Synergistic depolymerisation of alginate and chitosan by high hydrostatic pressure (HHP) and pulsed electric fields (PEF) treatment in the presence of H2O2. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2023.120999
Robert, P., Marquis, M., Barron, C., Guillon, F., & Saulnier, L. (2005). FT-IR investigation of cell wall polysaccharides from cereal grains. Arabinoxylan infrared assignment. Journal of Agricultural and Food Chemistry, 53(18), 7014–7018. https://doi.org/10.1021/jf051145y
Roubroeks, J. P., Andersson, R., & Åman, P. (2000). Structural features of (1→ 3), (1→ 4)-β-D-glucan and arabinoxylan fractions isolated from rye bran. Carbohydrate Polymers, 42(1), 3–11. https://doi.org/10.1016/S0144-8617(99)00129-0
Šandula, J., Kogan, G., Kačuráková, M., & Machová, E. (1999). Microbial (1→ 3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydrate Polymers, 38(3), 247–253. https://doi.org/10.1016/S0144-8617(98)00099-X
Schalk, K., Lexhaller, B., Koehler, P., & Scherf, K. A. (2017). Isolation and characterization of gluten protein types from wheat, rye, barley, and oats for use as reference materials. PLoS ONE. https://doi.org/10.1371/journal.pone.0172819
Shams, R., Manzoor, S., Shabir, I., Dar, A. H., Dash, K. K., Srivastava, S., Pandey, V. K., Bashir, I., & Khan, S. A. (2024). Pulsed electric field-induced modification of proteins: A comprehensive review. Food and Bioprocess Technololgy, 17, 351–383. https://doi.org/10.1007/s11947-023-03117-x
Shoukat, M., & Sorrentino, A. (2021). Cereal β-glucan: A promising prebiotic polysaccharide and its impact on the gut health. International Journal of Food Science and Technology, 56, 2088–2097. https://doi.org/10.1111/ijfs.14971
Skendi, A., Biliaderis, C. G., Papageorgiou, M., & Izydorczyk, M. S. (2010). Effects of two barley β-glucan isolates on wheat flour dough and bread properties. Food Chemistry, 119, 1159–1167. https://doi.org/10.1016/j.foodchem.2009.08.030
Sourki, A. H., Koocheki, A., & Elahi, M. (2017). Ultrasound-assisted extraction of β-d-glucan from hull-less barley: Assessment of physicochemical and functional properties. International Journal of Biological Macromolecules, 95, 462–475. https://doi.org/10.1016/j.ijbiomac.2016.10.111
Su, S., & Higashiyama, T. (2018). Arabinogalactan proteins and their sugar chains: Functions in plant reproduction, research methods, and biosynthesis. Plant Reproduction, 31(1), 67–75. https://doi.org/10.1007/s00497-018-0329-2
Voiniciuc, C. (2022). Modern mannan: A hemicellulose’s journey. New Phytologist, 234(4), 1175–1184. https://doi.org/10.1111/nph.18091
Wang, J., Hou, G. G., Liu, T., Wang, N., & Bock, J. (2018). GlutoPeak method improvement for gluten aggregation measurement of whole wheat flour. LWT, 90, 8–14. https://doi.org/10.1016/j.lwt.2017.11.059
Zambrano, J. A., Thyagarajan, A., Sardari, R. R. R., & Olsson, O. (2023). Characterization of high arabinoxylan oat lines identified from a mutagenized oat population. Food Chemistry. https://doi.org/10.1016/j.foodchem.2022.134687
Zannini, E., Núñez, A. B., Sahin, A. W., & Arendt, E. K. (2022). Arabinoxylans as functional food ingredients: A review. Foods, 11(7), 1026. https://doi.org/10.3390/foods11071026
Zhang, F., Tian, M., Du, M., & Fang, T. (2017). Enhancing the activity of pectinase using pulsed electric field (PEF) treatment. Journal of Food Engineering, 205, 56–63. https://doi.org/10.1016/j.jfoodeng.2017.02.023
Zhang, H., Zhang, N., Xiong, Z., Wang, G., Xia, Y., Lai, P., & Ai, L. (2018). Structural characterization and rheological properties of β-D-glucan from hull-less barley (Hordeum vulgare L. var. nudum Hook. f.). Phytochemistry, 155, 155–163. https://doi.org/10.1016/j.phytochem.2018.08.004
Zhang, K., Li, X., Ma, Z., & Hu, X. (2019). Solvent retention capacity of oat flour: Relationship with oat β-glucan content and molecular weight. Food Hydrocolloids, 93, 19–23. https://doi.org/10.1016/j.foodhyd.2019.02.001
Zhao, Y., Zhou, H. M., Huang, Z. H., & Zhao, R. Y. (2020). Different aggregation states of barley β-glucan molecules affects their solution behavior: A comparative analysis. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2019.105543
Acknowledgements
PhD student Tomislava Grgić is thankful for a received mobility grant within the Central European Exchange Program (CEEPUS) at the University of Chemistry and Technology (UCT), Prague, Czech Republic.
Funding
Open access publishing supported by the National Technical Library in Prague. This paper is supported by the PRIMA program under grant agreement No. 2031 project “FLAT BREAD MINE.” This project is part of the PRIMA program, which is an Art.185 initiative supported and funded under Horizon 2020, the European Union’s Framework Program for Research and Innovation. This study was funded by the Republic of Croatia Ministry of Science and Education through the European Regional Development Fund through the project (KK.01.1.1.02.0001) “Equipping the semi-industrial practice for the development of new food technologies.”
Author information
Authors and Affiliations
Contributions
Tomislava Grgić: formal analysis, data curation, writing original draft, and preparation of all tables and figures; Roman Bleha: methodology and formal analysis; Petra Smrčková: formal analysis; Višnja Stulić: formal analysis; Tomislava Vukušić Pavičić: formal analysis and methodology; Andriy Synytsya: data curation, resources, writing original draft, review and editing, and revision of the manuscript; Damir Iveković: methodology, formal analysis, resources, and writing original draft; Dubravka Novotni: validation, revision of the manuscript, and supervision. All authors read the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Disclaimer
The results and content found in this paper/report reflect only the author’s view. The PRIMA Foundation is not responsible for any use that may be made of the information it contains.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grgić, T., Bleha, R., Smrčkova, P. et al. Pulsed Electric Field Treatment of Oat and Barley Flour: Influence on Enzymes, Non-starch Polysaccharides, Dough Rheological Properties, and Application in Flat Bread. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03383-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03383-3