Abstract
In contrast to zygotic embryogenesis, somatic embryogenesis culture systems, not limited in tissue quantity and accessibility, were found to be useful models with which to investigate the role of phytohormones during induction, development and maturation of somatic embryos. Artificial seed technology is one of the important efficient methods of in vitro propagation of a number of agronomically important plants, including Medicago species, with Medicago truncatula serving as a Fabaceae experimental model. The review will focus on the role of exogenous and endogenous plant growth regulators /phytohormones in different phases of somatic embryogenesis in Medicago species. There is evidence that induction and development of M. sativa somatic embryos require endogenous levels not only of growth stimulants (e.g. gibberellins, auxins and cytokinins), but also of phytohormones known as growth inhibitors, i.e. ABA, jasmonates and ethylene. Any alterations in the contents of these plant growth inhibitors during the distinct phases of M. sativa SE, proved unfavorable for the subsequent somatic embryo production, suggesting that the level of biosynthetic activity in tissues is optimal for sustaining an appropriate course of this developmental process. It turned out that, in the case of ethylene, its synthesis in and activity towards M. sativa is not linked to the induction, but is very important in the proliferation and differentiation phases. An ABA content lower than that of active GAs (GA4, GA7, GA1, GA5, GA3, GA6) and IAA was proven to enable the embryo formation in the M. truncatula callus. The importance of some phytohormones in the germination and conversion of somatic embryos is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis (SE) is a non-sexual process in plants in which somatic or gametic (e.g. microspore) cells develop into structures that resemble zygotic embryos. The process can take place both in vivo and in vitro. The occurrence of SE under natural conditions has also been observed in a few plant species, including Ranunculus sceleratus, Malaxis paludosa, Pryophylium calycium and Kalanchoë diagremontiana (Brown et al. 1995; Garcês et al. 2007). Since the first description of SE in an in vitro tissue culture 65 years ago (Steward et al. 1958), the process has been the subject of many studies and provides an ideal in vitro experimental system for research on early plant development at the morphological, physiological and molecular levels. Moreover, somatic embryos, immediately after their obtaining and drying, can be used as the so-called artificial seeds (somseeds; synthetic seeds) which constitute a healthy and uniform seed material that can be stored for a long time without any loss to its germination potential. Obtaining of these seeds proves independent from, e.g. unfavorable climatic conditions, which are an obstacle for obtaining fully viable seed material; under appropriate conditions, such seeds can develop functional shoot and root systems. Somatic embryo formation can be induced in vitro in many plants from various taxonomic groups, including several agronomically important species, such as those representing the family Fabaceae which includes Medicago. The ability to undergo an in vitro embryogenesis has been found in many species of Medicago, but most researchers have focused on economically important species and varieties of the Medicago sativa-falcata complex which includes M. sativa, M. falcata, M. varia, M. caerula, M. glandulosa and M. hemicycle, and which are generally lumped together as M. sativa (Brown et al. 1995). The perennial species of the Medicago complex are usually open-pollinated and autotetraploids. In addition to the cultivated species mentioned, embryogenesis was induced in wild, diploid, self-pollinating Medicago species such as M. arborea, M. lupulina, M. polymorpha and M. truncatula (Iantcheva et al. 2001). The embryogenic properties of Medicago are correlated with the genotype of the mother plants, are inherited and could be enhanced by selection (Saunders and Bingham 1972; Bingham et al. 1975; Wan et al. 1988; Neves et al. 1999; Elmaghrabi and Ochatt 2006). The first report on the in vitro regeneration, using tissue cultures, in a representative of the Medicago group, i.e. Medicago sativa L., was published in 1972 by Saunders and Bingham. Since then, several dozen papers on embryogenesis in the Medicago sativa - falcata complex have been published (reviewed by Brown et al. 1995, McKersie et al. 1995). Bingham (1989, 1991) registered both alfalfa Regen-S and hybrid Regen-SY germplasm for use in tissue culture and transformation. In turn, McKersie et al. (1989) showed that the genotype RL-34 of the Medicago sativa L. cultivar Rangelander is highly embryogenic and genetically stable during the in vitro culture. A diploid Medicago truncatula, a genetic and genomic model for legumes, was first regenerated using SE in 1989 by the group of professor Rose in Australia (Nolan et al. 1989) and required a special seed line called Jemalong 2HA (Rose et al. 1999). In the same year, the group of Professor Fevereiro in Portugal obtained a highly embryogenic line of M. truncatula, the M9-10a, derived from the non-embryogenic line M9 of the Jemalong cultivar (Neves et al. 1999; Santos and Fevereiro 2002). Acquisition of embryogenic lines from non-embryogenic ones by the researchers mentioned above has been a great step forward to the extensive use of those lines in studied related to the SE regulation at the physiological and molecular level.
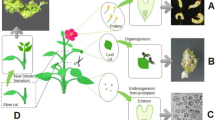
As in other plant species, emergence of somatic embryos of Medicago in in vitro cultures involves several stages, each controlled by a set of various internal (genotype, explant type) and external factors (physical, such as temperature, light, pH; chemical, such as macro- and micronutrients, growth regulators, sugars). The embryogenic properties of Medicago spp. are correlated not only with the donor plant genotype but also with the explant type; the choice of explants has a great effect on the success of an embryogenesis protocol (Brown et al. 1995). Different explants have been selected in Medicago spp. Generally, mature vegetative tissues such as leaves and petioles are often used (Meijer and Brown 1987; McKersie et al. 1989; Neves et al. 1999), as are cotyledons and hypocotyls (Iantcheva et al. 1999). Nolan et al. (1989) observed a significantly larger embryogenesis in leaf explants taken from regenerated plantlets of M. truncatula than in those from the original plant. The choice of the explant type does not seem to be of a decisive importance for obtaining Medicago embryogenic cultures, but – due to the state of primary cell competence – it affects the occurrence of a specific type of somatic embryogenesis. Embryos can form in two ways, either directly from pre-embryogenic determined cells (PEDC), e.g. an embryo, or indirectly from induced embryogenic determined cells (IEDC), which were not embryogenic before (e.g. leaves, petiole explants). Several different protocols for direct and indirect Medicago spp. SE have been developed; they are based on the ability of the embryogenic cells to form embryos after exogenous application of auxins, mainly synthetic 2,4-D and cytokinins, also mainly synthetic, such as kinetin or BA (Denchev et al. 1991; Pasternak et al. 2000; Meijer and Brown, 1987; McKersie et al. 1989). Depending on the method of somatic embryo production, different explants and media are used and two or more phases of embryogenesis are distinguished. In the direct embryogenesis without the callus phase, young trifoliate leaves were used as initial explants to obtain the embryos of M. falcata L. (Denchev et al.1991); in M. sativa subs. varia A2, leaf protoplasts (Pasternak et al. 2000) or in M. truncatula genotype R-108-1 leaf and root (Iantcheva et al.2006) were used. In the indirect embryogenesis of M. sativa cv. Rangelander or M. truncatula cv. Jemalong mutant 2HA petioles and leaves are undoubtedly, most important (Meijer and Brown, 1987, McKersie et al. 1989; Neves et al. 1999) [Figs. 1 and 2]. In turn, leaves or roots were used for the induction of callus or cell suspension of M. truncatula genotype R-108-1 and genotype M9-10a (Iantcheva et al. 2005; Duque et al. 2006; Ochatt et al. 2013).
Depending on the explants and the number of SE phases (2 or more), the process of embryo formation in these plants lasts from 4 to about 6 weeks. The development of two different systems for obtaining M. sativa cv. Rangelander embryos by Meijer and Brown (1987) and McKersie et al. (1989), differing in the number of phases, turned out to be very useful for studies on involvement of hormones in individual SE stages (Figs. 1 and 2). The two-stage system, with an induction phase (IP) and differentiation phase (DP) on solid media allows to obtain, relatively quickly and in an uncomplicated manner, somatic embryos from the callus, although most of the embryos reach the globular and heart-shaped stage, and only a few develop into the cotyledonary stage (Fig. 1). This system allowed to accurately determine the contribution of each of the phytohormones of interest to the induction of embryogenesis and initiation of the somatic embryo formation. But apart from the number of embryos produced, it was difficult to accurately trace the stages of their development. The longer, more labor-intensive system using solid (induction, differentiation, maturation phases) media and liquid media (proliferation phase) was more appropriate for this purpose (Fig. 2). The use of both systems facilitated the detailed and thorough examination of the role of phytohormones in the distinct phases of SE. To follow the role of plant phytohormones in the induction of embryogenesis, the use of a regeneration system with non-embryogenic and embryogenic genotypes (Fig. 3) proved appropriate. It is generally accepted that the competence for SE is genetically controlled and is mediated through the actual ontogenetic state of the initial explants (cytological, physiological, biochemical status) which respond to a specific inductive signal. In most plant species, including those of Medicago, the signal is auxin, mainly synthetic 2,4-dichlorophenoxyacetic acid (2,4D), a known herbicide, usually applied in concentrations of at 4.5 to 22.5 µM (Meijer and Brown 1987; McKersie and Brown 1996). Other synthetic auxins include 1-naphthalene acetic acid (NAA), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) and (2,4-dichlorophenoxy)-propanoic acid which have been shown to be effective in the SE induction in Medicago spp. (Nolan et al. 1989; Brown et al. 1995). Depending on the plant system, also cytokinins, regulators responsible for plant cell divisions, may be required to enable embryogenesis to occur in cultures of Medicago species, including M. sativa and M. truncatula (Brown et al. 1995). Mainly synthetic cytokinins such as kinetin, applied in the range of 1–5 µM, and less often 6-benzyladenine (BA) and 6-benzylaminopurine (BAP) are used together with auxins for embryo induction. The kinetin used during IP significantly increases the production of embryos on the differentiation medium (Meijer and Brown 1987). To induce SE in M. truncatula, mainly BAP (Nolan et al. 1989; Iantcheva et al. 2014) or a natural cytokinin, zeatin, are used (Araujo et al. 2004). Thus, importance of exogenous stimulants such as auxins and cytokinins, commonly used in the form of their synthetic analogues, in the induction of Medicago spp. SE has been amply documented. However, knowledge on the role of endogenous classical plant growth phytohormones such as the growth stimulators gibberellins (GAs) and IAA and growth inhibitors such as abscisic acid (ABA), jasmonic acid (JA) and ethylene present in initial explants and tissues during different stages of Medicago spp. SE have been described only in few original works. Generally, the approaches illustrated in Fig. 2 were used in an attempt to understand the role of hormones in SE regulation. The first step, used commonly, is to add phytohormones to the culture medium during individual stages of SE, the next step involving addition of inhibitors of their biosynthesis and action (as far as it is known). Finally, determining endogenous hormone levels and the ratio of inhibitors to stimulants may provide a picture from which to assess the participation of individual phytohormones in SE regulation in plants, including Medicago spp. Additionally, some approaches listed above have been used to assess the importance of GAs, ABA, JA and ethylene during regeneration of Medicago spp. somatic embryos, i.e. during their germination and conversion into seedlings.
Thus, the aim of this review is to summarize information on the involvement of phytohormones (IAA, cytokinin, ethylene, ABA, GAs and jasmonic acid) in in vitro indirect Medicago spp. somatic embryogenesis in different SE phases. Studies that involve molecular research on SE regulation in Medicago spp. will not be referred to here; an extensive review of Rose (2019) provides wide adequate information on this topic with respect to Medicago spp.
Indole-3-acetic acid (IAA)
The literature information regarding the key importance of auxins, especially synthetic ones, in direct and indirect SE in plants, including Medicago spp. is very abundant, compared to information on the levels of endogenous IAA, a natural auxin, during individual stages of the process (Jiménez 2005).
Pasternak et al. (2002) showed that the level of endogenous IAA increased transiently in all protoplast cultures during the first 4–5 days of direct SE from leaf protoplast-derived cells of the embryogenic alfalfa genotype (Medicago sativa, subsp. varia A2), the increase being correlated with cell division and differentiation, hence with the development of the embryogenic cell type. More information on changes in the IAA content at individual stages of indirect SE from petiole leaf explants of the M. sativa cv. Rangelander embryogenic genotype (Fig. 2), such as the induction phase (in primary explant and callus), embryogenic suspension, differentiation phase (embryos: globular, torpedo, early and late cotyledonary) and maturation phase (matured dormant embryos without chlorophyll) was provided by Ruduś et al. (2009). The very low IAA levels were observed in the callus and cell suspension in the presence of synthetic auxins, 2.4-D at 4.5 µM, followed by quite constant but high levels in embryos developing from globular to early-cotyledonary stage in the absence of any exogenous growth regulators in the differentiation medium. Subsequently, the IAA content decreased substantially in late-cotyledonary embryos, and continued to decline further in mature embryos. To investigate in detail the importance of IAA, recognized as a key hormone for SE induction, not only its biosynthesis, but also its catabolism was studied on two genotypes of M. truncatula Jemalong: embryogenic M9-10a (E) and non-embryogenic M9 (NE) [Fig. 3] (Kępczyńska and Orłowska 2021). Although the same level of IAA was found in the initial leaf explants of both lines, during the 21-day induction phase in the presence of 2,4-D auxin (0.5 µM) in the tissues of the embryogenic line, by day 21 the IAA content increased in the callus by a factor of 6. The differences observed were related to different IAA catabolism during the 21 days of the induction phase; the highest level of 2-oxinoindole-3-acetic acid (oxIAA) was found in the 21-day-old NE callus. The IAA catabolism is considered to be the major pathway of IAA inactivation (Zhao et al. 2013).
The results presented indicate that IAA participates not only in SE induction, but also in the development of somatic embryos in Medicago species.
Cytokinin
Cytokinins responsible for cell division are also used in the SE induction phase in Medicago spp. (Meijer and Brown 1987, Nolan et al. 1989). In M. sativa, the most commonly used kinetin was applied in concentrations ranging within 1–5 µM. BA, zeatin and zeatin riboside have been used much less frequently (Brown et al. 1995). BAP (Nolan et al. 1989) or zeatin, a natural cytokinin (Araujo et al. 2004) is applied to the induction medium for M. truncatula.
Application of cytokinin, especially kinetin, at a concentration of 1–5 µM, together with auxins, produces a strong stimulation of embryo induction in M. sativa (Meijer and Brown 1987). However, the current data on endogenous tissue cytokinin levels during Medicago spp. SE are highly insufficient. No differences in the contents of cytokinins (zeatin, zeatin riboside, N-isopentyladenine, N-isopentyladenosine) in initial leaf explants during direct SE were observed in M. falcata L. (Ivanova et al. 1994). Higher levels of cytokinins were detected in the M. arborea NE callus than in the E callus (Pintos et al. 2002).
Ethylene
Ethylene, the only gaseous phytohormone having, among all known phytohormones, the simplest structure, produced by almost all parts of the plant and involved in regulation of many plant development processes, is particularly associated with in vitro cultures (Biddington 1992). The gas accumulates, to a different extent, in airtight culture vessels and can thus modify physiological and morphogenetic processes there. Early studies on the ethylene involvement in regulation of the indirect SE from petiole leaf explants of the highly embryogenic (F1.1) and virtually non-embryogenic (F.1.6) genotypes of Medicago sativa L. ssp. falcata SE were conducted in a system consisting of two distinct phases: induction (10 days, 2,4-D kinetin) and differentiation (20-day growth, regulator-free medium) (Meijer and Brown 1988; Meijer 1989). The ethylene-producing capacity increased in the cultures of both genotypes during the first 10 days on the induction medium and then decreased significantly during the initiation of somatic embryo formation on the differentiation medium (Meijer 1989). Cobalt and nickel ions, inhibitors of the conversion of 1-aminocyclopropane-1-carboxylic acid (ACC), a direct precursor of ethylene biosynthesis, strongly inhibited synthesis of the hormone during IP, but did not affect formation of the embryos during DP. However, cobalt ions present during DP did inhibit ethylene production and the embryo formation. Application of other known ethylene biosynthesis inhibitors blocking the ACC formation, such as aminooxyacetic acid (AOA) and aminoethoxyvinylglycine (AVG) during the induction phase did not affect ethylene production or somatic embryos, which proved the necessity of the ethylene biosynthesis during the embryo formation. In the same regeneration system, Meijer and Brown (1988) used the M. sativa ssp. falcata genotype F.1.1 and showed that out of four ACC biosynthesis inhibitors [AOA, AVG, 2,4-dinitrophenol (DNP) and salicylic acid (SA)], three – when applied in the differentiation phase - did not affect ethylene production, and SA increased it, but all inhibited the embryodifferentiation.
These results suggested that the production of ethylene during the SE induction was not relevant to the process. The suggestion is in agreement the outcome of using 2,5-norbornadiene (NBD), a specific inhibitor of ethylene action, for the first time in the SE research with a similar regeneration system (Kępczyński et al 1992). The inhibitor in question did not significantly affect the embryogenesisinduction, but – like the ethylene biosynthesis inhibitors (AOA, AVG, cobalt and nickel ions, SA) present during differentiation of M. sativa cv. Rangelander –inhibited the somatic embryo formation. Later on, application of an ethylene biosynthesis and action inhibitors, AVG and Ag+, respectively, during development of the embryogenic callus in M. truncatula cv. Jemalong mutant 2HA mesophyll protoplasts confirmed the essential role of ethylene in Medicago spp. (Mantiri et al. 2008). Expression of the ethylene biosynthesis gene ACC synthase (ACS) and ACC oxidase (ACO) was inhibited in the presence of the compounds mentioned; in addition, the embryo production was strongly inhibited as well.
The rate of ethylene production is known to be dependent on the presence of 2,4-D and kinetin in the culture medium; the two compounds can act synergistically to promote ethylene biosynthesis (Lieberman 1979). The two regeneration protocols used, the 2-phase (RPI, Fig. 1) and the 4-phase one (RPII, Fig. 2) for somatic embryo production in M. sativa cv. Rangelander confirmed that higher concentrations of 2.4-D and kinetin in the induction medium contributed to higher ethylene production (Kępczyńska et al. 2009). The highest ethylene production was detected in both regeneration protocols, compared to production in the differentiation medium free from 2,4-D and kinetin; this confirmed results reported previously by Meijer (1989) with M. sativa ssp. falcata. Moreover, the use of the 4-step regeneration system allowed, for the first time, to trace ethylene production by the embryogenic suspension and, most importantly, to trace the production during various embryonic development stages and during embryo maturation in the presence of ABA (Kępczyńska et al. 2009). Much less ethylene was produced by the embryogenic suspension compared to the callus, but after transfer to the growth regulator-free differentiation medium, the production of the gas increased, although still by about almost half as much as during the callus growth. Ethylene production was drastically reduced following transfer of the embryos to the maturation medium supplemented with 20 µM ABA (Kępczyńska et al. 2009). Tracing the production of ethylene, endogenous ACC (a direct precursor of ethylene biosynthesis) and the in vivo activity of ACO (the enzyme responsible for ACC to ethylene conversion) during the 2- and 4-phase SE led to the conclusion that the activity of the enzyme, but mainly the availability of ACC, is responsible for changes in the ethylene production during individual SE phases. Huang et al. (2001) showed that when the callus obtained from leaf petiole of M. sativa L. cv. Jin nan was transferred to a liquid medium for the induction of embryogenesis, the ACC level sharply increased to decrease as embryogenesis proceeded. They noted that the decrease in the ACC content during differentiation was related to conversion of this ethylene precursor to 1-malonyloamino-cyclopropane-1-carboxylic acid (MACC), and suggested that such conversion may be beneficial for the formation of embryos. A high ethylene accumulation in the culture vessels due to rubber caps as well as ethylene removal by KMnO4 and HgClO4 during the induction and differentiation phases in M. sativa cv. Rangelander SE had no effect on induction of the callus and its growth, nor was the formation and development of somatic embryos affected (Kępczyńska et al. 2009). Reduction of ethylene biosynthesis by AVG during the induction phase in RPI had no effect on callus growth and somatic embryo production, but when AVG was added to differentiation phase, the decrease in ethylene production was accompanied by inhibition of embryo production. Application of AVG and other SA during the proliferation and differentiation phases in RPII not only reduced the production of embryos, but also their development; more embryos were arrested at the globular or torpedo stage (Kępczyńska et al. 2009; Kępczyńska and Zielińska 2011). Mantiri et al. (2008), who studied the M. truncatula SE, showed that the ACS and ACO genes, encoding ACC- synthase and ACC-oxidase, the key enzymes in ethylene biosynthesis, are consistently expressed at higher levels in tissues of the super-embryogenic mutant 2HA than in the non-embryogenic callus of the Jemalong cultivar. Regulation of M. sativa L.cv. Rangelander somatic embryo formation was shown to involve not only ethylene biosynthesis, but also ethylene action; effects of a blocked ethylene action during SE in RPI (Kępczyński et al. 1992) was confirmed in experiments with an extended number of SE stages (RPII system, Fig. 2) by using not only NBD, but also 1-methylcyclopropane (1-MCP) another ethylene binding inhibitor, during the proliferation and differentiation phases of indirect SE in M. sativa cv. Rangelander (Kępczyńska and Zielińska 2011). Both had an inhibitory effect on the suspension’s multiplication rate and embryogenic capacity, production of embryos and their development; development of more embryos was arrested at the globular stage. Moreover, both ethylene biosynthesis and action inhibitors present during the proliferation and differentiation phases diminished the lateral germination and conversion of cotyledonary embryos obtained, i.e. their vigor, on an MS solid medium (Kępczyńska and Zielińska 2011).
All these studies conducted on Medicago spp. showed that both ethylene biosynthesis and action were necessary for SE in the proliferation and differentiation phases and, moreover, that disturbance of these processes adversely affected germination cotyledonary embryos obtained and their conversion to seedlings. Ethylene, a known seed germination stimulant (Kępczyński and Kępczyńska 1997), participates also in germination and conversion of somatic M. sativa cv. Rangelander embryos (Kępczyńska and Zielińska 2013). The inhibitory effect of AVG and SA, two ethylene biosynthesis inhibitors, on somatic embryo germination and conversion to plantlets indicates that the processes require endogenous ethylene.
Although somatic embryos of M. sativa and other species kept in vitro do not produce endosperm-like zygotic seeds containing α-amylase responsible for starch hydrolysis (Kohno and Namori 1991), they also produce the enzyme discussed (Kępczyńska and Zielińska 2006). The inhibitory effect of AVG or SA present in the germination medium on α-amylase activity as well as on starch hydrolysis and acceleration of the disappearance of soluble sugars in the presence of these inhibitors in the conversion medium indicate involvement of ethylene biosynthesis in regulation of sugar metabolism of M. sativa, germination of embryos and their conversion to seedlings (Kępczyńska and Zielińska 2013).
Abscisic acid
Abscisic acid (ABA) is a classical plant growth inhibitor which inhibits the plant growth and development; it also regulates embryo maturation and seed dormancy, and inhibits germination, cell division and elongation (Finkelstein and Rock 2002). Few studies have focused on the role of exogenous ABA in the induction of SE in Medicago spp. Its use in two phases of the regeneration system in M. sativa cv. Rangelander (Fig. 1), i.e. the induction and differentiation, resulted in reduction of the callus and embryo production and development (Ruduś et al. 2001). In another regeneration system (Fig. 2) in M. sativa cv. Rangelander, the use of ABA in the induction, proliferation of suspension and differentiation phases resulted in reduction of growth of the callus and embryogenic tissue suspension as well as in production and development of somatic embryos (Ruduś et al. 2006). ABA added to the differentiation medium was observed to inhibit embryo development; more globular and torpedo embryos than cotyledonary ones were produced. Such retardation of the embryo development by ABA present in the differentiation medium can be related to the compound’s inhibitory effect on ethylene production through reduction of the ACO activity, which confirms earlier findings that ethylene biosynthesis (Meijer 1989; Kępczyńska et al. 2009; Kępczyńska and Zielińska 2011) and action (Kępczyński et al. 1992; Kępczyńska and Zielińska 2011) are involved in the control of individual phases of SE in M. sativa cv. Rangelander.
ABA inhibited also germination of mature embryos and their conversion to plantlets (Ruduś et al. 2006). When was applied in at the differentiation phase, exogenous ABA was a stimulant of somatic embryo production in M. truncatula 2HA (1 µM) (Nolan and Rose 1998). ABA application at a low ABA / GA3 ratio to the induction medium was found to have a stimulatory effect on embryo production in M. truncatula 2HA (Nolan et al. 2014). Fluridone (FL), an ABA biosynthesis inhibitor, present in the induction, proliferation or differentiation phase in Medicago sativa SE was observed to exert a negative effect on the somatic embryo production and development (Ruduś et al. 2009), which suggests that indirect somatic embryogenesis in alfalfa cultures requires some level of the plant growth inhibitor discussed. FL decreased ABA levels in developing somatic embryos of M. sativa (Ruduś et al. 2009.
Thus, the data obtained so far from experiments involving applications of both ABA and its biosynthesis inhibitor point to the participation of endogenous ABA in SE regulation in Medicago spp. plants and suggest that a certain amount of endogenous ABA is needed both during the induction of embryogenesis and for the proper course of the process.
Using the Elisa method to determine the ABA level during the direct M. falcata embryogenesis, Ivanova et al. (1994) found that the initial leaf explants of fast embryogenic lines showed lower ABA levels than the initial explants of slow-embryogenic line did, and that the contents at different stages of SE differed, depending on the line. The first detailed ABA content analysis with the multiplex GC-MS/MS technique was performed on various tissues of M. sativa L. cv Rangelander: intact petioles without sterilization, initial explants after sterilization, callus, cell suspension, embryos (globular, torpedo, early and late cotyledonary, mature), at specific phases of the indirect SE (Ruduś et al. 2009). During the entire SE, ABA was synthesized in all the tissues listed above, but the intensity of the process differed. The relatively high ABA content in the sterilized initial explants drastically decreased during the callus growth and suspension proliferation, but it increased considerably in the differentiating embryos, although the fully developed late-cotyledonary embryos showed a decreased ABA synthesis. Any alteration in the ABA content during distinct SE stages proved unfavorable for the subsequent embryo formation, suggesting that the level of biosynthetic activity in tissues is optimal for sustaining an appropriate course of the process, and that endogenous ABA is involved in regulation of the embryo induction and development in the embryogenic M. sativa cv. Rangelander. That endogenous ABA is important in regulating initiation of the direct embryogenesis in M. falcata was suggested by Ivanova et al. (1994); they showed that a higher ABA level in the initial explant of the non-embryogenic line, compared with the content in the embryogenic line was correlated with inhibition of the somatic embryo induction. Later on, Kępczyńska and Orłowska (2021) reported a higher level of ABA during the indirect embryogenesis of M. truncatula cv. Jemalong (Fig. 3) not only in initial explants of the NE genotype (M9), compared to that in the E genotype (M9-10a), but also in the NE callus on the last day of the induction phase (day 21 – the callus tissues of both genotypes were transferred to the differentiation medium) to be related to the absence of embryo production. Differences in the ABA contents between tissues of the E and NE M. truncatula genotypes during the indirect SE induction phase are probably due to differing contents of ABA catabolites and conjugates (Kępczyńska and Orłowska 2021). All these results show that a certain amount of endogenous ABA is needed for the induction of indirect embryogenesis in Medicago sativa and M. truncatula and for development of the somatic embryos.
Jasmonates
The phytohormones jasmonic acid (JA) and its methyl ester (MeJA), the main representatives of jasmonates, occur in different plant tissues and organs and affect many physiological processes. Although they exhibit chemical and biological similarities to ABA, in some cases they do not mimic the ABA effects (Parthier 1990). The role of these compounds in somatic embryogenesis in Medicago spp. has been discussed in few reports only. Exposure to JA and MeJA during a two-phase regeneration system in M. sativa cv. Rangelander (Fig. 1), i.e. the induction and differentiation, reduced the callus growth and the number of embryos; the inhibitory effect was weaker than that of ABA (Ruduś et al. 2001). MeJA used in the second regeneration system of M. sativa (Fig. 2) also inhibited the callus induction and growth as well as proliferation of the embryogenic suspension, but did not have any significant effect on embryo production and development when applied during the differentiation phase, in contrast to ABA which caused a 50% reduction in the production of cotyledonary embryos (Ruduś et al. 2006). Moreover, MeJA could not replace the routinely used ABA as a somatic embryo maturation inducer and, in contrast to ABA, only slightly reduced germination of the cotyledonary embryos and their conversion to plantlets. Manipulation of the endogenous jasmonate levels with indoprofen, a specific inhibitor of octadecanoid biosynthesis, was found to modify the course of the M. sativa somatic embryogenesis (Ruduś et al. 2009). Inhibition of individual stages of the indirect M. sativa ES by indoprofen during the induction phase, suspension multiplication and differentiation proved the necessity of jasmonates for those processes to occur. Biosynthesis of jasmonates and their precursor, 12-oxophytodienoic acid (OPDA), took place in tissues of M. sativa cv. Rangelander during SE proceeding in accordance to both RPI and RPII (Ruduś et al. 2005, 2009). Exposing the initial explants to mechanical and chemical stresses led to a strong accumulation of JA/ MeJA and OPDA, the concentrations of which dropped markedly during callus growth and suspension proliferation. Their levels increased again during the formation of early-cotyledonary embryos; however, when embryos reached the late-cotyledonary stage, the JA, MeJA and OPDA levels decreased considerably (Ruduś et al. 2005, 2009). An indoprofen-driven reduction of the levels of both JA and its biosynthetic precursor, OPDA, in late-cotyledonary somatic embryos confirmed the compound to be a specific jasmonate biosynthesis inhibitor. Any changes in the JA contents during individual SE phases turned out to be unfavorable for the subsequent embryo formation, which suggested that the jasmonate biosynthetic activity level in tissues is optimal for the appropriate course of SE.
Gibberellins
Compared to the high number of studies explaining the role of phytohormones from the group of stimulants, such as auxins and cytokinins, in SE regulation, research focused on gibberellins (GAs), important plant growth stimulants, is modest in volume (Jiménez 2005). Gibberellins comprise over 136 natural plant constituents, among which biological activity is exhibited by GA1, GA3, GA4, GA5, GA6, and GA7. They are a major class of phytohormones which regulate plant growth and development from seed germination to seed-fruit set (Sponsel 2016). Most information on the role of these phytohormones in SE derives from exogenous GA3 application during different phases; the outcomes are species-specific. The gibberellin A3, applied in the two-step protocol to M. sativa cv. Rangelander SE (Fig. 1) during the induction and differentiation phases reduced the callus growth and increased the number of somatic embryos; embryo production increased strongly when the hormone was present in the differentiation medium (Ruduś et al. 2002). Positive effects of GA3 addition to the induction medium, but together with ABA (a high GA3 and a low ABA concentration), on somatic embryo production have been reported for the M. truncatula 2 H line (Nolan et al. 2014). GA3 applied, even at low concentrations, during the induction phase inhibited the callus growth and the subsequent embryo production in the M. truncatula M9-10a genotype (Igielski and Kępczyńska 2017). These different effects of exogenous GA3 may suggest that the level of endogenous GAs may be sufficient for the embryogenic callus growth and embryo development in some cases and insufficient in other instances. In the M. truncatula M9-10a genotype, the amounts of endogenous GAs were probably adequate for the progress of SE. Inclusion of paclobutrazol, a triazole inhibitor of ent-kauirene oxidase, in the induction and differentiation medium during SE of M. sativa Rangelander had an inhibitory effect on the callus growth and embryo production (Ruduś et al. 2002). Also, the inhibitor of this gibberellin biosynthesis, present during embryogenesis induction in the M. truncatula cv. Jemalong embryogenic genotype M9-10a strongly inhibited the callus growth and subsequent production of embryos (Igielski and Kępczyńska 2017). Those effects indicate that endogenous GAs are required for both induction and differentiation processes. Extensive research conducted by Igielski and Kępczyńska (2017) answered the question whether gibberellins, and which of the biologically active ones (GA4, GA7, GA1, GA5, GA3, GA6) are involved in induction of the somatic embryogenesis in M. truncatula cv. Jemalong. In these studies, traced during three week-long induction phase, when cells were forming callus from leaf explants of NE (M9) and E (M9-10a) genotypes, production of intermediates and active gibberellins from 2 pathways (13-hydroxylation and non-13 hydroxylation) of gibberellins biosynthesis as well as expression of the identified in M. truncatula genome 20 most important genes responsible for the biosynthesis of active gibberellins. The 13-hydroxylation pathway was found to be activated only in the embryogenic tissues (M9-10a), with a significant increase in GA53 and GA19 intermediates after the first week of the 21-day induction phase. This was associated with an increase, after the second week, in only one bioactive gibberellin, GA3. Of all the 20 genes tested, expression only three (MtCPS, MtGA3ox, MtGA3ox2) was specific to the embryogenic tissues and reflected changes in the GA53, GA19 and GA3 contents.
Summing up
All the endogenous phytohormones described play an important role in the induction and differentiation of somatic embryos in Medicago spp., but the tissue sensitivity to individual hormones varies with the species, genotype and the system for SE induction used. Interaction between exogenous plant growth regulators added to the medium (different concentrations have been applied) and endogenous levels in primary explants used for initiation of SE must be taken into account when the differences observed are being addressed.
The use of two regenerative systems: M. sativa cv. Rangelander (embryogenic genotype) and M. truncatula cv. Jemalong (non-embryogenic M9 and embryogenic M9-10a genotypes) provided new data on the involvement of endogenous growth stimulants such as IAA, gibberellins and the growth inhibitors, known as the stress-related hormones, i.e. ethylene, ABA and jasmonates in the induction of SE and embryo development in these plant species.
During the SE induction (Pasternak et al. 2002; Ruduś et al. 2009; Kępczyńska and Orłowska 2021) and early embryo development in Medicago spp., the synthesis of IAA, known as the main SE inducer, takes place, the IAA level increasing during the processes mentioned.
In embryogenic lines of M. sativa and M. truncatula, endogenous GAs play a stimulatory role during the SE induction (Ruduś et al. 2002; Igielski and Kępczyńska 2017), and also during embryo differentiation in M. sativa (Ruduś et al. 2002). During the induction phase of indirect SE in M. truncatula, when leaf explants of the non-embryogenic and embryogenic lines formed calluses, all the six bioactive GAs from two biosynthetic pathways were present, but the SE induction was mainly accompanied by the biosynthesis of GA3 (Igielski and Kępczyńska 2017).
Ethylene, ABA and jasmonates are synthesized in tissues of embryogenic M. sativa cv. Rangelander during the whole process of indirect SE, the biosynthetic capacity differing substantially between the individual SE phases (Ruduś et al. 2006; Kępczyńska et al. 2009). ABA synthesis takes place also in M. truncatula tissues of both genotypes during the induction phase (Kępczyńska and Orłowska 2021). Any changes in the contents of these plant inhibitors during the individual phases of M. sativa SE proved unfavorable for the subsequent somatic embryo production, suggesting that the level of biosynthetic activity in embryogenic tissues is optimal for sustaining an appropriate course of the process. The control of individual phases of SE in M. sativa involves not only biosynthesis, but also ethylene action; biosynthesis and action of the hormone are not linked to the induction, but are very important in the proliferation and differentiation phases (Kępczyński et al.1992, Kępczyńska et al. 2009, Kępczyńska and Zielińska 2013). Moreover, disturbance of ethylene biosynthesis and binding during the proliferation phase of the embryogenic suspension and embryo development adversely impacted germination and conversion of cotyledonary embryos. Endogenous ethylene may be also involved in the regulation of M. sativa L. embryo germination and conversion by controlling starch hydrolysis through the influence on α-amylase activity and soluble carbohydrates metabolism (Kępczyńska and Zielińska 2013).
We believe that the best way to study the role of endogenous hormones in the SE induction in plants is to have the non-embryogenic and embryogenic genotypes of the cultivar available and simultaneously analyze their levels and their catabolites during the callus induction, as described for Medicago truncatula cv. Jemalong. Our studies showed that two genotypes of the same species (M. truncatula) and the same variety (Jemalong) differ in their GAs, IAA and ABA metabolism (Igielski and Kępczyńska 2017; Kępczyńska and Orłowska 2021). Moreover, induction of embryo formation is related to the ABA content lower than that of all the bioactive GAs (GA4, GA7, GA1, GA5, GA3, GA6) and IAA at the time of callus transfer to a hormone-free differentiation medium.
Conclusion and perspectives
The possibility of in vitro embryogenesis is of a great importance for both the basic research and practice. It allows to study the mechanism of the somatic embryogenesis and the factors controlling it, and is also of a great value for the production of artificial seeds used for large-scale vegetative propagation of plants. Thus, knowledge on changes in the levels of hormones and their metabolites throughout SE can be applied in practice on account of the research on the crosstalk between the signaling pathways that influence phytohormones. We address the importance of hormones in various physiological processes by tracing the pathways of their biosynthesis and their binding to receptors. The discovery of specific inhibitor binding in all phytohormones is of great importance for acquiring complete information on the role of the hormones in the process under study. Studies on the role of endogenous hormones in the SE induction in Medicago spp. considered only the ethylene-binding inhibitors (NBD, Ag+, 1-MCP) (Kępczyński et al. 1992, Mantri et al. 2008, Kępczyńska and Zielińska 2011). There are no corresponding data for inhibitors of auxin, gibberellins, ABA and cytokinins. Acquisition of such data will have a strong functional implication as it will allow to improve the SE protocol and efficiency of the process. In addition, there is a need to use a system for M. truncatula SE similar to that used for M. sativa (RPII), involving the propagation of embryogenic cells from callus in a liquid medium and a sequential sieving of the cell suspension obtained through 500 and 250 μm nylon screens to separate the prioembryogenic aggregates and spreading it on a 200 μm nylon screen in a differentiation hormone-free medium. The latter allow for following the development of somatic embryos on the differentiation medium from the globular stage to the final one, the cotyledonary stage. Such system is necessary for acquiring a larger greater number of well-developed cotyledonary embryos. In the Medicago truncatula Handbook (http://www.noble.org/Medicago handbook) are some protocols (Duque et al. 2006; Iantcheva et al. 2006) that can be used for the study the effects of manipulating endogenous hormone levels on the development of individual somatic stages of M. truncatula embryos.
Data availability
All data generated or analysed in this study are included in this article.
Abbreviations
- ABA:
-
Abscisic acid
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACO:
-
ACC oxidase
- ACS:
-
ACC synthase
- AOA:
-
Aminooxyacetic acid
- AVG:
-
Aminoethoxyvinylglycine
- DNP:
-
2,4-dinitrophenol
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- 2,4,5:
-
T- 2,4,5-trichlorophenoxyacetic acid
- BA:
-
6- benzyladenine
- BAP:
-
6- Benzyloaminopurine
- IAA:
-
Indole-3-acetic acid
- GAs:
-
Gibberellins
- GA3 :
-
Gibberellin acid
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- 1-MCP:
-
1-methylcyclopropane
- MACC:
-
1- (malonylamino)cyclopropane-1-carboxylic acid
- NAA:
-
1-Naphthalene acetic acid
- NBD:
-
2,5- Norbornadiene
- OPDA:
-
12-Oxophytodienoic acid
- Ox-IAA:
-
2-Oxinoindole-3-acetic acid
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- E:
-
Embryogenic
- NE:
-
Non-embryogenic
- DP:
-
Differentiation phase
- IP:
-
Induction phase
- RPI:
-
Regeneration protocol I
- RPII:
-
Regeneration protocol II
- SE:
-
Somatic embryogenesis
References
Araujo SS, Duque ASR, Santos DMMF, Fevereiro MPS (2004) An efficient transormation method to regenerate a high number of transgenic plants using a new embryogenic line of Medicago truncatula cv. Jemalong. Plant Cell Tiss Org Cult 78:123–131. https://doi.org/10.1023/B:TICU.0000022540.98231.f8
Biddington NL (1992) The influence of ethylene in plant tissue culture. Plant Growth Regul 11:173–187. https://doi.org/10.1007/BF00024072
Bingham ET (1989) Registration of Regen-S-alfalfa germplasm useful in tissue culture and transformation research. Crop Sci 29:1095–1096. https://doi.org/10.2135/cropsci1989.0011183X002900040071x
Bingham ET (1991) Registration of alfalfa hybrid Regen-SY germplasm for tissue culture and transformation research. Crop Sci 31:1098. https://doi.org/10.2135/cropsci1991.0011183X003100040075x
Bingham ET, Hurley LV, Kaatz DM, Saunders JW (1975) Breeding alfalfa which regenerate from callus tissue culture. Crop Sci 15:719–721. https://doi.org/10.1007/BF00033736
Brown DCW, Finstad KI, Watson EM (1995) Somatic embryogenesis in herbaceous dicots. In: Thorpe TA (ed) Vitro Embryogenesis in plants. Kluwer Academic Publishers, Dordrecht., pp 345–415. https://doi.org/10.1007/978-94-011-0485-2_10
Denchev P, Velcheva M, Atanasov A (1991) A new approach to direct somatic embryogenesis in Medicago Plant Cell, Rep 10:338–341 https://doi.org/10.1007/BF00193154
Duque AS, Araújo S, dos Santos DF, Fevereiro P (2006) Long-term embryogenic cell suspension cultures of Medicago truncatula cv. Jemalong line M9-10a. In: Mathesius U, Journet EP, Sumner LW (eds) The Medicago truncatula handbook. The Samuel Roberts Noble Foundation, pp 6–12
Elmaghrabi A, Ochatt S (2006) Isoenzymes and flow cytometry for the assessment of true-to-typeness of calluses an cell suspension of barrel medic prior to regenerayion. Plant Cell Tiss Organ Cult 85:31–43. https://doi.org/10.1007/s11240-005-9046-2
Finkelstein RR, Rock CD (2002) Abscisic acid biosynthesis and response. In Somerville CR, Meyerowitz EM (eds) The Arabidopsis book. American Society of Plant Biologist, Rockville. Doi/https://doi.org/10.1199/tab.0058
Garcês HM, Champagne CE, Townsley BT, Park S, Malho R, Pedroso MC, Harada JJ, Sinha NR (2007) Evolution of asexual reproduction in leaves of the genus Kalanchoë. Proc Natl Sci USA 104:15578–15583. https://doi.org/10.1073/pnas.0704105104
Huang XL, Li XJ, Li Y, Huang LZ (2001) The effect of AOA on ethylene and polyamine metabolism during early phases of somatic embryogenesis in Medicago sativa. Physiol Plant 53:319–326. https://doi.org/10.1034/j.1339-3054.2001.1130317x
Iantcheva A, Vlahova M, Bakalova E, Kondorosi E, Elliott MC, Atanassov A (1999) Regeneration of diploid annual medics via direct somatic embrtogenesis promoted by thidiazuron and benzylaminopurine. Plant Cell Rep 18:904–910. https://doi.org/10.1007/s002990050682
Iantcheva A, Vlahova M, Trinh, Brown SC, Slater C, Elliott C, Atanassov A (2001) Assessment of polysomaty embryo formation and regeneration in liquid media for various species of diploid annual. Medicago Plant Sci 160:621–627. https://doi.org/10.1016/s01168-9452(00)00432-5
Iantcheva A, Slavov S, Prinsen E, Vlahova M, van Onckelen H, Atanassov A (2005) Embryo induction and regeneration from root explants of Medicago truncatula after osmotic pre-treatment. Plant Cell Tiss Organ Cult 81:37–43. https://doi.org/10.1007/s11240-004-2774x
Iantcheva A, Vlahova M, Atanassov A (2006) Cell suspension culture of M. truncatula cv. R 108 1 initiated from leaf and root explants. In: Mathesius U, Journet EP, Sumner LW (eds) The Medicago truncatula handbook. The Samuel Roberts Noble Foundation, pp 2–5
Iantcheva A, Revalska M, Zehirow G, Vassilieva V (2014) Agrobacterium – mediated transformation of Medicago truncatula cell suspension culture provides a system for functional analysis. In Vitro Cell Dev Biol Plant 50:149–157. https://doi.org/10.1007/s11627-013-9554-4
Igielski R, Kępczyńska E (2017) Gene expression and metabolite profiling of gibberellin biosynthesis during induction of somatic embryogenesis in Medicago truncatula Gaertn. PLoS ONE 12(7):e0182055. https://doi.org/10.1371/journal.pone.0182055
Ivanova A, Velcheva M, Denchev P, Atanassov A, Van Onckelen HA (1994) Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant 92:85–89
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–11. https://doi.org/10.1007/s10725-005-3478-x
Kępczyńska E, Orłowska A (2021) Profiles of endogenous ABA, bioactive GAs, IAA and their metabolites in Medicago truncatula Gaertn. Non-embryogenic and embryogenic tissues during induction phase in relation to somatic embryo formation. Planta 253:67. https://doi.org/10.1007/s00425-021-03582-8
Kępczyńska E, Zielińska S (2006) Regulation of Medicago sativa L. somatic embryos regeneration by gibberelin A3 and abscisic acid in relation to starch content and α-amylase activity. Plant Growth Regul 49:209–217. https://doi.org/10.1007/s10725-006-9106-6
Kępczyńska E, Zielińska S (2011) Disturbance of ethylene biosynthesis and perception during somatic embryogenesis in Medicago sativa L. reduces embryos’ ability to regenerate. Acta Physiol Plant 33:1969–1980 https://doi.orgJournal of Experimental Botany, /. https://doi.org/10.1007/s11738-011-0745-5
Kępczyńska E, Zielińska S (2013) The role of endogenous ethylene in carbohydrate metabolism in Medicago sativa L. somatic embryos in relation to their ability to regeneration. J Plant Growth Regul 32:191–199. https://doi.org/10.1007/s00344-012-9288-2
Kępczyńska E, Ruduś I, Kępczyński J (2009) Endogenous ethylene in indirect somatic embryogenesis of Medicago sativa L. Plant Growth Regul 59:63–73. https://doi.org/10.1007/s10725-009-9388-6
Kępczyński J, Kępczyńska E (1997) Ethylene in seed dormancy and germination. Physiol Plant 101:720–726. https://doi.org/10.1111/j.1399-3054.1997.tb01056
Kępczyński J, McKersie BD, Brown DCW (1992) Requirement of ethylene for growth of callus and somatic embryogenesis in Medicago sativa L. J Exp Bot 43:1199–1202. https://doi.org/10.1093/jxb/43.9.1199
Kohno A, Nanmori T (1991) Changes in α- and β-amylase activities during germination of alfalfa (Medicago sativa L). Plant Cell Physiol 32:459–466. https://doi.org/10.1007/BF0248941
Lieberman M (1979) Biosynthesis and action of ethylene. Ann Rev Plant Physiol 30:533–591
Mantiri FK, Kurdyukov S, Lohar DP, Sharopova N, Saeed NA, Wang X-D, VandenBosch KA, Ray J, Rose RJ (2008) The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol 146:1622–1636. https://doi.org/10.1104/pp.107.110379
McKersie BD, Brown DCW (1996) Somatic embryogenesis and artificial seeds in forage legumes. Seed Sci Res 6:109–126. https://doi.org/10.1017/S0960258500003135
McKersie BD, Senaratna T, Bowley SR, Brown DCW, Krochko F, Bewley JD (1989) Application of artificial seed technology in the production of hybrid alfalfa (Medicago sativa L). In Vitro Cell Dev Biol 25:1183–1188. https://doi.org/10.1007/BF02621272
McKersie BD, van Acker S, Lai FM (1995) In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry somatic embryogenesis and synthetic seeds. Springer -Verlag, Berlin, Heidelberg, pp 153–169. https://doi.org/10.1007/978-3-662-03091-2_10
Meijer EGM (1989) Developmental aspects of ethylene biosynthesis during somatic embryogenesis in tissue cultures of Medicago sativa. J Exp Bot 40:479–484. https://doi.org/10.1093/jxb/40.4.479
Meijer EGM, Brown DCW (1987) A novel system for rapid high frequency somatic embryogenesis in Medicago sativa. Plant Physiol 69:591–596. https://doi.org/10.1111/j.1399-3054.1987.tb01971.x
Meijer EGM, Brown DCW (1988) Inhibition of somatic embryogenesis in tissue cultures of Medicago sativa by aminoethoxyvinylglycine, amino-oxyacetic acid, 2,4-dinitrophenol and salicylic acid at concentrations which do not inhibit growth or ethylene production. J Exp Bot 39:263–270
Neves LO, Duque SRL, Almeida JS, Fevereiro PS (1999) Repetitive somatic embryogenesis in Medicago truncatula ssp. Narbonensis and M.truncatula Gaertn.cv. Jemalong. Plant Cell Rep 18:398–405. https://doi.org/10.1007/s002990050593
Nolan KE, Rose RJ (1998) Plant regeneration from cultured Medicago truncatula with particular reference to abscisic acid and light treatments. Aust J Bot 46(1):151–160. https://doi.org/10.1071/BT96138
Nolan KE, Rose RJ, Gorst JR (1989) Regeneration of M. truncatula from tissue culture: increased somatic embryogenesis using explants from regenerated plants. Plant Cell Rep 8:278–281. https://doi.org/10.1007/BF00274129
Nolan KE, Song Y, Liao S, Saeed NA, Zhang X, Rose RJ (2014) An unusual abscisic acid and gibberellic acid synergism increases somatic embryogenesis, facilitates its genetic analysis and improves transformation Medicago truncatula. PLoS ONE 9(6):e99908. https://doi.org/10.1371/journal.pone.0099908
Ochatt S, Jacas L, Patat-Ochatt EM, Djenanne S (2013) Flow cytometric analysis and molecular characterization of Agrobacterium tumefaciens- mediated transformants of Medicago truncatula. Plant Cell Tiss Organ Cult 113:237–244. https://doi.org/10.1007/s11240-012-0263-1
Parthier B (1990) Jasmonates—hormonal regulators or stress factors in leaf senescence. J Plant Growth Regul 9:57–63. https://doi.org/10.1007/BF02041942. A
Pasternak TP, Miskolczi P, Ayaydin F, Mészáros T, Dudis D, Fehér A (2000) Exogenous auxin and cytokinin dependent activation of CDKs and cell division in leaf protoplast-derived cells alfalfa. Plant Growth Regul 32:129–141. https://doi.org/10.1023/A:1010793226030
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen HA, Dudis D, Feher A (2002) The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol 129:1807–1819. https://doi.org/10.1104/pp.000810
Pintos B, Martin JP, Centeno ML, Villalobos N, Guerra H, Martin L (2002) Endogenous cytokinins level in embryogenic and non- embryogenic calli of Medicago arborea L. Plant Sci 163:955–996. https://doi.org/10.1016/S0168-9452(02)00244-3
Rose RJ (2019) Somatic embriogenesis in the Medicago truncatula model:cellular and molecular mechanism. Front Plant Sci 10:267. 103389/fpls.2019.00267
Rose RJ, Nolan KE, Bicego L (1999) The development of the highly regenerable seed line Jemalong 2HA fortransformation of M.truncatula – implications for regenerability via somatic embryogenesis. J Plant Physiol 155:788–791. https://doi.org/10.1016/S0176-1617(99)80097-2
Ruduś I, Kępczyńska E, Kępczyński J (2001) The influence of the jasmonates and abscisic acid on callus growth and somatic embryogenesis in Medicago sativa L. tissue culture. Acta Physiol Plant 23:103–107. https://doi.org/10.1007/s11738-001-0029-6
Ruduś I, Kępczyńska E, Kępczyński J (2002) Regulation of Medicago sativa L. somatic embryogenesis by gibberellins. Plant Growth Regul 36:91–95. https://doi.org/10.1023/A:1014751125297
Ruduś I, Kępczyńska E, Kępczyński J, Wasternack C, Miersch O (2005) Changes in jasmonates and 12-oxophytodienoic acid contents of Medicago sativa L. during somatic embryogenesis. Acta Physiol Plant 27:317–324. https://doi.org/10.1007/s11738-005-0055-x
Ruduś I, Kępczyńska E, Kępczyński J (2006) Comparative efficiency of abscisic acid and metyl jasmonate for indirect somatic embryogenesis in Medicago sativa L. Plant Growth Regul 48:1–11. https://doi.org/10.10 07/s10725-005-5136-8
Ruduś I, Weiler EW, Kępczyńska E (2009) Do stress–related phytohormones, abscisic acid and jasmonic acid play a role in the regulation of Medicago sativa L. somatic embryogenesis? Plant Growth Regul 59:159–169. https://doi.org/10.1007/s10725-009-9399-3
Santos D, Fevereiro P (2002) Loss of DNA methylation affects somatic embryogenesis in M. truncatulaPlant. Cell Tissue Org Cult 70:155–161. https://doi.org/10.1023/A:1016369921067
Saunders JW, Bingham ET (1972) Production of alfalfa plants from callus tissue. Crop Sci 12:804–808. https://doi.org/10.2135/cropsci1972.0011183X001200060026x
Sponsel VM (2016) Signal achievements in gibberellin research: the second half- century. Ann Plant Rev 49:1–. https://doi.org/10.1002/9781119210436.chl
Steward FC, Mapes MO, Mears K (1958) Growth and organized development of cultured cells. II: organization in cultures grown from freely suspended cells. Am J Bot 45:705–708. https://doi.org/10.1002/j.1537-2197.1958.tb10599.x
Wan Y, Sorensen EL, Liang GH (1988) Genetic control of in vitro regeneration in alfalfa Medicago sativa L. Euphytica 39:3–9. https://doi.org/10.1016/0304-4211(79)90004-X
Zhao Z, Zhang Y, Liu X, Zhang X, Liu S, Yu X, Ren Y, Zheng X, Zhou K, Jiang L, Guo X, Gai Y, Wu C, Zhai H, Wang H, Wan J (2013) A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev Cell 27(1):113–122. https://doi.org/10.1007/BF02318910
Acknowledgements
We thank Katarzyna Łagowska for technical assistance and to Teresa Radziejewska for linguistic assistance.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
EK and JK developed the concept and wrote the manuscript. Both authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kępczyńska, E., Kępczyński, J. Hormonal regulation of somatic embryogenesis in Medicago spp.. Plant Cell Tiss Organ Cult 155, 613–625 (2023). https://doi.org/10.1007/s11240-023-02593-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02593-5