Abstract
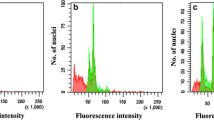
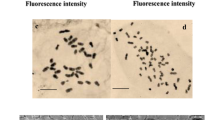
A simple and efficient procedure was established for in vitro propagation of lattice henbane (Hyoscyamus reticulatus L.) using shoot tip explants cultured on Murashige and Skoog (MS) medium supplemented with N6-benzylaminopurine (BAP) and indolyl-3-acetic acid (IAA). In vitro-induced autotetraploid plants of H. reticulatus L. exhibiting high yield of scopolamine were successfully induced by different concentrations of colchicine solutions (0.00, 0.05, 0.1, 0.2 and 0.5 %) which were applied for 24, 48 and 72 h. Treated shoot tips were regenerated on MS medium supplemented with 8.8 mM BAP and 2.2 mM IAA and rooted on ½MS medium containing 2.2 mM indolyl-3-butyric acid, then were acclimatized and transferred to soil. According to the results, 0.1 % (w/v) of colchicine for 48 h can be effective for induction of polyploidy in H. reticulatus L. The induced tetraploid plants represented a different structural, physiological and biochemical characteristics. Autotetraploid plants of H. reticulatus also showed a higher conversion of hyoscyamine to scopolamine as scopolamine content was increased from 0.23 in diploids to 8.66 % in the induced tetraploid plants. Regarding the higher commercial value of scopolamine than the other tropane alkaloids, tetraploidy can efficiently be used to improve scopolamine production of H. reticulatus.







Similar content being viewed by others
References
Ahuja A, Sambyal M, Koul S (2002) In vitro propagation and conservation of Atropa acuminata Royle ex Lindl-An indigenous threatened medicinal plant. J Plant Biochem Biotechnol 11(2):121–124
Al-Humaid AI (2003) Effects of compound fertilization on growth and alkaloids of Datura innoxia Mill. plants. J Agr Rural Dev Trop. 104:151–165
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. J Plant Physiol 24(1):1
Asha Rani NS, Prasad MP (2013) Studies on the organogenesis of Atropa Belladonna in in-vitro conditions. J Biotechnol Bioeng 4:457–464
Bahmanzadegan JA, Sefidkon F, Sanboli A (2009) Determination of hyoscyamine and scopolamine in Four Hyoscyamus species from Iran. Iranian J Pharm Res 8(1):65–70
Bennett M (1972) Nuclear DNA content and minimum generation time in herbaceous plants. Proc Roy Soc Lon Ser B Biol Sci 181(1063):109–135
Bhattacharyya R, Bhattacharya S (2001) High frequency in vitro propagation of Phyllanthus amarus Schum. & Thom. by shoot tip culture. Indian J Exp Biol 39(11):1184–1187
Chavadej S, Becker H (1984) Influence of Colchicine treatment on chromosome number and growth rate of tissue Culture of Valeriana wallichii DC. Plant Cell Tiss Organ Cult. 3:265–272
Chen LL, Gao SL (2007) In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci Hort 112(3):339–344
Cheng ZM, Korban SS (2011) In vitro ploidy manipulation in the genomics era. Plant Cell Tiss Organ Cult 104:281–282
Dehghan E, Häkkinen ST, Oksman-Caldentey KM, Ahmadi FS (2012) Production of tropane alkaloids in diploid and tetraploid plants and in vitro hairy root cultures of Egyptian henbane (Hyoscyamus muticus L.). Plant Cell Tissue Organ Cult 110(1):35–44
Deivis TH (1978) Flora of Turkey, vol 6. University Press, Edinburg, pp 454–455
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011) Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv Sciella. Plant Cell Tissue Organ Cult 106(3):485–493
Gao S, Zhu D, Cai Z, Xu D (1996) Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell Tissue Organ Cult 47(1):73–77
Ghafarzadegan R, Khadiv PP, Khalighi Sigaroudi F, Pirali Hamedani M, Kadkhoda Z, Rezazadeh SA (2010) Optimization of extraction method of hyoscine from hyoscyamus niger L. J Med Plants 9(36):87–95
Ghotbi Ravandi E, Rezanejad F, Zolala J, Dehghan E (2013) The effects of chromosome-doubling on selected morphological and phytochemical characteristics of Cichorium intybus L. J Hortic Sci Biotech 88(8):701–709
Gopi C, Sekhar YN, Ponmurugan P (2006) In vitro multiplication of Ocimum gratissimum L. through direct regeneration. Afr J Biotechnol 5:9
Gu X, Yang A, Meng H, Zhang J (2005) In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill. cv. Zhanhua. Plant Cell Rep 24(11):671–676
Hashimoto T, Yamada Y (1994) Alkaloid biogenesis: molecular aspects. Annu Rev Plant Phys 45:257–285
Heping H, Shanlin G, Lanlan C, Xiaoke J (2008) In vitro induction and identification of autotetraploids of Dioscorea zingiberensis. In Vitro Cell Dev-Pl 44(5):448–455
Ionkova I (2010) Tropane alkaloids in Hyoscyamus reticulatus L. In: Awaad AS, Govil JN, Singh VK Drug plants I. pp 373–389
Kaensaksiri T, Soontornchainaksaeng P, Soonthornchareonnon N, Prathanturarug S (2011) In vitro induction of polyploidy in Centella asiatica (L.) Urban. Plant Cell Tissue Organ Cult 107(2):187–194
Kamada H, Okamura N, Satake M, Harada H, Shimomura K (1986) Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep 5(4):239–242
Kang Y, Min J, Moon H, Karigar C, Prasad D, Lee C, Choi M (2004) Rapid in vitro adventitious shoot propagation of Scopolia parviflora through rhizome cultures for enhanced production of tropane alkaloids. Plant Cell Rep 23(3):128–133
Kayani HA, Khan S, Naz S, Chaudhary MI (2013) Micropropagation of Agastache anisata using nodal segments as explants and cytotoxic activity of its methanolic extracts. Pak J Bot 45(6):2105–2109
Koul S, Sambyal M, Kitchlu S, Bakshi S, Kaul M (2010) Development, micropropagation and characterization of colchiploid of Echinacea purpurea (L.) Moench. Indian J Biotech 9:221–224
Kutchan TM (1995) Alkaloid biosynthesis [mdash] the basis for metabolic engineering of medicinal plants. Plant Cell 7(7):1059
Lavania U (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Acta Hortic 3(2):170–177
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122(1):1–25
Lin X, Zhou Y, Zhang J, Lu X, Zhang F, Shen Q, Wu S, Chen Y, Wang T, Tang K (2011) Enhancement of artemisinin content in tetraploid Artemisia annua plants by modulating the expression of genes in artemisinin biosynthetic pathway. Biotechnol Appl Bioc 58(1):50–57
Liu Z, Gao S (2007) Micropropagation and induction of autotetraploid plants of Chrysanthemum cinerariifolium (Trev.) Vis. In vitro Cell Dev-Pl 43(5):404–408
Liu S, Chen S, Chen Y, Guan Z, Yin D, Chen F (2011) In vitro induced tetraploid of Dendranthema nankingense (Nakai) Tzvel. shows an improved level of abiotic stress tolerance. Sci Hort 127(3):411–419
Maharana SB, Mahato V, Behera M, Mishra RR (2012) In vitro regeneration from node and leaf explants of Jatropha curcas L. and evaluation of genetic fidelity through RAPD markers. Indian J Biotechnol 11:280–287
Manske RRHF, Holmes HHL (1950) The alkaloids: chemistry and physiology V1. J Chem Physiol 1:1–525
Mathura S, Fossey A, Beck SL (2006) Comparative study of chlorophyll content in diploid and tetraploid black wattle (Acacia mearnsii). Forestry 79(4):381–388
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Omidbaigi R (1997) Approaches to production and processing of medicinal plants, vol 2. Tarrahane Nashr Public Tehran 14:70–78
Ovečka M, Bobák M, Šamaj J (1997) Development of shoot primordia in tissue culture of Papaver somniferum L. Biol Plant 39(4):499–506
Philipov S, Berkov S (2002) GC-MS investigation of tropane alkaloids in Datura stramonium. Z Naturforsch C 57(5/6):559–561
Pryor R, Frazier L (1968) Colchicine-induced tetraploid azaleas. Sci Hort 3(4):283–286
Roy A, Leggett G, Koutoulis A (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep 20(6):489–495
Shahriari-Ahmadi F, Dehghan E, Farsi M, Azizi M (2009) Tetraploid induction of Hyoscyamus muticus L. using colchicine treatment. Pak J Biol Sci 11(24):2653–2659
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84(3):263–268
Sundari M, Benniamin A, Manickam V (2010) Micropropagation and in vitro flowering in Solanum nigrum Linn. a medicinal plant. Int J Biotechnol 1(1):29–32
Swanson CP (1957) Cytology and cytogenetics. Carl P, Swanson
Urwin NA, Horsnell J, Moon T (2007) Generation and characterisation of colchicine-induced autotetraploid Lavandula angustifolia. Euphytica 156(1–2):257–266
Viehmannová I, Cusimamani EF, Bechyne M, Vyvadilová M, Greplová M (2009) In vitro induction of polyploidy in yacon (Smallanthus sonchifolius). Plant Cell Tissue Organ Cult 97(1):21–25
Wendel J, Doyle J (2005) Polyploidy and evolution in plants. Plant diversity and evolution: genotypic and phenotypic variation in higher plants, p 97
Wolfe KH (2001) Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet 2(5):333–341
Zhang Z, Dai H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159(1–2):59–65
Zhang Q, Luo F, Liu L, Guo F (2010) In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.). Plant Cell Tissue Organ Cult 101(1):41–47
Zia M, Rehman R, Chaudhary MF (2007) Hormonal regulation for callogenesis and organogenesis of Artemisia absinthium L. Afr J Biotechnol 6:16
Acknowledgments
This work was supported by Urmia University, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Y. Paek.
Rights and permissions
About this article
Cite this article
Madani, H., Hosseini, B., Dehghan, E. et al. Enhanced production of scopolamine in induced autotetraploid plants of Hyoscyamus reticulatus L.. Acta Physiol Plant 37, 55 (2015). https://doi.org/10.1007/s11738-015-1795-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1795-x