Abstract
Urban dust is a reservoir of potentially toxic elements (PTEs) that can be incorporated into aquatic ecosystems where they bioaccumulate and biomagnify causing toxic effects. The aim of this work was to assess the PTEs’ concentrations and toxicity to Caenorhabditis elegans of inorganic extracts from urban dust of Barranquilla, the largest Colombian Caribbean city. Trace elements were analyzed by inductively couple plasma-mass spectrometry. PTEs concentration decreased in the order Sr > Cu > Ba > Mo > Se > Cr > V > Ni > As > Zn > Rb > Mn > Sb > Co > Sn > Cd > La > Ce >Tl ≈ Bi > Ag ≈ Pb. Inorganic extracts from urban dust affected physiological parameters in the nematode, such as survival, growth and locomotion. Lethality showed a positive relation with Sr and negative with V. Growth displayed a negative association with Mo. Expression of mtl-2, sod-4, and unc-25 genes was induced by PTEs. The results suggest that C. elegans is a sensitive organism capable of responding to exposure to urban dust extracts, being a suitable sensor for the implementation of warning systems related to risks to biota associated with air pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dust deposited and accumulated on different external surfaces in the urban area of a city are fine solid particles that originate as a mixture of substances from various natural and anthropogenic sources (Atiemo et al., 2011). Increased anthropogenic activities, including industrial, vehicle and domestic emissions (Yadav et al., 2019), construction and demolition contribute to greater concentrations of potentially toxic elements (PTEs), polynuclear aromatic hydrocarbons (PAHs) and other pollutants (Ali et al., 2019) in urban dust. In addition, these particles are characterized by their high contaminant adsorption capacity and environmental mobility through runoff waters and wind (Rahman et al., 2019).
The PTEs present in the dust particles are persistent in the environment, becoming incorporated into different matrices and the trophic network (Palacios-Torres et al., 2018; Queirós et al., 2019; Tejeda-Benítez et al., 2018). The absorption and bioaccumulation of PTEs can cause toxic effects to humans and wildlife (Yokel et al., 2006), mostly associated with endocrine disruption, neurotoxicity, DNA damage, and participation as a secondary factor to disease (Ali et al., 2019; Rahman et al., 2019). Environmental pollution has increased significantly worldwide in recent decades due to urbanization and industrialization processes (Ali et al., 2019; Kurt-Karakus, 2012; McDonald et al., 2013). This is particularly complex in emerging economies where the lack of environmental protection and poor health systems may accentuate the problem (Li & Lin, 2019; Molina et al., 2015; Romieu et al., 1990). In Colombia, 65% of premature deaths are related to urban pollution, making it a public health problem (SDD, 2012). Although there are some studies about pollution by PTEs in urban areas, those have focused on the characterization of pollutants in environmental matrices and identification of sources (Osorio-Martinez et al., 2021; Romero et al., 2015; Silva et al., 2020; Trujillo-González & Torres-Mora, 2015), existing the need to carry out investigations to elucidate the toxic effects that pollutants present in urban dust can cause in biota. Thus, relevant ecotoxicological information could be useful to obtain scientific support for developing policies to regulate, monitor and mitigate exposure to PTEs.
Barranquilla is the most industrialized city in the Colombian Caribbean, located in the north of the country and is the fourth most populated city (DNP, 2014). Its industrial and urban development has led to an increase in pollution indicators (Aldana-Domínguez et al., 2018; Silva et al., 2020). In addition, this area is exposed to atmospheric pollutants from road traffic, industrial activities, construction activities, waste burning and even emissions from forest fires (Gallego-Cartagena et al., 2020; Osorio-Martinez et al., 2021).
In recent years, studies have focused on evaluating PTE concentrations and estimating risks to the environment and human health from exposure to urban dust and surface soils (Cortés et al., 2017; Du et al., 2013; Gabarrón et al., 2017; Han et al., 2020; Škrbić et al., 2018; Zhang et al., 2019). Some of them have evaluated the actual toxicity of the bioavailable fraction of pollutants associated with urban dust particles, employing different biological models such as Caenorhabditis elegans, Eisenia fetida, Sphaerechinus granularis, Arbacia lixula, and Heterocypris incongruens (Khanal et al., 2015; Trifuoggi et al., 2019; Yadav et al., 2019).
The use of the nematode Caenorhabditis elegans as a biological model in environmental assessments is widespread (Anbalagan et al., 2012; Queirós et al., 2019). This organism is characterized by its small size, transparent body, short life cycle, easy handling in the laboratory, shares about 80% of homologous genes with humans, and has well-known physiology (Aschner et al., 2013; Leung et al., 2008). Therefore, it facilitates the development of robust, efficient and economical toxicological experiments (Machado et al., 2020; Tejeda-Benitez & Olivero-Verbel, 2016; Williams & Dusenbery, 1990).
Transgenic strains of C. elegans carrying fluorescent proteins, e.g., green fluorescent protein (GFP), associated to genes of different metabolic pathways have permitted gene expression studies (Tejeda-Benitez & Olivero-Verbel, 2016). These are frequently employed in environmental assessment. For instance, mtl-1 and mtl-2 respond to metals by producing metallothionein (MT), a protein related to metal detoxification and protection against oxidizing agents (Aschner et al., 2013). Sod-1 to sod-5 code for superoxide dismutase (SODs), a key molecular protection against oxidative stress; and unc-25, a neurotoxicity marker that encodes the enzyme glutamic acid decarboxylase (GAD), fundamental for the synthesis of γ-aminobutyric acid (GABA), the most abundant inhibitory neurotransmitter in vertebrates and invertebrates (Jorgensen, 2005; Mclntire et al., 1993); among others. Transgenic nematodes containing these genes have been used in toxicity studies of PTEs (Anbalagan et al., 2012; Ma et al., 2009; Tejeda-Benitez et al., 2016) and other xenobiotics that pollute different environmental matrices (De la Parra-Guerra et al., 2020; García-Espiñeira et al., 2018; Polak et al., 2014; Rui et al., 2013). It has been reported that the urban dust in Barranquilla presents concentrations of V, Cr, Mn, Co, Ni, Cu, Zn, As, Se, Mo, Ag, Cd, Sn, Sb, Pb, and Bi above background values, with ecological risk associated mainly with Cd, Cu, As, Hg, Pb, and Ni (Osorio-Martinez et al., 2021). Thus, it has been hypothesized that the extracts of urban dust from this city cause toxic effects in C. elegans associated with the PTEs contained in the particles.
Therefore, the objective of this study was to quantify the PTEs in the inorganic extract of urban dust deposited in the Barranquilla and to evaluate their toxicity using C. elegans as a pollution sensor. This establishes a baseline for further studies on the risk to public health that exposure to PTEs may imply.
2 Materials and Methods
2.1 Study Area
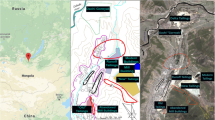
Barranquilla (10°59'16" N, 74°47'20" W) has an area of 154 km2 and 1.4 million inhabitants (DANE, 2019). It is the largest city in the Colombian Caribbean, administratively divided into five locations: Riomar, North - Historical Centre, Southwest, Metropolitan, and Southeast (Fig. 1). It is located between 0 and 142 m.a.s.l, with dry tropical climate. The average temperature range varies from 24.5 °C to 32.4 °C. The average annual relative humidity, solar brightness and rainfall are 80%, 2562 h and 821 mm, respectively (Ramírez-Cerpa et al., 2017). The weather is governed by a dry (December to April) and a rainy period (April to early December). From November to April, the trade winds blow from the northeast, and towards the end of June they appear from the southeast (Angulo, 2017).
2.2 Sampling
During the dry season (February 2019), 35 dust samples were collected in the urban area of Barranquilla (Fig. 1). The sampling sites were geo-referenced using a Garmin eTrex® GPS (Garmin International, Inc., USA). Approximately 300 g of dust particles were taken from the external surfaces of residences and commercial sites (awnings, balconies) in an area between 400-800 cm2. This corresponds roughly to the dry deposition occurred during the last 8-12 weeks (Osorio-Martinez et al., 2021). Dust samples were placed in sterile glass vials and transported to the laboratory. Subsequently, particles smaller than 75 μm were obtained through a sieve and stored at -20 °C until the extracts were obtained (Palacios-Torres et al., 2018).
2.3 Quantification of Trace Elements in Inorganic Extract of Urban Dust
The dust extracts were prepared by mixing 1.0 g of urban dust with 3.0 mL of ultrapure water (18.2 MΩ cm, Milli-Q system, Millipore, Billerica, MA, USA), shaking the suspension at 300 rpm for 24 h at 30 °C. Tube with ultrapure water was used as a control. Then, the mixtures were centrifuged at 5000 rpm for 5 min. The supernatants were filtered and acidified with a 5% HNO3 solution (Merck, Germany) for analysis.
Trace elements were determined utilizing an inductively coupled plasma mass spectrometer (model NexION 300X, PerkinElmer, USA), using conditions published elsewhere (Santos et al., 2019). Multi-element standard solutions (10 mg/L, PlasmaCal calibration solution, SCP33MS, SCP Science, Quebec, Canada) were employed to construct the calibration curves (0.1-10 μg/L). The calibration of the equipment was monitored by measuring the 5% HNO3 solution (blank) and standard solutions of known concentration every 10 samples. The limits of detection (LODs) and quantification (LOQs) are shown in Table S1.
2.4 Evaluation of Toxicity in C. elegans
2.4.1 Inorganic Extracts
A mixture of 15 g of urban dust and 45 mL K-medium (53 mM NaCl, 32 mM KCl) was shaken at 300 rpm for 24 h at 30 °C. Subsequently, the suspension was centrifuged at 5000 rpm for 5 min. The inorganic extracts obtained were stored at 4 °C.
2.4.2 Strains and Culture
The wild type N2 strain was used for the lethality, growth, and locomotion tests. The transgenic strains sod-4::GFP, mtl-2::GFP, and unc-25::GFP were employed to identify changes in gene expression. Nematodes were synchronized using a bleach solution (NaOH 0.5 M; HClO 0.8%) (Kenyon, 1988; Williams & Dusenbery, 1990). The strains were kept at 20 °C in Petri dishes with K-agar. This was prepared with KCl (0.03 M), NaCl (0.05 M), agar (17 g/L), peptone (2.5 g/L), cholesterol (25.8 μM), CaCl2 (1 mM), and MgSO4 (1 mM). The larvae were sown with Escherichia coli OP50 as food (Brenner, 1974; Williams & Dusenbery, 1990).
2.4.3 Lethality Assay
L4 larval stage nematodes were exposed for 24 h to the entire extracts and K-medium at 20 °C. Approximately 10 ± 1 nematodes were used for each treatment. Subsequently, the number of living and dead organisms was recorded by inspection with a dissecting microscope (Williams & Dusenbery, 1990; Zhuang et al., 2014). Death was assumed when there was no movement during a period of 30 s (Rui et al., 2013; Wu et al., 2013). Three experiments were carried out, with three technical replicas each.
2.4.4 Growth Assay
L1 larval stage nematodes were exposed to the inorganic extracts and K-medium for 72 h at 20 °C, and E. coli OP50 was added as food. Photographic recording of the worms was made with a Nikon SMZ 745T dissecting microscope. The body length was measured with ImageJ software (Arnold et al., 2013; Tejeda-Benitez & Olivero-Verbel, 2016). Approximately thirty nematodes were examined per treatment. Each treatment was evaluated three times (De la Parra-Guerra et al., 2020; Höss et al., 2009; Tejeda-Benitez et al., 2016).
2.4.5 Locomotion Assay
Synchronized L4 larval worms were exposed to inorganic extracts and K-medium. After 24 h, the number of body bends movements in 20 s was counted as 10 ± 1 nematodes for each treatment. A Nikon SMZ 745T dissecting microscope was used in the assay (Roh et al., 2010). The average number of movements was obtained per treatment. The experiment was repeated three times, each with three technical replicates.
2.4.6 Quantification of Gene Expression
The GFP transgenic strains of C. elegans were used to assess the effects associated with expression of genes related to metal exposure (mtl-2) (Ma et al., 2009), oxidative stress (sod-4) (Doonan et al., 2008; Fujii et al., 1998), and neurotoxicity (unc-25) (Jin et al., 1999). Aliquots of synchronized L4 nematodes were placed in 96-well microplates with non-fluorescent background and exposed to the extracts. The microplates were incubated at 20 °C in the dark, for 6, 12 and 24 h. Subsequently, fluorescence readings were taken on a Perkin-Elmer Victor 1420 plate reader at 485/525 nm for excitation/emission, respectively (Tejeda-Benitez & Olivero-Verbel, 2016). Normalization of the data was carried out against the vehicle control, K-medium. Three replicates were performed per treatment and the experiment was repeated three times.
2.4.7 Statistical Analysis
The data are presented as the mean ± standard error. Normal distribution and homoscedasticity were verified with the Shapiro-Wilk and Bartlett tests, respectively. Significant differences between means were determined with ANOVA/Kruskal-Wallis. Subsequently, the Dunnet/Dunn test was used to compare each treatment with the control. Spearman correlation, multiple linear regression (Forward method), and principal component (PCA) analyses were performed to determine associations between variables. Standard kriging and linear interpolation methods were employed to create the spatial distribution maps of the toxicity test results, using Surfer 15.3.307 (Golden Software, LLC, CO, USA). The statistical analyses were performed with GraphPad Prism 5 for Windows, version 5.01 (GraphPad Software, San Diego, CA) and IBM SPSS Statistics version 25 (IBM SPSS Statistics, NY, EE. UU.). The significance criterion was set at p < 0.05.
3 Results
3.1 Trace Element Content in Extracts from Urban Dust
The PTEs content (ng/mL) in urban dust extracts are presented in Table 1, Table S2, and Figure S1. The decreasing order of mean concentration was: Sr > Cu > Ba > Mo > Se > Cr > V > Ni > As > Zn > Rb > Mn > Sb > Co > Sn > Cd > La > Ce. Elements V, Cr, Co, Ni, Cu, As, Sr, Mo, and Ba were determined in more than 80% of the urban dust extracts. The results for Ag, Tl, Pb, and Bi were lower than the LOQ. Standard deviation (SD) data indicated wide variability in content PTEs in the dust extracts.
The average concentrations of PTEs for each location of Barranquilla are presented in Table 2. Metropolitan locality showed the highest values for V, Ni, Cu, Zn, As, Se, Sr, Mo, Cd, Sb, and Ba. Additionally, Cr, Mn, Co, Rb, and Sn were greater in the Southeast; while La and Ce in the Southwest. North - Historical Centre location had the lowest concentrations of V, Cr, Co, Ni, As, Rb, Sr, Mo, Ba, and La.
The comparison of the content of V, Cr, Co, Ni, Cu, As, Sr, Mo, and Ba between the locations with respect to the North - Historical Centre are presented in Fig. 2. The Metropolitan location showed differences for V, Cu, As, and Ba. In addition, the Metropolitan and Southeast locations exhibited differences with respect to Cr, Ni, Mo, and Sr. Likewise, the Riomar and Metropolitan locations presented differences for Co (p<0.05).
3.2 Toxicity of Urban Dust Extracts on Physiological Parameters in C. elegans
The results of the lethality, growth and locomotion tests in C. elegans are presented in Fig. 3. Twenty percent of the extracts caused lethality in the nematodes. The highest mortality was observed with the extracts of samples 25, 1, and 32, with values of 98.7%, 90.0%, and 89.6%, from the Southwest, Riomar and Southeast locations, respectively. The extracts 27, 29, 30, and 31 caused a mortality between 20.5% and 48.3%.
Nematode size was inhibited by 11.4% of the extracts, while 60% of the extracts induced an increase in length. While worm locomotion was reduced by up to 61.6%. Forty-three percent of the extracts induced decreased body bend movements.
Toxicity of urban dust extracts by Barranquilla locations on physiological parameters in C. elegans are shown in Fig. 4. Southwest, Metropolitan and Southeast localities presented lethality of 22.8%, 9.31%, and 28.9%, respectively (p<0.05). While the North - Historical Centre locality presented the lowest lethality in the biological model. The locations did not differ from each other.
Toxicity of inorganic extracts of urban dust samples collected in Barranquilla on physiological parameters in C. elegans. Control (Ctrl). Locations: Riomar (Rm), North - Historical Centre (CH), Southwest (SW), Metropolitan (Mt), and Southeast (SE). ●. Minimum and maximum values. *. Significant difference when compared to control. #. Significant difference when compared to Riomar (p < 0.05). The mean control values for lethality, body length and locomotion inhibition were 0, 100 and 100%, respectively
Extracts from the Southeast samples inhibited growth by an average of 5.60%. Worm size increased by 32.0% and 18.0% for the Riomar and North - Historical Centre locations, respectively. The average length of the worms exposed to the Riomar extracts showed significant differences with the control. While the Southeast location presented differences with respect to the Riomar locality.
North - Historical Centre and Southwest locations showed a significant reduction in nematode mobility with respect to control (p<0.05). However, the localities between them did not present differences in this parameter.
3.3 Gene Expression
The gene expression of C. elegans mutant strains by exposure to urban dust extracts are shown in Fig. 5, Figure S2, and S3. GFP strains exhibited the highest gene expression at 6 h of exposure to the extracts. Forty-nine percent of the extracts induced significant expression of the mtl-2 gene up to approximately four-fold compared to the control at 6 h and 12 h exposure. Besides, Riomar, Metropolitan, and Southeast locations presented significant expression of mtl-2 gene (p<0.05).
The sod-4 transgenic nematodes exposed to the inorganic extracts exhibited increased fluorescence expression up to a maximum of seven-fold that of the control. The expression of this gene was induced by 88.6% of the extracts. During the time of exposure the response decreased. Dust extracts from Riomar, Southwest, Metropolitan, and Southeast locations showed significant fluorescence in the strain (p<0.05).
Expression of the unc-25 gene in the mutant strain presented a higher expression up to approximately seven-fold that of the control and the levels decreased with time. Fluorescence expression was induced by 54.3% of the extracts. Riomar, Southwest, Metropolitan, and Southeast locations showed a significant increase in the expression of unc-25 in the first 6 h of exposure (p<0.05).
3.4 Multivariate Analysis
Spearman's correlation analysis between PTEs concentrations, physiological parameters, and gene expression are shown in Table S3. High positive associations were observed between Cr and Mo (0.929); V and As (0.916); Ni and Sr (0.888); Sr and Mo (0.791); Co and Ni (0.789); Mo and Ni (0.785); Cr and Ni (0.758); As and Co (0.755); and Cr and Sr (0.752).
Lethality test was positively associated with Cr (0.630), Ni (0.395), Sr (0.406), and Mo (0.559). In addition, growth was negatively associated with Cr (-0.648), Ni (-0.398), Cu (-0.389), Sr (-0.432), and Mo (-0.685). Likewise, GFP strain expression showed positive associations at different exposure times as follows, mtl-2 gene with Cr, Co, Ni, Cu, Sr, Mo, and Ba; sod-4 gene with Co, Ni, Cu, As, Sr, and Ba; and unc-25 with the elements Cr, Co, Ni, Cu, As, Sr, Mo, and Ba. In addition, lethality was related to growth (-0.526), locomotion (-0.336), mtl-2 gene (0.337), and unc-25 gene (0.412). While, mtl-2 gene was associated with sod-4 (0.671) and unc-25 (0.682), and sod-4 with unc-25 (0.810) (Table S3).
Multiple regression analysis indicated that the lethality is strongly associated with the elements V and Sr (r = 0.644, p = 0.001). While the growth of the nematode showed a high relationship with the metals Cr, Co, and Mo (r = 0.782, p = 0.039). As for locomotion, it showed no association with PTEs. In addition, relationships were obtained between mtl-2 gene and Cu (r = 0.517, p = 0.001), sod-4 gene with V and Ni (r = 0.782, p = 0.039), and unc-25 gene with As (r = 0.355, p = 0.037) (Table S4).
The results of the PCA were presented in Fig. 6 and Table S5. Five components (PC) explained 86.4% of the variability of the variables. The PC1 representing 25.1% of the variance was made up of the PTEs V, Co, Ni, Cu, As, Sr, and Ba. While the PC2 explains 20.2% and corresponds to the expression of mtl-2 and sod-4 genes. The PC3 (17.4%) was conformed by the expression of the unc-25 gene. The 14.4% of the variability was defined by the PC4 (growth, Cr, and Mo). Lethality and locomotion were part of the PC5 (9.16%).
4 Discussion
4.1 Trace Element Content in the Aqueous Fraction of Urban Dust
The PTEs Sr, Cu, Ba, Mo, Cr, V, Ni, As, and Co were quantified in the aqueous extracts of urban dust from Barranquilla. In addition to the natural origin of the PTEs, anthropogenic activities contribute to the increased concentration of the elements in the environmental matrices. Industrial emissions related to metallurgy, coal and oil combustion, cement plants, ceramic and glass product manufacturing, chrome plating, leather tanning, paints, wood treatment, catalysts, pesticide manufacturing, among others, are sources of Sr, Ba, Ni, Cu, Mo, As, Co, and Cr (ATSDR, 2004, 2008; Du et al., 2013; Qie et al., 2018; Yongming et al., 2006; Zhang et al., 2019). Other activities such as motor transport, gasoline combustion and wear on vehicle parts are known sources of Ni, Cu, and Mo (Du et al., 2013; Hou et al., 2019; Zhang et al., 2019), whereas oil use and petroleum combustion are known emitters of V (Zhang et al., 2019).
In general, the content of PTEs in the aqueous extracts of urban dust from Barranquilla was lower than that reported for the same samples using microwave-assisted acid digestion (Osorio-Martinez et al., 2021). This indicates that several metals in the dust, such as Ag, Tl, Pb, and Bi, are not leaching into the water, probably as a result of their corresponding chemical species and distribution in the particles.
The urban area of Barranquilla is contaminated by PTEs in particulate matter (PM10) (Ramírez et al., 2020) and urban dust (Osorio-Martinez et al., 2021). For urban dust extracts, significant values of V, Cu, As, and Ba were registered at the Metropolitan location; Cr, Ni, Mo, and Sr at the Metropolitan and Southeast locations; and Co at the Metropolitan and Riomar areas. These sites are characterized by high traffic (private vehicles, motorcycles, public and cargo transport), industrial activities (foundries and metallurgy, plastics), and port areas (Osorio-Martinez et al., 2021).
Different investigations have reported that the total content of PTEs is variable in these particles (Han et al., 2020; Osorio-Martinez et al., 2021; Romero et al., 2015; Škrbić et al., 2018; Zhang et al., 2019). However, the inorganic fraction constitutes the pollutants that manage to enter ecosystems through urban runoff (Rahman et al., 2019) and/or direct solubilization from the dust. Therefore, urban dust represents environmental and toxicological hazards by acting as a reservoir for natural and anthropogenic PTEs (Han et al., 2020; Osorio-Martinez et al., 2021; Ramírez et al., 2020; Silva et al., 2020; Zhang et al., 2019).
4.2 Toxicity of Urban Dust Extracts on Physiological Parameters in C. elegans
Nematodes act as sensors responding with changes in their physiological, genetic, and phenotypic parameters when exposed to PTEs (Aschner et al., 2013; Jiang et al., 2016; Ma et al., 2009; Shen et al., 2009; Tejeda-Benitez et al., 2016; Wang & Xing, 2008). The lethality test showed the toxicological hazard of acute exposure to these particles and the relationship with V and Sr. The combined action of these PTEs could have affected the survival of nematodes. Previous studies have reported that individual PTEs have been less toxic than their mixtures (Chu & Chow, 2002; Moyson et al., 2019); however, toxicological effects from the interaction of multiple PTEs may depend on their speciation and concentration (Moyson et al., 2018).
Vanadium can cause toxicity by altering Na+ and Ca2+ homeostasis, and/or inducing oxidative stress in invertebrates and fish (Meina et al., 2020). In other models, such as rats, V stimulates H2O2 production, inhibits oxidative phosphorylation, causes enzymatic and respiratory process suppression, and can lead to lipid peroxidation. The genotoxicity exerted by V compounds (oxidation states +4 and +5) includes chromosomal aberrations, DNA breakage, and guanosine hydroxylation, likely involving the formation of free radicals (Tripathi et al., 2018).
It is known that Sr and its calcium-like chemical characteristics may underlie neurotoxic and neuromuscular disturbances in organisms (ATSDR, 2004), changing enzyme kinetic parameters, and interacting with secondary cellular messenger and transporter systems that utilize calcium. In algae, Sr is thought to mimic calcium sorption and compete with calcium-binding at ion exchange sites in the cell wall (FEQGs, 2020). In fish, strontium bioaccumulates in the otoliths, vertebrae, and operculum (Watts & Howe, 2010). In C. elegans it decreases pharyngeal pumping, reducing the ability to ingest food (Avery & You, 2018; Güner et al., 2018).
High concentrations of PTEs in inorganic extracts of urban dust inhibit the growth of C. elegans. This has also been reported for soils and river sediments (Höss et al., 2009; Tejeda-Benitez et al., 2016). The results suggest that Co and Cr are linked to the growth of the nematode, a role that has been widely documented (Moyson et al., 2019; Regoli et al., 2012; Rudel et al., 2013). In contrast, growth inhibition was dependent on Mo concentration. Molybdenum is an essential trace element associated with a variety of metalloenzymes (e.g., xanthine oxidase, sulfite oxidase, aldehyde oxidase, and DMSO reductase) (Barceloux & Barceloux, 1999; Eisler, 1989). This element presents considerable variability in toxicity, depending on the chemical form and the animal species (Kapp, 2014). At high concentrations, Mo could form complexes with copper affecting its absorption and metabolism (Bremner, 1979). In the Eastern oyster (Crassostrea virginica) Mo causes decreased growth (Knothe & Van Riper, 1988), and although freshwater fish are more sensitive than seawater fish, younger fish are usually more sensitive than older fish (Kapp, 2014). Toxicity in vertebrate animals includes anorexia, anemia, and bone lesions (Bremner, 1979; Eisler, 1989).
The body movements of C. elegans were inhibited by exposure to urban dust extracts. Locomotive behaviors are susceptible to being disrupted by a wide spectrum of environmental stressors (Wang & Xing, 2008). In this study, no relationships between PTEs and locomotion were observed; however, it has been reported that in the nematode locomotion is affected by Cr, Ni, and Cu (Tejeda-Benitez et al., 2016; Wang & Xing, 2008), with further impacts for binary and tertiary PETs mixtures (Moyson et al., 2018).
4.3 Quantification of Gene Expression
The PTEs present in the aqueous extracts of urban dust induced the expression of genes related to metal response, oxidative stress and neurotoxicity. The expression of the mtl-2 gene was Cu concentration-dependent. In C. elegans, MT expressed by the mtl-2 gene is strongly induced in intestinal cells after exposure to metals, implying that they are involved in homeostasis and/or metal ion detoxification (Höckner et al., 2011). Therefore, an excess of PTEs, induces the expression of the mtl-2 gene (Polak et al., 2014) and can overcome the detoxifying action of MT, causing consequent cytosolic damage (Ma et al., 2009; Polak et al., 2014; Tejeda-Benitez et al., 2016). In addition, it can cause an oxidizing effect in the cells of the organism (Höckner et al., 2011) and even death.
The presence of V and Ni in the inorganic fraction activates the defense responses against oxidative stress in C. elegans. It has been reported that PTEs, as a V and Ni, induce the production of reactive oxygen species (ROS) and activate the production of SOD, which is one of the main enzymes of defense against superoxide radicals (Meina et al., 2020; Tang et al., 2020). In addition, high concentrations of PTEs may exceed the detoxification capacity of C. elegans, causing observable effects on growth, reproduction, physiological behavior, and neurological dysfunctions (Ijomone et al., 2020; Jiang et al., 2016; Kumar et al., 2015; Ngwa et al., 2017). The sod-4 strain was also used to evaluate the toxicity of sediments extracts of the Magdalena River (Tejeda-Benitez et al., 2016), in this case the expression was two-fold compared to the control; results were lower than those obtained in this study.
In nematodes, GABA is an inhibitory and excitatory neurotransmitter mainly at neuromuscular synapses, since worms do not have a central nervous system (Aschner et al., 2013). unc-25 encodes the GAD which catalyzes the conversion of glutamate to GABA (Jin et al., 1999). Overexpression of this gene was associated with As. Arsenic is toxic in the four oxidation states (+V, +III, 0, -III), and trivalent and pentavalent arsenic are the most common species found in ecosystem (Rahman et al., 2014). This metalloid is persistent in the environment, enters the biota through primary consumers, and biomagnifies (Alvarado-Flores et al., 2019). Mitochondrial oxidative stress via ROS has been linked to arsenic-induced neurotoxicity (Vahidnia et al., 2007). This suggests that PTEs promote inhibition of the enteric system (Jorgensen, 2005), and GABAergic neurodegeneration in nematodes (Du & Wang, 2009), consequently altering locomotion, foraging, and defecation behavior (Aschner et al., 2013; Mclntire et al., 1993). Therefore, it may explain how the expression of unc-25 is related to mortality in C. elegans when exposed to urban dust extracts.
This study provides scientific evidence of the ecotoxicological risk of exposure to traces of V, Sr, Mo, Cu, and As present in extracts of dust deposited in urban areas of Barranquilla. Besides, it shows the nematode C. elegans as a sensor, of low cost and easy handling, for the monitoring and environmental impact of PTEs in urban areas and to evaluate the risk of other pollutants present in urban dust. In addition, it provides basic information to understand the impact that urban dust from industrialized cities can cause on nearby aquatic ecosystems such as rivers, marshes, and the sea. Therefore, this could support the implementation of strategies by national and local authorities for the surveillance of pollutant emissions and risks.
5 Conclusions
Caenorhabditis elegans is a biological model that can be used as a sensor to evaluate the toxicity of PTEs in inorganic extracts of urban dust from industrialized cities in the Colombian Caribbean. Metropolitan and Southeast locations of Barranquilla presented the highest concentrations of V, Cr, Co, Ni, Cu, As, Mo, Sr, and Ba in the extracts. Exposure to dust extracts affected physiological parameters such as survival and growth in C. elegans. Gene expression related to metal response, oxidative stress, and neurotoxicity in GFP transgenic nematodes was related to V, Ni, Cu, and As. Future studies could be oriented to determine the concentration, temporal variability, and toxicity of PTEs, organic compounds such as pesticides, PAHs, and emerging contaminants, among others, in urban dust and runoff from Barranquilla. This would allow the establishment of a monitoring system for the toxicity of deposited dust in urbanized areas.
Data Availability
All data generated or analyzed during this study are included in this publication [and its supplementary information files].
References
Agency for Toxic Substances and Disease Registry – ATSDR. (2004). Toxicological Profile for Strontium. Retrieved July 10, 2020, from https://www.atsdr.cdc.gov/ToxProfiles/tp159.pdf
Agency for Toxic Substances and Disease Registry – ATSDR. (2008). Chromium Toxicity. Retrieved July 10, 2020, from https://www.atsdr.cdc.gov/csem/chromium/docs/chromium.pdf
Aldana-Domínguez, J., Montes, C., & González, J. A. (2018). Understanding the Past to Envision a Sustainable Future: A Social–Ecological History of the Barranquilla Metropolitan Area (Colombia). Sustainability, 10(7), 2247. https://doi.org/10.3390/su10072247
Ali, M. U., Liu, G., Yousaf, B., Ullah, H., Abbas, Q., Munir, M. A. M., & Irshad, S. (2019). Biomonitoring and health risks assessment of trace elements in various age-and gender-groups exposed to road dust in habitable urban-industrial areas of Hefei, China. Environmental Pollution, 244, 809–817. https://doi.org/10.1016/j.envpol.2018.10.084
Alvarado-Flores, J., Rubio-Franchini, I., Sánchez-Ávila, A. S., Ramírez-Tlalolín, G. D. J., & Rico-Martínez, R. (2019). Arsenic toxicity, bioaccumulation and risk assessment: A case study in Tolimique Dam, Aguascalientes, Mexico. Cogent Environmental Science, 5(1), 1650630. https://doi.org/10.1080/23311843.2019.1650630
Anbalagan, C., Lafayette, I., Antoniou-Kourounioti, M., Haque, M., King, J., Johnsen, B., Baillie, D., Gutierrez, C., Rodriguez, J., & De Pomerai, D. (2012). Transgenic nematodes as biosensors for metal stress in soil pore water samples. Ecotoxicology, 21(2), 439–455. https://doi.org/10.1007/s10646-011-0804-0
Angulo, J. (2017). Barranquilla's Water Distribution System: A First Detailed Description. Procedia Engineering, 186, 12–19. https://doi.org/10.1016/j.proeng.2017.03.202
Arnold, M. C., Badireddy, A. R., Wiesner, M. R., Di Giulio, R. T., & Meyer, J. N. (2013). Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Archives of Environmental Contamination and Toxicology, 65(2), 224–233. https://doi.org/10.1007/s00244-013-9905-5
Aschner, M., Chen, P., Martinez-Finley, E. J., Bornhorst, J., & Chakraborty, S. (2013). Metal-induced neurodegeneration in C. elegans. Frontiers in Aging Neuroscience, 5, 18. https://doi.org/10.3389/fnagi.2013.00018
Atiemo, M. S., Ofosu, G. F., Kuranchie-Mensah, H. A., Tutu, O., Palm, L., & Blankson, S. A. (2011). Contamination assessment of heavy metals in road dust from selected roads in Accra, Ghana. Research Journal of Environmental and Earth Sciences, 3(5), 473–480. https://doi.org/10.1080/23311843.2017.1405887
Avery, L., & You, Y. J. (2018). C. elegans feeding. In WormBook: The Online Review of C. elegans Biology Retrieved August 6, 2020, from http://www.wormbook.org/chapters/www_feeding/feeding.html
Barceloux, D. G., & Barceloux, D. (1999). Molybdenum. Journal of Toxicology: Clinical Toxicology, 37(2), 231–237. https://doi.org/10.1081/CLT-100102422
Bremner, I. (1979). The toxicity of cadmium, zinc and molybdenum and their effects on copper metabolism. Proceedings of the Nutrition Society, 38(2), 235–242. https://doi.org/10.1079/PNS19790037
Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. https://doi.org/10.1093/genetics/77.1.71
Chu, K. W., & Chow, K. L. (2002). Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative. Aquatic Toxicology, 61(1-2), 53–64. https://doi.org/10.1016/s0166-445x(02)00017-6
Cortés, J. L., Bautista, F., Delgado, C., Quintana, P., Aguilar, D., García, A., Figueroa, C., & Gogichaishvili, A. (2017). Spatial distribution of heavy metals in urban dust from Ensenada, Baja California, Mexico. Revista Chapingo serie Ciencias Forestales y del Ambiente, 23(1), 47–60. https://doi.org/10.5154/r.rchscfa.2016.02.005
De la Parra-Guerra, A., Stürzenbaum, S., & Olivero-Verbel, J. (2020). Intergenerational toxicity of nonylphenol ethoxylate (NP-9) in Caenorhabditis elegans. Ecotoxicology and Environmental Safety, 197, 110588. https://doi.org/10.1016/j.ecoenv.2020.110588
Departamento Administrativo Nacional de Estadística – DANE. (2019). Resultados Censo Nacional de Población y Vivienda 2018 - Barranquilla. Atlántico - Julio 18 de 2019. Gobierno de Colombia Retrieved August 20, 2020, from https://www.dane.gov.co/files/censo2018/informacion-tecnica/presentaciones-territorio/180719-CNPV-presentacion-Atlantico.pdf
Departamento Nacional de Planeación – DNP. (2014). Mision Sistema de Ciudades; ONU-Habitat: Bogota. Colombia. Retrieved August 20, 2020, from https://wwwdnp.gov.co/programas/vivienda-agua-y-desarrollo-urbano/desarrollo-urbano/Paginas/sistema-de-ciudades.aspx
Doonan, R., McElwee, J. J., Matthijssens, F., Walker, G. A., Houthoofd, K., Back, P., Matscheski, A., Vanfleteren, J. R., & Gems, D. (2008). Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes & Development, 22(23), 3236–3241. https://doi.org/10.1101/gad.504808
Du, M., & Wang, D. (2009). The neurotoxic effects of heavy metal exposure on GABAergic nervous system in nematode Caenorhabditis elegans. Environmental Toxicology and Pharmacology, 27(3), 314–320. https://doi.org/10.1016/j.etap.2008.11.011
Du, Y., Gao, B., Zhou, H., Ju, X., Hao, H., & Yin, S. (2013). Health risk assessment of heavy metals in road dusts in urban parks of Beijing, China. Procedia Environmental Sciences, 18, 299–309. https://doi.org/10.1016/j.proenv.2013.04.039
Eisler, R. (1989). Molybdenum hazards to fish, wildlife, and invertebrates: a synoptic review (No. 19). Fish and Wildlife Service, US Department of the Interior Retrieved August 20, 2020, from https://www.nrc.gov/docs/ML1521/ML15212A086.pdf
Federal environmental quality guidelines – FEQGs. (2020). Strontium. Environment and Climate Change Canada. Government Canada Retrieved February 2, 2021, from https://www.canada.ca/content/dam/eccc/documents/pdf/pded/feqg-strontium/Federal-environmental-quality-guidelines-strontium.pdf
Fujii, M., Ishii, N., Joguchi, A., Yasuda, K., & Ayusawa, D. (1998). A novel superoxide dismutase gene encoding membrane-bound and extracellular isoforms by alternative splicing in Caenorhabditis elegans. DNA Research, 5(1), 25–30. https://doi.org/10.1093/dnares/5.1.25
Gabarrón, M., Faz, A., & Acosta, J. A. (2017). Soil or dust for health risk assessment studies in urban environment. Archives of Environmental Contamination and Toxicology, 73(3), 442–455. https://doi.org/10.1007/s00244-017-0413-x
Gallego-Cartagena, E., Morillas, H., Maguregui, M., Patiño-Camelo, K., Marcaida, I., Morgado-Gamero, W., Silva, L. F. O., & Madariaga, J. M. (2020). A comprehensive study of biofilms growing on the built heritage of a Caribbean industrial city in correlation with construction materials. International Biodeterioration & Biodegradation, 147, 104874. https://doi.org/10.1016/j.ibiod.2019.104874
García-Espiñeira, M. C., Tejeda-Benítez, L. P., & Olivero-Verbel, J. (2018). Toxic effects of bisphenol A, propyl paraben, and triclosan on Caenorhabditis elegans. International Journal of Environmental Research and Public Health, 15(4), 684. https://doi.org/10.3390/ijerph15040684
Güner, R., Berksoy, S., Özpinar, N., Akyol, M., Tosun, M., & Özçelik, S. (2018). Effects of strontium chloride hexahydrate in Caenorhabditis elegans individuals as a model for skin aging. European Journal of Pharmaceutical and Medical Research, 5(12), 67–72.
Han, Q., Wang, M., Cao, J., Gui, C., Liu, Y., He, X., He, Y., & Liu, Y. (2020). Health risk assessment and bioaccessibilities of heavy metals for children in soil and dust from urban parks and schools of Jiaozuo, China. Ecotoxicology and Environmental Safety, 191, 110157. https://doi.org/10.1016/j.ecoenv.2019.110157
Höckner, M., Dallinger, R., & Stürzenbaum, S. R. (2011). Nematode and snail metallothioneins. Journal of Biological Inorganic Chemistry, 16(7), 1057. https://doi.org/10.1007/s00775-011-0826-3
Höss, S., Jänsch, S., Moser, T., Junker, T., & Römbke, J. (2009). Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicology and Environmental Safety, 72(7), 1811–1818. https://doi.org/10.1016/J.ECOENV.2009.07.003
Hou, S., Zheng, N., Tang, L., Ji, X., Li, Y., & Hua, X. (2019). Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environment International, 128, 430–437. https://doi.org/10.1016/j.envint.2019.04.046
Ijomone, O. M., Miah, M. R., Akingbade, G. T., Bucinca, H., & Aschner, M. (2020). Nickel-induced developmental neurotoxicity in C. elegans includes cholinergic, dopaminergic and GABAergic degeneration, altered behaviour, and increased SKN-1 activity. Neurotoxicity Research, 37, 1018–1028. https://doi.org/10.1007/s12640-020-00175-3
Jiang, Y., Chen, J., Wu, Y., Wang, Q., & Li, H. (2016). Sublethal toxicity endpoints of heavy metals to the nematode Caenorhabditis elegans. PLoS One, 11(1), e0148014. https://doi.org/10.1371/journal.pone.0148014
Jin, Y., Jorgensen, E., Hartwieg, E., & Horvitz, H. R. (1999). The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. Journal of Neuroscience, 19(2), 539–548. https://doi.org/10.1523/JNEUROSCI.19-02-00539.1999
Jorgensen, E. M. (2005). Gaba. In WormBook: The Online Review of C. elegans Biology. WormBook Retrieved August 6, 2020, from http://www.wormbook.org/chapters/www_gaba/gaba.html
Kapp, R. W. (2014). Molybdenum. In P. Wexler (Ed.), Encyclopedia of Toxicology (3rd ed., pp. 383–388). Academic Press. https://doi.org/10.1016/b978-0-12-386454-3.00884-8
Kenyon, C. (1988). The nematode Caenorhabditis elegans. Science, 240(4858), 1448–1453. https://doi.org/10.1126/science.3287621
Khanal, R., Furumai, H., & Nakajima, F. (2015). Characterization of toxicants in urban road dust by Toxicity Identification Evaluation using ostracod Heterocypris incongruens direct contact test. Science of the Total Environment, 530, 96–102. https://doi.org/10.1016/j.scitotenv.2015.05.090
Knothe, D. W., & Van Riper, G. G. (1988). Acute toxicity of sodium molybdate dihydrate (Molyhibit 100) to selected saltwater organisms. Bulletin of Environmental Contamination and Toxicology, 40(5), 785–790. https://doi.org/10.1007/BF01697531
Kumar, R., Pradhan, A., Khan, F. A., Lindström, P., Ragnvaldsson, D., Ivarsson, P., Olsson, P., & Jass, J. (2015). Comparative analysis of stress induced gene expression in Caenorhabditis elegans following exposure to environmental and lab reconstituted complex metal mixture. PLoS One, 10(7), e0132896. https://doi.org/10.1371/journal.pone.0132896
Kurt-Karakus, P. B. (2012). Determination of heavy metals in indoor dust from Istanbul, Turkey: estimation of the health risk. Environment International, 50, 47–55. https://doi.org/10.1016/j.envint.2012.09.011
Leung, M. C. K., Williams, P. L., Benedetto, A., Au, C., Helmcke, K. J., Aschner, M., & Meyer, J. N. (2008). Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicological Sciences, 106, 5e28. https://doi.org/10.1093/toxsci/kfn121
Li, J., & Lin, B. (2019). The sustainability of remarkable growth in emerging economies. Resources, Conservation and Recycling, 145, 349–358. https://doi.org/10.1016/j.resconrec.2019.01.036
Ma, H., Glenn, T. C., Jagoe, C. H., Jones, K. L., & Williams, P. L. (2009). A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environmental Toxicology and Chemistry, 28(6), 1311–1318. https://doi.org/10.1897/08-496.1
Machado, M. L., Zamberlan, D. C., Arantes, L. P., Aschner, M., & Soares, F. A. A. (2020). Caenorhabitidis elegans as an animal model in toxicological studies. In C. Pope & J. Liu (Eds.), An Introduction to Interdisciplinary Toxicology (pp. 533–544). Academic Press. https://doi.org/10.1016/B978-0-12-813602-7.00038-7
McDonald, R. I., Marcotullio, P. J., & Güneralp, B. (2013). Urbanization and global trends in biodiversity and ecosystem services. In T. Elmqvist, M. Fragkias, J. Goodness, B. Güneralp, P. Marcotullio, R. McDonald, S. Parnell, M. Schewenius, M. Sendstad, K. Seto, & C. Wilkinson (Eds.), Urbanization, Biodiversity and Ecosystem Sevices: Challenges and Opportunities. A Global Assessment (pp. 31–52). Springer Nature.
Mclntire, S. L., Jorgensen, E., & Horvitz, H. R. (1993). Genes required for GABA function in Caenorhabditis elegans. Nature, 364(6435), 334–337. https://doi.org/10.1038/364334a0
Mclntire, S. L., Jorgensen, E., Kaplan, J., & Horvitz, H. R. (1993). The GABAergic nervous system of Caenorhabditis elegans. Nature, 364(6435), 337–341. https://doi.org/10.1038/364337a0
Meina, E. G., Niyogi, S., & Liber, K. (2020). Investigating the mechanism of vanadium toxicity in freshwater organisms. Aquatic Toxicology, 229, 105648. https://doi.org/10.1016/j.aquatox.2020.105648
Molina, L. T., Gallardo, L., Andrade, M., Baumgardner, D., Borbor-Córdova, M., Bórquez, R., Casassa, G., Cereceda-Balic, F., Dawidowski, L., Garreaud, R., Huneeus, N., Lambert, F., McCarty, J. L., Mc Phee, J., Mena-Carrasco, M., Raga, G. B., Schmitt, C., & Schwarz, J. P. (2015). Pollution and its Impacts on the South American Cryosphere. Earth’s Future, 3(12), 345–369. https://doi.org/10.1002/2015EF000311
Moyson, S., Town, R. M., Vissenberg, K., & Blust, R. (2019). The effect of metal mixture composition on toxicity to C. elegans at individual and population levels. Plos One, 14(6), e0218929. https://doi.org/10.1371/journal.pone.0218929
Moyson, S., Vissenberg, K., Fransen, E., Blust, R., & Husson, S. J. (2018). Mixture effects of copper, cadmium, and zinc on mortality and behavior of Caenorhabditis elegans. Environmental Toxicology and Chemistry, 37(1), 145–159. https://doi.org/10.1002/etc.3937
Ngwa, H. A., Ay, M., Jin, H., Anantharam, V., Kanthasamy, A., & Kanthasamy, A. G. (2017). Neurotoxicity of vanadium. In M. Aschner & L. Costa (Eds.), Neurotoxicity of Metals (pp. 287–301). Springer. https://doi.org/10.1007/978-3-319-60189-2_14
Osorio-Martinez, J., Silva, L. F., Flores, E. M., Nascimento, M. S., Picoloto, R. S., & Olivero-Verbel, J. (2021). Environmental and human health risks associated with exposure to hazardous elements present in urban dust from Barranquilla, Colombian Caribbean. Journal of Environmental Quality, 50(2), 350–363. https://doi.org/10.1002/jeq2.20200
Palacios-Torres, Y., Caballero-Gallardo, K., & Olivero-Verbel, J. (2018). Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere, 193, 421–430. https://doi.org/10.1016/j.chemosphere.2017.10.160
Polak, N., Read, D. S., Jurkschat, K., Matzke, M., Kelly, F. J., Spurgeon, D. J., & Stürzenbaum, S. R. (2014). Metalloproteins and phytochelatin synthase may confer protection against zinc oxide nanoparticle induced toxicity in Caenorhabditis elegans. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 160, 75–85. https://doi.org/10.1016/j.cbpc.2013.12.001
Qie, G., Wang, Y., Wu, C., Mao, H., Zhang, P., Li, T., Li, Y., Talbot, R., Hou, C., & Yue, T. (2018). Distribution and sources of particulate mercury and other trace elements in PM2.5 and PM10 atop Mount Tai. China. Journal of Environmental Management, 215, 195–205. https://doi.org/10.1016/j.jenvman.2018.03.050
Queirós, L., Pereira, J. L., Gonçalves, F. J. M., Pacheco, M., Aschner, M., & Pereira, P. (2019). Caenorhabditis elegans as a tool for environmental risk assessment: emerging and promising applications for a “nobelized worm”. Critical Reviews in Toxicology, 49(5), 411–429. https://doi.org/10.1080/10408444.2019.1626801
Rahman, M. A., Hogan, B., Duncan, E., Doyle, C., Krassoi, R., Rahman, M. M., Naidu, R., Lim, R. P., & Hassler, C. (2014). Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicology and Environmental Safety, 106, 126–135. https://doi.org/10.1016/j.ecoenv.2014.03.004
Rahman, M. S., Khan, M. D. H., Jolly, Y. N., Kabir, J., Akter, S., & Salam, A. (2019). Assessing risk to human health for heavy metal contamination through street dust in the Southeast Asian Megacity: Dhaka, Bangladesh. Science of the Total Environment, 660, 1610–1622. https://doi.org/10.1016/j.scitotenv.2018.12.425
Ramírez, O., da Boit, K., Blanco, E., & Silva, L. F. (2020). Hazardous thoracic and ultrafine particles from road dust in a Caribbean industrial city. Urban Climate, 33, 100655. https://doi.org/10.1016/j.uclim.2020.100655
Ramírez-Cerpa, E., Acosta-Coll, M., & Vélez-Zapata, J. (2017). Análisis de condiciones climatológicas de precipitaciones de corto plazo en zonas urbanas: caso de estudio Barranquilla, Colombia. Idesia, 35(2), 87–94. https://doi.org/10.4067/S0718-34292017005000023
Regoli, L., Van Tilborg, W., Heijerick, D., Stubblefield, W., & Carey, S. (2012). The bioconcentration and bioaccumulation factors for molybdenum in the aquatic environment from natural environmental concentrations up to the toxicity boundary. Science of the Total Environment, 435, 96–106. https://doi.org/10.1016/j.scitotenv.2012.06.020
Roh, J. Y., Park, Y. K., Park, K., & Choi, J. (2010). Ecotoxicological investigation of CeO2 and TiO2 nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environmental Toxicology and Pharmacology, 29(2), 167–172. https://doi.org/10.1016/J.ETAP.2009.12.003
Romero, M. D. P., Pinilla, R. D., & Zafra-Mejía, C. A. (2015). Temporal Assessment of the Heavy Metals (Pb and Cu) Concentration Associated with the Road Sediment: Fontibón-Barrios Unidos (Bogotá DC, Colombia). Ingeniería y Universidad, 19(2), 315–333. https://doi.org/10.1114/javeriana.iyu19-2.etcm
Romieu, I., Weitzenfeld, H., & Finkelman, J. (1990). Urban air pollution in Latin America and the Caribbean: health perspectives. World health statistics quarterly. World Health Statistics Report, 43(3), 153–167. https://doi.org/10.1080/10473289.1991.10466910
Rudel, D., Douglas, C. D., Huffnagle, I. M., Besser, J. M., & Ingersoll, C. G. (2013). Assaying environmental nickel toxicity using model nematodes. PloS One, 8(10), e77079. https://doi.org/10.1371/journal.pone.0077079
Rui, Q., Zhao, Y., Wu, Q., Tang, M., & Wang, D. (2013). Biosafety assessment of titanium dioxide nanoparticles in acutely exposed nematode Caenorhabditis elegans with mutations of genes required for oxidative stress or stress response. Chemosphere, 93(10), 2289–2296. https://doi.org/10.1016/J.CHEMOSPHERE.2013.08.007
Santos, R. F., Cruz, S. M., Krzyzaniak, S. R., Duarte, F. A., Mello, P. A., & Flores, E. M. (2019). Trace metal impurities determination in high-purity polyimide by plasma-based techniques. Microchemical Journal, 146, 492–497. https://doi.org/10.1016/j.microc.2019.01.039
Shen, L., Xiao, J., Ye, H., & Wang, D. (2009). Toxicity evaluation in nematode Caenorhabditis elegans after chronic metal exposure. Environmental Toxicology and Pharmacology, 28(1), 125–132. https://doi.org/10.1016/j.etap.2009.03.009
Silva, L. F. O., Milanes, C., Pinto, D., Ramirez, O., & Lima, B. D. (2020). Multiple hazardous elements in nanoparticulate matter from a Caribbean industrialized atmosphere. Chemosphere, 239, 124776. https://doi.org/10.1016/j.chemosphere.2019.124776
Škrbić, B. D., Buljovčić, M., Jovanović, G., & Antić, I. (2018). Seasonal, spatial variations and risk assessment of heavy elements in street dust from Novi Sad, Serbia. Chemosphere, 205, 452–462. https://doi.org/10.1016/j.chemosphere.2018.04.124
Sustainable Development Department – SDD. (2012). Colombia: Strengthening Environmental and Natural Resources Institutions. Retrieved May 9, 2020, from https://www.minambiente.gov.co/images/AsuntosambientalesySectorialyUrbana/pdf/contaminacion_atmosferica/Colombia_Strengthening_Environmental_and_Natural_Resources_Institutions.pdf
Tang, B., Williams, P. L., Xue, K. S., Wang, J. S., & Tang, L. (2020). Detoxification mechanisms of nickel sulfate in nematode Caenorhabditis elegans. Chemosphere, 260, 127627. https://doi.org/10.1016/j.chemosphere.2020.127627
Tejeda-Benitez, L., Flegal, R., Odigie, K., & Olivero-Verbel, J. (2016). Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environmental Pollution, 212, 238–250. https://doi.org/10.1016/j.envpol.2016.01.057
Tejeda-Benítez, L., Noguera-Oviedo, K., Aga, D. S., & Olivero-Verbel, J. (2018). Toxicity profile of organic extracts from Magdalena River sediments. Environmental Science and Pollution Research, 25(2), 1519–1532. https://doi.org/10.1007/s11356-017-0364-9
Tejeda-Benitez, L., & Olivero-Verbel, J. (2016). Caenorhabditis elegans, a Biological Model for Research in Toxicology. Reviews of Environmental Contamination and Toxicology, 237, 1–35. https://doi.org/10.1007/978-3-319-23573-8_1
Trifuoggi, M., Pagano, G., Oral, R., Gravina, M., Toscanesi, M., Mozzillo, M., Siciliano, A., Burić, P., Lyons, D. M., Palumbo, A., Thomas, P. J., D'Ambra, L., Crisci, A., Guida, M., & Thomas, P. J. (2019). Topsoil and urban dust pollution and toxicity in Taranto (southern Italy) industrial area and in a residential district. Environmental Monitoring and Assessment, 191(1), 43. https://doi.org/10.1007/s10661-018-7164-7
Tripathi, D., Mani, V., & Pal, R. P. (2018). Vanadium in biosphere and its role in biological processes. Biological Trace Element Research, 186(1), 52–67. https://doi.org/10.1007/s12011-018-1289-y
Trujillo-González, J. M., & Torres-Mora, M. A. (2015). Assessment of heavy metals in accumulated road dust in three sectors of the city of Villavicencio, Colombia. Luna Azul, 41, 296–308. https://doi.org/10.17151/luaz.2015.41.16
Vahidnia, A., Van der Voet, G. B., & De Wolff, F. A. (2007). Arsenic neurotoxicity-a review. Human & Experimental Toxicology, 26(10), 823–832. https://doi.org/10.1177/0960327107084539
Wang, D., & Xing, X. (2008). Assessment of locomotion behavioral defects induced by acute toxicity from heavy metal exposure in nematode Caenorhabditis elegans. Journal of Environmental Sciences, 20(9), 1132–1137. https://doi.org/10.1016/S1001-0742(08)62160-9
Watts, P., & Howe, P. (2010). Strontium and strontium compounds (No. 77). World Health Organization Retrieved May 6, 2021, from https://www.who.int/ipcs/publications/cicad/cicad77.pdf
Williams, P. L., & Dusenbery, D. B. (1990). Aquatic Toxicity Testing Using the Nematode, Caenorhabditis elegans. Environmental Toxicology and Chemistry, 9(10), 1285–1290. https://doi.org/10.1002/etc.5620091007
Wu, Q., Nouara, A., Li, Y., Zhang, M., Wang, W., Tang, M., Ye, B., Ding, J., & Wang, D. (2013). Comparison of toxicities from three metal oxide nanoparticles at environmental relevant concentrations in nematode Caenorhabditis elegans. Chemosphere, 90(3), 1123–1131. https://doi.org/10.1016/J.CHEMOSPHERE.2012.09.019
Yadav, I. C., Devi, N. L., Singh, V. K., Li, J., & Zhang, G. (2019). Spatial distribution, source analysis, and health risk assessment of heavy metals contamination in house dust and surface soil from four major cities of Nepal. Chemosphere, 218, 1100–1113. https://doi.org/10.1016/j.chemosphere.2018.11.202
Yokel, R. A., Lasley, S. M., & Dorman, D. C. (2006). The speciation of metals in mammals influences their toxicokinetics and toxicodynamics and therefore human health risk assessment. Journal of Toxicology and Environmental Health, Part B, 9(1), 63–85. https://doi.org/10.1080/15287390500196230
Yongming, H., Peixuan, D., Junji, C., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi'an, Central China. Science of the Total Environment, 355, 176–186. https://doi.org/10.1016/j.scitotenv.2005.02.026
Zhang, M., Li, X., Yang, R., Wang, J., Ai, Y., Gao, Y., Zhang, Y., Zhang, X., Yan, X., Liu, B., & Yu, H. (2019). Multipotential Toxic Metals Accumulated in Urban Soil and Street Dust from Xining City, NW China: Spatial Occurrences, Sources, and Health Risks. Archives of Environmental Contamination and Toxicology, 76(2), 308–330. https://doi.org/10.1007/s00244-018-00592-8
Zhuang, Z., Zhao, Y., Wu, Q., Li, M., Liu, H., Sun, L., Gao, W., & Wang, D. (2014). Adverse effects from clenbuterol and ractopamine on nematode Caenorhabditis elegans and the underlying mechanism. PLoS One, 9(1), e85482. https://doi.org/10.1371/journal.pone.0085482
Acknowledgments
The authors thank the Program for Doctoral Studies in Colombia (MINCIENCIAS, 785-2017. Res. 0093, February 2018); the International Student Mobility Program, the Initiative to Support Research Groups and Doctoral Programs (Vice-Rectory for Research of the University of Cartagena), and the National Council for Scientific and Technological Development (CNPq – Brazil).
Funding
Open Access funding provided by Colombia Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1676 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osorio-Martinez, J., Silva, L.F., Flores, E.M.M. et al. Toxicity Assessment of Urban Dust from Barranquilla, a Colombian Caribbean City, using Caenorhabditis elegans. Water Air Soil Pollut 234, 375 (2023). https://doi.org/10.1007/s11270-023-06332-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06332-6