Abstract
The presence and composition of soil microbial communities has been shown to have a large impact on plant–plant interactions and consequently plant diversity and composition. The goal of the present study was to evaluate impact of arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing bacteria, which constitutes an essential link between the soil and the plant’s roots. A greenhouse pot experiment was conducted to evaluate the feasibility of using selected microbes to improve Hieracium pilosella and Medicago sativa growth on Zn–Pb-rich site. Results of studies revealed that biomass, the dry mass of shoots and roots, increased significantly when plants were inoculated with mycorrhizal fungi and nitrogen-fixing bacteria. The addition of Azospirillum sp. and Nostoc edaphicum without mycorrhiza suppressed plant growth. Single bacterial inoculation alone does not have a positive effect on M. sativa growth, while co-inoculation with AMF improved plant growth. Plant vitality (expressed by the performance index) was improved by the addition of microbes. However, our results indicated that even dry heat sterilization of the substratum created imbalanced relationships between soil-plant and plants and associated microorganisms. The studies indicated that AMF and N2-fixers can improve revegetation of heavy metal-rich industrial sites, if the selection of interacting symbionts is properly conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental problems arising from tailings containing heavy metals are windblown dust dispersal, leaching of contaminants into surface and groundwaters. Phytoremediation strategies aims to decrease the environmental impact from the heavy metal laden waste by establishing vegetation cover over the degraded area. However, plant growth is often inhibited due to metal toxicity and a combination of factors including low nutrient levels, acidity or alkalinity, poor water holding capacity, and poor physical structure. Unfavorable air–water conditions lead to wind erosion in dry periods as well as soil erosion during rainfall (Bradl 2004; Turnau et al. 2012). Plants and plant-associated microbes are involved in many biogeochemical processes operating in the rhizosphere. Plants themselves alter soil chemistry through changes in pH and redox conditions (Alford et al. 2010). They also release various secondary metabolites including inorganic and organic compounds that contribute to nutrient acquisition, accelerating metal mobility or immobilization (Bais et al. 2006; Toljander et al. 2007). Plants are naturally associated with microorganisms whose microbial communities can directly or indirectly affect metal mobility, availability, and uptake of elements. Microbial consortia including mycorrhizal fungi and nitrogen-fixing bacteria could facilitate the survival of their host plants growing on metal-contaminated sites, by producing growth-stimulating substances and/or by conferring increased tolerance to stress (Doornbos et al. 2012; Rajkumar et al. 2012). It has been shown that co-inoculation with arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing bacteria is a promising approach to favor the establishment and survival of legume plants in poor soils (Tsimilli-Michael et al. 2000; Lin et al. 2007; Franzini et al. 2010). However, little is known about the effects of mixed microbial consortia on the establishment of legumes under heavy metal stress conditions. The aim of this study was to screen microbial interactions, which would take place in the rhizosphere of selected plant species grown on Zn–Pb-rich substrate. Hieracium taxon was chosen as a model plant genus since it occurs in a large variety of habitats and the genus have a worldwide distribution. In open environments, Hieracium pilosella shows vigorous clonal growth, resulting in the formation of dense mats. The leguminous species Medicago sativa has been suggested as a good candidate for remediation of metal-rich tailings primarily due to its ability to fix atmospheric N2 and increase the pH of acid soils (Gardea-Torresdey et al. 1998; Mar Vázquez et al. 2000; Turnau et al. 2012). The remediation research has been carried out in Zn–Pb Trzebionka tailing since 1990 (Turnau et al. 2012). The study employed a full factorial experiment on H. pilosella, legume M. sativa, and microbial communities derived from Zn–Pb-rich tailing. Substrate sterilization procedures have been used to study the effect of introduced microbes on plant growth and to eliminate the influence of other soil microbial communities including soil-borne plant pathogens. Plants were inoculated with combinations of selected microbes such as Rhizophagus irregularis (syn. Glomus intraradices) and indigenous strains of Azospirillum sp. and Nostoc edaphicum. The concept of single, dual, and/or multilevel co-inoculations was studied in relation to plant performance. The study was performed to (1) assess the effectiveness of microbial inoculation and co-cropping in the formation of a more stable vegetation cover on Zn–Pb tailings, (2) determine interaction between plants and microbial consortia, (3) determine possible interactions of co-cropped plants: alfalfa (M. sativa) and hawkweed (H. pilosella) growing together on contaminated site, (4) check whether microbial inoculation could be useful in establishment of plants on the Zn–Pb-rich substratum, (5) select the most efficient combination of microbial inoculation to improve plant growth on tailing, and (6) compare the effect of microbial inoculation on growth and photosynthetic parameters of plants grown on non-sterilized and dry heat sterilized tailing.
Materials and methods
Site characterization
The ZG Trzebionka Mine Company is located near Chrzanów (Southern Poland, 30 km west of Kraków, N 50° 09′, E 19° 25′). The Chrzanów district has a long history of metal ore mining. During the twentieth century, extraction of Zn–Pb ores was carried out by the ZG Trzebionka Mine Company. The Trzebionka ore field is located in the SE part of the Silesian–Cracow ore district. Zinc and lead ore were extracted from Mississippi Valley-type mines where deposits were located in Triassic dolomites. These are strata-bound tabular ore bodies with very complex internal structures. The primary ore minerals are sphalerite (ZnS) and galena (PbS) accompanied by iron sulfides, cerussite, smithsonite, and hemimorphite. The wall rock is crystalline dolostone, called “ore-bearing dolomite.” The ore grade is low: 4.2 % Zn and 1.7 % Pb (on average) (Mucha and Szuwarzyński 2004). Since 1970, excavated ores have been subjected to flotation processes. The waste material produced as a by-product of flotation was deposited 1 km from the ore extraction site over ca. 40 years and resulted in the formation of a 60 m high and 64 ha area of tailing. Each year, 2.2 million tons of ores were extracted (Szuwarzyński 1993). Extraction activity was terminated in 2009 when resources were exhausted. The slopes were constructed from the coarser grained fractions of wastes, separated in hydrocyclones (Turnau et al. 2012). The ponds are now a danger to the environment because of the eolian erosion taking place on the surrounding slopes and on the dried out portion of the plateaus above the slopes. Low porosity results in unfavorable air–water conditions, restricted water infiltration during rainfall, and restricted water recharge by capillary rise from deeper layers during dry periods. These conditions favor wind erosion in dry periods and water erosion during rainfall (Trafas 1996). The list of vascular plant species recorded on ZG Trzebionka zinc wastes were presented at Turnau et al. 2012.
Waste material characterization
The chemical composition of the tailing material is unfavorable for plant growth because the carbonate content exceeds 75 %. High concentrations of Ca2+ and SO4 2− ions together with low concentrations of Na+, K+, Mg2+, Cl−, and HCO3 − ions are also typical for this site. Original tailing material contains no organic matter and is P- and N-deficient. The pH of the waste ranges from 7 to 8. Growth substratum from the Zn–Pb tailing contained high concentrations of heavy metals: 468 μg g−1 cadmium, 7068 μg g−1 lead, and 53,303 μg g−1 zinc. The analysis of metal content in substratum was done using total reflection X-ray fluorescence (TXRF) (Turnau et al. 2008). The soil moisture ranged from 0.06 to 0.15 m3 m−3 (Ryszka and Turnau 2007).
AMF inoculation
The AMF inocula used was R. irregularis UNIJAG PL. 30/BR 1 obtained from the AMF collection (Jagiellonian University, Kraków). R. irregularis was propagated on stock cultures with Zea mays for 6 months. Colonized root fragments, mycelium, and a sand–soil mixture containing propagules (ca. 100 propagules g−1 of substratum) were used as inoculum.
Isolations of plant growth-promoting rhizobacteria
Both associated bacteria (Azospirillum sp. and N. edaphicum) were isolated from native ecotype of H. pilosella and M. sativa roots/rhizosphere that were collected at the Zn–Pb-rich tailing. The bacterial strains were isolated following protocols for PGPR isolation (Bashan et al. 1993). Fifteen strains of rhizobacteria and five strains of diazotrophic bacteria were obtained after isolation. In in vitro cultures, plants were subcultured with bacterial strains on the modified Strullu–Romand medium (MSR) (Promega Benelux, Leiden, The Netherlands). Cultures were cultivated for 2 months in a growth chamber at 24 °C, under a 12/12 h light regime. Two strains of N2-fixers: Azospirillum sp. and N. edaphicum were selected for further examination. Taxonomic identification was based on cell/colony morphologies using the following references: (Starmach 1966; Ettl and Gärtner 1995; Hoek 1995; Hindák 1996; Jeffery et al. 2010).
Azospirillum sp.
The bacterial inoculum of Azospirillum sp. was grown on nutrient broth (Difco Bacto, USA). Flasks were incubated at 26 °C, for 36 h on a rotary shaker (170 rpm). Actively growing cells were then washed three times with sterile phosphate-buffered saline (100 mM phosphate buffer; 0.85 % NaCl; pH 7.0) by centrifugation (10 min, 15,000×g) (Murty and Ladha 1988). The washed cells were resuspended in the same buffered saline as described earlier to a final concentration of about 108 colony-forming units (cfu)/ml. Bacterial suspension with an optical density at 600 nm (OD600) corresponded to a final concentration of ∼108 bacteria/ml. Twenty milliliters of the bacterial inoculation suspensions was poured onto the substrate surface of each pot to initiate infection and subsequent nodulation. Control treatments were inoculated with sterilized (121 °C, 20 min, 1 bar) inoculation suspension.
Nostoc edaphicum Kondrateva
Cyanobacteria, N. edaphium, were grown in 250-ml Erlenmeyer flasks containing 100 ml of Jaworski medium at pH 7.0. The flasks were incubated at 26 °C, for 3 weeks on a rotary shaker (120 rpm). Actively growing cells were harvested by centrifugation (10 min, 15,000×g) then washed three times with sterile physiological saline (9 g l−1 NaCl) and resuspended in sterile. Treatments without cyanobacteria were treated in the same way with sterilized (121 °C, 20 min, 1 bar) inoculation suspension. Twenty milliliters of the bacterial inoculation suspensions was poured onto the substrate surface of each pot.
Experimental design
A greenhouse experiment utilizing a full factorial randomized block design was implemented. The experiment was conducted with the use of two plant species (H. pilosella L. and M. sativa L.). H. pilosella was chosen as a model plant based on observations made on ZG Trzebionka site where this plant grows vigorously. Its main way of spreading is clonal growth. It usually formed new seedlings on the top of the partly dried tufts, and the new ramets were formed outside. Such ramets sometimes disappeared while it was hot and dry, but after the rain, they were usually rebuilt from the remaining parts. In the places where H. pilosella appeared in the following seasons, some other accompanying plants established, such as M. sativa. The growth of M. sativa was nearby H. pilosella patches, very rarely growing alone on this particular tailing. Due to N2-fixing abilities, Medicago species were proposed as good candidates for remediation strategies to enrich poor in nutrient substrata. There were several attempts to introduce M. sativa, but this plant was not able to survive there. Therefore, an experiment under laboratory conditions on zinc–lead waste was carried out to discover if the growth of alfalfa can be improved by the co-cropping and introduction of mycorrhizal fungi and N2-fixing bacteria.
Control plants H. pilosella (Hp) and M. sativa (Ms) were grown separately, and they were also co-cropped (Hp + Ms). No inoculation was provided for control plants. Eight different inocula variant were applied: (1) AMF; (2) Azospiriullum sp.; (3) Azospiriullum sp. + AMF; (4) Nostoc edaphicum; (6) N. edaphicum + AMF; (7) Azospirillum sp. + N. edaphicum; (8) Azospiriullum sp. + N. edaphicum + AMF. Plants were grown on the Zn–Pb-rich tailing. Experiment was conducted on the non-sterile (NS), where no treatments were provided, and on the sterile substratum (S), where dry heat sterilization was applied to sterilized substratum. Waste material was sterilized at 100 °C for 2 h, over 3 days in a row and then allowed to cool for 72 h to eliminate biotic communities, but still retain abiotic tailing traits. Next, the plants were inoculated with arbuscular mycorrhizal fungi R. irregularis (M), Azospirillum sp. (A), and N. edaphicum (N) in different combinations or left non-inoculated (controls). Each combination was replicated five times for a total of 100 pots. Two compartmented cultivation systems with 37-μm polyester mesh (Sefar LFM, Switzerland) were provided to separate H. pilosella and M. sativa roots. Seeds of H. pilosella ecotype were obtained from populations grown at an industrial waste disposal area ZG Trzebionka. Seeds of M. sativa (V29814/04/001) were obtained from Malopolska Hodowla Roslin (HBP, Poland, Krakow). Both types of seeds were surface sterilized with (1 % chloramine T (3 min), 6 % sodium hypochlorite (2 min), and 90 % ethanol (2 min)) and washed three times with distilled sterile water between each step. Seeds of H. pilosella were pregerminated on 3 % water agar under greenhouse conditions. Fourteen-day-old seedlings were transferred into plastic pots (14 cm tall by 11 cm wide) and filled with 500 g of tailing material. Each pot was sown with 100 seeds of Alfalfa. Plants were watered three times a week with deionized water. Pots were regularly weighed to maintain moisture content at 80 % of water-holding capacity. The plants were not supplied with any nutrient solution. The experiment was conducted in a controlled environment in growth chamber (PaNELTECH, Poland with an in-built TAC Xenta/Vista system) maintained at 24 °C, under a 12/12 h light regime. Maximum photosynthetic photon flux (PPF) was 110 ± 10 μmol (s m2)−1, and supplementary light was not needed. Relative humidity ranged from 20 to 25 %. Plants were harvested after 17 weeks of growth.
Plant vitality
Plant vitality was evaluated before harvesting using a Plant Efficiency Analyzer (Hansatech Instruments, UK) estimating chlorophyll a fluorescence transient of intact leaves. Measurements were taken on the upper surface of fully expanded leaves. For each soil/microbe combination, three leaves of each H. pilosella plant and 15 M. sativa plants per pot were measured. The collected data set was used for the JIP test analysis (analysis of O-J-I-P fluorescence transient) (Strasser and Srivastava 1995; Tsimilli-Michael et al. 2000; Strasser et al. 2004). Although indirect, the JIP test allows information about the structure and function of the photosynthetic apparatus (mostly related to PSII) to be obtained. The photosynthetic efficiencies, i.e., the maximum quantum yield of PSIIat t = 0, φPo = TR0 / ABS = 1 − (Fo / Fm) = Fv / Fm was measured. The specific flux parameters chosen to be calculated in the present study, all referring to the condition of the sample at time zero, expressed per reaction center (RC) were analyzed: (1) ABS/RC—the average absorption per RC; (2) TR0/RC—the specific trapping flux per RC; (3) DI0/RC—the dissipated energy flux per RC; (4) ET0/RC—the maximal specific flux for electron transport per RC. Parameters were derived from the theory of energy flux from biomembranes (Strasser et al. 2000, 2004). Also, the performance indexes which are products of terms expressing “potentials” for photosynthetic performance where PIABS: performance index (PI) on absorption basis PIABS = [RC / ABS] [TR0 / (ABS − TR0] [ET0 / (TR0 − ET0)] and PITOTAL: total PI, measuring the performance up to the PSI end electron acceptors, PITOTAL = PIABS [RE0 / ET0 − RE0)] were analyzed. The logarithms of performance indexes at t = 0 log (PITOTAL, PIABS) were defined as the total driving force (DFTOTAL, DFABS) for photosynthesis of the observed system, created by summing the partial driving forces for each of several energy bifurcations (Strasser et al. 2004).
Harvest and sample preparation
Each plant was carefully removed from the pot. Loosely adhering waste material was removed from the roots, and larger root pieces remaining in the substrate were manually picked out. Root and aerial part biomass of harvested plant was weighted separately. Total fresh biomass of roots was weighted; after it, one fourth of roots was taken for staining to estimate mycorrhizal colonization. Collected biomass was frozen at −20 °C and then freeze-dried for 72 h, at −55 °C (Christ Beta 1–8 LD plus, SciQuip Ltd., UK). Dry weights of shoots and roots were determined after the freeze-dried procedure.
Arbuscular mycorrhizal colonization
At harvest, roots were removed from substrate and then gently rinsed with running tap water and then with deionized water (DI). Mycorrhizal root parts were randomly collected and cleared in 10 % KOH for 24 h at room temperature. Subsequently, after careful washing in tap water, the roots were acidified for 1 h in 5 % lactic acid and stained for 24 h at room temperature in 0.05 % aniline blue in lactic acid, in order to visualize the fungal structures inside the roots. Mycorrhizal colonization was estimated in the roots of H. pilosella and M. sativa. Root colonization by R. irregularis was determined according to protocol provided in Mycorrhiza Manual (www2.dijon.inra.fr/mychintec/Protocole/Workshop_Procedures.html). Since experiment was carried out in plant growth chamber, the possibilities of contamination by airborne spores from other AMF was limited. The following parameters were assessed: intensity of the mycorrhizal colonization in the root system (M%), intensity, and arbuscule abundance in the root system (A%). From each pot, approximately 80 cm of fine root fragments were randomly taken for mycorrhizal estimation. Mycorrhizal colonization and arbuscular richness were estimated microscopically in about 60 1-cm-long root fragments per pot.
Medicago sativa nodules
Roots were shaken gently to remove substrate, then washed gently and dried with moist tissue. Nodules were removed and fixed in formaldehyde–acetic acid–ethanol (FAA). The number of nodules was counted per pot. Different nodules: white, pink, brown, hard, and soft were collected form roots.
Statistical analysis
For biomass, dry mass, and mycorrhizal parameters, means were compared using ANOVA The Tukey’s test was used as a post hoc test when ANOVA showed significance. If assumptions were not met, we used non-parametric tests (Kruskal–Wallis test).
For photosynthetic parameters, first was performed the principal component analysis (PCA) to select variables with the highest loadings for first and second axis. Then, for selected variables, ANOVA analysis was performed. If assumptions were not met, we used non-parametric tests (Kruskal–Wallis test). For PCA, we used CANOCO 5 software (Ter Braak and Šmilauer 2012). For ANOVA and other tests, we used STATISTICA (version 10 StatSoft, 2010) software.
Results
Biomass production
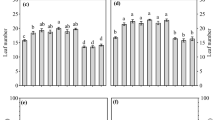
Plants grown (M. sativa and H. pilosella) in NS Zn–Pb-rich tailing demonstrated stunted growth consistent with S substratum in comparison with plants grown on NS substrate (Fig. 1). Significant differences between microbial treatments regarding biomass were observed. Growth of H. pilosella was significantly lower in the dry heat sterilized tailing material than on the non-sterile substratum. Significantly higher (p < 0.05) shoots biomass/dry mass was observed when H. pilosella was inoculated with microbes. Especially when plants were grown on sterile substrate, AMF inoculation together with diazotrophs: Azospirillum sp. and N. edaphicum exerted a positive effect on Hieracium growth (Fig. 1a). No significant changes of H. pilosella biomass were observed in the non-sterile substrate compared to the control plants (Fig. 1a). Although, stimulation of H. pilosella growth was observed when plants were co-inoculated with AMF and N2-fixers while compared to single inoculation with N2-fixers (Nostoc or Azospirillum) (Fig. 1a). Similar trends were also obtained for root biomass and dry weights (data not shown).
Biomass in (g) of a Hieracium pilosella shoots (Hp), b Medicago sativa shoots (Ms), c Medicago sativa roots; plants grown on NS non-sterilized substrate, S dry heat sterilized substrate, and inoculated with different microorganisms: M Rhizophagus irregularis, A Azospirillum sp., N Nostoc edaphicum; different letters above bars indicate statistically significant differences (P < 0.05)
Mycorrhizal inoculation together with N2-fixers always stimulated alfalfa growth, while single and double inoculation with Azospirillum sp. or N. edaphicum did not have a positive effect on M. sativa shoot growth (Fig. 1b). In S substrate, single inoculation with Azosporillum sp. was as high as in the case of control plants (Fig. 1b). Biomass of M. sativa grown on both (S) and (NS) substrates increased, when plants were inoculated with R. irregularis. On sterile substrate, positive effect of mycorrhizal inoculation was even more pronounced (Fig. 1b). Root growth of H. pilosella was greatly reduced when plants were supplied with a single inoculation with a N. edaphicum in NS treatment and double inoculated with both N2-fixers in S substrate (Fig. 1c).
To conclude, growth of non-inoculated plants in both substrates (S and NS variant) was reduced. Dry heat sterilization procedure had the greatest effect, reducing shoots and root growth of both plant species. In general, shoot biomasses as well as dry weights (not shown) of H. pilosella and M. sativa were higher in non-sterile substrate than in sterile one (Fig. 1a, b). Always, presence of AMF positively affected fresh biomass of both tested plant species (p < 0.05). There was significant interaction between sterilization procedure and various microbial treatments for H. pilosella. Co-inoculation (AMF + N 2 -fixers) was effective and increased productivity of H. pilosella grown on the Trzebionka tailing. The greatest M. sativa shoot development was reached in plants dually inoculated with R. irregularis + N. edaphicum on both substratum (S and NS). Non-inoculated control plants and inoculated plants did not show any differences in root growth except inoculation with N. edaphicum (on non-sterile substrate) and double N2-fixer inoculation (on sterile substrate), which negatively affects alfalfa root growth (Fig. 1c).
Root AMF colonization
Intensity of the mycorrhizal colonization in the root system (M%)
Roots of inoculated plants were extensively colonized by R. irregularis, and percentage root colonization of M. sativa was comparable with that of H. pilosella. Dry heat sterilization procedure caused decreases of mycorrhizal colonization of both plants (Fig. 2a, b). In the case of M. sativa, no statistically important interactions were found between substratum treatment and inoculation option, while significant interactions were observed for H. pilosella. The most efficient treatments were those when H. pilosella was co-inoculated with Azospirillum sp. as well as when double inoculation with both N2-fixers was provided (Fig. 2a). Alfalfa inoculated with N. edaphicum (N) and R. irregularis (M) grown on sterile as well as on non-sterile substratum were significantly different from all other treatments (Fig. 2b). Addition of N. edaphicum significantly decreased intensity of the mycorrhizal colonization in the root system of both plant species whether plants were grown on sterilized substrate or not (Fig. 2a, b).
Relative mycorrhizal root length (M%) evaluated for a Hieracium pilosella (Hp), b Medicago sativa (Ms), when plants were grown on NS non-sterilized substrate, S dry heat sterilized substrate; plants were inoculated with M Rhizophagus irregularis, A Azospirillum sp., N Nostoc edaphicum; different letters beside the values indicate statistically significant difference (P < 0.05)
Arbuscular richness: arbuscule abundance in the root system (A%)
On sterilized substrate, mycorrhizal colonization was significantly lower. Significant decreases in A% values were observed for both plant species. Arbuscule richness in root fragments where the arbuscules were abundant (A%) showed important differences between substrate sterilization treatment and microbial inoculation variant for H. pilosella species. When H. pilosella was grown on non-sterile substrate, the highest rate of arbuscular richness was observed for plants inoculated with both N2-fixers (Fig. 3a). While, single inoculation of H. pilosella with cyanobacteria, as well as double with N. edaphicum and Azospiruillum sp. negatively affected arbuscule abundance (A%) in the root system on sterile substrate (Fig. 3a). The arbuscule abundance in the alfalfa root system (A%) was significantly higher when plants were grown on non-sterile substrate when compared to sterile system (Fig. 3b). Inoculation with N2-fixers caused decrease in the arbuscule richness in root fragments where the arbuscules were abundant (A%) in the M. sativa root system (Fig. 3b). However, in non-sterile substrate, other microbes present in the substrate probably attenuated negative effect of N2-fixers on arbuscular richness. Sterilization procedure of the substratum prior to decreased mycorrhizal colonization. Inoculation with N2-fixers affected the percentage of AMF colonization. The response of H. pilosella was different depending on sterilization procedure of the substrate. When plants were grown on non-sterile substrate, nitrogen-fixing microbial inoculants positively influenced H. pilosella root colonization, while on sterile substrate, N2-fixers negatively affected arbuscule abundance (Fig. 3a). The lowest survival rate and lowest arbuscule abundance that were found for both plants were single inoculation with N. edaphicum was applied (Fig. 3a, b).
Relative arbuscular richness (A%) evaluated for a Hieracium pilosella (Hp), b Medicago sativa (Ms), when plants were grown on NS non-sterilized substrate, S dry heat sterilized substrate; plants were inoculated with M Rhizophagus irregularis, A Azospirillum sp., N Nostoc edaphicum; different letters beside the values indicate statistically significant difference (P < 0.05)
Nodule number
The number of nodules in alfalfa inoculated with R. irregularis grown on non-sterile were significantly higher, whilst a very few nodules were observed in sterile treatments (Fig. 4). A beneficial effect of applied microbial inoculations was found for both S and NS substrates (Fig. 4). The +AMF treatments significantly enhanced the development of root nodules. Dual inoculation with the R. irregularis and the associated diazotrophs (Azospirillum sp. and N. edaphicum) also resulted in a synergistically increased nodule number. The highest nodule number on non-sterile substrate was found when both N2-fixers and AMF was applied (Fig. 4). When M. sativa was grown on dry heat sterilized substrate, nodules were formed just when plants were co-inoculated with mycorrhiza and N2-fixing bacteria. Otherwise, nodules where not present (Fig. 4).
Number of nodules per 50 cm of Medicago sativa roots (Ms) co-cropped with Hieracium pilosella (Hp). Number of nodules was estimated after 3 months of growth on a Zn–Pb-rich tailing: NS non-sterilized substrate, S dry heat sterilized substrate. Plants were inoculated with M Rhizophagus irregularis, A Azospirillum sp., N Nostoc edaphicum
Plant vitality
Hieracium pilosella
PCA analysis showed that there was no differentiation into two plant groups collected from sterile and non-sterile substrate when we consider photosynthetic parameters for M. sativa. In the case of H. pilosella, two groups of samples were recognized: sterile and non-sterile one (Supplement 1).
Statistical analysis of photosynthetic parameters obtained for H. pilosella showed interaction between plants grown on sterilized substratum/non-sterile and provided inoculation treatment. The result of ANOVA for experimental data showed Fv/Fm, TR0/RC, ET0/RC, RE0/RC, and PITOTAL, PIABS, DFABS, and DFTOTAL changed significantly after inoculation (Fig. 5a, b). Microbial inoculation resulted in increase of F v/F m parameter for H. pilosella grown on sterile substrate, except the case when H. pilosella was only co-cropped with M. sativa. Measured values of Fv/Fm were in the range of 0.72–0.75. On non-sterilized substrate, Fv/Fm values were significantly lower (0.67–0.78) only when triplicate co-inoculation was provided (mycorrhiza and both bacterial strains). Single and triplicate AMF inoculation (Myc and Myc + Bact) induced a decrease by 23–10 % of ABS/RC and 38–23 % of DI0/RC on sterile substrate; this decrease is counterbalanced in the single or dual inoculation with diazotrophs (Bact). On sterilized substrate, AMF single and triple inoculation (Myc and Myc + Bact) significantly increased PIABS by (45–90 %) and PITOTAL by (25–50 %) of H. pilosella, while double inoculation with N2-fixers caused a decrease of PI parameters (Fig. 5b). Inoculation with AMF and both N2-fixers increased DFABS and DFTOTAL by 25–30 % compared to control (Fig. 5b). On NS substrate, single inoculation with AMF (Myc) and triplicate inoculation with mycorrhiza and diazotrophs (Myc + Bact) decreased both ABS/RC (20 %) and TR0/RC parameter (40 %). Figure 5b demonstrates that single inoculation with AMF (Myc) increased PIABS by 25 %, and the triplicate inoculation with AMF inoculation and both diazotrophs (Myc + Bact) increased PITOTAL around 10 % (Fig. 5). Single mycorrizal inoculation (Myc) increased DFTOTAL of H. pilosella (Fig. 5).
Deviation of the fluxes as expressed relative to control plants Hieracium pilosella. The values are expressed in percents (%) and reflect the deviation flux differences between non-inoculated plants and plants after single inoculation with the AM fungus Rhizophagus irregularis (Myc), dual inoculation with the diazotrophs bacteria: Azospirillum sp. and Nostoc edaphicum (Bact), triplicate co-inoculations with AMF fungus R. irregularis and diazotroph bacteria (Myc + Bact), plants grown on NS non-sterilized substrate, S dry heat sterilized substrate. ABS/RC the average absorption per RC, DI 0 /RC the dissipated energy flux per RC, TR 0 /RC the specific trapping flux per RC, ET 0 /RC the maximal specific flux for electron transport per RC, PI ABS performance index on absorption basis, PI TOTAL total performance index (PI), DF ABS driving force on absorption basis, DF TOTAL total driving force
Medicago sativa
The result of ANOVA evaluated for alfalfa showed no interactions observed between sterilization procedure (plants grown on sterile or non-sterile substrate) and provided microbial inoculation. Microbial inoculation did not cause any significant changes of Fv/Fm parameter in alfalfa sample, neither when plants were grown on sterile and non-sterile substrate. Fv/Fm values obtained for alfalfa were in the range of 0.83 and 0.85. The analysis of specific energy fluxes per QA− reducing reaction center revealed differences in absorption (ABS/RC), trapping (TR0/RC), reduction of end acceptors at PSI electron acceptor side (ET0/RC), and in the dissipated energy flux (DI0/RC) between controls and inoculated with microbe plants (Fig. 6). Microbial inoculation affected the performance index PIABS of alfalfa. PIABS parameter increased when plants were inoculated with AMF (Myc and Myc + Bact), while bacterial inoculation (Bact) caused a decrease of this parameter (Fig. 6). Figure 6a showed that on sterile substratum, a decrease of ABS/RC, TR0/RC, and RE0/RC by 4–7 % was observed in the case of single inoculation with AMF (Myc) and triplicate inoculations with mycorrhiza and diazotrophs (Myc + Bact). Different effect was observed for M. sativa grown on NS case, where single inoculation with R. irregularis (Myc) increased ABS/RC, TR0/RC, and RE0/RC by 9–22 % (Fig. 6). Although, there was no significant changes in phenomenological energy flux parameters observed in non-sterile treatment when M. sativa was double inoculated with diazotrophs (Bact) and triplicate with AMF + N2-fixers (Myc + Bact). In NS substrate, just double inoculation with N2-fixers (Bact) and triplicate inoculation with AMF + diazotrophs (Myc + Bact) resulted in the decrease of PITOTAL parameter by 23–20 %. Although, inoculation with mycorrhizal fungi R. irregularis (Myc) caused an increase of performance indexes PITOTAL by 10 %. The same results were observed for DFTOTAL parameter (Fig. 6b).
Deviation of the fluxes as expressed relative to control plant Medicago sativa. The values are expressed in percents (%) and reflect the deviation flux differences between non-inoculated plants and plants after single inoculation with the AM fungus Rhizophagus irregularis (Myc), dual inoculation with the diazotrophs bacteria: Azospirillum sp. and Nostoc edaphicum (Bact), tripartite co-inoculations with AMF fungus R. irregularis and diazotrophs bacteria (Myc + Bact), plants grown on NS non-sterilized substrate, S dry heat sterilized substrate. ABS/RC the average absorption per RC, DI 0 /RC the dissipated energy flux per RC, TR 0 /RC the specific trapping flux per RC, ET 0 /RC the maximal specific flux for electron transport per RC, PI ABS performance index on absorption basis, PI TOTAL total performance index (PI), DF ABS driving force on absorption basis, DF TOTAL total driving force
Discussion
Co-cropping
The polluted sites require combination of different restoration approaches such as intercropping, co-cropping, or pre-cropping to improve the establishment of a plant cover on metal-rich industrial wastes (Khan 2005; Sprocati et al. 2014). H. pilosella and M. sativa were selected because their potential ability to be used in vegetation practices on heavy metal-rich polluted sites. Clonal plants such as H. pilosella spread horizontally within their habitat by means of stolons, rhizomes, and ramets. Connection between ramets allow for translocation of resources within the clone. Through a spatial division of labor, clonal species were able to perform specific tasks and closely co-operate by potentially independency including reproduction (Stueffer et al. 1996). Developing vegetative reproduction system and exhibiting phenotypic plasticity allows H. pilosella to overcome the establishment risk and regenerate under unfavorable conditions such as dry periods and lack of nutrients (Salzman 1985; Winkler and Stöcklin 2002; Roiloa and Retuerto 2012). H. pilosella populations usually almost exclusively depend on clonal reproduction (Bishop and Davy 1985). It has been claimed that H. pilosella is allelopatic species (Murphy and Aarssen 1995; Murphy 2000; Jankowska et al. 2014). However, our experiment reveals suppression of H. pilosella by M. sativa rather than the reverse. Similar results were obtained for H. pilosella and Arrhenatherum elatius in colliery spoils of north of France (Henn et al. 1988). The leguminous species M. sativa has been suggested as a good candidate for remediation of contaminated soils, due to its rhizobial symbionts and ability to fix atmospheric N2 (Gardea-Torresdey et al. 1998, 1999; Peralta-Videa et al. 2002; Lin et al. 2007). Co-cropping different species may enhance the overall capabilities of a phytoremediation. However, under unfavorable conditions like the presence of heavy metals, co-cropping of H. pilosella (non-fixers) and M. sativa (legume) caused a negative effect regarding H. pilosella growth and performance (Figs. 1a and 5a). Environmental conditions may disturb the benefits from co-cropping of non-fixing plants with legumes, which under certain conditions, like a presence of heavy metals, might exhibit a limited capacity to fix nitrogen. On the other hand, potential benefits gained by a neighboring non-fixer plant will strongly depend on their capacity to use effectively the extra N input (Temperton et al. 2007). This could be caused by the competition for other macroelements and microelements as well as for water and light (Tilman et al. 1997).
Substrate sterilization procedure
In general, different microbial populations may establish themselves under the influence of root exudates and bioavailability of essential nutrients. However, the presence of toxic metals strongly determines the size and composition of microbial populations in the rhizosphere (Bever et al. 2010). Different soil sterilization procedures are proposed in biological researches concerning microbial influence or heavy metal impact on plant growth. Without the assurance of sterile conditions, obtained metal data sets can be misinterpreted as sorption of metals to solid substrate or losses due to biological activities. Of concern is the need to eliminate biological activity in a soil sample where single interactions between plants and selected microorganisms are investigated. Since many sterilization methods can dramatically change physical and chemical properties of the soil, it is therefore important to choose the least destructive. Where chemical stability is required, air-dry sterilization rather than moisture is recommended (Lotrario et al. 1995; Trevors 1996; Egli et al. 2006).
However, our results indicate that even dry heat sterilization disturbed primarily established relationships between soil-plant and plants and associated microorganisms. In our studies, the plants’ response to microbial inoculation was significant when the substrate was dry heat sterilized. In non-sterile substrate, the microbial communities were more complex from the beginning. Already established relationships between native soil microbe communities and roots in non-sterile prevent plants from metal stress. Significantly higher biomass and dry mass production of plants growing on non-sterile substrate indicate better starting conditions. For this reason, additional inoculation of plants growing on non-sterile substrate did not cause such significant plant responses. This sterilization appears to imbalance already established microbial relationships in the soil, which is why we cannot draw the same conclusions from S and non-sterile treatment regarding additional mycorrhizal and N2-fixer’s inoculations.
Arbuscular mycorrhiza interaction with N2-fixers
The presence and composition of soil microbial communities have been shown to have large impacts on plant–plant and plant–microbial interactions and consequently plant diversity and composition. The rhizosphere is a specialized niche defined as the root–soil interface, where associated microorganisms, roots, and soil come together (Alford et al. 2010). Root colonization is a competitive process that is affected by environmental conditions such as soil moisture, soil texture and pH, organic matter content, access to the nutrients, as well as specificity of host. Thus, microbial population is one of the essential parts of dynamic rhizosphere system that affect the rhizosphere soil properties such as pH, redox potential, metal concentration, water content, bulk density, root exudation, and all the biological transformations (Barea et al. 2002; Bais et al. 2006; Bakker et al. 2013). However, unfavorable conditions on polluted sites impose the need to compete for nutrients and other resources which are limited. Therefore, individual PGPR strains exhibit specificity in sensitivity to environmental parameters to promote host growth and/or suppress plant pathogens (Hibbing et al. 2010). Depending on the strains used, they may perform a number of tasks ranging from narrow to broad (Doornbos et al. 2012; Bakker et al. 2013). Increasing attention is focused on the interactions between PGPR bacteria and mycorrhizal fungi (Biró et al. 2000; Mar Vázquez et al. 2000; Tsimilli-Michael et al. 2000; Barea et al. 2005a, b). Possible application of AM fungi and different soil bacteria in bioremediation processes like phytostabilization and phytoextraction has been a great concern (Barea et al. 2002, 2005a; Weyens et al. 2009). Studies show that dual inoculation AM fungus with nitrogen-fixing soil bacteria enhanced nitrogen fixation in legumes (Bagyaraj et al. 1979; Biró et al. 2000; Tsimilli-Michael et al. 2000; Scheublin et al. 2004; Temperton et al. 2007). Azospirillum brasilense and Glomus intrarradices were capable of co-existing in sugar cane roots both intracellularly and intercellularly, causing changes in the cell wall. Sugar cane plant biomass, the number of endophytic microorganisms, and nitrogen-fixing activity increased with joint inoculation (Bellone and de Bellone Silvia 2012). Inoculation with native bacterial and fungal strains ensures best performance under harsh conditions such as high metal content. Beside AMF, diazotrophs, free-living nitrogen-fixing bacteria strongly attributed to nitrogen fixation and cause an increase of nitrogen amount available to plants (Bethlenfalvay et al. 1982; Toro et al. 1998; Biró et al. 2000; Mar Vázquez et al. 2000; Franzini et al. 2010). In case of M. sativa, formation of nodules would be the most desirable; however, on Zn–Pb Trzebionka tailings, nodules are rarely found (Turnau et al. 2012), and this was also proved during the present investigation as shown in case of control plants grown on non-sterile substratum. Nodulation is an energetically costly process, and legumes balance the nitrogen demand with the energy expense by limiting the number of nodules (Kassaw et al. 2015). In addition, toxic metals present in the substratum are known to induce morphogenic responses and as a consequence reduce the formation of rhizobial infection (Potters et al. 2007; Lafuente et al. 2010). Those metals can also lower down the expression of several nodulation genes (Lafuente et al. 2010). Bacterial activity and the rhizobial symbiosis are influenced by mycorrhiza, both qualitatively and quantitatively (Barea et al. 2005a, b). Our results shows that additional inoculation with selected microbes increased number of nodules; however, we have to underline that total number of nodules is not as important as number of active one. H. pilosella responded positively to mycorrhizal inoculation but not to single inoculation with N2-fixing bacterial symbionts. On the other hand, mycorrhizal inoculation together with N2-fixers always stimulates alfalfa growth, while single as well as double inoculation with N. edaphicum or Azospirillum sp. did not have a positive effect on shoot growth. This result may indicate that poor substrate such as Zn–Pb-rich tailings used in our studies may have precluded a net benefit from the mycorrhizal symbiosis more strongly than from N2-fixing bacteria. This can be explained by the fact that symbiotic nitrogen fixation is very phosphorus intensive due to the high ATP requirement. AM fungi increased mineral and nutrient uptake where up to 80 % of plant’s phosphorus (P) needs and 25 % of its nitrogen (N) is obtained via the fungus (Marschner and Dell 1994; Smith et al. 2004; Govindarajulu et al. 2005; Parniske 2008). The mycorrhization percentage of plants growing on this particular Zn–Pb tailing was shown to be usually high (Orłowska et al. 2005). While under non-polluted conditions, usually lower percentage of mycorrhization is efficient to obtain plant growth promoting effect (Russo et al. 2005). On industrial tailings, this might be not enough due to involvement of fungal hyphae in sequestering toxic metals, what results in increased metal concentration within roots which is one of the known avoidance mechanisms (Leyval et al. 1997). Overall, mycorrhizal inoculation with AMF always had a positive effect on plant growth. It has been proposed that the presence of their exudates low and high molecular weight compounds including carbohydrates and organic acids. This may create a favorable environment around the mycorrhizal fungal hyphae and cell surface structures, which could have supported microbial growth (Scheublin et al. 2004; Toljander et al. 2007; Miransari 2011). Rhizodephozition is a dynamic and extremely complex process, where there is a loss of reduced C for the plant and where the organic C pool enters the soil. This stimulates the biological activity in the rhizosphere (Jones et al. 2009). Microbial activity in soil is greatly influenced by the loss of carbon-containing metabolites where up to 40 % of photosynthetically fixed carbon is secreted into the rhizosphere by plant roots (Bais et al. 2006). In non-polluted soils, the higher C costs to plants of maintaining both fungal and bacterial symbionts may result in indirect antagonistic interactions between symbionts (Bethlenfalvay et al. 1982; Mortimer et al. 2008; Franzini et al. 2010). However, under unfavorable conditions, plants prefer co-inoculation strategy with AMF and N2-fixers.
Extracellular polysaccharides (EPS) were purposed by Bianciotto 2001 to play a crucial role in the anchoring of A. brasilense and R. leguminosarum and in the formation of biofilms on the root and the AM fungus (Bianciotto et al. 2001). Biofilm microorganisms may play an essential role as mediators in the transfer of heavy metals (García-Meza et al. 2005). Extracellular polymeric substances potentially act as detoxification agents against metals, acting as metal-binding sites. Biofilm occurrence seems to enhance complexation and immobilization of Cr, Ni, Cu, Zn, As, and Pb. Due to the organic matter improvement, the biofilms could also be conceptualized as an organic cover, which controls pH (Lukešová 2001; García-Meza et al. 2006). The persistence of the photosynthetically active forms such as cyanobacteria might result in a natural fertilization of the tailing substratum building up an appropriate environment for further plant establishment. The importance of soil cyanobacteria in increasing soil fertility, through the input of nitrogen, promotes the release of nutrients from insoluble compounds (Maxwell 1991). Studies conducted by Trzcińska and Pawlik-Skowrońska 2008 refer to the cyanobacteria isolated from Zn–Pb-loaded soils, including N. edaphicum strain. Those isolated strains are Zn-Pb-resistant ecotypes, which have been rarely reported, in other terrestrial environment (Trzcińska and Pawlik-Skowrońska 2008). N. edaphicum can utilize various inorganic and organic nitrogen sources for growth. Those nitrogen sources are utilized in the hierarchical order of NH4+ > NO3− > N2. This flexibility allows Nostocales to colonize and compete as a phototroph in illuminated habitats, irrespective of the specific nitrogen source (Meeks et al. 2001). This ability seems to be important especially in poor nutrient environment such as waste tailing. The present studies reveal positive effect on plant growth by dual inoculation with native strain of N. edaphicum and AM fungi. This might be related to nitrogen level available for AMF symbionts. However, single inoculation with cyanobacteria or with nitrogen-fixing bacteria did not have a positive effect on plant growth. In both types of substrate treatments (S and NS), AMF colonization stimulated the formation of root nodules in the alfalfa roots. AMF colonization has been known to enhance the formation of nodules, by increase P which then attributed to better N uptake, because of higher nitrogenase-fixation activity in mycorrhizal plants (Toro et al. 1998; Andrade et al. 2004; Barea et al. 2005b; Lin et al. 2007). Also, this positive role of AMF might be related to biofilm formation. Bacteria living in the biofilm forms have the ability to survive much harsher environmental conditions (Costerton et al. 1995). On the other hand, dual symbiosis formed by AMF with Rhizobium strain has been shown to inhibit nodule development and N2-fixation of Phaseolus vulgaris (Lin et al. 2007; Franzini et al. 2010). Given this case that the formation of root nodules may be strongly inhibited not just by heavy metals present in the soil, but also by other microorganisms, therefore, a selection of appropriated symbionts to specific plants and environmental condition is needed to improve successful phytoremediation. This may be due to the competition for nutrients between mixed microorganisms and the ability of one organism to deal with heavy metals more than the other (Hudek et al. 2012).
Plant photosynthesis
The JIP test is presently widely accepted for evaluation of PSII behavior. Organisms exposed to stress such as high light level or heat showed pronounced decrease of φPo = TR0/ABS, measured as Fv/Fm (Strasser et al. 2004). Plants grown under optimal conditions show values of Fv/Fm in the range 0.79–0.85 (Maxwell and Johnson 2000; Kalaji et al. 2014). Similar values were observed in case of plants studied in the present research. In comparison to control plants (grown alone without addition of inocula), microbial inoculation resulted in significant increase of Fv/Fm parameter for H. pilosella, except the case when H. pilosella was only co-cropped with M. sativa. On non-sterilized substrate, Fv/Fm values were significantly lower, only when triplicate co-inoculation was provided. Decrease of Fv/Fm already suggests negative effect of inoculation on photosynthesis functioning. M. sativa did not exhibit any changes regarding Fv/Fm parameters. Previous studies indicated that performance indexes (PITOTAL, PIABS) and driving forces (DFTOTAL, DFABS) are sensitive measures of plant responses to different kinds of environmental stresses such as irradiance, drought, heat, salt, biotic-stressed, and exposure to heavy metals (Tsimilli-Michael et al. 2000; Strasser et al. 2000; Strauss et al. 2006; Christen et al. 2007; Yusuf et al. 2010; Kalaji et al. 2011; Oukarroum et al. 2014). Therefore, these parameters are consistent to evaluate the plant performance, especially when plants were exposed to the stress (Strasser et al. 2000; Strauss et al. 2006). Findings that microbes can cause a stress have been also reported earlier (Tsimilli-Michael et al. 2000; Tsimilli-Michael and Strasser 2002). Similarly to abovementioned parameters, the test showed differences in (ABS/RC), trapping (TR0/RC), and reduction of end acceptors at PSI electron acceptor side (ET0/RC) and the dissipated energy flux (DI0/RC) between controls and plants inoculated with microbes as was shown in Tsimilli-Michael et al. 2000 and Strasser et al. 2004. ABS/RC that gives the total absorption of chlorophylls in PSII antennae per reaction centers is a good measure for average functional antenna size (Tsimilli-Michael et al. 2000). Higher values of ABS/RC together with significant increase in DI0/RC observed in control plants (M. sativa and H. pilosella) could be considered as indicators of photoinhibitory damages to PSII complexes (Force et al. 2003). Co-cropping system that includes M. sativa and H. pilosella without microbial inoculation is not sufficient for successful remediation. Under laboratory conditions, plants are not exposed to such extreme stresses as the one observed on industrial wastes. Therefore, the chances for plant survival are even lower. The use of microbes visibly attenuates negative effect of co-cropping and additionally can be useful for nutrient cycling. Figures 6 and 5 indicate similar trends in plant behavior depending on the use of substrate sterilization procedure. Under sterile conditions, both plants exhibited higher PI parameters when triple inoculation was performed. If substrate was not sterilized, the addition of bacterial inoculation had even more negative effect on plant performance. As shown above, JIP test provides a useful tool for evaluation of the effectiveness of microbial inoculation that has to be taken into account, especially when phytoremediation of polluted site has to be optimized.
Conclusions
Future bioremediation research should strive toward an improved understanding of the functional mechanisms behind such microbial interactions, so that optimized combinations of microorganisms can be applied as effective inoculants within sustainable remediation systems. Appropriate bioremediation practices may include these inoculates to obtain a beneficial effect on plant establishment on heavy metal-polluted sites. Our experimental tests confirm that both negative and positive feedbacks occur between plants and microbial communities. An improved understanding of the interactions taking place in the rhizosphere will help to translate the results of simplified experiments into field application. The characteristics of a microbial network in laboratory conditions could help to design specific remediation strategy on metal-rich industrial wastes.
References
Alford ÉR, Pilon-Smits EAH, Paschke MW (2010) Metallophytes—a view from the rhizosphere. Plant Soil 337:33–50
Andrade SAL, Abreu CA, de Abreu MF, Silveira APD (2004) Influence of lead additions on arbuscular mycorrhiza and Rhizobium symbioses under soybean plants. Appl Soil Ecol 26:123–131
Bagyaraj D, Manjunath A, Patil R (1979) Interactions between a vesicular-arbuscular mycorrhiza and Rhizobium and their effects on soybean in the field. New Phytol 82:141–145
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interations with plants and other organisms, Annual Review of Plant Biology. Annual Review of Plant Biology. Annual Reviews, Palo Alto, pp. 233–266
Bakker PA, Berendsen RL, Doornbos RF, Wintermans PC, Pieterse CM (2013) The rhizosphere revisited: root microbiomics. Front Plant Sci 4:1–7
Barea J-M, Azcón R, Azcón-Aguilar C (2002) Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek 81:343–351
Barea J-M, Pozo MJ, Azcón R, Azcón-Aguilar C (2005a) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Barea J, Azcón R, Azcón-Aguilar C (2005b) Interactions between mycorrhizal fungi and bacteria to improve plant nutrient cycling and soil structure. In: Varma A, Buscot F (eds) Microorganisms in soils: roles in genesis and functions. Soil biology. Springer, Berlin
Bashan Y, Holguin G, Lifshitz R (1993) Isolation and characterization of plant growth-promoting rhizobacteria. In: Glick BR, Thompson JE (eds) Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton, pp 331–345
Bellone CH, de Bellone Silvia C (2012) Interaction of Azospirillum brasilense and Glomus intrarradix in sugar cane roots. Indian J Microbiol 52:70–75
Bethlenfalvay GJ, Pacovsky RS, Bayne HG, Stafford AE (1982) Interactions between nitrogen fixation, mycorrhizal colonization, and host-plant growth in the Phaseolus-Rhizobium-Glomus symbiosis. Plant Physiol 70:446–450
Bever JD, Dickie IA, Facelli E, Facelli JM, Klironomos J, Moora M, Rillig MC, Stock WD, Tibbett M, Zobel M (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25:468–478
Bianciotto V, Andreotti S, Balestrini R, Bonfante P, Perotto S (2001) Extracellular polysaccharides are involved in the attachment of Azospirillum brasilense and Rhizobium leguminosarum to arbuscular mycorrhizal structures. Eur J Histochem 45:39–49
Biró B, Köves-Péchy K, Vörös I, Takács T, Eggenberger P, Strasser RJ (2000) Interrelations between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. Appl Soil Ecol 15:159–168
Bishop GF, Davy AJ (1985) Density and the commitment of apical meristems to clonal growth and reproduction in Hieracium pilosella. Oecologia 66:417–422
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Christen D, Schönmann S, Jermini M, Strasser RJ, Défago G (2007) Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ Exp Bot 60:504–514
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Doornbos RF, van Loon LC, Bakker PA (2012) Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron Sustain Dev 32:227–243
Egli M, Mirabella A, Kägi B, Tomasone R, Colorio G (2006) Influence of steam sterilisation on soil chemical characteristics, trace metals and clay mineralogy. Geoderma 131:123–142
Ettl H, Gärtner G (1995) Syllabus der Boden-, Luft-und Flechtenalgen. Stuttgard. New York, 699
Force L, Critchley C, van Rensen JS (2003) New fluorescence parameters for monitoring photosynthesis in plants. Photosynth Res 78:17–33
Franzini VI, Azcón R, Mendes FL, Aroca R (2010) Interactions between Glomus species and Rhizobium strains affect the nutritional physiology of drought-stressed legume hosts. J Plant Physiol 167:614–619
García-Meza JV, Barrangue C, Admiraal W (2005) Biofilm formation by algae as a mechanism for surviving on mine tailings. Environ Toxicol Chem 24:573–581
García-Meza JV, Carrillo-Chávez A, Morton-Bermea O (2006) Sequential extractions on mine tailings samples after and before bioassays: implications on the speciation of metals during microbial re-colonization. Environ Geol 49:437–448
Gardea-Torresdey JL, Gonzalez JH, Tiemann KJ, Rodriguez O, Gamez G (1998) Phytofiltration of hazardous cadmium, chromium, lead and zinc ions by biomass of Medicago sativa (Alfalfa). J Hazard Mater 57:29–39
Gardea-Torresdey JL, Tiemann KJ, Gamez G, Dokken K (1999) Effects of chemical competition for multi-metal binding by Medicago sativa (alfalfa). J Hazard Mater 69:41–51
Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Henn H, Petit D, Vernet P (1988) Interference between Hieracium pilosella and Arrhenatherum elatius in colliery spoils of north of France. Oecologia 76:268–272
Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 8:15–25
Hindák F (1996) Key to the unbranched filamentous green algae (Ulotrichineae, Ulotrichales, Chlorophyceae). Bulletin Slovenskey Botanickej Spolocnosti pri SAV, Bratislava, Suplement 1
Hoek C (1995) Algae: an introduction to phycology. Cambridge university press
Hudek L, Rai S, Michalczyk A, Rai LC, Neilan BA, Ackland ML (2012) Physiological metal uptake by Nostoc punctiforme. BioMetals 25:893–903
Jankowska J, Ciepiela GA, Jankowski K, Kolczarek R, Sosnowski J, Wiśniewska-Kadżajan B (2014) The allelopathic influence of Taraxacum officinale on the initial growth and development of Festuca rubra (L.). J Ecol Eng 15:38–44
Jeffery S, Gardi C, Jones A, Montanarella L, Marmo L, Miko L, Ritz K, Peres G, Römbke J, Van der Putten W (2010) European atlas of soil biodiversity. European Commission
Jones D, Nguyen C, Finlay R (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Kalaji HM, Govindjee BK, Kościelniak J, Żuk-Gołaszewska K (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73:64–72
Kalaji MH, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dąbrowski P, Elsheery NI, Ferroni L, Guidi L, Hogewoning SW, Jajoo A, Misra AN, Nebauer SG, Pancaldi S, Penella C, Poli DB, Pollastrini M, Romanowska-Duda ZB, Rutkowska B, Serôdi J, Suresh K, Szulc W, Tambussi E, Yanniccari M, Zivcak M (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122(2):121–58
Kassaw T, Bridges W Jr, Frugoli J (2015) Multiple autoregulation of nodulation (AON) signals identified through split root analysis of medicago truncatula sunn and rdn1 mutants. Plants 4:209
Khan AG (2005) Rote of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elem Med Biol 18:355–364
Lafuente A, Pajuelo E, Caviedes MA, Rodríguez-Llorente ID (2010) Reduced nodulation in alfalfa induced by arsenic correlates with altered expression of early nodulins. J Plant Physiol 167:286–291
Leyval C, Turnau K, Haselwandter K (1997) Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7:139–153
Lin A-J, Zhang X-H, Wong M-H, Ye Z-H, Lou L-Q, Wang Y-S, Zhu Y-G (2007) Increase of multi-metal tolerance of three leguminous plants by arbuscular mycorrhizal fungi colonization. Environ Geochem Health 29:473–481
Lotrario JB, Stuart BJ, Lam T, Arands RR, O’Connor OA, Kosson DS (1995) Effects of sterilization methods on the physical characteristics of soil: implications for sorption isotherm analyses. Bull Environ Contam Toxicol 54:668–675
Lukešová A (2001) Soil algae in brown coal and lignite post-mining areas in Central Europe (Czech Republic and Germany). Restor Ecol 9:341–350
Mar Vázquez M, César S, Azcón R, Barea JM (2000) Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl Soil Ecol 15:261–272
Marschner H, Dell B (1994) Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
Maxwell C (1991) Floristic changes in soil algae and cyanobacteria in reclaimed metal-contaminated land at Sudbury, Canada. Water Air Soil Pollut 60:381–393
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Meeks JC, Elhai J, Thiel T, Potts M, Larimer F, Lamerdin J, Predki P, Atlas R (2001) An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium. Photosynth Res 70:85–106
Miransari M (2011) Interactions between arbuscular mycorrhizal fungi and soil bacteria. Appl Microbiol Biotechnol 89:917–930
Mortimer P, Pérez-Fernández M, Valentine A (2008) The role of arbuscular mycorrhizal colonization in the carbon and nutrient economy of the tripartite symbiosis with nodulated Phaseolus vulgaris. Soil Biol Biochem 40:1019–1027
Mucha J, Szuwarzyński M (2004) Sampling errors and their influence on accuracy of zinc and lead content evaluation in ore from the Trzebionka mine (Silesian–Cracow Zn–Pb ore district, Poland). Chemom Intell Lab Syst 74:165–170
Murphy SD (2000) Field testing for pollen allelopathy: a review. J Chem Ecol 26:2155–2172
Murphy SD, Aarssen LW (1995) In vitro allelopathic effects of pollen from three Hieracium species (Asteraceae) and pollen transfer to sympatric Fabaceae. Am J Bot 82:37–45
Murty M, Ladha J (1988) Influence of Azospirillum inoculation on the mineral uptake and growth of rice under hydroponic conditions. Plant Soil 108:281–285
Orłowska E, Ryszka P, Jurkiewicz A, Turnau K (2005) Effectiveness of arbuscular mycorrhizal fungal (AMF) strains in colonisation of plants involved in phytostabilisation of zinc wastes. Geoderma 129:92–98
Oukarroum A, Bussotti F, Goltsev V, Kalaji MH (2014) Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ Exp Bot 109:80–88
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6:763–775
Peralta-Videa J, Gardea-Torresdey J, Gomez E, Tiemann K, Parsons J, Carrillo G (2002) Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environ Pollut 119:291–301
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574
Roiloa S, Retuerto R (2012) Clonal integration in Fragaria vesca growing in metal-polluted soils: parents face penalties for establishing their offspring in unsuitable environments. Ecol Res 27:95–106
Russo A, Felici C, Toffanin A, Götz M, Collados C, Barea J, Moënne-Loccoz Y, Smalla K, Vanderleyden J, Nuti M (2005) Effect of Azospirillum inoculants on arbuscular mycorrhiza establishment in wheat and maize plants. Biol Fertil Soils 41:301–309
Ryszka P, Turnau K (2007) Arbuscular mycorrhiza of introduced and native grasses colonizing zinc wastes: implications for restoration practices. Plant Soil 298:219–229
Salzman AG (1985) Habitat selection in a clonal plant. Science 228:603–604
Scheublin TR, Ridgway KP, Young JPW, Van Der Heijden MG (2004) Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70:6240–6246
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
Sprocati AR, Alisi C, Pinto V, Montereali MR, Marconi P, Tasso F, Turnau K, De Giudici G, Goralska K, Bevilacqua M (2014) Assessment of the applicability of a “toolbox” designed for microbially assisted phytoremediation: the case study at Ingurtosu mining site (Italy). Environ Sci Pollut Res 21:6939–6951
Starmach K (1966) Cyanophyta-Sinice. In: Flora słodkowodna Polski. 2. (Eds), Warszawa: PAN, Państwowe Wydawnictwo Naukowe
Strasser RJ, Srivastava A (1995) Polyasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing photosynthesis: mechanisms, regulation and adaptation, 445–483
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. Springer
Strauss AJ, Krüger GHJ, Strasser RJ, Heerden PDRV (2006) Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I.-P. Environ Exp Bot 56:147–157
Stueffer J, De Kroon H, During H (1996) Exploitation of environmental hetergeneity by spatial division of labor in a clonal plant. Functional Ecology, 328–334
Szuwarzyński M (1993) The lead and zinc ore deposits in the vicinity of Chrzanów. Geol Q 37:209–228
Temperton V, Mwangi P, Scherer-Lorenzen M, Schmid B, Buchmann N (2007) Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 151:190–205
Ter Braak CJF, Šmilauer P (2012) Canoco reference manual and user’s guide: software for ordination, version 5.0. Microcomputer Power, Ithaca, USA, 496 pp.
Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E (1997) The Influence of functional diversity and composition on ecosystem processes. Science 277:1300–1302
Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD (2007) Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol Ecol 61:295–304
Toro M, AzcÓN R, Barea JM (1998) The use of isotopic dilution techniques to evaluate the interactive effects of Rhizobium genotype, mycorrhizal fungi, phosphate-solubilizing rhizobacteria and rock phosphate on nitrogen and phosphorus acquisition by Medicago sativa. New Phytol 138:265–273
Trafas M (1996) Changes in the properties of post-flotation wastes due to vegetation introduced during process of reclamation. Appl Geochem 11:181–185
Trevors J (1996) Sterilization and inhibition of microbial activity in soil. J Microbiol Methods 26:53–59
Trzcińska M, Pawlik-Skowrońska B (2008) Soil algal communities inhabiting zinc and lead mine spoils. J Appl Phycol 20:341–348
Tsimilli-Michael M, Strasser R (2002) Mycorrhization as a stress adaptation procedure, Mycorrhizal technology in agriculture. Springer, pp. 199–209
Tsimilli-Michael M, Eggenberg P, Biro B, Köves-Pechy K, Vörös I, Strasser RJ (2000) Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol 15:169–182
Turnau K, Anielska T, Ryszka P, Gawroński S, Ostachowicz B, Jurkiewicz A (2008) Establishment of arbuscular mycorrhizal plants originating from xerothermic grasslands on heavy metal rich industrial wastes–new solution for waste revegetation. Plant Soil 305:267–280
Turnau K, Gawroński S, Ryszka P, Zook D (2012) Mycorrhizal-based phytostabilization of Zn–Pb tailings: lessons from the Trzebionka mining works (Southern Poland). In: Varma A, Kothe E (eds) Bio-geo interactions in metal-contaminated soils. Soil biology. Springer, Berlin, pp 327–348
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009) Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598
Winkler E, Stöcklin J (2002) Sexual and vegetative reproduction of Hieracium pilosella L. under competition and disturbance: a grid-based simulation model. Ann Bot 89:525–536
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee SNB (2010) Overexpression of γ-tocopherol methyl transferase gene in transgenic brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta (BBA) - Bioenerg 1797:1428–1438
Acknowledgments
This work was supported by the Foundation for Polish Science, International PhD Projects Programme co-financed by the EU European Regional Development Fund (MPD/2009-3/5) and by project MAESTRO contract no. 2011/02/A/NZ9/00137. Funding was also provided by the Małopolskie Centre of Entrepreneurship-Programme DOCTUS (ZS. 4112-129/2010). The authors would like to thank Prof. Konrad Wołowski (Institute of Botany Polish Academy of Science, Krakow) for identification of cyanobacteria and Prof. Zbigniew Miszalski for constructive comments. Special thanks to Dr. Rafał Ważny from The Malopolska Center of Biotechnology for his help in preparation of materials for analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 53 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ogar, A., Sobczyk, Ł. & Turnau, K. Effect of combined microbes on plant tolerance to Zn–Pb contaminations. Environ Sci Pollut Res 22, 19142–19156 (2015). https://doi.org/10.1007/s11356-015-5094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5094-2