Abstract
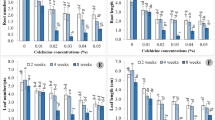
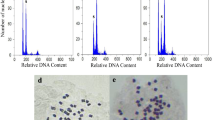
In vitro conditions for Japanese quince polyploidisation were investigated. Microshoots and isolated cotyledons were treated with colchicine and oryzalin. Morphogenesis was more dependent on the concentration of colchicine or oryzalin than on the duration of exposure, genotype differences were observed. Low oryzalin concentrations had no impact on morphogenesis. Plants with changed chromosome numbers were obtained at 0.25–38 mM colchicine and 10–50 μM oryzalin concentrations. It was determined that stomata length is a suitable parameter for identifying putative Japanese quince tetraploids. Stomata of tetraploid shoots of the same clone were approximately 1/3 longer than in the diploids. It was shown that through polyploidisation gigas effect was not obtained in fruit size but tetraploids have reduced seed set and an increased proportion of fruit flesh.
Similar content being viewed by others
Abbreviations
- BA:
-
6-benzylaminopurine
- DMSO:
-
dimethyl sulfoxide
- GA:
-
gibberellic acid
- IBA:
-
indole-butyric acid
- PPFD:
-
photosynthetic photon flux density
References
Arumuganathan K, Earle ED, (1991) Estimation of nuclear DNA content of plants by flow cytometryPlant Mol. Biol. Rep. 9(3): 229–241
Bouvier L, Fillon FR, Lespinasse Y, (1994) Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitroPlant Breeding 113:343–346
Bouvier L, Guerif Ph, Djulbic M, Durel ChE, Chevreau E, Lespinase Y, (2002) Chromosome doubling of pear haploid plants and homozygosity assessment using isozyme and microsatellite markersEuphytica 123(2):255–262
Bukhari YM, (1997) Nuclear DNA-amounts in Acacia and Prosopis (Mimosaceae) and their evolutionary implicationsHereditas 126: 45–51
Chakraborti SP, Vijayan K, Roy BN, Qadri SMH, (1998) In vitro induction of tetraploidy in mulberry (Morus alba L.)Plant Cell Rep. 17: 799–803
Dhawan OP, Lavania UC, (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: A review Euphytica 87: 81–89
Dikson EE, Arumuganathan K, Kresovich S, Doyle JJ, (1992) Nuclear DNA content variation within the RosaceaeAm. J. Bot. 79(9): 1081–1086
Murashige T, Skoog F, (1962) A revised medium for rapid growth and bioassays with tobacco culturesPhysiol. Plant 15: 473–497
Nitsch JP, Nitsch C, (1969) Haploid plants from pollen grainsScience 163: 85–87
Roberts AV, Lloyd D, Short KC, (1990) In vitro procedures for the induction of tetraploidy in a diploid roseEuphytica 49: 33–38
Rose JB, Kubba J, Tobutt KR, (2000) Chromosome doubling in sterile Syringa vulgaris × S. pinnatifolia hybrids by in vitro of nodal explantsPlant Cell, Tissue and Organ Cult 63(2):127–132
Roy AT, Leggett G, Koutoulis A, (2001) In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.)Plant Cell Rep. 20:489–495
Rumpunen K, (2001) Diversity in the plant genus Chaenomeles Acta Universitatis Agriculturae Sueciae Agraria 293:7–53
Sedov EN, Zhdanov ZA & Sedova ZA (1996) Apple Breeding, Moscow (in Russian)
Tambong JT, Sapra VT, Garton S, (1998) In vitro induction of tetraploids in colchicine-treated cocoyam plantletsEuphytica 104(3): 191–197
Väinölä A, (2000) Polyploidization and early screening of Rhododendron hybrids Euphytica 112(3): 239–244
Weber C, (1963) Cultivars in the genus ChaenomelesArnoldia 23: 17–75
Acknowledgements
This study was carried out with the financial assistance of the Commission of the European Communities, Agriculture and Fisheries (FAIR) specific RTD programme, CT 97–38–94, “Japanese quince (Chaenomeles japonica) – a new European fruit crop for production of juice, flavour and fibre”. It does not necessarily reflect its views and in no way anticipates the Commissions’ future policy in this area.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stanys, V., Weckman, A., Staniene, G. et al. In vitro induction of polyploidy in japanese quince (Chaenomeles japonica). Plant Cell Tiss Organ Cult 84, 263–268 (2006). https://doi.org/10.1007/s11240-005-9029-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-005-9029-3