Abstract
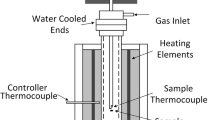
A CaO-Fe2O3 system is a fundamental binary system for the iron ore sintering process. Although the basic reactions have been investigated since the 1960s, melting and solidification caused by the combustion of coke results in an unstable state owing to extreme temperature variations. In this study, using a hot thermocouple method, samples of 10 pct CaO-90 pct Fe2O3 and 20 pct CaO-80 pct Fe2O3 were melted on a thermocouple and quenched with several techniques. The obtained samples were precisely examined by XRD. It was found that the sample containing 10 pct CaO-90 pct Fe2O3 changed to 10 pct CaO-13 pct FeO-77 pct Fe2O3 under an oxygen partial pressure (\( P_{{{\text{O}}_{2} }} \)) of 0.21 during melting. For the 10 pct CaO sample, the crystal phases found at a low cooling rate (509 K/s) were WFss, C4WF8 (C: CaO, W: FeO, F: Fe2O3), and C2W4F9. When the sample composition was 20 pct CaO, the precipitated crystal phases were C4WF4, C4F7, and C4WF8. On the other hand, the crystal phases for high cooling rates (1590 and 7900 K/s) with 10 pct CaO were WFss (solid solution of WF and F), F, and C2W4F9. The formation of the equilibrium phases WFss, F, C4WF4, and C4WF8 can be understood by examining the isothermal section of the phase diagrams, while the unstable phases C2W4F9 and C4F7 are discussed on the basis of the reactions under an equilibrium state.

















Similar content being viewed by others
References
K. Sanbongi, Y. Omori, K. Toita and M. Asada, Tetsu-to-Hagane, 1964, vol. 50, pp. 1574–1577.
M. Asada, Y. Omori and K. Sanbongi, Tetsu-to-Hagane, 1968, vol. 54, pp. 14-19.
K. Kojima, K. Nagano, T. Inazumi, K. Takagi and K. Shinada, Tetsu-to-Hagane, 1969, vol. 55, pp. 669–681.
R. Gerardin, E. Millon, A. Bonazebi, J. F. Brice, F. Jeannot and O. Evrard, J. Phys. Chem. Solids, 1988, vol. 49, pp. 343–348.
B. Bergman and C. Song, J. Am. Ceram. Soc., 1989, vol. 72, pp. 1364–1367.
M. Hillert, M. Selleby and B. Sundman, Metall. Trans. A, 1990, vol. 21A, pp. 2759–2776.
H. Chessin and E. T. Turkdogan, J. Am. Ceram. Soc., 1962, vol. 45, pp. 597–599.
P.B. Braun and W. Kywestroo, Philips. Res. Rep., 1960, vol. 1, pp. 394–397.
B. Phillips and A. Muan, Trans. Metall. Soc. AIME, 1960, vol. 218, pp. 1112–1118.
B. Phillips and A. Muan, J. Am. Ceram. Soc., 1958, vol. 41, pp. 445–454.
S.B. Holmquist, Nature, 1960, vol. 185, p. 640.
Y. Kashiwaya, C. E. Cicutti and A. W. Cramb, ISIJ Int., 1998, vol. 38, pp. 357–365.
Y. Kashiwaya, C. E. Cicutti, A. W. Cramb and K. Ishii, ISIJ Int., 1998, vol. 38, pp. 348–356.
P. Kahn Son and Y. Kashiwaya, ISIJ Int., 2008, vol. 48, pp. 1165–1174.
J. Yang, Y. Cui, L. Wang, Y. Sasaki, J. Zhang, O. Ostrovski and Y. Kashiwaya, Steel Res. Int., 2015, doi:10.1002/srin.201400346.
J. Yang, Y. Sasaki, O. Ostrovski, C. Zhang, D. Cai, Y. Kashiwaya and J. Zhang, ISIJ Int., 2016, vol. 56, pp. 574–583. doi:10.2355/isijinternational.ISIJINT-2015-583.
J. Yang, J. Zhang, Y. Sasaki, O. Ostrovski, C. Zhang, D. Cai and Y. Kashiwaya (2016) Metall. Mater. Trans. B. doi:10.1007/s11663-016-0715-9.
Y. Kashiwaya, T. Nakauchi, P. Khanh Son, S. Akiyama and K. Ishii, ISIJ Int., 2007, vol. 47, pp. 44–52.
A. Arakcheeva, O. Karpinski, Sov. Phys – Crystallogr. (Eng. Trans.), 1990, vol. 35, pp. 650–652.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted April 5, 2017.
Rights and permissions
About this article
Cite this article
Kashiwaya, Y. Crystal Phases Formed in a CaO-Fe2O3 System Under a High Cooling Rate in Air. Metall Mater Trans B 48, 3228–3238 (2017). https://doi.org/10.1007/s11663-017-1094-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-017-1094-6