Abstract
Marine organisms possess bioactive potential with tremendous pharmaceutical promise. The purpose of the present study was to investigate the antioxidant (peroxyl radical-scavenging and reducing capacities) and antiosteoporotic activities of isolated constituents (1–26) from the soft corals Sinularia maxima and Lobophytum crassum on pre-osteoblastic MC3T3-E1 cells and pre-osteoclastic RAW 264.7 cells. Among the isolated compounds, 7, 9, 17, 18, and 20 (10.0 μM) exhibited significant peroxyl radical-scavenging capacity, with TE values ranging from 4.00 ± 0.14 to 9.06 ± 0.33 μM. In addition, compounds 1, 5, 7, 20, and 24 significantly stimulated the differentiation of pre-osteoblastic MC3T3-E1 cells by increasing collagen synthesis and/or mineralization functions of osteoblasts, while compounds 1, 5–8, 12, 14–22, 24, and 26 showed significant inhibition of TRAP in NF-κB ligand-induced osteoclastic RAW 264.7 cells, with values ranging from 99.11 ± 1.36 to 61.54 ± 1.61 %. These results indicate that the soft corals S. maxima and L. crassum are excellent sources among the antioxidant and antiosteoporotic marine invertebrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is caused by an imbalance between the generation of reactive oxygen species (ROS) and antioxidant defense. Severe oxidative stress is hypothesized to play a prominent role in many diseases, including osteoporosis, cancer, aging, and neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis. These effects are believed to be caused by damage to many important biological molecules, such as lipids, proteins, carbohydrates, and DNA (Rao and Rao, 2013; Young and Woodside, 2001). Natural antioxidants in food have also attracted interest due to their safety and potential nutritional and therapeutic effects (Kaur and Kapoor, 2001).
Osteoporosis, one of the most frequently encountered degenerative diseases in aging populations, is characterized by a decrease in bone mass and density, causing bones to become fragile and prone to fracture (Simon, 2007). Bone development in vertebrate animals is maintained by the coordinated actions of osteoblasts (bone formation) and osteoclasts (bone resorption). Disease occurs when there is an imbalance between bone resorption and bone formation during the bone remodeling process. Inadequate formation of new bone or excessive bone resorption is often association with osteoporosis (McCormick, 2007; Soni et al., 2013).
Free radicals also play an important role in bone remodeling by promoting differentiation and bone resorptive activity in osteoclasts. However, high levels of free radical may lead to oxidative stress and must be converted to less reactive forms by antioxidant enzymes (Ellis et al., 1997). Osteoporotic subjects exhibit low antioxidant levels (Maggio et al., 2003) and high ROS levels, suggesting that they are under increased oxidative stress (Nazrun et al., 2011; Sontakke and Tare, 2002).
Soft corals are widely distributed but have a marked preference for tropical waters of depths between 5 and 30 m relative to temperate reefs. The genera of Sinularia and Lobophytum (Phylum: Coelenterata, Family: Alcyoniidae, Class: Anthozoa, Order: Alcyonacea) are two of the most widely distributed of their genera, constituting a dominant portion of the biomass in tropical reef environments (Anjaneyulu and Rao, 1997; Lakshmi and Kumar, 2009). Previous studies have indicated that diterpenes, a main constituent of the genus Sinularia and Lobophytum (most are derived from the 14-membered cembrane nucleus), exhibit various biological activities, such as anti-inflammatory (Chao et al., 2011; Cheng et al., 2010; Lu et al., 2010; Su and Wen, 2011), antiviral (Cheng et al., 2010), and cytotoxic (Grote et al., 2008; Kamel et al., 2007; Lo et al., 2009; Su et al., 2009) effects.
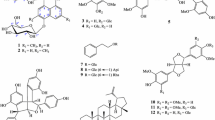
In the search for active components from Vietnamese marine organisms, soft corals S. maxima and L. crassum have proved to harbor antiosteoporotic agents. Herein, we report various diterpenoids that may contribute to biological activities in bone disease therapies. For biological evaluation of these isolates, we utilized peroxyl radical-scavenging and reducing assays and assessed their effects on osteoblast and osteoclast differentiation of twenty-six diterpenoids (1–26, Fig. 1) from soft corals S. maxima and L. crassum in osteoblastic MC3T3-E1 cells and osteoclastic RAW 264.7 cells.
Materials and methods
Compounds
From the methanolic extracts of the soft corals S. maxima and L. crassum, twenty-six compounds (1–26) were isolated and structurally elucidated. Stock solutions of tested compounds in DMSO were prepared, kept at −20 °C, and diluted to the final concentration in fresh media before each experiment. For not to affect cell growth, the final DMSO concentration did not exceed 0.5 % in all experiments.
Reagents and cell culture materials
2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH), neocuproine, Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), β-glycerophosphate, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Triton X-100, Hank’s balanced salt solution (HBSS), RANKL, leukocyte acid phosphatase assay kit, sodium tartrate, p-nitrophenylphosphate (PNPP), phosphate-buffered saline (PBS, pH 7.4), dihydroethidium dihydrorhodamine (DHR), and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). MC3T3-E1 cells and RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). All solvents used were of analytical grade and supplied by SK Chemicals (Ulsan, Korea).
Reducing capacity
The electron-donating capacities of each compound to reduce Cu(II) to Cu(I) ions were assessed according to the method of Aruoma et al. (1998). Forty microliters of different concentrations of each compound dissolved in ethanol was mixed with 160 μL of a mixture containing 0.5 mM CuCl2 and 0.75 mM neocuproine, a Cu(I) ions specific chelator, in 10.0 mM phosphate buffer, pH 7.4. Absorbance was measured using a microplate reader at 454 nm for 1 h. Increased absorbance of the reaction mixture indicated greater reducing power.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay, which has been employed extensively in previous antioxidant studies (Kurihara et al., 2004), was carried out using a Tecan GENios multifunctional plate reader (Salzburg, Austria) with fluorescent filters (excitation wavelength: 485 nm, emission filter: 535 nm). In the final assay mixture, fluorescein (40.0 nM) was used as a target of free radical attack with AAPH (20.0 mM) as a peroxyl radical generator in the peroxyl radical-scavenging capacity assay (Kurihara et al., 2004). The analyzer was programmed to record fluorescein fluorescence every 2 min after AAPH had been added. All fluorescence measurements were expressed relative to the initial reading. Final values were calculated based on the difference in the area under the fluorescence decay curve between the blank and test samples. All data are expressed as net protection area (net area). Trolox (1.0 μM) was used as the positive control to scavenge peroxyl radicals. It was used as a control standard and prepared fresh daily. The ORAC value is calculated by dividing the area under the sample curve by the area under the Trolox curve, with both areas being corrected by subtracting the area under the blank curve. One ORAC unit is assigned as the net area of protection provided by Trolox at a final concentration of 1.0 µM. The area under the curve of the sample is compared to the area under the curve for Trolox, and the antioxidative value is expressed in micromoles of TE per liter.
Cell culture
MC3T3-E1 (pre-osteoblasts from C57BL/6 mouse) cells were cultured at 37 °C in a 5 % CO2 atmosphere in an α-modified minimum essential medium (α-MEM). Unless otherwise specified, the medium contained 10 % heat-inactivated FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. The cells were treated, at 90 % confluence, with a culture medium containing 10.0 mM β-glycerophosphate and 50.0 µg/mL ascorbic acid to initiate differentiation. The differentiation medium was changed every 3 days.
RAW 264.7 [macrophages (pre-osteoclasts) from BALB/c mouse] cells were cultured in 96-well plates (1 × 104 cells/mL) containing DMEM supplemented with 10 % (v/v) FBS for 2 days. The medium was then replaced with test samples in a differentiation medium containing 50 ng/mL RANKL. The differentiation medium was changed every 2 days.
Cell cytotoxicity by MTT assay
RAW 264.7 cells were cultured in 24-well plates (2 × 104 cells/mL) containing DMEM supplemented with 10 % (v/v) FBS and 1 % (v/v) antibiotics in a humidified atmosphere of 5 % CO2 at 37 °C for 5 days, washed with PBS, and pretreated with different concentrations (1.0–20.0 μM) of samples to be tested. After 5 days’ incubation, MTT reagent was added to each well, and the plate was incubated at 37 °C for 1 h. The medium was removed, and the plate was washed twice with PBS. The intracellular insoluble formazan was dissolved in DMSO. The absorbance of each cell was recorded in DMSO. The absorbance of each cell was then measured at 570 nm using an ELISA (Tecan, Salzburg, Austria) reader, and the percentage proliferation was calculated (Loosdrecht et al., 1994).
Collagen content
After differentiating the MC3T3-E1 cells into osteoblasts for 8 days, the cells were cultured with a medium containing 0.3 % BSA and samples individually for 2 days. On harvesting, the medium was removed and the cell monolayer was gently washed twice with PBS. Collagen content was quantified by Sirius Red-based colorimetric assay (Tullberg-Reinert and Jundt, 1999). Cultured osteoblasts were washed with PBS, followed by fixation with Bouin’s fluid for 1 h. After fixation, the fixation fluid was removed and the culture dishes were washed by immersion in running tap water for 15 min. The culture dishes were air-dried and stained by Sirius Red solution for 1 h under mild shaking on a shaker. Thereafter, the solution was removed, and the cultures were washed twice with acidic water (acetic acid/distilled water, 1:200, v/v) to remove non-bound dye. The stained material was dissolved in 0.1 N NaOH, and absorbance was measured at 540 nm using ELISA reader.
Calcium deposition assay
After differentiating the MC3T3-E1 cells into osteoblasts for 15 days, the cells were cultured with a medium containing 0.3 % BSA and samples individually for 2 days. Staining with Alizarin Red S is a standard method for the visualization of nodular patterns and calcium depositions of osteoblast cultures in vitro. At harvest, the medium was removed and the cell monolayer was gently washed twice with PBS. The cells were fixed with 70 % ethanol for 1 h and stained with 40.0 mM Alizarin Red S for 10 min with gentle shaking. To quantify the bound dye, the stain was solubilized with 1.0 mL 10 % (v/v) cetylpyridinium chloride for 15 min while shaking shielded from light. The absorbance of the solubilized stain was measured at 562 nm using ELISA reader (Bi et al., 2006).
TRAP staining
RAW 264.7 cells were seeded in 12-well plates (3 × 104 cells/well) containing DMEM medium plus 10 % FBS, and the medium was replaced with test samples in differentiation medium containing 50.0 ng/mL RANKL. The differentiation medium was changed every 2 days. After 5 days, the medium was removed, and the cell monolayer was gently washed twice using PBS. The cells were fixed in 3.5 % formaldehyde for 10 min and washed with distilled water. The cells were incubated at 37 °C in a humid and light-protected incubator for 1 h in the reaction mixture of a leukocyte acid phosphatase assay kit (Sigma-Aldrich, St. Louis, MO, USA, Cat. No. 387), as directed by the manufacturer. The cells were washed three times with distilled water, and TRAP-positive multinucleated cells containing three or more nuclei were counted under a light microscope (Kim et al., 2013; Lee et al., 2013).
TRAP activity
After differentiating the RAW 264.7 cells into osteoclasts for 5 days, the medium was removed, and the cell monolayer was gently washed twice using ice-cold PBS. The cells were fixed in 3.5 % formaldehyde for 10 min and ethanol–acetone (1:1) for 1 min. Subsequently, the dried cells were incubated in 50.0 mM citrate buffer (pH 4.5) containing 10.0 mM sodium tartrate and 6.0 mM PNPP. After 1 h incubation, the reaction mixtures were transferred to new well plates containing an equal volume of 0.1 N NaOH. Absorbance was measured at 405 nm using an enzyme-linked immunoassay reader, and TRAP activity was expressed as the percent of the untreated control (Kim et al., 2013; Lee et al., 2013).
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of at least three independent experiments performed in triplicate. Statistical analysis was carried out using the SPSS statistical package (SPSS, Chicago, IL, USA) program, and the significances between the control and experimental groups were analyzed using Student’s t test. Differences were considered to be significant at *P < 0.05 and **P < 0.01.
Results and discussion
In addition to estrogen deficiency, the role of oxidative stress in the pathogenesis of osteoporosis has drawn considerable interest since there is now ample evidence to suggest that ROS-induced oxidative stress is associated with its development (Rao and Rao, 2013; Srinivasan et al., 2010). Although oxidative stress is believed to be one of the primary contributors to the pathogenesis of osteoporosis via its role in osteoclastic resorption and detrimental effects on bone-forming osteoblasts (which could be prevented by supplementation with antioxidants (Arai et al., 2007; Chen et al., 2013; Muhammad et al., 2012; Rao and Rao, 2013), the detailed mechanism by which oxidative stress leads to osteoporosis through osteoclasts and osteoblasts remains controversial and unclear. Under these conditions, an imbalance between ROS production and antioxidant mechanisms may be disturbed, resulting in oxidative stress that could lead to osteoporosis (Abdollahi et al., 2005). More recent studies have suggested that the intracellular antioxidant activity of scavenging ROS may be responsible for enhanced suppression (Abdollahi et al., 2005; Wilson, 2014) of pre-osteoclastic RAW 264.7 cell differentiation into osteoclasts (Brayboy et al., 2001; Lee et al., 2013; Muhammad et al. 2012; Suh et al., 2013; Yan et al., 2014). To our knowledge, there is a lack of scientific data regarding the antiosteoporotic activity of marine-derived compounds from soft corals, prompting us to undertake the present study (Cuong et al., 2008).
Determination of antioxidant activity
Several methods have been developed to estimate the antioxidant capacity of phytochemicals in foods. The most popular and well-characterized one is the ORAC assay, in which AAPH is used to generate peroxyl radicals. This assay is widely used for the evaluation and comparison of antioxidant capacities in natural products and has also been successfully applied in bioavailability (BA) studies (Apak et al., 2013; Cao and Prior, 1998; Prior et al., 2003). The reducing capacity assay, based on electron transfer, is also popular and frequently used for measuring the capacity of food antioxidants in the reduction in an oxidant, which changes color when reduced (Huang et al., 2005).
Peroxyl radical-scavenging capacity of diterpenoid compounds
Our previous work on the constituents of soft corals S. maxima and L. crassum led to the identification of several novel cembranoid derivatives, which were evaluated for their anti-inflammatory properties. A full assignment of all nuclear magnetic resonance (NMR) data was also reported (Cuong et al., 2014; Thao et al., 2012, 2013, 2014). In this study, the antioxidant capacity of secondary metabolites (1–26) was determined by measuring their peroxyl radical-scavenging and reducing activities. With regard to peroxyl radical-scavenging activity, Fig. 2 shows the scavenging effects of diterpenoids at concentrations of 1.0 and 10.0 μM on peroxyl radicals generated by AAPH. Furthermore, compounds 7–9, 13, 14, 18, 20, and 21 exhibited the highest activities among the isolated compounds, with TE values of 4.65 ± 0.16, 3.98 ± 0.11, 5.23 ± 0.07, 4.32 ± 0.10, 4.95 ± 0.23, 4.00 ± 0.14, 9.06 ± 0.33, and 4.02 ± 0.17 μM, respectively, at a concentration of 10.0 μM. Remarkably, scabrolide A (20) exhibited the greatest peroxyl radical-scavenging capacity relative to other diterpenoids, with a TE value of 9.06 ± 0.33 μM, which is based on the fusion of five-, six-, and seven-membered carbocyclic rings linked to a five-ring lactone. In addition, compounds 1–6, 10–12, 15–17, 25, and 26 exhibited moderate peroxyl radical-scavenging capacity against peroxyl radicals at the same concentration (TE values ranged from 2.27 ± 0.10 to 3.62 ± 0.19 μM). The remaining compounds (23 and 24) exerted weak activities (<1.80 μM TE), even at a concentration of 10.0 μM (Fig. 2). These results suggest that the peroxyl radical-scavenging capacities of compounds are dependent on the location and number of hydroxy groups.
Peroxyl radical-scavenging capacity of diterpenes (1–26). Data are expressed as the mean ± SD of three individual experiments. Statistical analysis was carried out using the SPSS statistical package (SPSS, Chicago, IL, USA) program, and the significances between the control and experimental groups were analyzed using Student’s t test. Differences were considered to be significant at *P < 0.05 and **P < 0.01
Reducing capacity of diterpenoid compounds
The reducing capacities of diterpenoids (1–26) were evaluated by the amount of Cu(I) ions reduced with neocuproine, which interacts specifically with Cu(I) ions. Reducing capacity appears to be associated with electron transfer, which is a well-established mechanism contributing to antioxidant capacity (Huang et al., 2005). The ability of the diterpenoids (1–26) to stimulate the reduction in copper ions was investigated to determine whether their peroxyl radical-scavenging activity, which involves donating a hydrogen atom, was related to their reducing capacity, which entails donating electrons. Compounds 7, 9, 17, 18, and 20 (10.0 μM) showed meaningful reducing capacity, with 3.27 ± 0.17, 3.87 ± 0.06, 3.55 ± 0.17, 3.35 ± 0.11, and 9.14 ± 0.30 μM generated Cu(I) ions, respectively. Noticeably consistent with the peroxyl radical-scavenging capacity, scabrolide A (20) demonstrated the highest reducing capacity of all the diterpenoids. In addition, compounds 1, 3, 5, 6, 11, 14–16, and 22 exhibited significant reducing capacity, with values ranging from 1.89 ± 0.06 to 2.51 ± 0.06 μM. The remaining compounds showed negligible reducing capacity, with <1.80 μM Cu(I) ions generated at various concentrations (Fig. 3).
Reducing capacity of diterpenes (1–26). Data are expressed as the mean ± SD of three individual experiments. Statistical analysis was carried out using the SPSS statistical package (SPSS, Chicago, IL, USA) program, and the significances between the control and experimental groups were analyzed using Student’s t test. Differences were considered to be significant at *P < 0.05 and **P < 0.01
Considering the structure–activity relationship (SAR) of these compounds, our results suggest that the ability of compounds 7–9, 13, 14, 18, 20, and 21 to donate hydrogen atoms or electrons to peroxyl radicals and convert them into relatively stable forms may contribute to their peroxyl radical-scavenging capacities. In addition, compounds 7, 9, 18, and 20 exhibited stronger peroxyl radical-scavenging capacities and reducing capacities from Cu(II) ions to Cu(I) ions compared with compounds 8, 13, 14, 18, and 21. This suggests that the hydrogen-donating ability of compounds 8, 13, 14, and 21 is strong, but their single electron transfer activity is weak. The results were not sufficient for discussion regarding the SARs of diterpenoid derivatives and/or other components. However, more studies may be required for the understanding of their selective potential antioxidant activities.
Determination of stimulatory effect on osteoblast differentiation
Osteoblasts are derived from mesenchymal stem cells, whereas multinuclear osteoclasts are formed by the fusion of mononuclear macrophages derived from hematopoietic stem cells (Boyle et al., 2003). Osteoblasts play a crucial role in bone formation through proliferation and differentiation. Osteoblast differentiation, an important process for their function, confers marked rigidity and strength to the bone while maintaining some degree of elasticity. One model commonly used to study osteogenic development is the MC3T3-E1 osteoblast-like cell line derived from C57BL/6 mouse calvarial. MC3T3-E1 cells provide a useful model of osteoblast differentiation. In culture, these cells are characterized by distinct proliferative and differentiation stages, thereby reproducing a temporal program consistent with osteoblast differentiation as it occurs during in vivo bone formation (He et al., 2013; Quarles et al., 1992). Collagen content and mineralization are the most widely recognized biochemical markers for osteoblast activity. Thus, the effects of the isolated compounds on collagen synthesis and mineralization of osteoblastic MC3T3-E1 cells were examined. Collagen content was quantified using a Sirius Red-based colorimetric assay.
We found that compounds 1, 5, 7, 20, and 24 (10.0 μM) from S. maxima significantly increased collagen synthesis. Relative to the control, compounds 20 and 24 remarkably increased collagen synthesis up to 111.83 ± 1.69 and 109.08 ± 0.46 %, respectively (Table 1). The effects of compounds 1–26 on mineralization, another important process in differentiation, were examined by measuring calcium deposition via Alizarin Red staining. Relative to the control, compounds 1, 5, 7, and 20 (10.0 μM) significantly increased mineralization up to 106.68 ± 0.16–111.98 ± 0.41 %. Thus, compounds 1, 5, 7, and 20 significantly stimulated the differentiation of pre-osteoblastic MC3T3-E1 cells to increase collagen synthesis and/or the mineralization function of osteoblasts, and may therefore play an important role in bone disease therapy. Compounds from L. crassum showed weak or no activity to increase collagen synthesis or mineralization at the same concentration. This is consistent with a recent report of the osteoblastic MC3T3-E1 inhibitory activities of other diterpenes (Cuong et al., 2008).
Determination of inhibitory effect on osteoclast differentiation
Estrogen deficiency enhances the genesis and activity of osteoclasts, resulting in an unbalanced increase in bone resorption. Osteoclasts play a central role in pathologic bone loss, including postmenopausal osteoporosis (Kousteni et al., 2001). Despite a variety of mechanisms through which estrogen deficiency induces bone loss, upregulation of the osteoclastogenic cytokines receptor activator of RANKL and TNFα plays a significant role (Fatourechi et al., 2003; Roggia et al., 2001). RANKL is highly expressed in osteoblast/stromal cells, primitive mesenchymal cells surrounding the cartilaginous anlagen, and hypertrophying chondrocytes. In addition to its role in osteoclast differentiation from their precursor cells, RANKL also promotes increased activity and survival of these cells by exerting an anti-apoptotic effect (Lacey et al., 1998). Therefore, RANKL inhibition appears to be the most rational and advisable strategy to prevent bone destruction in multiple diseases and possibly eradicate major human diseases, such as osteoporosis.
The inhibitory effects of diterpenes 1–26 on osteoclast differentiation were also investigated to determine their antiosteoporotic activity through suppressing excessive bone resorption by osteoclasts. TRAP is highly expressed in osteoclasts and widely used as a phenotypic marker. Osteoclast differentiation from murine macrophage RAW 264.7 cells was induced by RANKL, which is essential for terminal differentiation of monocytes/macrophages into osteoclasts. Treatment with RANKL strongly induced osteoclast formation from RAW 264.7 pre-osteoclast cells and enhanced TRAP activity up to 51.32 ± 0.08 % relative to the control. Compounds 1, 5–8, 12, 14–22, 24, and 26 showed significant inhibitory effects on TRAP activity in multinucleated TRAP-positive cells at 10.0 μM, with values ranging from 99.11 ± 1.36 to 61.54 ± 1.61 %. Among them, compounds 7, 20, and 24 exhibited the highest activities and suppressed TRAP, with values of 65.16 ± 0.62, 65.10 ± 1.86, and 61.54 ± 1.61 %, respectively (Fig. 4; Table 2). However, these results could be regarded as inhibitory effects of the compounds on cell viability. To address this, the compounds (20.0 μM) were tested for their cytotoxic activity on RAW 264.7 macrophage cells during a 5-day differentiation period. However, no significant cytotoxic effects were observed (data not shown). Thus, these results suggest that compounds 7, 20, and 24 possess antiosteoclastogenic activities, but do not affect cell viability.
Inhibitory effects of diterpenes 1–26 on TRAP activity in RANKL-induced osteoclastic RAW 264.7 cells. TRAP activity was measured from cultures after 5 days of treatment with RANKL and test compounds (10.0 μM). The treated control was obtained from RAW 264.7 cells cultured with RANKL stimulation and without test compounds. Data are expressed as percentages of the control (mean ± SD, n = 3, *P < 0.05 and **P < 0.01 vs TC. C control, which was not treated; TC treated control, which was treated with RANKL)
The SARs of diterpenes 1–26 indicate that the presence of an epoxy group at C-7/C-8 and/or C-5/C-8 is necessary for antiosteoclastogenic activity. This information may facilitate the identification of other antiosteoporotic lead compounds from diterpenoids and provides support for additional studies.
Conclusions
In conclusion, the present study shows that many diterpenoids from soft corals S. maxima and L. crassum possess peroxyl radical-scavenging, reducing capacities, and antiosteoclastogenic activities. These findings indicate that compounds 1, 5, 7, 20, and 24 significantly stimulate the differentiation of pre-osteoblastic MC3T3-E1 cells, while the effect of compounds 5, 7, 20, and 24 on the differentiation of pre-osteoclastic RAW 264.7 cells is due, in part, to their intracellular antioxidant capacity, since they can scavenge ROS and play an important signaling role in the differentiation process. This is the first report to describe the antioxidant and antiosteoporotic activities of pure isolates from S. maxima and L. crassum. Although further studies are required to confirm their efficacy in vivo, it is the contention of the authors that these compounds could be used in the development of therapeutic targets for osteoporosis.
Abbreviations
- AAPH:
-
2,2′-Azobis(2-amidinopropane) dihydrochloride
- CUPRAC:
-
Cupric ion reducing antioxidant capacity
- DMSO:
-
Dimethylsulfoxide
- ORAC:
-
Oxygen radical absorbance capacity
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB:
-
Nuclear factor-κB
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- ROS:
-
Reactive oxygen species
- TE:
-
Trolox equivalents
- TNFα:
-
Tumor necrosis factor alpha
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Abdollahi M, Larijani B, Rahimi R, Salari P (2005) Role of oxidative stress in osteoporosis. Therapy 2:787–796
Anjaneyulu ASR, Rao GV (1997) Chemical constituents of the soft coral species of Sarcophyton genus: a review. J Indian Chem Soc 74:272–278
Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K (2013) Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl Chem 85:957–998
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y (2007) Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 59:27–33
Aruoma OI, Deiana M, Jenner A, Halliwell B, Kaur H, Banni S, Corongiu FP, Dessí MA, Aeschbach R (1998) Effect of hydroxytyrosol found in extra virgin olive oil on oxidative DNA damage and on low-density lipoprotein oxidation. J Agric Food Chem 46:5181–5187
Bi Y, Nielsen KL, Kilts TM, Yoon A, Karsdal MA, Wimer HF, Greenfield EM, Heegaard AM, Young MF (2006) Biglycan deficiency increases osteoclast differentiation and activity due to defective osteoblasts. Bone 38:778–786
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Brayboy JR, Chen XW, Lee YS, Anderson JJB (2001) The protective effects of Ginkgo biloba extract (EGb 761) against free radical damage to osteoblast-like bone cells (MC3T3-E1) and the proliferative effects on EGb 761 on these cells. Nutr Res 21:1275–1285
Cao G, Prior RL (1998) Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem 44:1309–1315
Chao CH, Chou KJ, Huang CY, Wen ZH, Hsu CH, Wu YC, Dai CF, Sheu JH (2011) Bioactive cembranoids from the soft coral Sinularia crassa. Mar Drugs 9:1955–1968
Chen Q, Yang L, Zhang G, Wang F (2013) Bioactivity-guided isolation of antiosteoporotic compounds from Ligustrum lucidum. Phytother Res 27:973–979
Cheng SY, Chuang CT, Wang SK, Wen ZH, Chiou SF, Hsu CH, Dai CF, Duh CY (2010) Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J Nat Prod 73:1184–1187
Cuong NX, Tuan TA, Kiem PV, Minh CV, Choi EM, Kim YH (2008) New cembranoid diterpenes from the Vietnamese soft coral Sarcophyton mililatensis stimulate osteoblastic differentiation in MC3T3-E1 cells. Chem Pharm Bull 56:988–992
Cuong NX, Thao NP, Luyen BTT, Ngan NTT, Thuy DTT, Song SB, Nam NH, Kiem PV, Kim YH, Minh CV (2014) Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chem Pharm Bull 62:203–208
Ellis ZK, Osdoby PC, Li L, Brandi MLML, Osdoby P (1997) A human homolog of the 150 kD avian osteoclast membrane antigen related to superoxide dismutase and essential for bone resorption is induced by developmental agents and opposed by estrogen in FLG 29. Calcif Tissue Int 60:187–193
Fatourechi GE, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL (2003) Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 111:1221–1230
Grote D, Dahse HM, Seifert K (2008) Furanocembranoids from the soft corals Sinularia asterolobata and Litophyton arboreum. Chem Biodivers 5:2449–2456
He L, Lee J, Jang JH, Sakchaisri K, Hwang J, Molstad HJC, Kim KA, Ryoo IJ, Lee HG, Kim SO, Soung NK, Lee KS, Kwon YT, Erikson RL, Ahn JS, Kim BY (2013) Osteoporosis regulation by salubrinal through eIF2α mediated differentiation of osteoclast and osteoblast. Cell Signal 25:552–560
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Kamel HN, Ferreira D, Fernandez LFG, Slattery M (2007) Cytotoxic diterpenoids from the hybrid soft coral Sinularia maxima × Sinularia polydactyla. J Nat Prod 70:1223–1227
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables—the millennium’s health. Int J Food Sci Technol 36:703–725
Kim SN, Kim MH, Min YK, Kim SH (2013) Licochalcone A inhibits the formation and bone resorptive activity of osteoclasts. Cell Biol Int 32:1064–1072
Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC (2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730
Kurihara H, Fukami H, Asami S, Toyoda Y, Nakai M, Shibata H, Yao XS (2004) Effects of oolong tea on plasma antioxidative capacity in mice loaded with restraint stress assessed using the oxygen radical absorbance capacity (ORAC) assay. Biol Pharm Bull 27:1093–1098
Lacey DL, Timms E, Tan HL (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Lakshmi V, Kumar R (2009) Metabolites from Sinularia species. Nat Prod Res 23:801–850
Lee SH, Ding Y, Yan XT, Kim YH, Jang HD (2013) Scopoletin and scopolin isolated from Artemisia iwayomogi suppress differentiation of osteoclastic macrophage RAW 264.7 cells by scavenging reactive oxygen species. J Nat Prod 76:615–620
Lo KL, Khalil AT, Kuo YH, Shen YC (2009) Sinuladiterpenes A-F, new cembrane diterpenes from Sinularia flexibilis. Chem Biodivers 6:2227–2235
Loosdrecht AA, Beelen RHJ, Ossenkoppele GJ, Broekhoven MG, Langenhuijsen MMAC (1994) A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174:311–320
Lu Y, Su JH, Huang CY, Liu YC, Kuo YH, Wen ZH, Hsu CH, Sheu JH (2010) Cembranoids from the soft corals Sinularia granosa and Sinularia querciformis. Chem Pharm Bull 58:464–466
Maggio D, Pierandrei M, Polidori MC, Catani M (2003) Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab 88:1523–1527
McCormick RK (2007) Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev 12:113–145
Muhammad N, Luke DA, Shuid AN, Mohamed N, Soelaiman IN (2012) Two different isomers of vitamin E prevent bone loss in postmenopausal osteoporosis rat model. Evid Based Complement Alternat Med Article ID 161527:7
Nazrun AS, Norazlina M, Norliza M, Nirwana SI (2011) Tocotrienols as an anti osteoporotic agent: the progress so far. Int J Osteoporos Metab Disord 4:1–14
Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R (2003) Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J Agric Food Chem 51:3273–3279
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res 7:683–690
Rao, LG and Rao AV (2013) Oxidative stress and antioxidants in the sisk of osteoporosis—role of the antioxidants lycopene and polyphenols. Topics in osteoporosis Rijeka, Croatia: InTech, pp 117–161
Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R (2001) Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98:13960–13965
Simon LS (2007) Osteoporosis. Rheum Dis Clin N Am 33:149–176
Soni HK, Kandachia JM, Jani DK, Patel GR (2013) Pharmacological investigation of bonton capsule for anti-osteoporotic activity in ovariectomized rat. Int J Pharm Phytopharmacol Res 3:52–56
Sontakke AN, Tare RS (2002) A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chem Acta 318:145
Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M, Avadhani NG (2010) Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann NY Acad Sci 1192:245–252
Su JH, Wen ZH (2011) Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar Drugs 9:944–951
Su JH, Lin YF, Lu Y, Yeh HC, Wang WH, Fan TY, Sheu JH (2009) Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem Pharm Bull 57:1189–1192
Suh KS, Lee YS, Kim YS, Choi EM (2013) Sciadopitysin protects osteoblast function via its antioxidant activity in MC3T3-E1 cells. Food Chem Toxicol 58:220–227
Thao NP, Nam NH, Cuong NX, Quang TH, Tung PT, Tai BH, Luyen BTT, Chae D, Kim S, Koh YS, Kiem PV, Minh CV, Kim YH (2012) Diterpenoids from the soft coral Sinularia maxima and their inhibitory effects on lipopolysaccharide-stimulated production of proinflammatory cytokines in bone marrow-derived dendritic cells. Chem Pharm Bull 60:1581–1589
Thao NP, Nam NH, Cuong NX, Quang TH, Tung PT, Dat LD, Chae D, Kim S, Koh YS, Kiem PV, Minh CV, Kim YH (2013) Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorg Med Chem Lett 23:228–231
Thao NP, Luyen BTT, Ngan NTT, Song SB, Cuong NX, Nam NH, Kiem PV, Kim YH, Minh CV (2014) New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg Med Chem Lett 24:228–232
Tullberg-Reinert H, Jundt G (1999) In situ measurement of collagen synthesis by human bone cells with a Sirius Red-based colorimetric microassay: effects of transforming growth factor β2 and ascorbic acid 2-phosphate. Histochem Cell Biol 112:271–276
Wilson C (2014) Bone: oxidative stress and osteoporosis. Nat Rev Endocrinol. doi:10.1038/nrendo.2013.1225
Yan XT, Lee SH, Li W, Sun YN, Yang SY, Jang HD, Kim YH (2014) Evaluation of the antioxidant and anti-osteoporosis activities of chemical constituents of the fruits of Prunus mume. Food Chem 156:408–415
Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54:176–186
Acknowledgments
This study was supported by the Priority Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2009–0093815), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Thao, N.P., Luyen, B.T.T., Lee, S.H. et al. Antiosteoporotic and antioxidant activities of diterpenoids from the Vietnamese soft corals Sinularia maxima and Lobophytum crassum . Med Chem Res 24, 3551–3560 (2015). https://doi.org/10.1007/s00044-015-1395-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1395-8