Abstract
Osteoporosis is a disease that affects the quality of life of elderly people. The balance between bone formation mediated by osteoblasts and bone resorption by osteoclasts is important to maintain the normal bone condition. Therefore, the promotion of osteoblast differentiation and the suppression of osteoclastogenesis are effective strategies for osteoporosis treatment. Marine organisms are a promising source of biologically active and structurally diverse secondary metabolites, and have been providing drug leads for the treatment of numerous diseases. We describe the marine-derived secondary metabolites that can inhibit receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis and promote osteoblast differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the European Union, the International Osteoporosis Foundation reported that 22 million women and 5.5 million men aged 50–84 had osteoporosis in 2010 [1]. In the United States, 53.6 million women and men over 50 years old had osteoporosis in 2010, which is considered to be a major public health threat [2]. In Japan, a nationwide survey of hip fractures, the most prominent sign of osteoporosis, is conducted every 5 years. This survey revealed that the total number of hip fractures was 193,400, consisting of 44,100 men and 149,300 women in 2017 [3]. Although the mortality rate is lower than that of other diseases, such as cancer and cardiovascular diseases, osteoporosis affects the quality of life of elderly people [4].
Several approaches have been adopted for the treatment of osteoporosis. Hormonal replacement therapy with estrogen has been applied for the prevention and treatment of osteoporosis [5]. However, risks of breast and endometrial cancers are increased with the prolonged use of estrogen [6]. Calcitonin is used for the suppression of bone resorption, but it has side effects, including flushing, nausea, and diarrhea [7]. Bisphosphonates are common for osteoporosis [8], but recently they were reported to lead to pathological conditions such as osteonecrosis of the jaw, atrial fibrillation, excessive suppression of bone turnover, hypocalcemia, and acute inflammatory response.
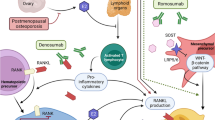
The balance between bone formation by osteoblasts and bone resorption by osteoclasts is important to maintain the normal bone condition. Therefore, the suppression of bone resorption by osteoclasts is an effective strategy for the treatment of osteoporosis in addition to the promotion of osteoblast differentiation. The monocyte/macrophage lineage differentiates into osteoclasts by stimulation with receptor activator of nuclear factor-κB ligand (RANKL) (Fig. 1). On the other hand, osteoblasts produce a decoy receptor, osteoprotegerin (OPG), that binds to excess RANKL [9].
RANKL stimuli are known to activate several downstream signaling pathways such as nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs). The activation of these pathways upregulates the expression of osteoclast-specific genes, including those encoding tartrate-resistant acid phosphatase (TRAP) and other enzymes involved in cell fusion and acidification of the subcellular space. These processes lead to the degradation of bone matrix proteins and dissolution of bone minerals [10]. Therefore, the compounds that affect osteoclastogenesis have attracted much attention for the treatment of osteoclast-related diseases [11, 12].
Marine organisms exhibit wide diversity such as plants, microorganisms, and animals including sponges, cnidarians, bryozoans, mollusks, tunicates, and echinoderms. For decades, these marine invertebrates have been considered to be a potential source of unique drug leads with diverse structures that are unobtainable by combinatorial syntheses. Marine secondary metabolites have fascinating biological activities and have been used for numerous biological functions such as repelling predators, protection against infection, and communication among individuals of inter/intraspecies. This suggests that marine organisms have survived using their metabolites, thereby constructing the ecological system [13,14,15]. The marine environment varies in depth, current, temperature, light, and nutritional status, and produces extraordinary phenomena, such as hydrothermal deposit and effusion of poisonous gas, which may influence marine organisms to produce secondary metabolites with different structures and biological activities. Many reports revealed their high potential for medicinal, agrochemical, nutraceutical, and cosmetic uses [16]. We review the marine natural products that inhibit osteoclastogenesis and induce osteoblast differentiation for use as interventions to improve osteoporosis.
Marine natural products that inhibit osteoclastogenesis
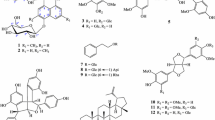
Biselyngbyaside is an 18-membered macrolide glycoside, first isolated from the marine cyanobacterium Lyngbya sp. collected from the reef of Bise, Okinawa, Japan, as a cytotoxic compound against a panel of human tumor cell lines (Fig. 2) [17]. Afterward, biselyngbyaside was demonstrated to inhibit RANKL-induced osteoclastogenesis in murine macrophages, RAW264 cells, and primary bone marrow-derived macrophages. It inhibited the activity of TRAP, which is an osteoclast-specific enzyme, in a dose-dependent manner with an IC50 value of 6 nM without cytotoxicity to RAW264 macrophages. Biselyngbyaside inhibited RANKL-induced osteoclast formation at 30 nM. In addition, it inhibited osteoblast-mediated osteoclast formation in co-cultures of osteoclasts with osteoblastic cells (UAMS-32) [18].
Largazole, a 16-membered cyclic depsipeptide, was isolated from a marine cyanobacterium Symploca sp. collected from Key Largo, Florida Keys, as an antiproliferative agent (Fig. 2) [19]. The same authors synthesized largazole, indicated to be a class I HDAC inhibitor [20], and reported antitumor activity in a xenograft mouse model [21]. Furthermore, it exhibited both in vitro and in vivo osteogenic activity mediated through the increased expression of Runx2 (a Runt protein) and bone morphogenetic proteins (BMPs) [22].
Irijimasides A–E, 14-membered macrolide glycosides, were isolated from a marine cyanobacterium Okeania sp. collected from Irijima, Okinawa, Japan (Fig. 2). These compounds dose-dependently inhibited RANKL-induced TRAP activity of RAW264 macrophage cells without significant cytotoxicity [23].
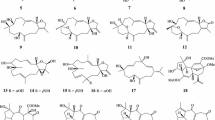
Macrolactins A–F, 24-membered ring lactones and related β-glucopyranosides, were isolated from an unidentified deep-sea marine bacterium collected in the North Pacific (Fig. 3). Macrolactin A exhibited antibacterial, antiviral, and cytotoxic activities [24]. Macrolactin F inhibited RANKL-induced osteoclastogenesis in primary bone marrow-derived macrophages (BMMs) by suppressing Akt, MAPK, and nuclear factor of activated T cells c1 (NFATc1) pathways. Moreover, the compound promoted osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway [25].
Mycoepoxydiene, an epoxycyclooctadiene-δ-1actone, was obtained from the solid-state fermentation of the fungus (OS-F66617) isolated from twig litter collected from a deciduous alluvial forest near Curitiba, Brazil (Fig. 4) [26]. Thereafter, mycoepoxydiene was reported to inhibit RANKL-induced osteoclast differentiation and to prevent bone loss in ovariectomized mice [27].
Stachybotrysin A and stachybotrylactone B, phenylspirodrimane derivatives, were isolated from the liquid cultures of the marine-derived fungus Stachybotrys sp. KCB13F013 collected from the sediment of Wi-island, South Korea (Fig. 4). Stachybotrysin A inhibited osteoclast differentiation in BMMs by suppressing RANKL-induced expression of the osteoclast-specific genes p-ERK, p-JNK, p-p38, c-Fos, and NFATc1 [28].
Insulicolide A is a benzoyl sesquiterpenoid containing a nitro group isolated from the marine fungus Aspergillus insulicola (Fig. 4) [29]. Insulicolide B and its derivatives were successively isolated from the marine-derived fungus A. ochraceus Jcma1F17 and demonstrated cytotoxicity [30]. The effects of the nitrobenzoyl sesquiterpenoids on osteoclastogenesis were examined, and 6β,9α-dihydroxy-14-p-nitrobenzoylcinnamolide exhibited the most potent suppression of RANKL-induced osteoclastogenesis and bone resorption at 0.5 µM [31].
Notoamides A–D are prenylated indole alkaloids isolated from the marine-derived fungus Aspergillus protuberus MF297-2 along with a known compound (+)-stephacidin A (Fig. 4) [32]. Later, two antipodes, (–)-stephacidin A and (+)-notoamide B, were isolated from the terrestrial fungi A. amoenus NRRL 35,600 [33]. Biological activities were assessed with natural and synthetic analogues, and a series of the (–)-enantiomers of notoamides A and B, 6-epi-notoamide T, and stephacidin A inhibited RANKL-induced osteoclastogenesis more strongly than their respective (+)-enantiomers [34]. Among the tested compounds, a synthetic compound (–)-6-epi-notoamide T was the most potent, with an IC50 value of 1.7 µM.
Meroterpenoids, austalides V − X, were isolated from the culture of the marine-derived fungus Penicillium rudallense as inhibitors of osteoclast differentiation (Fig. 4) [35]. Austalide V was the most potent inhibitor of RANKL-induced osteoclast differentiation, with an IC50 value of 1.9 μM.
Symbioimine is a unique amphoteric sulfated iminium compound isolated from the symbiotic marine dinoflagellate Symbiodinium sp. The compound inhibited RANKL-induced osteoclastogenesis in RAW264 cells with an IC50 value of 44 µg/mL without cytotoxicity (Fig. 5) [36].
Fucoxanthin is an oxygenated carotenoid contained in edible brown algae such as Undaria pinnatifida (wakame), Laminaria japonica (kombu), and Eisenia bicyclis (arame) (Fig. 6). Several health benefits of fucoxanthin have been reported such as suppression of adipocyte differentiation, antimutagenicity, and antiproliferative activity against cancers. Fucoxanthin inhibited RANKL-induced osteoclastogenesis in RAW264.7 macrophages at a concentration of 2.5 µM without cytotoxicity [37].
Sargachromanol G was first isolated from the brown alga Sargassum siliquastrum as antioxidant compounds with 15 new meroterpenoid congeners (Fig. 6) [38]. Later, the inhibitory effects of sargachromanol G on osteoclast formation from RANKL-treated RAW264.7 cells were reported, accompanied by suppression of the expression of osteoclast-specific markers such as TRAP, cathepsin K (CTSK), matrix metalloproteinase 9 (MMP9), and calcitonin receptor (CTR). Further examination of the mechanism of action revealed that sargachromanol G inhibited RANKL-induced activation of NF-κB by suppressing IκB-α degradation and inhibition of RANKL-induced phosphorylation of MAPKs (p38, JNK, and ERK) [39].
Agelasines were isolated from the marine sponge Agelas nakamurai Hoshino collected in Okinawa (Fig. 7) [40,41,42]. They were identified as monocyclic and bicyclic diterpenes with a 9-methyladeninium, and exhibited inhibitory effects against Na,K-ATPase. Later, agelasine D was reported to inhibit RANKL-induced osteoclastogenesis in BMMs through inhibiting the expression of osteoclastic markers, TRAP, cathepsin K, and MMP9. Furthermore, it suppressed RANKL-induced mRNA expression of dendritic cell-specific transmembrane protein (DC-STAMP) and osteoclast-stimulatory transmembrane protein (OC-STAMP). Moreover, RANKL-induced expression and protein production of both c-FOS and NFATc1 were downregulated by agelasine D [43].
Placotylenes A and B were isolated from a sponge Placospongia sp. collected in the South Sea, Korea and identified as unique iodinated linear polyacetylenes (Fig. 7). Placotylene A inhibited RANKL-induced osteoclastogenesis in BMMs at 10 µM, whereas its regioisomer placotylene B did not up to 100 µM. This inhibition was accompanied by the suppression of transcriptional and translational expression of NFATc1 [44].
During screening of osteoclastogenesis inhibitors from the extracts of marine sponges and marine-derived fungi, halenaquinone was isolated from the extract of the marine sponge Petrosia alfiani collected in Indonesia (Fig. 7). Halenaquinone inhibited RANKL-induced upregulation of TRAP activity of RAW264 cells, with an IC50 value of 2 µM. Studies on the inhibitory mechanism suggested that halenaquinone suppresses the NF-κB and Akt signaling pathways [45].
Ceylonamides A–F, nitrogenous spongian diterpenes, were isolated from the marine sponge Spongia ceylonensis collected in North Sulawesi, Indonesia (Fig. 7). Among these, ceylonamides A and B inhibited TRAP activity in RANKL-induced RAW264 cells, with an IC50 value of 13 and 18 μM, respectively, and formation of TRAP-positive multinuclear osteoclasts without cytotoxicity. Structure–activity relationship (SAR) studies revealed that the compounds having the amide carbonyl at C-16 inhibited more potently than those with the amide carbonyl at C-15. In addition, the presence of a bulkier substituent at the amide nitrogen resulted in more potent inhibitory activity [46]. Subsequently, six modified spongian diterpenes, ceylonins A–F, were isolated from another collection of the same sponge (Fig. 7). They contained an ether-bridged bicyclic ring system, which may be derived from spongia-13(16),14-dien-19-oic acid, a major metabolite of this sponge, and a C3 unit through intermolecular Diels–Alder reaction. Ceylonin A inhibited the formation of TRAP-positive multinuclear osteoclasts in RAW264 cells in a dose-dependent manner without cytotoxicity [47].
Aaptamine, bearing a 1H-benzo[de]-1,6-naphthyridine scaffold, was isolated from the marine sponge Aaptos aaptos collected in Okinawa, Japan, as an α-adrenoceptor blocking agent (Fig. 7) [48]. After its isolation, more than thirty congeners have been isolated from sponges thus far with differing biological activities. Among these, aaptamine, demethyl(oxy)aaptamine, and isoaaptamine inhibited RANKL-induced multinuclear osteoclast formation at 5 µM [49].
Seven triterpenes were isolated from the marine sponge Siphonella siphonochalina collected in the Red Sea. Among them, the pentacyclic neviotane-type triterpenes, neviotines A and D, inhibited multinuclear osteoclast formation with an IC50 value of 32.8 and 12.8 µM, respectively, whereas the IC50 values of other sipholane- and siphonellane-type triterpenes were higher than 50 µM (Fig. 7) [50].
Hymenialdisine is a bromopyrrole alkaloid isolated from the sponges Axinella verrucosa in the Mediterranean and Acanthella aurantiaca in the Red Sea (Fig. 7) [51]. This compound was selected from the library of marine natural products by screening of RANKL-induced osteoclastogenesis activity. It inhibited RANKL-induced osteoclast formation, bone resorption activity, and osteoclast-related gene expression by blocking the NF-κB and MAPK signaling pathways and NFATc1 expression. Furthermore, hymenialdisine was suggested to induce osteoblast differentiation by activating alkaline phosphatase (ALP) and promoting osteoblast matrix mineralization. In addition, hymenialdisine prevented the decrease in bone volume and trabecular thickness in a female C57BL/6j mouse model of ovariectomy-induced systematic bone loss. Thus, hymenialdisine is a notable compound that both inhibits osteoclast-related osteolysis and promotes osteoblast-induced ossification, with in vivo efficacy [52].
Recently, a simple methylenedioxy dibromoindole alkaloid, amakusamine, was isolated from a marine sponge belonging to the genus Psammocinia collected in Amakusa, Kumamoto, Japan (Fig. 7). Amakusamine inhibited the formation of multinuclear osteoclasts in RANKL-stimulated RAW264 cells, with an IC50 value of 10.5 µM, via the suppression of Nfatc1 expression. A series of amakusamine analogues was synthesized to examine their SAR, revealing that replacement of a methylenedioxy group with two methoxy groups slightly promotes activity. Hydrogenation of the Δ2 double bond reduced the activity. A bromine at C-4 is essential and bromination at C-7 slightly promoted activity. Replacement of the bromines with chlorines significantly reduced activity. Evaluation of the potencies of N-acyl derivatives demonstrated that those with C2 − C8-alkyl chains were equipotent or slightly more potent, but those with a C14-alkyl chain and benzoyl derivative were inactive even at 50 µM [53].
Marine natural products that induce osteoblast differentiation
Although the therapeutic agents for osteoporosis are expected to be developed on the basis of compounds that suppress osteoclast differentiation or promote osteoblast differentiation, the number of osteoblast differentiation promotors isolated from natural sources is less than that of osteoclast differentiation inhibitors. In addition to hymenialdisine described above, the following compounds have been reported thus far.
Phorbasones A and B, sesterterpenes, were isolated from the marine sponge Phorbas sp. collected at Gageo Island, Korea. Phorbasone A induced calcium deposition in mesenchymal C3H10T1/2 cells and the most potent effect was observed at 0.5 μg/mL (Fig. 8). Phorbasone A increased gene expression of the osteoblast differentiation markers, Runx2, ALP, OSX (osterix), PTH (parathyroid hormone), and PTHrP (PTH-related peptide) [54]. Furthermore, the same group screened a library of marine natural products and found that phorboketal A, previously isolated from the marine sponge Phorbas sp. collected at Gageo Island [55], promoted osteoblast differentiation in a concentration-dependent manner via ERK activation (Fig. 8) [56].
Majusculamides A and B are C-19 epimeric lipodipeptides isolated as the major products of the marine cyanobacterium Lyngbya majuscula Gomont collected at Kahala Beach, Oahu, Hawaii (Fig. 8) [57]. The same compounds were reisolated from the cyanobacterium Moorea producens collected at Bise, Okinawa, Japan, and induced osteoblast differentiation in MC3T3-E1 cells [58]. As majusculamide A was more potent than majusculamide B, the authors synthesized the analogues to assess SAR, and found that the numbers of methyl groups, configuration at C-19, and the functional groups at C-20 affected the activity. However, the carbon chain length of fatty acids and types of amino acid residues slightly affected the level of mineralization.
Conclusion
More than half of the small molecules approved as drugs are either natural products or those derived from a natural product or based on a natural product pharmacophore [59]. Among natural products reported thus far, those discovered from marine environment comprise diverse chemical scaffolds accompanied by potent biological activities [16]. We reviewed marine natural products that inhibit osteoclastogenesis or promote osteoblast differentiation. Continuous effort may result in the discovery of drug leads for the treatment of osteoporosis.
References
Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 8(1):136–251
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526
Takusari E, Sakata K, Hashimoto T, Fukushima Y, Nakamura T, Orimo H (2021) Trends in hip fracture incidence in Japan: estimates based on nationwide hip fracture surveys from 1992 to 2017. JBMR Plus 5(2):e10428
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Mcgowan JA, Stefanick ML. (2008) Estrogen therapy: prevention and treatment of osteoporosis. In: Marcus R, Feldman D, Nelson D, Rosen C (eds) Osteoporosis, vol I. Academic Press, New York, pp 1687–1703
Cagnacci A, Venier M (2019) The controversial history of hormone replacement therapy. Medicina 55(9):602
Stevenson JC, Evans IM (1981) Pharmacology and therapeutic use of calcitonin. Drugs 21(4):257–272
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. In Mayo Clinic Proc (Elsevier) 83(9):1032–1045
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7(4):292–304
Kawatani M, Osada H (2009) Osteoclast-targeting small molecules for the treatment of neoplastic bone metastases. Cancer Sci 100(11):1999–2005
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287
Lei J, Zhou J (2002) A marine natural product database. J Chem Inf Comput Sci 42(3):742–748
Newman DJ, Cragg GM (2004) Marine natural products and related compounds in clinical and advanced preclinical trials. J Nat Prod 67(8):1216–1238
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75(3):311–335
Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR (2021) Marine natural products. Nat Prod Rep 38(2):362–413
Teruya T, Sasaki H, Kitamura K, Nakayama T, Suenaga K (2009) Biselyngbyaside, a macrolide glycoside from the marine cyanobacterium Lyngbya sp. Org Lett 11(11):2421–2424
Yonezawa T, Mase N, Sasaki H, Teruya T, Hasegawa SI, Cha BY, Yagasaki K, Suenaga K, Nagai K, Woo JT (2012) Biselyngbyaside, isolated from marine cyanobacteria, inhibits osteoclastogenesis and induces apoptosis in mature osteoclasts. J Cell Biochem 113(2):440–448
Taori K, Paul VJ, Luesch H (2008) Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J Am Chem Soc 130(6):1806–1807
Ying Y, Taori K, Kim H, Hong J, Luesch H (2008) Total synthesis and molecular target of largazole, a histone deacetylase inhibitor. J Am Chem Soc 130(26):8455–8459
Liu Y, Salvador LA, Byeon S, Ying Y, Kwan JC, Law BK, Hong J, Luesch H (2010) Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J Pharmacol Exp Ther 335(2):351–361
Lee SU, Kwak HB, Pi SH, You HK, Byeon SR, Ying Y, Luesch H, Hong J, Kim SH (2011) In vitro and in vivo osteogenic activity of largazole. ACS Med Chem Lett 2(3):248–251
Yamano A, Natsume N, Yamada M, Sumimoto S, Iwasaki A, Suenaga K, Teruya T (2020) Irijimasides A-E, macrolide glycosides from an Okeania sp. marine cyanobacterium. J Nat Prod 83(5):1585–1591
Gustafson K, Roman M, Fenical W (1989) The macrolactins, a novel class of antiviral and cytotoxic macrolides from a deep-sea marine bacterium. J Am Chem Soc 111(19):7519–7524
Li L, Sapkota M, Gao M, Choi H, Soh Y (2017) Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Eur J Pharmacol 815:202–209
Cai P, McPhail AT, Krainer E, Katz B, Pearce C, Boros C, Caceres B, Smith D, Houck DR (1999) Mycoepoxydiene represents a novel class of fungal metabolites. Tetrahedron Lett 40(8):1479–1482
Zhu J, Chen Q, Xia X, Mo P, Shen Y, Yu C (2013) Mycoepoxydiene suppresses RANKL-induced osteoclast differentiation and reduces ovariectomy-induced bone loss in mice. Appl Microbiol Biotechnol 97(2):767–774
Kim JW, Ko SK, Kim HM, Kim GH, Son S, Kim GS, Hwang GJ, Jeon ES, Shin KS, Ryoo IJ, Hong YS (2016) Stachybotrysin, an osteoclast differentiation inhibitor from the marine-derived fungus Stachybotrys sp. KCB13F013. J Nat Prod 79(10):2703–2708
Rahbæk L, Christophersen C, Frisvad J, Bengaard HS, Larsen S, Rassing BR (1997) Insulicolide A: a new nitrobenzoyloxy-substituted sesquiterpene from the marine fungus Aspergillus insulicola. J Nat Prod 60(8):811–813
Tan Y, Yang B, Lin X, Luo X, Pang X, Tang L, Liu Y, Li X, Zhou X (2018) Nitrobenzoyl sesquiterpenoids with cytotoxic activities from a marine-derived Aspergillus ochraceus fungus. J Nat Prod 81(1):92–97
Tan Y, Deng W, Zhang Y, Ke M, Zou B, Luo X, Su J, Wang Y, Xu J, Nandakumar KS, Liu Y (2020) A marine fungus-derived nitrobenzoyl sesquiterpenoid suppresses receptor activator of NF-κB ligand-induced osteoclastogenesis and inflammatory bone destruction. Br J Pharmacol 177(18):4242–4260
Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S (2007) Notoamides A-D: prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew Chem Int Ed 46(13):2254–2256
Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM (2008) Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (−)-stephacidin a and (+)-notoamide B from Aspergillus versicolor NRRL 35600. Angew Chem Int Ed 47(19):3573–3577
Kato H, Kai A, Kawabata T, Sunderhaus JD, McAfoos TJ, Finefield JM, Sugimoto Y, Williams RM, Tsukamoto S (2017) Enantioselective inhibitory abilities of enantiomers of notoamides against RANKL-induced formation of multinuclear osteoclasts. Bioorg Med Chem Let 27(22):4975–4978
Wang W, Lee J, Kim KJ, Sung Y, Park KH, Oh E, Park C, Son YJ, Kang H (2019) Austalides, osteoclast differentiation inhibitors from a marine-derived strain of the fungus Penicillium rudallense. J Nat Prod 82(11):3083–3088
Kita M, Kondo M, Koyama T, Yamada K, Matsumoto T, Lee KH, Woo JT, Uemura D (2004) Symbioimine exhibiting inhibitory effect of osteoclast differentiation, from the symbiotic marine dinoflagellate Symbiodinium sp. J Am Chem Soc 126(15):4794–4795
Das SK, Ren R, Hashimoto T, Kanazawa K (2010) Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264.7 cells. J Agric Food Chem 58(10):6090–6095
Jang KH, Lee BH, Choi BW, Lee HS, Shin J (2005) Chromenes from the Brown Alga Sargassum siliquastrum. J Nat Prod 68(5):716–723
Yoon WJ, Kim KN, Heo SJ, Han SC, Kim J, Ko YJ, Kang HK, Yoo ES (2013) Sargachromanol G inhibits osteoclastogenesis by suppressing the activation NF-κB and MAPKs in RANKL-induced RAW 264.7 cells. Biochem Biophys Res Commun 434(4):892–897
Nakamura H, Wu H, Ohizumi Y, Hirata Y (1984) Agelasine-A, -B, -C, and -D, novel bicyclic diterpenoids with a 9-methyadeninium unit possessing inhibitory effects on Na, K-ATPase from the Okinawan sea sponge Agelas sp. Tetrahedron Lett 25(28):2989–2992
Wu H, Nakamura H, Kobayashi J, Ohizumi Y, Hirata Y (1984) Agelasine-E and -F, novel monocyclic diterpenoids with a 9-methy adeninium unit possessing inhiboty effects on Na, K-ATPase isolated from the Okinawan sea sponge Agelas nakamurai Hoshino. Tetrahedron Lett 25(34):3719–3722
Wu H, Nakamura H, Kobayashi JI, Kobayashi M, Ohizumi Y, Hirata Y (1986) Structures of agelasines, diterpenes having a 9-methyladeninium chromophore isolated from the Okinawan marine sponge Agelas nakamurai Hoshino. Bull Chem Soc Jpn 59(8):2495–2504
Kang MR, Jo SA, Yoon YD, Park KH, Oh SJ, Yun J, Lee CW, Nam KH, Kim Y, Han SB, Yu J (2014) Agelasine D suppresses RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1 and NF-κB. Mar Drugs 12(11):5643–5656
Kim H, Kim KJ, Yeon JT, Kim SH, Won DH, Choi H, Nam SJ, Son YJ, Kang H (2014) Placotylene A, an inhibitor of the receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation, from a Korean sponge Placospongia sp. Mar Drugs 12(4):2054–2065
Tsukamoto S, Takeuchi T, Kawabata T, Kato H, Yamakuma M, Matsuo K, El-Desoky AH, Losung F, Mangindaan RE, de Voogd NJ, Arata Y (2014) Halenaquinone inhibits RANKL-induced osteoclastogenesis. Bioorg Med Chem Lett 24(22):5315–5317
El-Desoky AH, Kato H, Angkouw ED, Mangindaan RE, de Voogd NJ, Tsukamoto S (2016) Ceylonamides A-F, nitrogenous spongian diterpenes that inhibit RANKL-induced osteoclastogenesis, from the marine sponge Spongia ceylonensis. J Nat Prod 79(8):1922–1928
El-Desoky AH, Kato H, Kagiyama I, Hitora Y, Losung F, Mangindaan RE, de Voogd NJ, Tsukamoto S (2017) Ceylonins A-F, spongian diterpene derivatives that inhibit RANKL-induced formation of multinuclear osteoclasts, from the marine sponge Spongia ceylonensis. J Nat Prod 80(1):90–95
Nakamura H, Kobayashi JI, Ohizumi Y, Hirata Y (1982) Isolation and structure of aaptamine a novel heteroaromatic substance possessing α-blocking activity from the sea sponge Aaptos aaptos. Tetrahedron Lett 23(52):5555–5558
Tsukamoto S, Fukumoto A, Hitora Y (2018) Isolation of aaptic acid from the marine sponge Aaptos lobata and inhibitory effect of aaptamines on RANKL-induced formation of multinuclear osteoclasts. Heterocycles 97(2):1219–1225
El-Beih A, El-Desoky AH, Al-Hammady MA, Elshamy AI, Hegazy ME, Kato H, Tsukamoto S (2018) New inhibitors of RANKL-induced Osteoclastogenesis from the marine sponge Siphonochalina siphonella. Fitoterapia 128:43–49
Cimino G, De Rosa S, De Stefano S, Mazzarella L, Puliti R, Sodano G (1982) Isolation and X-ray crystal structure of a novel bromo-compound from two marine sponges. Tetrahedron Lett 23(7):767–768
Wang Q, Chen D, Jin H, Ye Z, Wang C, Chen K, Kuek V, Xu K, Qiu H, Chen P, Song D (2020) Hymenialdisine: a marine natural product that acts on both osteoblasts and osteoclasts and prevents estrogen-dependent bone loss in mice. J Bone Miner Res 35(8):1582–1596
Maeyama Y, Nakashima Y, Kato H, Hitora Y, Maki K, Inada N, Murakami S, Inazumi T, Ise Y, Sugimoto Y, Ishikawa H (2021) Amakusamine from a Psammocinia sp sponge: Isolation, synthesis, and SAR study on the inhibition of RANKL-induced formation of multinuclear osteoclasts. J Nat Prod 84(10):2738–2743
Rho JR, Hwang BS, Joung S, Byun MR, Hong JH, Lee HY (2011) Phorbasones A and B, sesterterpenoids isolated from the marine sponge Phorbas sp. and induction of osteoblast differentiation. Org Lett 13(5):884–887
Rho JR, Hwang BS, Sim CJ, Joung S, Lee HY, Kim HJ (2009) Phorbaketals A, B, and C, sesterterpenoids with a spiroketal of hydrobenzopyran moiety isolated from the marine sponge Phorbas sp. Org Lett 11(24):5590–5593
Byun MR, Kim AR, Hwang JH, Sung MK, Lee YK, Hwang BS, Rho JR, Hwang ES, Hong JH (2012) Phorbaketal A stimulates osteoblast differentiation through TAZ mediated Runx2 activation. FEBS Lett 586(8):1086–1092
Marner FJ, Moore RE, Hirotsu K, Clardy J (1977) Majusculamides A and B, two epimeric lipodipeptides from Lyngbya majuscula Gomont. J Org Chem 42(17):2815–2819
Natsume N, Ozaki K, Nakajima D, Yokoshima S, Teruya T (2020) Structure-activity relationship study of majusculamides A and B and their analogues on osteogenic activity. J Nat Prod 83(8):2477–2482
Newman DJ, Cragg GM (2020) Natural products as sources of new drug over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):777–803
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Desoky, A.H.H., Tsukamoto, S. Marine natural products that inhibit osteoclastogenesis and promote osteoblast differentiation. J Nat Med 76, 575–583 (2022). https://doi.org/10.1007/s11418-022-01622-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-022-01622-5