Abstract
The aim of this study was to investigate the effect of autochthonous microorganisms present in soil collected from heavy metal (HM) uncontaminated (Pb ≈ 59 mg kg−1, Cd ≈ 0.4 mg kg−1, Zn ≈ 191 mg kg−1), moderately (Pb ≈ 343 mg kg−1, Cd ≈ 12 mg kg−1, Zn ≈ 1876 mg kg−1), and highly (Pb ≈ 1586 mg kg−1, Cd ≈ 57 mg kg−1, Zn ≈ 3280 mg kg−1) contaminated sites on Zea mays elemental composition, physiological status, and growth parameters. For this purpose, half of the collected soil was sterilized and soil characterization was performed. After 45 days of cultivation, the presence of HM in the soil negatively affected photosynthesis and transpiration rates, relative chlorophyll content, anthocyanins index, chlorophyll fluorescence parameters, and content of oxidative stress products (H2O2 and Malondialdehyde) of Zea mays, while soil sterilization had a positive effect on those parameters. Average percentage of colonization of root segments by arbuscular mycorrhiza fungi decreased with an increase of HM contamination in the soil. The increase in shoot concentration of HMs, particularly Cd and Zn, was a result of contaminated soils sterilization. Aboveground biomass of maize cultivated on sterilized soil was 3-fold, 1.5-fold, and 1.5-fold higher for uncontaminated, moderately contaminated and highly contaminated soils respectively when compared to nonsterilized soils. Contrary to our expectation, autochthonous microflora did not improve plant growth and photosynthetic performance; in fact, they had a negative effect on those processes although they did reduce concentration of HMs in the shoots grown on contaminated soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction between soil microbes and plant roots has been studied widely. The dynamics of this interaction depend on several factors, such as the physiological characteristics of plants and microorganisms, physicochemical soil properties, and even climatic conditions (Haney et al. 2015; Köhl et al. 2016; Mohite 2013; Pii et al. 2015). Plant associated microorganisms can play an important role in growth and nutritional status improvement (Cabral et al. 2016; Ercoli et al. 2017; Valliere and Allen 2016), as well as in the detoxification of harmful substances and maintenance of soil structure (Sułowicz et al. 2011; Thijs et al. 2014; Watts-Williams et al. 2017). Indigenous microorganisms living on heavy metal-contaminated sites have often adapted well to the presence of these elements, driven by long-term exposure to site-specific stress factors, and these adaptations can provide useful opportunities for bioremediation at such sites (Giller et al. 2009; Oliveira et al. 2014; Touceda-González et al. 2017; Vivas et al. 2003; Yu et al. 2014).
Many authors reported a connection between reduction of dehydrogenase activity and presence of pollutants in soil at high concentrations, which correlates with a decrease in microbial community diversity and biomass (Baćmaga et al. 2015; Wang et al. 2014; Wolińska and Stępniewska 2012).
An important component in the rhizosphere are the arbuscular mycorrhizal fungi (AMF), belonging to the phylum Glomeromycota, which live in symbiosis with 70–90% of plant species (Bonfante and Genre 2010; Bothe et al. 2010; Gianinazzi et al. 2010; Huang et al. 2009; Zhu et al. 2012). Recently, an increasing number of reports have emphasized the role of mycorrhiza in reducing stress reactions associated with environmental pollution by heavy metals (Firmin et al. 2015; Gucwa-Przepióra 2012; Li et al. 2016). AMF associated with plants may contribute to the accumulation of heavy metals in roots in a nontoxic form inside hyphal cell walls or bind them into phosphate compounds inside the fungal cells (Andrade and da Silveira 2008).
High concentrations of heavy metals in the soil negatively affect many plant physiological processes through the production of reactive oxygen species (ROS). This in turn results in lipid peroxidation, mostly irreversible oxidation of proteins and DNA damage. There are many reports indicating that plant-microbial interactions can alleviate oxidative stress caused by toxic concentrations of heavy metals (Islam et al. 2014; Islam et al. 2016; Nadeem et al. 2014). Islam et al. (2016) observed improving gas exchange parameters, such as photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci), as well as reduction of H2O2 and malondialdehyde (MDA) content in lentils (Lens culinaris Medik) grown in heavy metal-contaminated soil inoculated with microorganisms, in comparison with non-inoculated ones.
To date, most experiments have investigated the influence of selected microbes on plant growth and development, considering either selected strains alone on sterilized soil or a combined effect of selected microbes with autochthonous microorganisms on nonsterilized soil (Bai et al. 2008; Guo et al. 2013; Liang et al. 2009; Rostami et al. 2016; Wang et al. 2007; Verma et al. 2014; Zhu et al. 2010). However, the influence of autochthonous microorganisms, particularly on plant growth and physiological processes, is poorly described in the literature (Liu et al. 2014; Miransari et al. 2009; Yang et al. 2015). Experiments conducted on sterilized and nonsterilized soil without inoculation can offer an opportunity to assess the impact of these autochthonous microorganisms on plants cultivated on soils with different levels of heavy metal contamination. We hypothesize that presence of autochthonous microorganisms in soils should improve plant growth and photosynthesis performance of Zea mays plants. Thus, the aim of this study was to investigate the relationships between plant growth and the chlorophyll a fluorescence parameters, gas exchange parameters, pigments content, mineral nutrient status, heavy metals concentration, and oxidative stress in maize plants cultivated on contaminated and noncontaminated soils with and without autochthonous microorganisms.
Materials and methods
Site description and soil sampling
Soil for the pot experiment was sampled from three experimental sites located in Upper Silesia, Poland. Sites were selected according to heavy metal (HM) contamination status: fallow land in Piekary Śląskie (50° 22′ 06.2” N 18° 57′ 52.9″ E) with a high degree of pollution (HMhigh), caused by close vicinity to the former Pb/Zn smelter; arable land in Bytom (50° 20′ 41.9” N 18° 57′ 19.9″ E) with a moderate level of contamination (HMmod), resulting from being a short distance (about 2.5 km) from the same smelter and arable land in Gliwice (50° 15′ 16.9” N 18° 40′ 46.6″ E), located about 24 km from the smelter, classified as a nonpolluted area (HMlow). The zinc and lead smelter was operated from 1927 until 1990 when significant soil contamination occurred due to dust fall of particles containing HM (Fig. S1). At the Bytom site, cereals have been cultivated commercially for the last 20 years, while at the Gliwice site between 1970 and 2000, vegetables were cultivated without the use of any chemical fertilizer, and from 2000, this field was left fallow. The soil collected from each site was air-dried, passed through a 4-mm sieve, which removed stones and plant residues, mixed and divided into two parts. Half of each soil sample remained unsterilized while the second was sterilized in an autoclave at 120 °C and 1.2 bar for 20 min; this sterilization procedure was repeated four times with a 1-day interval.
Plant growth experiment
Subsamples for each soil were put into plastic pots. For each variant, four pots (0.0015 m3 volume) were established. Experimental treatments were as follows:
-
G_NS—nonsterilized soil from Gliwice, (HMlow)
-
G_S—sterilized soil from Gliwice, (HMlow)
-
B_NS—nonsterilized soil from Bytom, (HMmod)
-
B_S—sterilized soil from Bytom, (HMmod)
-
P_NS—nonsterilized soil from Piekary Śląskie, (HMhigh)
-
P_S—sterilized soil from Piekary Śląskie, (HMhigh)
Caryopses of maize (Zea mays L., cv. ‘Lokata’) were germinated in the dark at 28 °C. After 2 weeks of germination, three seedlings per pot were planted. The plants were cultivated in a phytotron for 45 days under controlled conditions: temperature 24 °C, light intensity PAR = 300 μmol (photons) m−2 s−1 and humidity 40%. The measurements of plant gas exchange parameters and pigment content were conducted after the 18th day from planting and consequently repeated every 2 weeks until the end of the experiment. Additionally, at the end of the experiment, chlorophyll a fluorescence and plant height measurements were taken. Following this, plant and soil samples were collected for further analysis. The plants were divided into shoots and roots, washed in tap water to remove soil particles, and then washed again with deionized water. The rest of the plant growth measurements were then carried out along with assessments of malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents in leaf samples. In addition, the intensity of root colonization by arbuscular mycorrhiza fungi was assessed. The rest of the shoots and roots biomass was dried for 72 h at 70 °C and then ground for elemental analysis. Soil samples collected from the pots after the experiment were examined for dehydrogenase activity.
Soil physicochemical parameters
Soil samples were collected before the experiment, air-dried and sieved to 2 mm (for pH, electrical conductivity, organic matter content) and then ground to < 0.25 mm for further analysis (total metal concentration, bioavailable metal concentration, nutrients: N, P, K).
Soil pH was measured in H2O (ratio 1:2.5 m/v) with a combination glass/calomel electrode (OSH 10-10, METRON, Poland) and a pH-meter (CPC-551, Elmetron, Poland) at 20 °C. The electrical conductivity was determined using the same device as for pH measurements using an ESP 2ZM electrode (EUROSENSOR, Poland) following the standard Polish protocol, PN-ISO 11265:1997.
Soil texture was evaluated by the sieve method according to protocol ISO 11277:2009. Soil organic matter content (OM) was measured by loss on ignition as follows: air-dry soil was dried at 105 °C for 24 h and then (5 g) treated with 550 °C for 4 h (Pogrzeba et al. 2017).
Analysis of elements concentration in the soil and plants samples
The bioavailable forms of Pb, Cd, and Zn in the soil were extracted using 0.01 M CaCl2 (for a review, see Peijnenburg et al. 2007). Extraction was conducted with 5 g of air-dried soil (< 0.25 mm) and 50 ml 0.01 M CaCl2 for 2 h. Bioavailable metal concentrations (Cd, Pb, Zn) were measured in filtrate using flame atomic absorption spectrometer (iCE 3500 FAAS, Thermo Scientific).
Total concentrations of Pb, Zn, Cd, Mg, Fe, and Ca in the soil (< 0.25 mm) and plant samples were obtained by flame atomic absorption spectrometry (iCE 3500 FAAS, Thermo Scientific) after microwave sample digestion in concentrated HNO3 and H2O2 (4:1 v/v) (ETHOS 1, Milestone, Italy).
The total nitrogen (N) concentration in soil was determined using the dry combustion method (ISO 13878:1998). Available phosphorus (P) and available potassium (K) concentrations in the soil were assessed according to the method described by Egner et al. (1960). Total N concentration in plant shoots and roots was measured using the titration method (Bremner 1996), whereas total phosphorus (P) and potassium (K) concentration in plant shoots and roots were estimated in previously mineralized samples using ICP (Liberty 220,Varian, USA).
Dehydrogenase activity determination
Dehydrogenase activity was determined according to the modified method of Casida et al. (1964). Soil samples (3 g) were placed in test tubes and incubated at 25 °C for 96 h. After that, the substrate (3% v/w TTC) was added and the tubes were incubated at 25 °C for 24 h. The samples were vortexed, filtered using acetone as an extractor agent, and read at λ = 485 nm using UV-Vis spectrophotometer (BioPhotometer® D30, Eppendorf, Germany).
Arbuscular mycorrhizal fungi colonization rate
For the estimation of mycorrhizal development, the roots were prepared according to a modified method of Phillips and Hayman (1970). After washing in tap and deionized water, the roots were purified in 7% KOH at 90 °C for 10 min and then rinsed in a few changes of deionized water. The root samples were acidified in 5% lactic acid for 24 h and stained with 0.01% aniline blue in lactic acid for 24 h. Subsequently, the evaluation of arbuscular mycorrhizal fungi (AMF) colonization was conducted according to Trouvelot et al. (1986) using Mycocalc software (http://www.dijon.inra.fr/mychintec/Mycocal-prg/download.html). Fifteen root fragments on one slide were observed under the microscope and rated according to the range of classes indicated in the Trouvelot et al. (1986) method. These classes give a rapid estimation of the level of mycorrhizal colonization of each root fragment and the abundance of arbuscules. The following parameters were evaluated: F%, percentage of segments showing internal colonization; M%, average percentage of colonization of root segments; A%, percentage of arbuscules in the whole root system.
Physiological parameters
Plant gas exchange measurements
Plant gas exchange parameters, such as photosynthesis rate (A), stomatal conductance (gs) transpiration rate (E), and intracellular CO2 concentration (Ci) were conducted on the first fully developed leaf (mostly the third from the apex). Measurements were carried out on the 18th, 32nd, and 45th day of plant cultivation in the soil using an infrared gas analyzer (LCpro+, ADC Bioscientific, UK) under controlled climate conditions (T = 24 °C, PAR = 1000 μmol m−2 s−1). Measurements were performed between 9 am and noon.
Plant pigment contents
Relative chlorophyll and anthocyanins content was measured using a plant pigment meter (DUALEX SCIENTIFIC+™, Force-A, France). The plant pigment content assay was performed immediately following the gas exchange measurements and using the same leaves as for the gas exchange measurements.
Chlorophyll a fluorescence measurements
Chlorophyll a fluorescence was measured using Handy Plant Efficiency Analyzer (Hansatech Instruments Ltd., UK). Before analysis, leaves were dark-adapted for 25 min using specially designed clips (LC, Hansatech Instruments, Ltd., UK). Measurements were conducted on the 45th day using the same leaves as for the plant pigment content and gas exchange measurements.
Plant growth measurements
At the end of the experiment, the shoot height (h) and dry biomass of plant shoots and roots were measured.
Oxidative stress assessment
The H2O2 content was determined in leaves using the modified procedure described by Loreto and Velikova (2001) while lipid peroxidation, an indicator of oxidative cell damage, was assessed by measuring MDA content in plant leaves according to the Dhindsa et al. (1981) method.
Statistical analysis
All data were analyzed using one-way ANOVA with LSD post hoc test (P < 0.05) for comparison of more than two independent groups, while Wilcoxon test (P < 0.05) was used for comparison of two independent groups. Principal component analyses (PCA) were performed on a correlation matrix to detect clusters of cases and correlation between investigating parameters corresponding to plant physiological status (plant growth measurements, elements concentration in shoots, gas exchange measurement, plant pigment content, mycorrhizal colonization, oxidative stress indicators). Statistical analyses were performed using Statistica v13.1 (Dell Inc., USA).
Results
Soil characteristics
The soil from Gliwice (HMlow) was classified as medium loam, while from Bytom (HMmod) and Piekary Śląskie (HMhigh) as Silty Loam (Table 1, Table S1). The lowest soil pH was found for Bytom site, while the highest was observed in Gliwice site. Analysis of soil electrical conductivity (EC) did not show significant differences between sterilized and nonsterilized soil, demonstrating similar tendencies as pH values (Table 1). Bytom soil showed a significantly lower content of organic matter (OM) by about 1%, compared to the soil from Gliwice and Piekary Śląskie. The elemental concentration in the soils is presented in Table 1. The significantly higher Ntotal concentration (by about 20%) was found in Gliwice and Piekary Śląskie when compared to Bytom soil. No significant differences were observed in Pavailable concentration in Bytom and Gliwice. The lowest Pavailable concentration was found in Piekary Śląskie soil and it was about 63% lower in comparison with the other soils. Kavailable concentration was higher in soils from Bytom and Piekary Śląskie, where there were no significant differences, while Kavailable concentration in soil from Gliwice was nearly 50% lower. The highest Mg and Cu concentration was observed in the P_NS soil while there were no significant differences between G_NS and B_NS. G_NS soil had the highest Ca concentration (75% higher than the other soils).
Concentration of bioavailable forms of the investigated heavy metals, Pb in each variant and Cd in control variants (G_NS and G_S) were below detection limit. The highest bioavailable Zn and Cd concentration was found in Piekary Śląskie soil, while moderate and the lowest were found in Bytom and Gliwice soil respectively. Sterilization did not affect soil elemental composition except for Pavailable concentration. It was found that sterilization of soil from Gliwice and Bytom resulted in a decrease of Pavailable concentration in those experimental variants.
Dehydrogenase activity (DHA), measured at the end of the experiment, was very much lower in the sterilized soils compared to nonsterilized which showed that sterilization was successful (Table 1). Sterilization decreased DHA in noncontaminated G_S soil by 96.7%, whereas in soils contaminated by heavy metals DHA, it was diminished by 87–88%. In nonsterilized soil, the highest DHA was found for noncontaminated soil (G_NS), while the lowest for the most contaminated soil (P_NS).
Concentration of elements in shoots and roots
The concentrations of investigated elements in plants are presented in Table 2. Concentrations of elements in root and shoots, in most cases, correspond to the values obtained during soil elemental analysis; however, the most notable differences were driven by comparisons between plants cultivated in sterilized or nonsterilized soils.
There were significant differences in N, K, and Mg root concentration, with a decrease of these elements concentration in roots of plants cultivated on B_S soil when compared to its nonsterilized counterpart. In addition, a decrease in roots Mg concentration by about 37% was found for G_S soil in comparison to its nonsterilized counterpart.
Nitrogen concentration in shoots was significantly lower (10%) for plants cultivated in G_S soil and significantly higher (37%) for plants cultivated in P_S soil as compared to their nonsterilized counterparts. Significantly, lower values for K shoot concentration were found in variants with sterilized soil. Interestingly, Ca concentration in shoots was significantly lower in sterilized soil, despite the fact that differences between concentrations of this element in roots were statistically insignificant. Sterility of soil significantly affected Cd concentration in shoots; it caused higher concentrations in plants cultivated in B_S and P_S soil by about 54 and 70% when compared to plants cultivated in B_NS and P_NS soil, respectively. A similar relationship was found for Zn shoot concentration; however, this was only seen in the Piekary Śląskie (HMhigh) soil.
Mycorrhizal parameters
Mycorrhizae colonization levels were significantly affected by HM concentration in soil (Fig. 1). The lowest mycorrhizal intensity (M%) was found in P_NS and B_NS plant roots which were lower by 86 and 70% in comparison G_NS, respectively. G_S, B_S, and P_S plant roots were devoid of AMF colonization. Moreover, in contrast to P_NS (HMhigh) and B_NS (HMmod) plants, G_NS (HMlow) showed the Arum-type mycorrhizal colonization. In plants grown on the HMC soils, AMF, when present, exhibited Paris-type mycorrhizal colonization (Fig. S2).
Degree of arbuscular mycorrhizal fungi colonization of roots and arbuscules formation. Values are means ± SE. (n = 4). Lower case letters (a, b, c) denote significant difference between means according to Fisher LSD test at P ≤ 0.05. G, soil from Gliwice site (HMlow); B, soil from Bytom site (HMmod); P, soil from Piekary Śląskie site (HMhigh); NS, nonsterilized soil; S, sterilized soil; F%, percentage of segments showing internal colonization; M%, average percentage of colonization of root segments; A%, percentage of arbuscules in the whole root system
Dark septate endophytes (DSE) were found in plants cultivated on HM-contaminated soils (P_NS and B_NS), while they were absent in root samples from G_NS. The mycelium was brownish and occurred mainly in root fragments where arbuscules were not present. Both dematiaceous septate hyphae and microsclerotia were observed (Fig. S3).
Chlorophyll and anthocyanins content
Relative chlorophyll content in Z. mays leaves decreased during the experiment, irrespectively of the studied experimental treatment (Fig. 2a). No significant difference in relative chlorophyll content was observed on the 18th day from planting across all experimental variants. The largest decrease of relative chlorophyll content was observed on the 45th day after planting. Among plants cultivated on nonsterilized soil, the greatest decrease of relative chlorophyll content was found in B_NS (HMmod) and P_NS (HMhigh) plants, which were 63% lower relative to that measured on the 18th day. In addition, by the 45th day, G_NS (HMlow) plants had significantly higher relative chlorophyll content than plants cultivated on the other nonsterilized soils. In contrast to measurements taken on the 18th day, significant differences were seen in relative chlorophyll content in leaves on the subsequent measuring dates (32nd and 45th days) with chlorophyll tending to be significantly higher in plants cultivated on sterilized soils compared to nonsterilized ones. Among plants cultivated on sterilized soil, by the 32nd and 45th days, the highest relative chlorophyll content was found in G_S and P_S plants, with no significant differences noted between them, while the lowest relative chlorophyll content was found for B_S plants.
Effect of autochthonous microorganisms on chlorophyll (a), flavonoids (b) and anthocyanins (c) leaves content. Values are means ± SE, (n = 16). Lower case letters (a, b, c) denote significant difference between means according to Fisher LSD test at P ≤ 0.05. A statistical analysis was performed for each day separately. G, soil from Gliwice site (HMlow); B, soil from Bytom site (HMmod); P, soil from Piekary Śląskie site (HMhigh); NS, nonsterilized soil; S, sterilized soil; DAP, day after planting
Anthocyanin levels also increased during cultivation (Fig. 2b). Analysis carried out on the 18th day sampling showed no significant differences between any of the treatments. By the 32nd day, a large increase in anthocyanin index was observed across all soils, about 64% higher than earlier measurements in plants cultivated on the nonsterilized soils. Leaves of plants grown on sterilized soils also showed an increased anthocyanin index, but the values were lower in comparison with their nonsterilized counterparts by 74%, 29%, and 55% for G_S, B_S, and P_S plants respectively. This 32nd day sampling revealed significant differences between sterilized and nonsterilized soils. Within the HM status groups, nonsterilized soils were not different to each other; in contrast, all three soil types within the sterilized group were significantly different to each other at this time point.
With the exception of G_NS, all treatments had again increased in anthocyanin index by the 45th day sampling. Among plants cultivated on nonsterilized soil, the highest values were found in leaves of P_NS plants, which was ten times higher than that of the first (18th day) measurements. The lowest values were found in leaves of G_NS. Among plants cultivated on sterilized soil, the highest values were found in leaves of B_S and P_S plants. Across all treatments, the lowest anthocyanin index tended to be found on the uncontaminated sterilized and unsterilized soils.
Chlorophyll a fluorescence
Chlorophyll a fluorescence induction curve
The transient sector between bands (O, J, I, and P) of the chlorophyll induction curve provides important information about the kinetic energy transfer and conversion between the different components of photosynthetic apparatus related to the light-dependent phase of photosynthesis (for review, see Goltsev et al. 2016; Kalaji et al. 2014; Strasser et al. 2004).
Across all experimental treatments, the only differences in the fluorescence transient curve were found at K- and J-band (Fig. 3). No differences were found at I-band. Different doses of HM in soil as well as soil sterility did not influence I-P phase.
Relative chlorophyll a fluorescence induction curve. Values are means ± SE. (n = 16). G, soil from Gliwice site (HMlow); B, soil from Bytom site (HMmod); P, soil from Piekary Śląskie site (HMhigh); NS, nonsterilized soil; S, sterilized soil; 0, minimal fluorescence intensity (FO); K, band at 0.0003 s; J, band at 0.002 s; I, band at 0.002 s; P, maximal fluorescence intensity (FM)
Chlorophyll a fluorescence parameters
The significant differences in most of JIP-test parameters (Table 3) were visible between sterilized and nonsterilized variants of the same soil, as well as between variants of different concentration of heavy metals in soil. Significantly, higher values of minimal fluorescence intensity (FO), maximal fluorescence intensity (FM), maximum quantum yield of primary photochemical reactions (ϕPo – FV/FM), indicator of photosystem II (PSII) functional activity normalized to the absorbed energy (PIABS) and total performance index; indicating the integral functional activity of PSII, photosystem I (PSI), and intersystem electron transport chain (PItotal) found in Z. mays leaves cultivated in soil from Gliwice (HMlow) and Piekary Śląskie (HMhigh). However, no significant differences were found between these parameters for plants cultivated in the Bytom (HMmod) sterilized and nonsterilized soil. In contrast, a significant decrease of absorption energy flux per active reaction centers (ABS/RC) and dissipated energy flux per RC (DIo/RC) was found in the same experimental variants, containing soil from Gliwice (HMlow) and Piekary Śląskie. Interestingly, ABS/RC, DIo/RC, PIABS, and PItotal in the nonsterilized soil did not show any differences between Gliwice (HMlow) and Bytom (HMmod) (simultaneously with lower values for Piekary Śląskie (HMhigh)). On sterilized soil significantly higher values were found for plants cultivated in Gliwice (HMlow) soil as compared to the B_S and P_S and no significant differences were found between the variants containing excessive level of heavy metals. Regarding differences in FO values between nonsterilized experimental variants, no significant differences were found between B_NS and other nonsterilized variants, while plants grown on Gliwice (HMlow) soil showed 25% higher values when compared to P_NS. However, on sterilized variants, the highest Fo was found for P_S, while 10 and 34% lower values were found for G_S and B_S respectively. Similar pattern was seen for FM; however, B_NS differ significantly from other nonsterilized variants, and on sterilized variants, no significant differences were found between G_S and P_S. The most common JIP-test parameter, ϕPo, also known as FV/FM, showed the same pattern of changes in the sterilized and nonsterilized soil. No significant differences were found between plants cultivated in soil from Gliwice (HMlow) and Bytom (HMmod), while significantly lower values were found for plants cultivated on soil from Piekary Śląskie (HMhigh).
Gas exchange parameters
Measurements taken on the 18th day after planting showed significant differences in photosynthetic rate (A) between plants grown on nonsterilized and sterilized soils (Fig. 4a). Plants from sterilized soils showed a higher photosynthetic rate in comparison with those of nonsterilized soils, by 25%, 26%, and 31% for G_S (HMlow), B_S (HMmod) and P_S (HMhigh) plants, respectively. Among the plants cultivated on sterilized soils, the highest rate was found for B_S plants and the lowest for P_S plants. Plants cultivated on moderately HM-contaminated (HMmod) and -sterilized soil (B_S) did not differ significantly from their uncontaminated control (HMlow) (G_S); results were similar for plants grown on the sterilized soils. Analysis carried out on the 32nd day showed a decreasing trend in photosynthetic rate; here, the highest rate was observed for G_S plants with this treatment showing the smallest decrease between the 18th and 32nd day of sampling (18%). There were no significant differences between G_NS and P_NS plants on the 32nd day. The lowest photosynthetic rate was observed for both B_NS and B_S (57 and 60%, respectively). After the 45th day of cultivation the highest rate was noted for the uncontaminated G_NS and G_S plants with no significant difference between the two soil sterilization treatments. Across all soils (sterilized and nonsterilized) plants from the HM-contaminated treatments tended to have lower photosynthetic rates than those from the uncontaminated one. However, values obtained for plants cultivated on highly contaminated sterilized soil were significantly higher when compared to the other values obtained for plants grown on contaminated soils.
Effect of autochthonous microorganisms on gas exchange parameters in maize plants. Values are means ± SE (n = 16). Lower case letters (a, b, c) denote significant difference between means according to Fisher LSD test at P ≤ 0.05. A statistical analysis was performed for each day separately. G, soil from Gliwice site (HMlow); B, soil from Bytom site (HMmod); P, soil from Piekary Śląskie site (HMhigh); NS, nonsterilized soil; S, sterilized soil; DAP, day after planting; A, photosynthesis rate (a); E, transpiration rate (b); gs, stomatal conductance (c); ci, intracellular CO2 concentration (d)
Transpiration rate (E) and stomatal conductance (gs) showed a similar declining trend over time (Fig. 4b, c). Measurements taken on the 18th day showed significant differences between plants grown on nonsterilized and sterilized soils. Plants from nonsterilized soils showed a lower transpiration rate in comparison with these of sterilized soils by 13%, 19%, and 31% for G_NS, B_NS, and P_NS plants, respectively. Among plants cultivated on sterilized soils, the highest rate was found for P_S plants and the lowest for B_S plants. For plants cultivated on nonsterilized soil, E value was the highest in G_NS plants and the lowest in P_NS plants. The highest differences in E and gs values were found between the 18th and 45th day after planting—by about 60% for all variants.
Intracellular concentration of CO2 (Ci) increased over time (Fig. 4d). Analysis carried out on the 18th day sampling showed no significant differences within the HM treatments in the nonsterilized soils but did show differences between all three HM levels in the sterilized soils. Among these plants cultivated on sterilized soils, the highest rate was found for G_S (HMlow) plants and the lowest for B_S plants. After 32 days, a large increase in intracellular concentration of CO2 was seen compared to values recorded on the 18th day, particularly in G_NS plants (by 120%). In other variants, there was also high increase of Ci value by 65%, 98%, 103%, and 127%, for B_NS, B_S, P_NS, and P_S plants, respectively. On the 45th day, Ci values were generally significantly higher on the sterilized soils though the highest value here individually was seen on the nonsterilized G_NS soil. Furthermore, among plants cultivated on nonsterilized soil there were no significant differences between the two HM contamination levels B_NS and P_NS, which recorded the lowest intracellular concentration of CO2. Among plants cultivated on sterilized soils, there were no significant differences in Ci concentrations between the different HMC levels.
Plant growth parameters
Shoot length
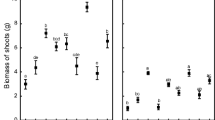
Plants grown on sterilized soils had longer shoots than those on nonsterilized soils, with the exception of P_NS and P_S, where differences in plant height were insignificant (Table 2). Among plants cultivated on sterilized soils, the highest shoot length was noted in G_S (81 ± 3 cm) plants, and the lowest in P_S plants, both were significantly different from B_S (73 ± 2 cm) plants. Among plants cultivated on nonsterilized soils, no significant differences between treatments were found.
Root and shoot biomass
Shoot biomass was generally lower for plants cultivated on nonsterilized soil in comparison with those cultivated on sterilized ones by 63%, 17%, and 36% for G_NS, B_NS, and P_NS, respectively (Table 2). Similar trends were observed for root biomass, which was lower in plants cultivated on nonsterilized soil when compared to plant cultivated on sterilized soil by 70%, 37%, and 63% for G_NS, B_NS, and P_NS experimental variants respectively. Among plants from nonsterilized soils, the highest root and shoot biomass was found for plant cultivated in Bytom soil (HMmod); however, on sterilized soil, the highest root biomass was found for plant cultivated on G_S and B_S soil while the highest shoot biomass was found for plants cultivated on G_S soil.
Oxidative stress assessment
H2O2 content in plant leaves
H2O2 content in the leaves was generally lower for plants grown in sterilized soils compared to nonsterilized soils though for B_S specifically the opposite was true (Fig. 5a). Among plants cultivated on nonsterilized and sterilized soil, the highest content of H2O2 was found on the HMhigh soils (P_NS and P_S), while the lowest content of H2O2 was found in the HMlow controls (G_NS and G_S). Maize leaves from contaminated soils typically showed H2O2 levels twice as high as their corresponding controls. Between the soil sterilization treatments there were no significant differences with the exception of HMmod, where the sterilized treatment did show a significantly higher H2O2 value compared to the nonsterilized.
Effect of autochthonous microorganisms on MDA (a) and H2O2 (b) leaves content. Values are means ± SE. (n = 4). Lower case letters (a, b, c) denote significant difference between means according to Fisher LSD test at P ≤ 0.05. G, soil from Gliwice site (HMlow); B, soil from Bytom site (HMmod); P, soil from Piekary Śląskie site (HMhigh); NS, nonsterilized soil; S, sterilized soil
MDA content in plant leaves
Similarly to the H2O2 results, MDA content in the leaves was lower in plants cultivated on sterilized soils compared to nonsterilized soils. Again, as heavy metal contamination in the soil increased, the MDA content in the leaves of both groups increased correspondingly (Fig. 5b).
Principal component analysis (PCA)
Principal component analysis (Fig. 6a, b) was performed on correlation matrix of all parameters to detect relationships between those corresponding to the efficiency of the photosynthetic apparatus (photosynthesis rate, plant pigments content), plant growth parameters, HM concentration in plant shoots (Pb, Cd, Zn), primary mineral macronutrients concentration in shoots (N, P, K), oxidative stress indicators in plant leaves (H2O2, MDA), and microbiological indicators (F%, M%, A%, DHA). The first principal component (PC1) is conditioned mostly by parameters of photosynthetic apparatus efficiency, element concentrations, and oxidative stress indicators, while the second principal component (PC2) is conditioned by plant growth parameters and microbial indicators (Fig. 6a). Parameters included in the PCA were assigned to four different correlated parameter groups. The first group contains microbial indicators (F%, M%, DHA, A%), the second group contains gas exchange measurements (A, gs, E), chlorophyll content in leaves (Chl) and two primary mineral macronutrients concentration in shoots (N and P). The third group consists of plant growth parameters with the last group consisting of HMs concentration in shoots (Pb, Cd, Zn), K concentration in shoots, anthocyanins, and oxidative stress indicators (MDA and H2O2).
Principal component analysis (PCA) distinguished into two parts: a correlation between variables along two PCA axis (PC1 × PC2) and b ordination of case along two PCA axis (PC1 × PC2). PCA was performed on correlation matrix. Closed figures correspond to plant cultivated in nonsterilized soil, while open figures corresponds to plant cultivated in sterilized soil. A white circle indicates a plant cultivated in soil from Gliwice site. A white triangle indicates plants cultivated in soil from Bytom site, a white square indicates plants cultivated in soil from Piekary Śląskie site, a blue ellipse indicates cases corresponding to plant cultivated on uncontaminated soil, a red ellipse indicates cases corresponding to plants cultivated on two contaminated soils, a green ellipse indicates cases corresponding to plants cultivated on sterilized soil, a black ellipse indicates cases corresponding to plants cultivated on nonsterilized soil, F% indicates percentage of segments showing internal colonization, M% indicates average percentage of colonization of root segments, A% indicates percentage of arbuscules in the whole root system. DHA, dehydrogenase activity; A, photosynthesis rate; E, transpiration rate; gs, stomatal conductance; Chl, leaves chlorophyll content; Anth, leaves anthocyanins content; N, shoot N concentration; P, shoot P concentration; K, shoot K concentration; Cd, shoot Cd concentration; Pb, shoot Pb concentration; Zn, shoot Zn concentration; MDA, leaves MDA concentration; H2O2, leaves H2O2 concentration; H, shoot height
According to their location in a coordination system (Fig. 6b) based on the two principal components (PC1 × PC2), it is possible to distinguish four clusters. The first two clusters distinguish between plants cultivated on HM-contaminated and -uncontaminated soils. The next two distinguish between nonsterilized and sterilized soil. It can be seen in Fig. 6b that the distance between cases corresponding to plants cultivated on sterilized and nonsterilized contaminated soils is shorter when compared to distance between cases corresponding to plants cultivated on sterilized and nonsterilized uncontaminated soil. A similar result was also seen for distance between sterilized contaminated and uncontaminated variants, which was shorter when compared to distance between nonsterilized contaminated and uncontaminated variants.
Discussion
In general, Z. mays plants were characterized by better growth and physiological status on sterilized soil as compared to the nonsterilized soil. Bowen and Rovira (1961) obtained similar results for root development on sterilized and nonsterilized soils for Solanum lycopersicon Mill., Trifolium sp. and Phalaris arundinacea L., suggesting that the presence of microorganisms reduced the primary root growth in those plants. This phenomenon could be associated with competition between plant roots and rhizosphere microorganisms for mineral N and other mineral nutrients. At the initial stage of plant development microorganisms outcompete plant roots for mineral nutrients, because of rapid growth rates and high surface-area-to-volume ratios compared with those of root hairs. However, over longer periods of cultivation microorganisms cannot compete so effectively with plants for nutrients (Kuzyakov and Xu, 2013). Another possible explanation for better growth is that nutrients could be released into soils from dead microorganisms following soil sterilization (Passioura 2002). To address this possibility, an elemental analysis was carried out on both sterilized and nonsterilized soils assuming that there is no significant fertilization effect of the sterilization.
The results obtained for nonsterilized soil indicated that with an increase of heavy metal (HM) contamination, a reduction in dehydrogenase activity (DHA) occurs. However, the differences between values obtained for the two contaminated soils were statistically insignificant. Chen et al. (2014) reported that long-term exposure of microorganisms to heavy-metal pollution results in a decrease of microbial biomass and corresponding activity as well as a reduction of microbial community diversity. This suggestion is in agreement with our observation of significantly lower arbuscular bycorrhiza fungi (AMF) root colonization and DHA on heavy metal-contaminated (HMC) soils as compared to the uncontaminated soil. Previous studies from Zn- and Pb-mining regions showed a strong negative effect of soil HM contamination on AMF abundance and diversity (Zarei et al. 2010). In addition, our results suggest that AMF on the contaminated soils displayed Paris-type mycorrhiza, while on uncontaminated soil AMF followed the Arum type, agreeing with results reported by Vogel-Mikuš et al. (2005). Microscopic root observation in our study also indicated the presence of DSE and microsclerotia on both contaminated soils, while there were no visible such structures on samples from uncontaminated soil. Zhang et al. (2013) reported that in most plants grown on lead and zinc slag heaps the colonization of roots by DSE appeared. In addition, presence of DSE in Piekary Śląskie (HMhigh) soil was previously reported by Gucwa-Przepióra et al. (2013). Those endophytes may be integral to the function of HM-contaminated ecosystems due to their function as replacements or complements of mycorrhizae damaged by pollutants (Zhang et al. 2013). Despite successful sterilization of soil, low DHA was observed for these sterilized soils which could be caused by secondary soil contamination by deposition of microbes from the air (Despres et al. 2012).
Besides soil specificity, there were almost no differences in mineral macronutrient concentration in shoots between nonsterilized and sterilized variants, except K and Ca. The lower K and Ca concentrations in shoots were observed on sterilized soils as compared to nonsterilized soils. However, due to higher biomass production on sterilized soils, competition between plants and microorganisms for mineral nutrients cannot be excluded. The phenomenon of competition between plants and microorganisms for N was previously reviewed by Kuzyakov and Xu (2013). In addition, it was found that on HMhigh-sterilized soil (P_S), there were considerably higher shoot N concentrations when compared to the nonsterilized equivalent (P_NS). Although lower K and Ca concentration in plant shoots grown on sterilized soil could be associated with higher Cd shoot concentrations in plants cultivated on HMC soils (Ghnaya et al. 2007; Małkowski et al. 2005), the considerably higher shoot N concentrations on HMhigh-sterilized soil when compared to HMhigh-nonsterilized soil was an unexpected result. We hypothesize that such differences between P_NS and P_S could be driven by a combined effect of HM toxicity and plant-microbe competition for mineral nutrients on the nonsterilized HMhigh soil.
Higher concentration of Cd in shoots was found in both contaminated sterilized soils, whereas it was not observed in HMlow soil (G_S). This phenomenon may be associated with a low concentration of Cd in a bioavailable forms in uncontaminated soil. Distinct results were observed for Zn shoot concentrations, where significantly higher concentrations were obtained only in soil where Zn concentration was the highest (P_S). There are many reports which show the impact of AMF on reduction of HM translocation due to their immobilization on root surface by AMF hyphae (Abdelmoneim et al. 2014, Ban et al. 2015, Wu et al. 2016). Thus, it is supposed that observed in our current study, the higher concentration of Cd and Zn (only for P_S) in shoots of maize grown on sterilized and contaminated soils is a result of very low root colonization by AMF due to soil sterilization.
Direct measurements of photosynthetic rate clearly indicated an inhibition of this process due to an accumulation of HM in plant tissues, which has been widely reported (Farooq et al. 2013; Hattab et al. 2009; Mobin and Khan 2007). Slightly higher photosynthetic rates and higher FM in plants grown on the P_S (HMhigh) soils when compared to the B_S (HMmod) could be related to higher N accumulation in shoots. Previous studies have suggested a correlation between chlorophyll content and N concentration in maize shoots (Schlemmer et al. 2013), which is with agreement to results obtained for chlorophyll content at the 45th day and the N concentration at the end of the experiment. Considerably higher concentrations of mineral nutrients, particularly N, could slightly mask the toxic effect of HM on the photosynthetic apparatus from sterilized HMhigh soils (P_S). The differences between trends obtained for direct photosynthetic rate (A) and the efficiency of the light-dependent photosynthesis phase, obtained from chlorophyll a fluorescence analysis, may be associated with a higher impact of HM on enzymatic activity in the light independent phase when compared to its influence on the electron transport chain during light-dependent phase (Dias et al. 2013; Rodriguez et al. 2015).
Despite the fact that slight differences appeared at absorption energy flux per active reaction center (ABS/RC) and dissipated energy flux per reaction center (DIo/RC) between contaminated and uncontaminated variants, which influenced on the other parameters (φPo, PIABS, PItotal), no differences were found in further electron transport chain at parameters related to flux of excitation energy trapped per active RC (TRo/RC) and electron transport flux per RC (ETo/RC) as well as on JIP-test parameters related to the PSI functionality (data not shown). Interestingly, OJIP-test parameters indicated that a more pronounced effect was found when comparing sterilized with nonsterilized experimental variants. However, within this comparison, changes were only visible between uncontaminated (HMlow) variants (G_NS vs. G_S) and highly contaminated (HMhigh) variants (P_NS vs. P_S). This observation may suggest that the competition between plant and microorganisms has a greater effect on plant growth and photosynthesis than the toxic effect of heavy metals.
This hypothesis explaining the slower growth of plants due to competition for nutrients between plants and autochthonous microorganisms seems to be reasonable taking into account the chlorophyll fluorescence analysis. This technique was previously used to assess the effect of nutrient deficiencies in the medium where plants were cultivated (Cetner et al. 2017; Kalaji et al. 2014; Kalaji et al. 2018; Tang et al. 2012). Kalaji et al. 2018 reported for rapeseed plants cultivated in soil with different mineral nutrients concentration that deficiencies of nutrients resulted in increase in ABS/RC even at low nutrients deficiencies; moreover, the authors found that there was increase in the K band at O-J phase. Those results are in agreement with phenomena observed between sterilized and nonsterilized soils in our study, i.e., nonsterilized soils may suffer a slight deficit due to competition. A decrease in the number of active reaction centers (RC) was considered as the mechanism of nutrient-deficient leaves against photo-oxidative damage and an excess of absorbed light energy, while the appearance of the K band denoted damage to the oxygen-evolving complex (Kalaji et al. 2014; Kalaji et al. 2018).
Transpiration rate, as well as stomatal conductance, decreased during the experiment, irrespectively of HM accumulation. The same trend was observed by Ci et al. (2010) and Anjum et al. (2016, 2017) for plants treated with different concentrations of Cd. The concentration of intracellular CO2 increased with the aging of plants and accumulation of HMs, which corresponds with results obtained by Tian et al. (2012). It has been shown that Cd concentration decreases the activity of enzymes involved in carboxylation reactions (e.g., RuBPCase) (Siedlecka et al. 1998), which could be related to an increased concentration of intracellular CO2. Values recorded for plant pigments and gas exchange parameters on the 18th and 32nd day could indicate the plants striving for an equilibrium state during acclimation in soil to varying environment conditions. This phenomenon is most visible in chlorophyll and anthocyanins content and photosynthesis rate. On the 18th day, sampling plant pigments did not differ significantly, while by the 32nd day, those parameters gave values characteristic for HM impact on plants, especially on sterilized experimental variants, while on nonsterilized variants this equilibrium was shifted in time. A similar phenomenon was observed for photosynthesis rate; however, on the 18th day, the highest values were recorded for plants cultivated on Bytom soils and the lowest for plants cultivated in Piekary Śląskie soils. On the 32nd day, the photosynthesis rate seems to stabilize at the levels which can suggest a toxic effect of HM on photosynthetic apparatus efficiency (Farooq et al. 2013). This phenomenon could be associated with a delay in plant reaction to the harsh environment due to reaching a critical concentration of ROS after some time from acclimation. It agrees with the suggestion by Poschenrieder et al. (2013) that metal induced ROS production can trigger antioxidant defenses that induce hormetic responses under certain conditions. However, once toxicity thresholds are passed, the ROS-induced scavenging system will be overcome and the negative effects on the antioxidant system and growth inhibition will occur.
The most common indicators of oxidative stress in plants are contents of H2O2 and malonedialdehyde (MDA) (Bidar et al. 2007; Rizwan et al. 2017; Wu et al. 2017; Zhang et al. 2007). However, there is also evidence for an increase of anthocyanin content under HM stress (Chen et al., 2015; Mobin and Khan 2007). Our results show clearly that cultivation of Z. mays on contaminated soil results in increased levels of H2O2, MDA and anthocyanin in leaves, corresponding to HM concentration in soil. Moreover, anthocyanin measurements suggest that differences between experimental variants appears earlier in sterilized treatments with anthocyanin content being lower in comparison to the nonsterilized treatments. Lower values of MDA and H2O2 concentrations in leaves were also obtained for sterilized soils at the end of the experiment. Zhang et al. (2007) reported that H2O2 accumulation in clover roots occurred in response to colonization by AMF. The similar tendencies obtained for MDA are likely driven by strong connectivity between H2O2 concentration and lipid peroxidation (Shahid et al. 2014).
Based on the results obtained from PCA, it could be assumed that autochthonous microorganisms are key players driving differences between plants cultivated in soil with different levels of contamination. In addition, differences between sterilized and nonsterilized variants were more apparent when higher microbial activity in the nonsterilized treatment was present, possibly demonstrating competition between plants and microorganisms.
Conclusions
Our results showed that steam sterilization did not have a significant effect on soil physicochemical properties. The average percentage of colonization of root segments by arbuscular mycorrhiza fungi (AMF) decreased with the increase of heavy metals (HM) concentration in soil by more than 50% depending on the level of contaminants. Alongside a decrease in AMF colonization due to sterilization there was significant increase in Cd shoot concentration by about 119 and 228% for B_S and P_S variants, respectively, when compared to nonsterilized counterparts. However, Zn plant shoot concentration was significantly higher by 68% only for P_S variant, when compare to nonsterilized counterpart. Dark septate endophytes were only present in roots of plants cultivated in HMhigh soils. Lower biomass yields on nonsterilized soil could be the result of competition between plants and microorganisms for mineral nutrients. Anthocyanins content could be good indicator of HM-induced oxidative stress due to its correlation with MDA and H2O2 leaf content. Autochthonous microflora seems to be a key player in determining differences between Zea mays physiological status cultivated on the sterilized and nonsterilized contaminated soils, having a negative impact on growth and photosynthetic performance, likely due to competition with plants for nutrients. This observation tends to overthrow the hypothesis that autochthonous microorganisms improve plant growth and photosynthetic performance. However, a lack of these microorganisms in the same soils considerably increased plants HM concentration, particularly Cd and Zn.
References
Abdelmoneim TS, Moussa TA, Almaghrabi OA, Abdelbagi I (2014) Investigation the effect of arbuscular mycorrhizal fungi on the tolerance of maize plant to heavy metals stress. Life Sci J 11:255–263
Andrade SA, da Silveira AP (2008) Mycorrhiza influence on maize development under Cd stress and P supply. Braz J Plant Physiol 20:39–50
Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L (2017) Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut 228:13. https://doi.org/10.1007/s11270-016-3187-2
Anjum SA, Tanveer M, Hussain S, Wang L, Khan I, Samad RA, Tung SA, Anam M, Shahzad B (2016) Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. CLEAN Soil Air Water 44:29–36
Baćmaga M, Borowik A, Kucharski J, Tomkiel M, Wyszkowska J (2015) Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican+mesosulfuron-methyl+iodosulfuron-methyl-sodium. Environ Sci Pollut Res Int 22:643–656
Bai J, Lin X, Yin R, Zhang H, Junhua W, Xueming C, Yongming L (2008) The influence of arbuscular mycorrhizal fungi on As and P uptake by maize (Zea mays L.) from As-contaminated soils. Appl Soil Ecol 38:137–145
Ban Y, Xu Z, Zhang H, Chen H, Tang M (2015) Soil chemistry properties, translocation of heavy metals, and mycorrhizal fungi associated with six plant species growing on lead-zinc mine tailings. Ann Microbiol 65:503–515
Bidar G, Garcon G, Pruvot C, Dewaele D, Cazier F, Douay F, Shirali P (2007) Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: plant metal concentration and phytotoxicity. Environ Pollut 147:546–553
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun 1:48. https://doi.org/10.1038/ncomms1046
Bothe H, Regvar M, Turnau K (2010) Arbuscular mycorrhiza, heavy metal, and salt tolerance. In: Sherameti I, Varma A (eds) Soil heavy metals. Springer, Berlin, Heidelberg, pp 87–111
Bowen GD, Rovira AD (1961) The effects of micro-organisms on plant growth. Plant Soil 15:166–188
Bremner JM (1996) Nitrogen – total. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis, part 3: chemical method. American Society of Agronomy and Soil Science Society of America, Madison, Wisconsin, USA, pp 1085–1121
Cabral C, Ravnskov S, Tringovska I, Wollenweber B (2016) Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 408:385–399
Casida LE Jr, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Cetner MD, Kalaji HM, Goltsev V, Aleksandrov V, Kowalczyk K, Borucki W, Jajoo A (2017) Effects of nitrogen-deficiency on efficiency of light-harvesting apparatus in radish. Plant Physiol Biochem 119:81–92
Chen J, He F, Zhang X, Sun X, Zheng J, Zheng J (2014) Heavy metal pollution decreases microbial abundance, diversity and activity within particle-size fractions of a paddy soil. FEMS Microbiol Ecol 87:164–181
Chen YE, Cui JM, Yang JC, Zhang ZW, Yuan M, Song C, Yang H, Liu HM, Wang CQ, Zhang HY, Zeng XY, Yuan S (2015) Biomonitoring heavy metal contaminations by moss visible parameters. J Hazard Mater 296:201–209
Ci D, Jiang D, Wollenweber B, Dai T, Jing Q, Cao W (2010) Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant 32:365–373
Després V, Huffman JA, Burrows SM, Hoose C, Safatov A, Buryak G, Frӧhlich-Nowoisky J, Elbert W, Andreae MO, Pӧschl U, Jaenicke R (2012) Primary biological aerosol particles in the atmosphere: a review. Tellus Ser B Chem Phys Meteorol 64:15598. https://doi.org/10.3402/tellusb.v64i0.15598
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Gonçalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant 35:1281–1289
Egnér H, Riehm H, Domingo WR (1960) Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. Kungliga Lantbrukshögskolans Annaler 26:199–215
Ercoli L, Schüßler A, Arduini I, Pellegrino E (2017) Strong increase of durum wheat iron and zinc content by field-inoculation with arbuscular mycorrhizal fungi at different soil nitrogen availabilities. Plant Soil 419:153–167
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Firmin S, Labidi S, Fontaine J, Laruelle F, Tisserant B, Nsanganwimana F, Pourrut B, Dalpe Y, Grandmougin A, Douay F, Shirali P, Verdin A, Lounes-Hadj Sahraoui A (2015) Arbuscular mycorrhizal fungal inoculation protects Miscanthus × giganteus against trace element toxicity in a highly metal-contaminated site. Sci Total Environ 527:91–99
Ghnaya T, Slama I, Messedi D, Grignon C, Ghorbel MH, Abdelly C (2007) Effects of Cd2+ on K+, Ca2+ and N uptake in two halophytes Sesuvium portulacastrum and Mesembryanthemum crystallinum: consequences on growth. Chemosphere 67:72–79
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giller KE, Witter E, McGrath SP (2009) Heavy metals and soil microbes. Soil Biol Biochem 41:2031–2037
Goltsev VN, Kalaji HM, Paunov M, Bąba W, Horaczek T, Mojski J, Kociel H, Allakhverdiev SI (2016) Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ J Plant Physiol 63:869–893
Gucwa-Przepiora E (2012) Udział mikoryzy arbuskularnej w procesach fitoremediacji-mikoryzoremediacja. Wiadomości Botaniczne 56
Gucwa-Przepióra E, Blaszkowski J, Kurtyka R, Malkowski L, Malkowski E (2013) Arbuscular mycorrhiza of Deschampsia cespitosa (Poaceae) at different soil depths in highly metal-contaminated site in southern Poland. Acta Soc Bot Pol 82:251–258
Guo W, Zhao R, Zhao W, Fu R, Guo J, Bi N, Zhang J (2013) Effects of arbuscular mycorrhizal fungi on maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) grown in rare earth elements of mine tailings. App Soil Ecol 72:85–92
Haney CH, Samuel BS, Bush J, Ausubel FM (2015) Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat Plants 1:15051. https://doi.org/10.1038/nplants.2015.51
Hattab S, Dridi B, Chouba L, Kheder MB, Bousetta H (2009) Photosynthesis and growth responses of pea Pisum sativum L. under heavy metals stress. J Environ Sci 21:1552–1556
Huang H, Zhang S, Wu N, Luo L, Christie P (2009) Influence of Glomus etunicatum/Zea mays mycorrhiza on atrazine degradation, soil phosphatase and dehydrogenase activities, and soil microbial community structure. Soil Biol Biochem 41:726–734
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol Environ Saf 104:285–293
Islam F, Yasmeen T, Ali Q, Mubin M, Ali S, Arif MS, Hussain S, Riaz M, Abbas F (2016) Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: potential for bacterial bioremediation. Environ Sci Pollut Res 23:220–233
ISO 11277:2009 - Soil quality - Determination of particle size distribution in mineral soil material - Method by sieving and sedimentation
ISO 13878:1998 - Soil quality - Determination of total nitrogen content by dry combustion (“elemental analysis”)
Kalaji HM, Bąba W, Gediga K, Goltsev V, Samborska IA, Cetner MD, Dimitrova S, Piszcz U, Bielecki K, Karmowska K, Dankov K, Kompała-Bąba A (2018) Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth Res 136:329–343
Kalaji HM, Oukarroum A, Alexandrov V, Kouzmanova M, Brestic M, Zivcak M, Samborska IA, Cetner MD, Allakhverdiev SI, Goltsev V (2014) Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol Biochem 81:16–25
Köhl L, Lukasiewicz CE, Heijden MG (2016) Establishment and effectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant Cell Environ 39:136–146
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Li H, Luo N, Zhang LJ, Zhao HM, Li YW, Cai QY, Wong MH, Mo CH (2016) Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Sci Total Environ 571:1183–1190
Liang CC, Li T, Xiao YP, Liu MJ, Zhang HB, Zhao ZW (2009) Effects of inoculation with arbuscular mycorrhizal fungi on maize grown in multi-metal contaminated soils. Int J Phytoremediat 11:692–703
Liu T, Wang C, Chen H, Fang F, Zhu X, Tang M (2014) Effects of arbuscular mycorrhizal colonization on the biomass and bioenergy production of Populus × canadensis ‘Neva’in sterilized and unsterilized soil. Acta Physiol Plant 36:871–880
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Małkowski E, Kurtyka R, Kita A, Karcz W (2005) Accumulation of Pb and Cd and its effect on Ca distribution in maize seedlings (Zea mays L.). Pol J Environ Stud 14:203–207
Miransari M, Bahrami HA, Rejali F, Malakouti MJ (2009) Effects of soil compaction and arbuscular mycorrhiza on corn (Zea mays L.) nutrient uptake. Soil Tillage Res 103:282–290
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Mohite B (2013) Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutri 13:638–649
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448
Oliveira T, Mucha AP, Reis I, Rodrigues P, Gomes CR, Almeida CMR (2014) Copper phytoremediation by a salt marsh plant (Phragmites australis) enhanced by autochthonous bioaugmentation. Mar Pollut Bull 88:231–238
Passioura JB (2002) Soil conditions and plant growth. Plant Cell Environ 25:311–318
Peijnenburg WJ, Zablotskaja M, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf 67:163–179
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158IN16–161IN18
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review Biol Fertil Soils 51:403–415
PN-ISO 11265:1997. Soil quality – Electrical conductance assessment (in Polish)
Pogrzeba M, Rusinowski S, Sitko K, Krzyżak J, Skalska A, Małkowski E, Werle S, McCalmont JP, Mos M, Kalaji HM (2017) Relationships between soil parameters and physiological status of Miscanthus x giganteus cultivated on soil contaminated with trace elements under NPK fertilisation vs. microbial inoculation. Environ Pollut 225:163–174
Poschenrieder C, Cabot C, Martos S, Gallego B, Barceló J (2013) Do toxic ions induce hormesis in plants? Plant Sci 212:15–25
Rizwan M, Ali S, Qayyum MF, Ok YS, Rehman MZ, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Hlth 39:259–277
Rodriguez E, Santos MDC, Azevedo R, Correia C, Moutinho-Pereira J, Miguel J, de Oliveira JMPF, Dias MC (2015) Photosynthesis light-independent reactions are sensitive biomarkers to monitor lead phytotoxicity in a Pb-tolerant Pisum sativum cultivar. Environ Sci Pollut Res Int 22:574–585
Rostami S, Azhdarpoor A, Rostami M, Samaei MR (2016) The effects of simultaneous application of plant growth regulators and bioaugmentation on improvement of phytoremediation of pyrene contaminated soils. Chemosphere 161:219–223
Schlemmer M, Gitelson A, Schepers J, Ferguson R, Peng Y, Shanahan J, Rundquist D (2013) Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int J Appl Earth Obs Geoinf 25:47–54
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, Springer international publishing, volume, vol 232, pp 1–44
Siedlecka A, Samuelsson G, Gardenstrom P, Kleczkowski LA, Krupa Z (1998) The activatory model of plant response to moderate cadmium stress-relationship between carbonic anhydrase and Rubisco. Photosynthesis: Mechanisms and Effects 4:2677–2680
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, (eds.) Chlorophyll a Fluorescenc. Springer, Netherlands, pp 321-362
Sułowicz S, Płociniczak T, Piotrowska-Seget Z, Kozdrój J (2011) Significance of silver birch and bushgrass for establishment of microbial heterotrophic community in a metal-mine spoil heap. Water Air Soil Pollut 214:205–218
Tang N, Li Y, Chen L-S (2012) Magnesium deficiency-induced impairment of photosynthesis in leaves of fruiting Citrus reticulata trees accompanied by up-regulation of antioxidant metabolism to avoid photo-oxidative damage. J Plant Nutr Soil Sci 175:784–793
Thijs S, Van Dillewijn P, Sillen W, Truyens S, Holtappels M, Haen J, Carleer R, Weyens N, Ameloot M, Ramos JL, Vangronsveld J (2014) Exploring the rhizospheric and endophytic bacterial communities of Acer pseudoplatanus growing on a TNT-contaminated soil: towards the development of a rhizocompetent TNT-detoxifying plant growth promoting consortium. Plant Soil 385:15–36
Tian Y, Zhang H, Guo W, Chen Z, Wei X, Zhang L, Han L, Dai L (2012) Assessment of the phytoremediation potential of bioenergy crop maize (Zea mays) in soil contaminated by cadmium: morphology, photosynthesis and accumulation. Fresenius Environ Bull 21:3575–3581
Touceda-González M, Prieto-Fernández Á, Renella G, Giagnoni L, Sessitsch A, Brader G, Kumpiene J, Dimitriou I, Eriksson J, Friesl-Hanl W, Galazka R, Jamssem J, Mench M, Muller I, Neu S, Puschenreiter M, Siebielec G, Vangronsveld J, Kidd PS (2017) Microbial community structure and activity in trace element-contaminated soils phytomanaged by Gentle Remediation Options (GRO). Environ Pollut 231:237–251
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi P (eds) Physiological and genetical aspects of mycorrhizae. INRA, Paris, pp 217–221
Valliere JM, Allen EB (2016) Interactive effects of nitrogen deposition and drought-stress on plant-soil feedbacks of Artemisia californica seedlings. Plant Soil 403:277–290
Verma JP, Yadav J, Tiwari KN, Jaiswal DK (2014) Evaluation of plant growth promoting activities of microbial strains and their effect on growth and yield of chickpea (Cicer arietinum L.) in India. Soil Biol Biochemist 70:33–37
Vivas A, Vörös I, Biró B, Campos E, Barea JM, Azcón R (2003) Symbiotic efficiency of autochthonous arbuscular mycorrhizal fungus (G. mosseae) and Brevibacillus sp. isolated from cadmium polluted soil under increasing cadmium levels. Environ Pollut 126:179–189
Vogel-Mikuš K, Drobne D, Regvar M (2005) Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonisation of pennycress Thlaspi praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environ Pollut 133:233–242
Wang FY, Lin XG, Yin R (2007) Effect of arbuscular mycorrhizal fungal inoculation on heavy metal accumulation of maize grown in a naturally contaminated soil. Int J Phytoremediation 9:345–353
Wang Y, Fang L, Lin L, Luan T, Tam NF (2014) Effects of low molecular-weight organic acids and dehydrogenase activity in rhizosphere sediments of mangrove plants on phytoremediation of polycyclic aromatic hydrocarbons. Chemosphere 99:152–159
Watts-Williams SJ, Tyerman SD, Cavagnaro TR (2017) The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: linking plant physiology and gene expression. Plant Soil 420:375–388
Wolińska A, Stępniewska Z (2012) Dehydrogenase activity in the soil environment. In: Canuto RA (ed) Dehydrogenases. InTech, https://doi.org/10.5772/48294. Available from: https://www.intechopen.com/books/dehydrogenases/dehydrogenase-activity-in-the-soil-environment
Wu S, Zhang X, Chen B, Wu Z, Li T, Hu Y, Sun Y, Wang Y (2016) Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ Exp Bot 122:10–18
Wu Z, Liu S, Zhao J, Wang F, Du Y, Zou S, Li H, Wen D, Huang Y (2017) Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ Exp Bot 133:1–11
Yang Q, Li B, Siemann E (2015) The effects of fertilization on plant-soil interactions and salinity tolerance of invasive Triadica sebifera. Plant Soil 394:99–107
Yu X, Li Y, Zhang C, Liu H, Liu J, Zheng W, Kang X, Leng X, Zhao K, Gu Y, Zhang X, Xiang Q, Chen Q (2014) Culturable heavy metal-resistant and plant growth promoting bacteria in V-Ti magnetite mine tailing soil from Panzhihua, China. PLoS One 9:e106618. https://doi.org/10.1371/journal.pone.0106618
Zarei M, Hempel S, Wubet T, Schäfer T, Savaghebi G, Jouzani GS, Nekouei MK, Buscot F (2010) Molecular diversity of arbuscular mycorrhizal fungi in relation to soil chemical properties and heavy metal contamination. Environ Pollut 158:2757–2765
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Zhang Y, Li T, Zhao ZW (2013) Colonization characteristics and composition of dark septate endophytes (DSE) in a lead and zinc slag heap in Southwest China. Soil Sediment Contam Int J 22:532–545
Zhu XC, Song FB, Liu SQ, Liu TD, Zhou X (2012) Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ 58:186–191
Zhu XC, Song FB, Xu HW (2010) Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 331:129–137
Funding
This work was supported by the Polish Ministry of Science and Higher Education (Institute for Ecology of Industrial Areas statutory funds).
Author information
Authors and Affiliations
Contributions
S.R. (60%)—designed and maintained most of the experiment, statistical analyses, wrote first draft of the manuscript, A.S.-B., P.Z.-R., G.W., K.S., M.S. (2%)—conducted the experiment (soil and plant material elemental analysis, AMF colonization), E.M., J.P.M., H.M.K. (4%)—manuscript improvements and language corrections, J.K., M.P. (9%)—experiment design, statistical analysis, wrote first draft of the manuscript. All authors contributed to revisions and gave final approval for publication
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gangrong Shi
Electronic supplementary material
ESM 1
(DOCX 2.22 mb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rusinowski, S., Szada-Borzyszkowska, A., Zieleźnik-Rusinowska, P. et al. How autochthonous microorganisms influence physiological status of Zea mays L. cultivated on heavy metal contaminated soils?. Environ Sci Pollut Res 26, 4746–4763 (2019). https://doi.org/10.1007/s11356-018-3923-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3923-9