Abstract
To study the final equilibrium state and process of CO2 injecting into the Fe–C melt with different initial carbon contents, a model was established based on the method of minimization of Gibbs free energy and the corresponding experiments were carried out in a high-temperature tube furnace. When CO2 is continuously injected into the Fe–C melt at 1873 K, the final equilibrium state of the system is such that the carbon and oxygen contents in the melt are 0.1977 and 0.0115 wt pct, respectively, and the volume ratio of CO in the gas phase is 85.75 vol pct. When the initial a[O] × a[C] in the melt is greater than those in equilibrium with CO under 0.8575 atm, the CO2 gas removes carbon from the melt. On the contrary, the role of CO2 gas is to add carbon and oxygen to the melt. At the same time, the variation of carbon and oxygen with time obtained by experiments was different from the theoretical calculation at extremely low carbon content, which requires further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a gaseous medium, CO2 is widely used in steelmaking processes as a replacement for O2/N2/Ar gases. At present, it is being used for the combined top and bottom blowing in the basic oxygen furnace (BOF) and bottom blowing in the electric arc furnace (EAF). It is also adopted in the ladle furnace (LF), and as the bottom blowing and lifting gas in the Rheinstahl–Heraeus (RH). Theoretical analyses and laboratory experiments were carried out in an argon-oxygen decarburization furnace (AOD) and a vanadium-extraction converter (de-V BOF), which can reduce the amount of dust and the total Fe content (TFe) in the slag and improve the dephosphorization efficiency. At the same time, CO2 can also improve the purity of molten steel in the LF and the stirring strength in the RH, and realize selective oxidation of chromium, vanadium, and other valuable elements with carbon. Table I gives an overview of the applications of CO2 in steelmaking processes and the results that have been obtained from the former research.[1,2,3,4,5,6,7,8,9,10,11,12,13,14] The theoretical basis for achieving the preceding effects is that CO2 exhibits weak oxidation, which occurs through the reaction CO2 + [C] = 2CO. The reaction characteristics mainly include the endothermic effect that reduces the temperature of the fire point zone and controls the heating rate of molten steel, generating nearly twice the amount of gas to improve the stirring intensity in the furnace, and selective oxidation of carbon and other valuable elements under different pressures and liquid steel compositions. However, these studies were carried out mostly in the industrial scale and did not go deep into theoretical analysis.
Besides, many researchers have studied the decarburization kinetics of CO2 as an oxidizing gas in liquid steel containing carbon in the laboratory.[15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] Most studies have evaluated the influence of the composition of multicomponent gaseous mixtures, such as O2–CO2–Ar, gas phase flow rate and pressure, temperature, and liquid steel composition (carbon, sulfur, silicon, manganese, chromium, vanadium, etc.) on the decarburization kinetics and the rate-limiting steps of the decarburization reaction, as shown in Table II. As can be seen from the summary in the table, most of the research was not referring to the low and extremely low carbon Fe–C melt.
The preceding studies mainly focus on the industrial applications and decarburization kinetics of CO2 gas used as an oxidant and stirring gas; however, the final equilibrium state of the system is not clear when CO2 is continuously injected into the Fe–C melt, and it needs to be clarified whether CO2 shows a decarburization effect in liquid steel under all carbon content ranges. In particular, there are no quantitative studies reporting the oxidation ability of CO2 when injecting it into molten steels.
In this case, we have carried out the study on the continuous injection of CO2 into Fe–C melts with different initial carbon contents to determine the effect law of CO2 on the decarburization process of the Fe–C melt, in order to enrich the database of CO2 injection in the steelmaking process.
Theoretical Calculation
To obtain a better understanding of how CO2 reacts with the Fe–C melt of different initial carbon contents, a model for continuous injecting CO2 will be set up and the composition change of components in the system will be simulated by the model.
Process Description
At steelmaking temperatures, the chemical reaction between the CO2 gas and Fe–C melt is a three-phase (gas–slag–metal) coupling reaction. This reaction mainly includes the decomposition reaction of the CO2 gas (Eq. [1]); equilibrium reaction of the CO, carbon, and oxygen in the melt (Eq. [2]); equilibrium reaction of O2 with oxygen in the melt (Eq. [3]); and equilibrium reaction of iron and oxygen in the melt to generate FeO in the slag phase (Eq. [4]). The gas phase includes O2, CO2, and CO; the liquid phase includes C, O, and Fe; and the slag phase is FeO. During the smelting process, the gas phase continuously enters the system. After the CO2 gas reacts with the components in the system, the gaseous reaction products escape from the slag–steel interface and a fresh stream of injected CO2 reacts with the melt to form a new state.
Modeling Assumptions
To simulate the reaction between the CO2 gas and the Fe–C melt, the following assumptions were made.
-
(1)
CO2 gas is input step by step.
-
(2)
At each step, the input CO2 gas and the system reach thermodynamic equilibrium.
-
(3)
Before the next calculation step, the gaseous components formed in the previous step are removed.
-
(4)
The pressure of the gas phase is 1 atm.
A schematic diagram of the reaction process between the CO2 gas and Fe–C melt is shown in Figure 1.
Model Setup
A single component in the reaction system may participate in two or more reactions during the reaction process. CO gas participates in the decomposition of CO2 gas and the equilibrium reaction of carbon and oxygen in the Fe–C melt, whereas the oxygen in the melt participates in all four of the reactions (Eqs. [1] through [4]) occurring in the system. Most of the existing research work only considers the reaction CO2 + [C] = 2CO and fails to describe the changes in all the components of the system.
The minimum Gibbs free energy method is effective for analyzing the thermodynamic equilibrium state of multiphase coupling systems.[37,38,39] For any multiphase closed system, when the temperature and pressure are determined, the necessary and sufficient conditions for all reactions in the system to reach equilibrium are that the total Gibbs free energy of the closed system reaches the minimum value and the mass of each element in the system is conserved. The objective function for this problem is presented in Eq. [5].
where \(\min \cdot G_{{\text{s}}}\) is the minimum value of Gs, J/mol; C is the total number of components in the system; \(G_{m,i}^{\Theta }\) is the standard Gibbs free energy of component i, J/mol; ni is the mole number of component i in the system when reaching the gas–slag–metal equilibrium state, mol; pi is the partial pressure of component i in the gas phase, atm; and ai is the activity of component i in the melt or slag with pure material or 1 wt pct as the standard state.
The constraint conditions are given subsequently. Equations [6] through [9] represent the matter conservation of oxygen, carbon, and iron in the system; the molar amount of each component is non-negative.
For calculating or modeling the variation of the gas–metal–slag phase during CO2 reacting with the Fe–C melt, a flow chart will be adopted in the current model, as shown in Figure 2.
In the beginning of the calculation, the volume of the injected gas and the volume ratio of the components are set at the current calculation step. Then, the mass of the two-phase (slag–metal) system is set, and the units of all the components in the gas–slag–metal system are converted into the corresponding molar amounts. Next, the initial value of the iterative calculation is set and is used in Eqs. [5] through [9]. The solution is obtained through achieving the minimum value of the nonlinear multivariate function with the constraint conditions. In this method, a barrier function is introduced in the original objective function to replace the inequality constraint. These problems are easier to solve than the original quality-constrained problem.[40] When the tolerance is less than 1e–6 or the maximum number of iteration steps reaches 10,000, the iterative calculation result is the system component composition within the current time-step. After removing the gas phase components, the molar weight of the slag in the two-phase system of slag–metal is taken as the initial condition for the t + 1th calculation. The preceding steps are repeated until the injection cutoff time is reached.
The standard molar Gibbs free energy and standard states of the system components are listed in Table III.[41] The standard state of the gas phase is a pure substance, that of carbon and oxygen in the metal phase corresponds to 1 wt pct Henrian activity, and that of iron in the metal phase and ferrous oxide in the slag phase is a pure liquid substance.
The activity calculation formula for carbon and oxygen in the melt[42,43] is shown in Eqs. [16] and [17]. The standard state of iron in the melt is a pure liquid substance, and the activity is the mass fraction of iron. Iron oxides in the slag phase coexist as ferrous and ferric oxides. The mass ratio of ferrous oxide to ferric oxide[44] is 0.9251:0.0749, and the activity of ferrous oxide is 0.9293. The model is also applicable for analyzing the injection of O2–CO2–CO–inert gas (N2/Ar) mixtures into the Fe–C melt.
where wi,pct is the mass percentage of i; fi is the Henrian activity coefficient of i; and T is the reaction temperature, K.
Calculating Results and Analysis
Reaction Process of CO2 Continuously Injected into the Fe–C Melt with Medium Carbon Content
To study the reaction phenomenon or process between the injected CO2 gas and Fe–C melt with medium carbon content, 2 wt pct was selected as the initial carbon content for the calculation. The injection time was 60 minutes, and the calculation step was 1 minute. The calculation boundary conditions are listed in Table IV.
Calculated with the model described in Section II, the variation of carbon and oxygen in the melt with time during blowing CO2 into the Fe–C melt with the initial carbon content of 2 wt pct is shown in Figure 3. The change of gas volume and the variation of the volume ratio of CO2 and CO in the gas phase with time are presented in Figures 4 and 5, respectively.
According to the calculation results shown in these figures, the reaction process can be divided into three stages. The first stage is from the beginning to the 34th minute, and the main feature is that the carbon content in the melt decreases continuously, whereas the oxygen content in the melt is maintained on the order of 10–3 wt pct. The second stage is from the 35th to the 40th minute; in this stage, the volume ratio of CO and the volume of the gas phase decrease rapidly, and the volume ratio of CO2 increases. Meanwhile these results show that the oxygen content in the melt increases rapidly. The third stage in from the 41st minute, where the system reaches the oxygen saturation state in the melt, which causes the volume and composition of the gas phase and the mass fraction of carbon and oxygen in the melt to remain unchanged. In this state, the gas phase volume is equal to the volume of CO2 injected into the system; the volume ratios of CO and CO2 are 85.75 and 14.25 vol pct, respectively; and the mass fractions of carbon and oxygen in the melt are 0.0115 and 0.1977 wt pct, respectively.
Figure 6 shows a schematic of the reaction process between the CO2 gas and Fe–C melt when the initial carbon content is 2 wt pct under thermodynamic equilibrium. The reasons leading to the phenomena shown in Figures 3, 4 and 5 are discussed in the following sections. When CO2 enters the melt, it firstly decomposes into CO and oxygen atoms. Under the condition of medium carbon content in the first stage, the oxygen content is extremely low (10–3 wt pct) when carbon and oxygen reach thermodynamic equilibrium with CO gas, and the oxygen content is almost unchangeable with time. Therefore, the oxygen atoms decomposed by CO2 mainly react with carbon in the melt to form CO gas, which is twice as much as the injection volume. At this stage, the CO2 gas facilitates the decarburization process and the overall reaction mechanism is CO2 + [C] = 2CO. In the second stage, with the continuous injection of CO2, the content of carbon in the melt decreases and the corresponding equilibrium oxygen concentration increases sharply (order of magnitude increases from 10–3 to 10–1 wt pct). Most of the oxygen atoms resulting from CO2 decomposition participate in the decarburization reaction, and a part of oxygen atoms dissolves in the melt, leading to the decrease of gas volume in the system.
In the third stage, that is, when the system reaches the oxygen saturation state, the oxygen partial pressure (\(p_{{{\text{O}}_{{2}} }}\)) is usually used to characterize the oxidation ability of the phase. One can compare the \(p_{{{\text{O}}_{{2}} }}\) of the gas and metal phases to explain the final equilibrium state of the CO2 injection process. The \(p_{{{\text{O}}_{{2}} }}\) of CO2–CO mixtures with different CO and CO2 proportions can be obtained by combining the isothermal equation of the chemical reaction (Eq. [22]), mass conservation equation (Eq. [23]), and calculation equation of partial pressure of the gas component (Eq. [24]). The \(p_{{{\text{O}}_{{2}} }}\) of the Fe–Osat–C melt (wO = 0.1977 wt pct) can be calculated as 4.75 × 10–9 atm with 0.5O2 = [O].
where \(n_{i}^{\ast}\) and \(p_{i}^{\ast}\) are the molar mass and partial pressure of i in thermodynamic equilibrium, respectively (mol/atm).
From Figure 7, it is seen that when the proportion of CO2 increases in the CO2–CO mixture, the corresponding \(p_{{{\text{O}}_{{2}} }}\) also increases and the \(p_{{{\text{O}}_{{2}} }}\) corresponding to the pure CO2 gas is 0.00348, which is much higher than that corresponding to oxygen saturation in the Fe–C melt (horizontal dash line in Figure 7). Therefore, in the third stage, as the reaction progresses, the pure CO2 gas continuously transfers oxygen atoms to the Fe–C melt, which will react with iron and exist as iron oxide in the system. When the proportion of CO2 in the CO2–CO mixture is 14.25 vol pct, the mixed gas is injected into the Fe–C melt with saturated oxygen content, the mixed gas no longer transfers oxygen to the melt, and the composition of each component in the system does not change. From the preceding analysis, we can infer that when the CO2–CO mixture is continuously injected into the Fe–C–O melt and when the proportion of CO2 in the gas mixture is more than 14.25 vol pct, the gas phase volume is equal to the volume of the CO2–CO mixture injected into the system under the equilibrium state. The volume ratio of CO in the gas phase is 85.75 vol pct, and that of CO2 maintains at 14.25 vol pct; the mass fractions of carbon and oxygen in the melt are 0.0115 and 0.1977 wt pct, respectively. When the proportion of CO2 in the CO2–CO mixture is less than 14.25 vol pct, the oxygen partial pressure corresponding to the melt is equal to the oxygen partial pressure corresponding to the CO2–CO mixture and the oxygen concentration in the melt will be lower than 0.1977 wt pct.
The preceding discussion shows that, when the carbon content in the metal phase is 0.05 wt pct, the volume of the gas phase is 1.67 times that of the initial injection gas volume during the CO2 gas continuously being injected into the melt and the volume of CO gas in the gas phase is 96.14 pct. During industrial production, the carbon content at the end of the smelting process is around 0.04 to 0.06 wt pct in the BOF and EAF. Therefore, it is appropriate to use CO2 gas as the bottom-blowing medium in the BOF and EAF because CO2 can react with the carbon in the melt to generate twice the volume of CO gas in most smelting processes, which can effectively enhance the stirring intensity of the molten pool.
Reaction Process of CO2 Continuously Injected into the Fe–C Melt with Extremely Low Carbon Content
The reaction process for CO2 gas injected into the Fe–C melt with extremely low carbon content was examined to fully understand the CO2 effect in the entire decarburization process, especially the change of components in the system. In this case, pure iron melt under ideal conditions was selected as the metal phase. The injection time was 60 minutes, and the calculation step was set as 1 minute. The boundary conditions used for the calculations are given in Table V, while the calculated results are shown in Figures 8 through 10.
As seen from Figures 8 through 10, the reaction process for CO2 injecting into the Fe–C melt with pure iron can be divided into three stages. The first stage is from the beginning to the 4th minute, which is characterized by the complete decomposition of CO2 in the melt, generating carbon and oxygen; in this case, the mass fractions of carbon and oxygen in the metal phase increase rapidly. The second stage is from the 5th to 28th minute. In the 5th minute, the gas phase volume is nearly twice and the carbon content in the melt reaches its maximum value. After the 5th minute, the volume of the gas phase and the volume fraction of CO in the gas phase gradually decrease, while the volume fraction of CO2 increases. Meanwhile, the carbon content decreases and the oxygen content continuously increases. From the 29th minute, the system reaches the oxygen saturation state in the melt, which is taken as the third stage.
Figure 11 shows the reaction mechanism or process of CO2 gas injected into the Fe–C melt when the initial metal phase is pure iron. The reasons leading to the phenomena shown in Figures 8 through 10 are explained subsequently. When CO2 enters the Fe–C melt, it first decomposes into CO and oxygen atoms and the a[O] × a[C] values in the pure iron melt are still considerably lower than those obtained in equilibrium with CO gas; thus, CO gas cannot be produced. Therefore, the overall reaction mechanism of CO2 gas reacting with elements in the melt can be represented as CO2 = [C] + 2[O]. In this case, the role of CO2 gas is to increase the amounts of oxygen and carbon. This situation is usually seen in the refining process. After the decarburization reaction in the primary refining furnace (BOF or EAF), the carbon content of molten steel at the end of smelting is relatively low. At the same time, owing to deoxidization and alloying during tapping, the dissolved oxygen content in the molten steel is extremely low. Therefore, if CO2 gas is injected into the molten steel during the refining process (LF, VD, or RH), it will be partially or completely dissolved in molten steel, thus adding oxygen and carbon to the molten steel, which reduces the effect of bottom blowing. Hence, using CO2 instead of Ar may not be suitable for the refining process, which needs to be verified by experiments.
With the rapid increase of carbon and oxygen content in the melt, a[O] × a[C] can be balanced with CO in the gas phase after 5 minutes. However, the oxygen partial pressure corresponding to the oxygen in the melt is still considerably lower than that corresponding to CO2 gas. Therefore, as the reaction progresses, CO2 gas continuously transfers oxygen atoms to molten steel. In this process, due to the continuous increase of oxygen content in molten steel, in order to maintain the reaction equilibrium between carbon and oxygen with CO gas, the carbon content will decrease accordingly. When the reaction lasted for 29 minutes, the oxygen content in the melt reached a saturation state.
Experiments and Discussion
To verify the accuracy of the model, experiments involving the injection of CO2 gas into the Fe–C melt with different initial carbon contents were carried out. In part of theoretical calculation, the Fe–C melt with 2 wt pct carbon content was selected to analyze the effect of CO2 on the reaction process. In this system, the CO2 gas mainly plays the role of decarburization for the medium carbon melt, which has been proved by previous research. Therefore, we chose the carbon content of 0.01 to 0.05 wt pct in the experimental scheme to mainly verify the carbon content in the equilibrium state when CO2 was continuously injected into the Fe–C alloy melt with low carbon content. In addition, there is no pure iron (the content of carbon and oxygen is 0), so two very low carbon contents of 0.004 and 0.006 wt pct were selected as experimental conditions to verify the recarburization behavior of CO2 gas in the Fe–C melt with extremely low carbon content. The experimental parameters are given in Table VI.
Experimental Apparatus and Process
The Fe–C alloy was prepared from electrolytic iron (1-s grade, produced by Toho Zinc Co., Ltd., Tokyo) and graphite powder (chemical pure, produced by Sinopharm Chemical Reagent Co., Ltd.). The composition of the industrial pure iron is given in Table VII.
The Fe–C alloy was prepared as follows.
-
(1)
600 g of electrolytic iron and graphite powder were weighed for preparing the alloy. The prepared Fe–C mixture was placed in an Al2O3 crucible with an outer diameter of 70 mm, an inner diameter of 50 mm, and a height of 195 mm. The crucible was placed in the constant-temperature zone of the tubular furnace. The experimental setup is shown in Figure 12.
-
(2)
High-purity argon was introduced into the corundum furnace tube from the bottom of the tube furnace and the temperature increased after 5 minutes of emptying.
-
(3)
During the heating and melting processes, the flow rate of argon at the bottom was maintained at 200 mL·min–1. After the temperature of the tubular furnace reached the reaction temperature, the temperature was maintained for 30 minutes so that the Fe and C elements were evenly mixed and the Fe–C alloy was formed.
CO2 injection into the Fe–C melt was performed as follows.
-
(1)
After the Fe–C alloy was prepared, the melt sample was extracted using the sampler as the initial sample.
-
(2)
A 6-mm corundum tube was used to connect the CO2 gas supply system, and the corundum tube was inserted into the bottom of the molten steel to blow CO2 for 5 minutes. After blowing for 5 minutes, the sample was collected with a quartz tube.
-
(3)
The sampling method was as follows: First, the quartz tube was inserted into the Fe–C melt (about 1 cm away from the bottom of the crucible); then, a pipette was used to extract a 6-mm-diameter rod with a length of approximately 5 to 8 cm.
The carbon content was determined using a carbon sulfur analyzer (EMIA-820V, Horiba, Kyoto), and the oxygen content in the sample was determined using an ONH instrument (TCH-600, LECOFootnote 1).
Experimental Results and Discussion
The changes of carbon content with time during CO2 injection into the Fe–C melt with different initial carbon contents were analyzed, and the results are shown in Figure 13. As seen in the figure, when the initial carbon content was greater than 0.01 wt pct, the carbon content decreased with the continuous blowing of CO2 gas into the melt and finally stabilized at approximately 0.011 wt pct. When the initial carbon content was 0.01 wt pct, the carbon content in the melt fluctuated from 0.01 to 0.011 wt pct after CO2 injection. This experimental result agrees with the theoretically calculated carbon content of the metal under final equilibrium state after injecting CO2 into the Fe–C melt with medium carbon content.
When the initial carbon contents were 0.004 and 0.006 wt pct, the carbon content increased linearly with time and finally stabilized to 0.011 wt pct. The calculation results prove that CO2 gas injection increases the carbon content into the Fe–C melt when the initial carbon content is lower than the final equilibrium carbon content.
To further study the variation of the melt composition when CO2 gas is injected into the Fe–C melt with an extremely low carbon content and determine the maximum carbon content in molten steel during theoretical calculations, another group of experiments was carried out. In these experiments, the injection intensity was reduced, that is to say, the liquid mass was increased, and the CO2 injection flow rate was reduced. The liquid mass was set at 1000 g, and the CO2 gas injection flow rate was set at 50 mL min–1. When the injection intensity was 0.05 mL min–1 g–1, the initial carbon and oxygen contents in the Fe–C melt were 0.0072 and 0.0270 wt pct, respectively. Argon is introduced into the bottom of the tubular furnace as a shielding gas, and the flow rate is 950 mL·min–1.
The variations of the carbon and oxygen contents in the melt with time are shown in Figures 14 and 15, respectively. It is seen from Figure 14 that the carbon content increases with time during the experiments and reaches the extreme peak in the 15th minute; then, it decreases to the final equilibrium value (0.011 wt pct). However, the peak value is less than the results obtained from the theoretical calculation, and the time to reach the maximum value is later than that of the theoretical calculation, which may be because the oxygen is the main surface-active element in the iron-based melt. The high oxygen concentration in the melt hinders the decomposition reactions of CO2 and CO in the Fe–C melt with an extremely low carbon content, resulting in the gas not reaching the thermodynamic equilibrium state during the bubble rising process of the molten pool. The kinetics of the process requires further study. Meanwhile, the oxygen content in the melt increased with time and reached the saturation concentration (0.2127 wt pct) in 75 minutes, which was slightly higher than the theoretical calculation results, as can be seen in Figure 15.
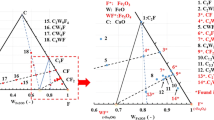
In order to more intuitively observe the change of carbon and oxygen activity when CO2 gas was continuously injected into the Fe–C melt under the condition of extremely low carbon content, Figure 16 is drawn and the change track with time (0 to 90 minutes) follows the red arrow in the figure. As seen in the figure, at 0 and 15 minutes, the carbon and oxygen activity products are all lower than the equilibrium line. After 30 minutes, the carbon and oxygen activity product of liquid steel is higher than the equilibrium line. Then, the carbon activity decreases and the carbon and oxygen activity product moves to the oxygen saturation point. Therefore, it can be concluded that, when CO2 gas is continuously injected into the Fe–C melt, whose initial carbon and oxygen activity point is above the equilibrium line, the carbon in the melt can be removed by CO2 gas, while, if the initial activity point of carbon and oxygen is below the equilibrium line, CO2 gas increases carbon and oxygen content into the melt. Finally, the Fe–C melt reaches the equilibrium point, that is, the oxygen element is saturated in the Fe–C melt.
Conclusions
Based on the thermodynamic calculation and experiments in the laboratory, the final reaction equilibrium state and process were studied by injecting CO2 into the Fe–C melt under different initial carbon contents. The following conclusions were obtained.[n12]
-
1.
At 1873 K, the oxygen partial pressure corresponding to CO2 gas is 0.0035 atm, which is much higher than that corresponding to the oxygen saturation in the Fe–C melt. Therefore, when CO2 is continuously injected into the Fe–C melt, the final equilibrium state of the system is such that the oxygen content reaches saturation (0.1977 wt pct) and the carbon content is 0.0115 wt pct. In the final equilibrium state, 85.75 vol pct of the injected CO2 gas is decomposed into CO gas and oxygen atoms, and the oxygen and iron atoms react to form iron oxide in the system.
-
2.
It is generally believed that CO2 plays a role in decarburization when it reacts with the Fe–C alloy. However, when the initial a[O] × a[C] in the melt is less than those in equilibrium with 0.8575 atm CO in 1873 K, the role of CO2 gas is to add carbon and oxygen to the melt until the system reaches equilibrium. Further injection of CO2 gas may lead to oxygen saturation in the melt.
-
3.
By comparing the theoretical calculation with the experimental data, it is verified that the final equilibrium state is carbon content 0.011 wt pct and oxygen concentration 0.2 wt pct when CO2 is continuously injected into the Fe–C melt at 1873 K. When the initial carbon and oxygen contents were 0.0072 and 0.0270 wt pct, the peak value of carbon content was less than the theoretical value and the time to reach the peak was behind the theoretical calculation in the process of continuously injecting CO2 into Fe–C melt. The reason for this requires further study.
-
4.
The industrial test results of the 300t converter bottom blowing different gas media show that the end carbon and oxygen product of the entire process of bottom blowing CO2 is higher than that of the entire process of bottom blowing inert gas. The end carbon and oxygen product of the bottom blowing CO2–Ar is 15.33 × 10–4, similar to the bottom blowing inert gas in the entire process.
Notes
LECO is a trademark of LECO Corporation, St. Joseph, MI.
References
C. Yi, R. Zhu, B.Y. Chen, C.R. Wang, and J.X. Ke: ISIJ Int., 2009, vol. 49, pp. 1694–99.
M. Lv, R. Zhu, X. Wei, H. Wang, and X. Bi: Steel Res. Int., 2012, vol. 83, pp. 11–15.
P. Blostein, D. Gortan, and K. Stephens: in 73rd Steelmaking Conf. Proc., Detroit, 1990, pp. 315–18.
X.L. Wang, R. Zhu, B.L. Zhang, J.M. Bian, Y.Q. Zhu, and W.H. Wu: in 2017 Mater. Sci. Technol. Conf., Chengdu, 2017, pp. 712–18.
M. Lv, R. Zhu, and L. Yang: Steel Res. Int., 2019, vol. 90, p. 1800454.
W. Du, Y. Wang, and X.P. Liang: JOM., 2017, vol. 69, pp. 1785–89.
W.T. Du, Q. Jiang, Z. Chen, X.P. Liang, and Y. Wang: JOM., 2019, vol. 71, pp. 4925–30.
G. Wei, R. Zhu, K. Dong, Z. Li, L. Yang, and X. Wu: Ironmak. Steelmak., 2018, vol. 45, pp. 839–46.
T. Bruce, F. Weisang, M. Allibert, and R. Fruehan: in 7th Proc, Electric Furnace Conf, Chicago, IL, 1987, pp. 293–97.
Y. Gu, H. Wang, R. Zhu, J. Wang, M. Lv, and H. Wang: Steel Res. Int., 2014, vol. 85, pp. 589–98.
B. Han, R. Zhu, Y. Zhu, R. Liu, W. Wu, Q. Li, and G. Wei: Metall. Mater. Trans. B., 2018, vol. 49B, pp. 3544–51.
B. Han, R. Zhu, C. Feng, W. Li, G. Wei, Z. Wang, and Q. Li: Vacuum, 2020, vol. 179, p. 109520.
B. Han, R. Zhu, G. Wei, C. Feng, and J. Dong: in Energy Technology 2020. Cham, 2020, vol. 2020, pp. 99–110.
R. Wang, Z. Yuan, S. Xie, J. Li, N. Hai, and J. Zhai: J. Iron Steel Res., 2018, vol. 30, pp. 874–80. (in Chinese).
P.A. Distin: Ph.D. Thesis, University of London, London, 1967.
Y. Niiri, K. Ito, and K. Sano: Tetsu-to-Hagané., 1969, vol. 55, pp. 437–45.
S. Anezaki, K. Shimizu, and T. Mori: Tetsu-to-Hagané, 1971, vol. 57, pp. 1109–22.
H. Nomura and K. Mori: Tetsu-to-Hagané, 1972, vol. 58, pp. 1603–11.
H. Nomura, K. Mori, and Y. Shirota: Tetsu-to-Hagané, 1974, vol. 60, pp. 361–71.
R.J. Fruehan and L.J. Martonik: Metall. Trans., 1974, vol. 5, pp. 1027–32.
N.H. El-kaddah and D.G.C. Robertson: Metall. Trans. B., 1978, vol. 9B, pp. 191–99.
H.G. Lee and Y.K. Rao: Metall. Trans. B., 1982, vol. 13B, pp. 403–09.
H.G. Lee and Y.K. Rao: Metall. Trans. B., 1982, vol. 13B, pp. 411–21.
F.J. Mannion and R.J. Fruehan: Metall. Mater. Trans. B., 1989, vol. 20B, pp. 853–61.
T. Nagasaka and R.J. Fruehan: ISIJ Int., 1994, vol. 34, pp. 241–46.
N. Simento, H.G. Lee, and P. Hayes: Steel Res. Int., 1998, vol. 69, pp. 318–24.
N.J. Simento, H.-G. Lee, and P.C. Hayes: ISIJ Int., 1999, vol. 39, pp. 1217–23.
H. Ono-Nakazato, Y. Morita, K. Tamura, T. Usui, and K. Marukawa: ISIJ Int., 2001, vol. 41, pp. S61–65.
K. Taguchi, H. Ono-Nakazato, T. Usui, and K. Marukawa: Metall. Mater. Trans. B., 2003, vol. 34B, pp. 861–67.
H.D. Zughbi: Scand. J. Metall., 2003, vol. 32, pp. 194–202.
H.D. Zughbi: Scand. J. Metall., 2004, vol. 33, pp. 242–50.
S.H. Jung: Master’s Thesis, Pohang University of Science and Technology, Pohang, 2010, pp. 68–70.
H. Wang: Ph.D. Thesis, Royal Institute of Technology, Stockholm, 2010, pp. 5–36.
C.P.P. Wu: Ph.D. Thesis, University of Toronto, Toronto, 2015.
Y. Fan, X. Hu, R. Zhu, and K. Chou: ISIJ Int., 2020, vol. 60, pp. 848–55.
Y. Fan, X. Hu, R. Zhu, and K. Chou: Steel Res. Int., 2020, vol. 91, p. 2000127.
I.-H. Jung, S.A. Decterov, and A.D. Pelton: Metall. Mater. Trans. B., 2004, vol. 35B, pp. 493–507.
R. Sarkar, P. Gupta, S. Basu, and N.B. Ballal: Metall. Mater. Trans. B., 2015, vol. 46B, pp. 961–76.
A. Kruskopf and V.-V. Visuri: Metall. Mater. Trans. B., 2017, vol. 48B, pp. 3281–300.
R.H. Byrd, M.E. Hribar, and J. Nocedal: SIAM J. Optim., 1999, vol. 9, pp. 877–900.
X.H. Huang: Principles of Ironmaking and Steelmaking, Metallurgical Industry Press, Beijing, 2013, p. 631.
Y. Lytvynyuk: Ph.D. Thesis, Montan University of Leoben, Leoben, 2013, p. 40.
G.K. Sigworth and J.F. Elliott: Met. Sci., 1974, vol. 8, pp. 298–310.
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin. J. Sangster, P. Spencer, and M.-A. Van Ende: FactSage Thermochemical Software and Databases—2010–2016. Calphad, 2016, vol. 54, pp. 35–53, http://www.factsage.com.
Acknowledgments
The authors acknowledge the support from the National Nature Science Foundation of China (Grant Nos. 51674021 and 52004023) and the Major Science and Technology Innovation Project of Shandong Province of China (Grant No. 2019JZZY010358).
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, W., Zhu, R., Wei, G. et al. Study on Final Equilibrium State and Process of CO2 Reacting with Fe–C Melt. Metall Mater Trans B 53, 1396–1410 (2022). https://doi.org/10.1007/s11663-022-02424-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-022-02424-6