Abstract
Acetolactate synthase genes (ALS) have successfully been modified for providing resistance to ALS-inhibiting herbicides in many plant species. Based on sequence and expression analyses, we confirmed VvALS1 as the best functional ALS candidate in grapevine. To develop an ALS-based herbicide selection system for facilitating grape transformation, we firstly evaluated the responses of Vitis vinifera cv Chardonnay callus and young in vitro shoots of Vitis vinifera cv Thompson Seedless to several representative ALS-inhibiting herbicides and found a typical linear response curves to some of the herbicides, including chlorsulfuron and imazapyr belonging to the sulfonylurea or imidazolinone families, respectively. Secondly, we created constructs containing amino acid substitutions in the domains which are known to be critical to herbicide resistance and generated transgenic plants for 3 amino acid substitutions using Agrobacterium-mediated transformation of meristematic bulk tissues of Thompson Seedless. Finally, we showed that ectopic expression of two amino acid substitutions (P191S and P191T) at the N-terminal region and another (W568L) at C-terminal region in VvALS resulted in high resistance to chlorsulfuron or imazapyr herbicides in transgenic in vitro shoots. Our work highlighted the potential use of VvALS mutations imparting herbicide resistance as a selectable marker in grapevine transformation research and as a means in fostering grapevine improvement via cisgenesis, paving the way for developing a selectable co-editing system to facilitate transgene-free gene-editing.
Key message
Amino substitutions in grapevine ALS gene including P191S, P191T and W568L confer resistance to ALS-inhibiting herbicides. Chlorsulfuron and imazapyr are suitable for plant selection in grapevine transgenic research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbicide resistance is an important agronomic trait for many crops. One of the key target plant genes for herbicide resistance is the acetolactate synthase (ALS) gene, which is well known for its natural mutations endowing resistance to ALS-inhibiting herbicides in various weedy biotypes and other plant species (Tranel et al. 2022). The evolution of numerous natural ALS mutations led to the emergence of many herbicide-resistant weed populations world-wide. According to the international herbicide resistance weed database (www.weedscience.com), thus far, a total of 180 amino acid substitutions at eight amino acid positions in ALS conferring resistance to various ALS-inhibiting herbicides have been identified in about 66 herbicide resistant weed species (Tranel et al. 2022). Moreover, about 35 site-specific mutations in ALS (some induced by gene-editing) endowing resistance to ALS-inhibiting herbicides in various crop species have so far been reported (reviewed in Han & Kim 2019).
These amino acid positions critical for herbicide resistance, including Alanine 122, Proline 197, Alanine 205, Aspartic acid 376, Arginine 377, Tryptophan 574, Serine 653 and Glycine 654 (numbering based on Arabidopsis ALS), belong to six conserved domains harboring herbicide binding sites responsible for the inhibitory effect (Powles and Yu 2010). Among these, Proline 197 is the most prominent mutation with 11 amino acid substitutions at this position conferring herbicide resistance, followed by Tryptophan 574 with four amino substitutions that endow resistance to ALS-inhibiting herbicides (Tranel et al. 2022). The most common substitutions at Proline 197 are Serine with 26 known substitutions conferring resistance to ALS-inhibiting herbicides followed by Threonine with 14 known substitutions conferring resistance to herbicides while the dominant substitution at Tryptophan 574 is Leucine (Table S1).
Information on fitness costs incurred by the ALS mutations is scarce. Studies involving resistant and susceptible species of annual ryegrass and wild radish showed that P-197-S (Proline substituted by Serine at the amino acid position 197) and W-574-L (Tryptophan substituted by Leucine at the amino acid position 574) substitutions have no significant effect on ALS kinetics nor plant fitness (Yu et al. 2010; Li et al. 2013). Similar responses were observed in kochia for these two amino acid substitutions (Légère et al. 2013). However, W-574-L substitution in green amaranth has been reported to have significant fitness costs (Tardif et al. 2006). On the other hand, P-190-S substitution in watermelon ALS through base editing was reported to show no significant fitness cost in gene edited plants (Tian et al. 2018).
Acetolactate synthase is a target for over 50 commercially available herbicides that are in use world-wide (Garcia et al. 2017). The ALS-inhibiting herbicides belong to five families namely, imidazolinones (IMI), pyrimidinylthiobenzoates (PTB), sulfonylaminocarbonyltriazolinone (SCT), sulfonylureas (SU) and triazolopyrimidines (TP). Some of the major attributes of these herbicides contributing to their dominance in the herbicide market include their high selectivity, potent herbicidal activity allowing very low rate of application as well as low toxicity to animals (Powles and Yu 2010). Different amino acid substitutions tend to impart variable levels of resistance to one or more of the ALS-inhibiting herbicides (Tranel et al. 2022). Trp-574 mutation for instance has been reported to impart high resistance to multiple herbicides including those belonging to the common SU, IMI and TP herbicide families (Bernasconi et al. 1995; Hattori et al. 1995) while Pro-197 mutations mainly impart high resistance to herbicides in the SU families but low-level resistance to either IMI or TP depending on the type of substitution (Harms et al. 1992; Gaines et al. 2020).
In addition to its potential of being an important agronomic trait, ALS mutation-derived herbicide resistance has also been explored as a plant selectable marker in transformation (Reviewed in Han and Kim 2019). The use of herbicide resistance traits as a plant selection marker is of paramount importance as it can potentially replace bacterial-derived markers and hence alleviating public concerns due to the presence of foreign genetic material in the traits or products generated through transformation. Numerous examples of such developments are available for various crop species including rice (Osakabe et al. 2005), apple (Yao et al. 2013) and wheat (Ogawa et al. 2008). Recently, herbicide resistance has also been used in transformation for co-editing genes in which one gene for herbicide resistance and one other gene for a trait of interest are simultaneously edited. By selecting against the non-edited cells using herbicides in the culture media, the frequency of edited cells can significantly be enriched, hence helping simplify the screening process. Based on this strategy, edited plants, including non-transgenic ones, have been successfully obtained through co-editing ALS and a gene of interest in some crops (Zhang et al. 2019; Malabarba et al. 2021).
To the best of our knowledge, so far there is only one report available on transgenic herbicide resistance trait development in grapevine i.e. the development of a 2,4-D resistant variety (Chancellor) using bacterial tfdA gene, which is known to involve in the breakdown of 2,4-D (Mulwa et al., 2007). Currently, there is no known report on grapevine ALS mutations conferring resistance to ALS-inhibiting herbicides. While herbicide resistance is a useful trait for grape production in certain regions, our primary objective in this study is to identify amino acid substitutions in Vitis vinifera ALS that can endow dominant resistance to ALS-inhibiting herbicides and serve as a selectable marker in grapevine transformation and gene editing. Here, we report our work on the evaluation of responses of grapevine embryogenic callus and in vitro meristematic tissue to ALS-inhibiting herbicides, the creation of transgenic vines expressing three VvALS1 single amino acid substitution mutations (P191S, P191T and W568L) and the demonstration of these substitution mutations for high resistance to ALS-inhibiting herbicides namely Imzarapyr or chlorsulfuron. Our results confirmed that mutations in the grapevine ALS gene can be effectively employed as a plant selectable marker in transformation. In addition, we report two ALS-inhibiting herbicides of different chemistry, which were determined to be suitable for resistant plant selection in grapevine.
Material and methods
Plant material and maintenance
Embryogenic calli and meristematic bulks (MBs) were used as experimental materials in this study. Embryogenic calli used for herbicide sensitivity tests were induced from inflorescences of field grown Vitis vinifera cv. Chardonnay following a published protocol (Perl et al. 1995). The calli were propagated on maintenance and proliferation medium 2 (MPM2), which is MS medium supplemented with 30 g L−1 sucrose and 8.28 µM picloram and stored in the dark at 26 °C (Dai et al. 2015).
Shoots from in vitro plants of Vitis vinifera cv. Thompson Seedless were used to initiate in vitro proliferating cultures on propagation medium (PR) composed of MS medium supplemented with 4.4 μM benzyl adenine (BA), 30 g L−1 sucrose, and 7 g L−1 agar (pH 5.6). MBs were generated by subjecting proliferating shoots from the in vitro culture to chemical (gradual increase in cytokinin concentration) and mechanical (elimination of the apical dome of the shoots) treatments according to published protocols (Mezzetti et al. 2002; Xie et al. 2016). Briefly, shoot tips were gently sliced from proliferating in vitro plants, apical dome removed and cultured on initiation medium 1 (IM1) containing 4.4 μM BA, 0.5 μM Naphthalene acetic acid (NAA), 30 g L−1 sucrose, and 8 g L−1 agar (pH 5.8) for 4 weeks to induce the formation of MB. After 4 weeks, apical domes were removed by carefully slicing the tops and bottoms and subcultured onto IM2 medium with increased concentration of cytokinin (6.6 μM BA) in the medium for an additional 4 weeks. The same procedure was repeated, and the sliced tissues were subcultured for further 4 weeks on IM3 medium containing 8.8 μM BA to generate MBs, which are characterized by a strong capacity to differentiate adventitious shoots and are good explants for genetic transformation. The MBs not used were maintained on IM3 medium by sub-culturing every 4 weeks for up to 3–4 months. The pH of the media is adjusted to 5.8 before autoclaving at 121 °C for 20 min. All cultures were maintained at 25 ± 1 °C under a 16 h photoperiod provided with cool white fluorescent lamps (40–60 µmol m−2 s−1).
Identification of grapevine ALS candidate genes and expression vector construction
To identify ALS candidate genes in grapevine, the amino acid sequence of Arabidopsis thaliana ALS (GI: AAK68759.1) was used as a query to search for ALS homologs in the grapevine reference genome (Jaillon et al. 2007; Canaguier et al. 2017) as well as other grapevine genomes in the NCBI database using the tblastn program. Analyses of the identified homologous sequences and phylogenetic trees supported VvALS1 (Vitvi14g01537) as the best ALS candidate gene in grapevine (Figs. 1 and 2). VvALS1 coding sequences containing different target point mutations were chemically synthesized using GenScript’s DNA synthesis service (GenScript Corp., Piscataway, NJ). Type II restriction enzyme (BsaI) sites were included in the synthesized sequence to facilitate direct cloning into Golden-Gate expression vector, pRNAi-GG (ABRC stock # CD3-1786) without the use of shuttle/entry vectors. The expression vectors include CaMV 35S promoter for the constitutive expression of VvALS1 transgenes and NOS promoter for the expression of KanR for plant selection. The vector constructs were transferred to Agrobacterium strain, EHA105 and used for transformation of Thompson Seedless’ MBs.
Sequence alignment of the candidate grapevine ALS (VvALS1-9) and functionally characterized ALS orthologs from other plant species including Thale cress (Arabidopsis thalaiana, AtALS), tomato (Solanum lycopersicum, SlALS1), potato (Solanum tuberosum, StALAS1), tobacco (Nicotiana tabacum, NtALS1), sunflower (Helianthus annuus, HAALS1), Sugar beet (Beta vulgaris, BvALS1), soybean (Glycine max, GmALS1), Rapeseed (Brassica napus, BnALS1), rice (Oryza sativa subsp. Japonica, OsALS1), maize (Zea mays, ZmALS1) and wheat (Triticum aestivum, TaALS). The amino acids whose substitutions are known to confer resistance to ALS-inhibiting herbicides are in bold and their positions indicated at the top (numbering standardized to AtALS sequence) with the corresponding positions in VvALS1 being Ala116, Pro191, Ala199, Asp370, Arg371, Trp568, Ser647 and Gly648). The shaded regions indicate the conserved domains harboring resistance substitutions. Genebank accession numbers and/or locus names of grapevine candidate ALS proteins: VvALS1 (XP_002274073.1/Vitvi14g01537), VvALS2 (XP_010663749.2), VvALS3 (CAN73760.1/Vitvi16g00747), VvALS4 (XP_019081497.1/Vitvi16g04298), VvALS5 (XP_010662475.2/ Vitvi16g00750), VvALS6 (Vitvi16g00753), VvALS7 (Vitvi16g04299.1), VvALS8 (RVW90609.1/Vitvi16g00759), VvALS9 (Vitvi16g043000.1), VvALS10 (Vitvi16g04301) and VvALS11 (Vitvi16g00765) and functionally characterized orthologs from other plant species: AtALS (AAK68759.1), SlALS1 (XP_004234664.1), StALS1 (XP_006361740.1), NtALS1 (XP_009769695.1), BvALS1 (XP_010695365.1), HaALS1 (AAT07323.1), BnALS1 (XP_013648961.1), GmALS1 (NP_001341804.1), OsJALS (AAX14282.1), TaALS (AAO53549.1) and ZmALS1 (PWZ20335.1)
Phylogenetic tree of putative grapevine ALS proteins and orthologs with confirmed herbicide resistance from tomato (Solanum lycopersicum, SlALS1), potato (Solanum tuberosum, StALAS1), tobacco (Nicotiana tabacum, NtALS1), Sugar beet (Beta vulgaris, BvALS1), sunflower (Helianthus annuus, HAALS1), soybean (Glycine max, GmALS1), Thale cress (Arabidopsis thalaiana, AtALS), Rapeseed (Brassica napus, BnALS1), wheat (Triticum aestivum, TaALS), rice (Oryza sativa subsp. Japonica, OsALS1), and maize (Zea mays, ZmALS1). The sequences were aligned using MUSCLE program by MEGA 6 and phylogenetic tree constructed by neighbor-joining. Analysis using 1000 bootstrap replicates was performed. The scale bar shows 0.05 amino acid substitutions per site. The locus names and/or GenBank accession numbers were as listed in Fig. 1
Plant transformation
Agrobacterium-mediated transformation of MBs of Thompson Seedless was performed as previously reported (Xie et al. 2016). Briefly, Agrobacterium tumefaciens strain EHA105 harboring binary vectors was cultured overnight at 28 °C and 200 rpm in YEP medium with the appropriate antibiotics to OD600 of about 0.6. The bacteria were pelleted by centrifuging at 5000 g at room temperature for 15 min and resuspended in liquid MS medium (4.4 g L−1 of MS salts including vitamins, 20 g L−1 sucrose, pH 5.2) supplemented with 100 µM Acetosyringone. The bacterial culture was adjusted to OD600 of 0.2–0.4 and placed on a shaker at 150 rpm for 5 h at 25 °C. The MB slices were cut into ~ 1 cm2 and 2 mm thickness and immersed in bacterial suspension and placed on a shaker at 80 rpm at 25 °C for 15 min. After inoculation, the slices were dried by blotting on sterile filter paper and transferred to hormone-free solid MS medium (4.4 g L−1 of MS salts including vitamins, 30 g L−1 sucrose, 7 g L−1 plant agar, pH 5.5) containing 100 µM acetosyringone for co-cultivation for 48 h in the dark at 25 °C. After co-cultivation, the slices were transferred to the selection medium (IM3 medium containing 100 mg L−1 carbenicillin and 70 mg L−1 kanamycin) and placed under light at 25 ± 1 °C under a 16 h photoperiod. The initial green shoots emerged about 20 days after inoculation were removed as these shoots were often not transgenic and the explants transferred to fresh selection medium. Inoculated MBs were transferred to fresh medium every 3–4 weeks until visible resistant shoots emerged. Antibiotic selection was carried out for about 2–3 months on 70 mg L−1 kanamycin or until shoots were regenerated. Any kanamycin-resistant new shoots were sliced out and transferred to MS medium supplemented with 2 µM BA without antibiotics for 3–4 weeks to aid shoot elongation. Subsequently, elongated putative transgenic shoots were isolated and transferred to rooting medium (MS salt and vitamins, 30 g L−1 sucrose, 7 g L−1 plant agar, 4.92 μM Indole-3-butyric acid (IBA), 50 mg L−1 kanamycin, pH 5.8). Rooted plantlets were transferred to magenta boxes containing hormone-free MS media with 50 mg L−1 kanamycin to establish whole plants.
DNA extraction and molecular analysis of transgenic plants
Genomic DNA was extracted from young leaves of putative transgenic plants as well as non-transgenic control using Qiagen DNAeasy Plant Kit (Qiagen, Hilden, Germany) as described by the supplier. Two pairs of primers were used for genomic PCR analysis to determine T-DNA integration into the plant genome. The first primer pairs were transgene (VvALS)-specific forward primer (ALSfN) 5′- ATGGTGGAGCACGACACAC-3′and reverse primer (ALSrN) 5′-CGAGAGCTTCCACGAGAACATC-3′while the second pairs were KanR-specific forward primer (KanF) 5′- GATGGATTGCACGCAGGTTCTC-3′ and reverse primer (KanR) 5′- TCGCTTGGTCGGTCATTTCG-3′. For amplification of housekeeping gene namely AP-2 complex subunit mu (AP2mu Gene ID: 100250494) as an internal control, forward primer (AP2muF) 5′ GTCCCAACTTAAATCCCGTCCTG-3′ and reverse primer (AP2muR) 5′ -CAATCTGGTGGCACAAAACTGAC-3′ were used (Tashiro et al. 2016). PCR reactions were conducted using GoTaq DNA polymerase (Promega) following manufacturer’s protocol.
Herbicide sensitivity test
Six widely used herbicides were selected in this study for determining the minimum inhibitory concentration of ALS-inhibiting herbicides in grapevine. The six selected herbicides belong to five ALS-inhibiting herbicide families based on the number of confirmed ALS mutations providing resistance in various plant species (Table S1 & S2), including imazapyr and imazaquin from the IMI family, chlorsulfuron and sulfosulfuron from the SU family, cloransulam-methyl from the TP family and bispyribac sodium salt from the PTB family. The six herbicides [active ingredients (AI)] were purchased from Sigma and stock solutions were prepared according to their solubility by dissolving the AI either in water (imazapyr and bispyribac sodium salt) or 10 mM potassium phosphate buffer with pH 7.5 (imazaquin, chlorsulfuron, sulfosulfuron and cloransulam methyl) while the working concentrations were all prepared in water and filter sterilized. The herbicide concentrations in the prepared stock solutions were 3.8 mM for Imazapyr, 1 mM for Imazaquin, 1.25 mM for chlorsulfuron, 1.7 mM for sulfosulfuron, 1.25 mM for cloransulam methyl and 2 mM for Bispyribac sodium salt.
Embryogenic calli of Vitis vinifera cv. Chardonnay were prepared by growing the calli on fresh MPM2 medium for 3 weeks to synchronize the growth before herbicide treatment. For the herbicide treatment, freshly prepared MPM2 medium was autoclaved, and the five or six respective levels of each herbicide were added after cooling the medium to about 55 °C. The range of herbicide concentrations were determined based on information from published reports and adjusted for grapevine based on results from the preliminary analysis (Shimizu et al. 2008; Schnell et al. 2012; Lee et al. 1988). Five or six different levels of herbicide concentrations were conducted for each of the six selected herbicides: imazapyr, 0.25, 0.5, 1.0, 5.0, 7.5 and 10 mg L−1; bispyribac sodium, 5.65, 11.3, 22.6, 45.2, and 113.0 µg L−1; imazaquin, 0.25, 0.5, 1.0, 2.5 and 5.0 mg L−1; chlorsulfuron, 1.0, 2.5, 5.0, 10.0, 25.0, and 50.0 µg L−1; Cloransulam methyl, 215, 645, 1290, 2580, and 5160 µg L−1; and sulfosulfuron, 62.5, 125, 250, 500, 1,000 µg L−1. The treatment was initiated by transferring the same number of homogenous-looking embryogenic calli (7 per plate) in three replications. Herbicide sensitivity response of the grapevine embryogenic callus was evaluated starting at 2 weeks after the initiation of treatment for 2–6 weeks depending on the level of toxicity. Response scores were based on a 0–10 scale, where zero is no necrotic response and 10 is whole callus death.
Similarly, Thompson Seedless’ MBs were evaluated for their responses to seven concentrations of imazapyr (1, 10, 50, 100, 500, 1000 & 2000 µg L−1), chlorsulfuron (0.1, 0.25, 1, 5, 10, 25 & 50 µg L−1), bispyribac sodium (1, 2, 5, 10, 22.6, 45.2 & 113 µg L−1) and Cloransulam methyl (78, 156, 312.5, 625, 1250, 2500 & 5000 µg L−1) based on the information from the embryogenic callus resistance test experiment described above and published reports (Shimizu et al. 2008; Schnell et al. 2012; veillet et al. 2019; Lee et al. 1988; Malabarba et al. 2021). The response data were collected starting at 2 weeks after treatment for up to 6 weeks.
Evaluation of transgenic VvALS1 in vitro shoots for herbicide tolerance
Shoots from the in vitro proliferating transgenic and control wild-type plants were cut and immediately placed on deep-well petri dish plates containing 50 ml PR medium with the appropriate levels of herbicides in three replications and kept under 16 h of light and 8 h of darkness. Transgenic in vitro shoots expressing mutant versions of VvALS1 gene were evaluated for resistance responses against three higher concentration levels for imazapyr (0.5, 1 & 2 mg L−1) and chlorsulfuron (10, 25 & 50 µg L−1) and 3 concentrations of bispyribac sodium salt (22.6, 45.2 and 113 µg L−1) as described above.
Results
Identification of VvALS candidate genes, domains, and amino acid positions conferring herbicide resistance
Previous work identified 10 ALS homologs in grapevine by blast search against the grapevine genome in the NCBI database (Li et al. 2015). In this study, we identified eleven grapevine ALS homologs, VvALS1-11, by blast searching the grapevine genomes in both the NCBI database and the published reference genome (Jaillon et al. 2007; Canaguier et al. 2017) using Arabidopsis AtALS (GI: AAK68759.1) as query (Figs. 1 & 2). Nine of them (VvALS3-11) are localized as clusters on chromosome 16 and code for partial ALS protein sequences (Fig. S1). The largest is VvALS11 with 671 amino acids while the shortest is VvALS9 with 128 amino acids (Fig. S1). Two other grapevine ALS homologs, VvALS1 (Vitvi14g01537) and VvALS2 (XP_010663749.2), codify 664 and 676 amino acids with the coding sequence being 1995 and 2031 base pairs, and are located on chromosomes 14 and 17, respectively. Phylogenetic tree and sequence analysis showed VvALS1 and VvALS2 as the top ALS candidates due to their higher homology to the functionally characterized ALS genes in other plant species including Arabidopsis, potato, tomato, tobacco, sunflower, sugar beet, soybean, and rapeseed (Fig. 2). These findings agree with the previous work (Li et al. 2015). In addition, both VvALS1 and VvALS2 have no introns like many other plant ALS genes.
We further examined the expression patterns of VvALS in embryogenic callus (unpublished) and other grapevine tissues (http://bar.utoronto.ca/efp_grape/cgi-bin/efpWeb.cgi) and found that VvALS1 was clearly the most prominent in all of the tissue types and developmental stages. VvALS1’s expression was several-fold higher than the next closest homolog, VvALS2 (Fig. 3). For instance, in callus tissues, only VvALS1 and VvALS2 were expressed with VvALS1 showing over 100-fold higher level of expression compared to VvALS2 (Fig. 3). All other ALS candidate genes were found to show no expression or only basal expression in all samples compared to VvLAS1 (Fig. 3).
Expression profiles of the candidate grapevine ALS genes in diverse plant tissues and developmental stages. Apart from embryogenic callus, transcript data from all other tissues were adapted from the Grapevine expression Atlas (http://bar.utoronto.ca/efp_grape/cgi-bin/efpWeb.cgi). PHWI-III refers to post-harvest withering stages of berry flesh, pericarp and skin
To determine potential sites within the amino acid sequences of candidate grapevine ALS proteins for herbicide resistance, we first analyzed published information for confirmed ALS amino acid substitutions in orthologous genes that have already been shown to confer resistance to ALS-inhibiting herbicides (Table S1). Moreover, we retrieved information on the different amino acid substitutions in ALS conferring resistance to ALS-inhibiting herbicides in herbicide resistant weed populations from the international herbicide resistance weed database (www.weedscience.com) (Table S1). This analysis showed eight amino acid positions, namely Alanine 122, Proline 197, Alanine 205, Asparagine 376, Arginine 377, Tryptophan 574, Serine 653, and Glycine 654 belonging to the 6 conserved domains in the Arabidopsis ALS (AAK68759.1) conferring resistance to ALS-inhibiting herbicides (Fig. 1 and Fig. S1). Subsequently, to determine the presence of these residues in grapevine, we conducted multiple protein sequence analysis of VvALS1-9 using the functionally characterized orthologs from other plant species including Arabidopsis, tomato, potato, tobacco, sunflower, sugar beet, soybean, rapeseed, rice, maize, and wheat as references. The results showed that the identified grapevine ALS homologs displayed variations in the number of conserved amino acid residues in their sequences, but VvALS1 is the only homolog with all 8 amino acid residues found in other plant species for herbicide resistance, which provides additional supporting evidence for VvALS1 as the most desirable target to modify for herbicide resistance. The corresponding positions of these 8 residues in VvALS1 include Ala116, Pro191, Ala199, Asp370, Arg371, Trp568, Ser647 and Gly648 (Fig. 1, Table S1). Based on large sets of possible combinations of amino acid substitutions confirmed to confer resistance to ALS-inhibiting herbicides in other plant species, we selected 11 amino acid substitutions belonging to 4 different positions, namely A116, P191, W568 and S647 (Table S1), as potential transgenic targets to evaluate for inducing herbicide resistance in grapevine.
Sensitivity responses to herbicides in embryogenic callus and in vitro shoots
There are many commercially available ALS-inhibiting herbicides belonging to the five herbicide families of IMI, PTB, SCT, SU and TP (Table S2). It is critical to determine which of those herbicides and their minimum inhibitory concentrations are suitable for use as a plant selectable marker in grapevine transformation. The ideal concentration should be the one which effectively kills the wild-type or untransformed cells in a defined time period. As only a few herbicides have so far been tested in grapevine tissue culture with inconclusive results (Li et al. 2015), we sought to evaluate six herbicides belonging to the five different ALS-inhibiting herbicide families. These herbicides were selected based on the number of previously confirmed ALS natural mutations in various weed species (www.weedscience.com) or artificially created mutations through transgenic approach that have shown resistance against respective herbicides (Table S2).
Evaluation of herbicide sensitivity responses of Chardonnay’ embryogenic callus revealed that for four of the six herbicides (imazapyr, chlorsulfuron, bispyribac sodium salt and cloransulam methyl) linear response curves were observed for the tested concentration ranges of the respective herbicides (Fig. 4, and Fig. S2). For imazapyr, at 0.25 mg L−1 concentration, the callus growth was barely affected except for slight discoloration. Callus growth inhibition is apparent only at 0.5 mg L−1 and gradually increases with 5 mg L−1 concentration causing significant discoloration and growth inhibition compared to the control. Chardonnay embryogenic calli seems to be highly sensitive to Chlorsulfuron as detectable level of toxicity is evident even at 1 µg L−1 in the form of growth inhibition and slight discoloration in the appearance of the callus. Twenty-five µg L−1 chlorsulfuron showed almost complete necrosis or discoloration of the callus. For Bispyribac sodium salt, spots of necrotic sectors on calli can be seen at the lowest concentration tested in this study (5.65 µg L−1) along with less pronounced level of growth inhibition. The necrotic sectors as well as the inhibition on callus growth gradually increase with the increase in concentration of the herbicide. Unlike the three herbicides, the effect of Cloransulam methyl was more profound on the rate of callus growth as even the lowest concentration tested in this study showed a dramatic inhibition of callus growth with less effect on the appearance of the callus. A substantial level of necrosis was observed starting at 1.29 mg L−1 concentrations of Cloransulam methyl. In contrast, imazaquin and sulfosulfuron were shown to be highly toxic to Chardonnay calli with severe growth inhibition as well as necrosis even at the lowest concentrations (Fig. 4 and Fig. S2). Based on these data, the minimal inhibitory concentration in embryogenic calli to achieve 50% or more necrosis or callus death is 5 mg L−1 for imazapyr, 10 µg L−1 for chlorsulfuron, 22.6 µg L−1 for bispyribac sodium salt and 2580 µg L−1 for cloransulam methyl (Fig. 4 and S2).
Herbicide sensitivity response curves in Chardonnay embryogenic calli. The treatment was done in three replications and sensitivity responses were scored 4 weeks after transferred to the herbicide containing medium and based on a 0–10 scale, where zero is no necrotic response and 10 is whole callus death. Error bars represent the standard error of the means (n = 3)
Similarly, sensitivity responses of Thompson Seedless’ in vitro shoots against a wide range of concentrations of four herbicides were evaluated to determine the minimum lethal concentrations suitable for tissue culture. Interestingly, shoots of non-transgenic Thompson Seedless were found to be highly sensitive to low doses of chlorsulfuron, imazapyr and bispyribac sodium salt while resistant to Cloransulam methyl herbicide (Fig. 5). For chlorsulfuron, visible growth inhibition was observed at 0.25 µg L−1 while 1 µg L−1 resulted in severe growth inhibition. For Imazapyr, substantial growth inhibition was observed at concentrations as low as 50 µg L−1 while 500 µg L−1 caused severe inhibition and eventual plant death. Thompson Seedless shoots appear to be more sensitive to imazapyr than embryogenic calli (Fig. 5). On the other hand, in vitro shoots were found to exhibit very high sensitivity to bispyribac sodium salt concentrations as low as 11.3 µg L−1 whereas high resistance to Cloransulam methyl herbicides at concentrations as high as 5 mg L−1. In comparison, in vitro shoots appear to be more sensitive to all tested herbicides but Cloransulam methyl. In general, it can be deduced from these results that imazapyr concentrations of 500 µg L−1 and chlorsulfuron concentrations of 10 µg L−1 are required to obtain complete inhibition of non-transgenic shoots (Fig. 5).
Determination of lethal concentrations of imazapyr, chlorsulfuron, bispyribac sodium and Cloransulam methyl using in vitro shoots of Thompson Seedless. Seven levels of imazapyr (1, 10, 50, 100, 500, 1000 & 2000 µg L−1), chlorsulfuron (0.1, 0.25, 1, 5, 10, 25 & 50 µg L−1), bispyribac sodium (1, 2, 5, 10, 22.6, 45.2 & 113 µg L−1) and Cloransulam methyl (78, 156, 312.5, 625, 1250, 2500 & 5000 µg L−1) were tested. The images for imazapyr and chlorsulfuron were taken 30 days after treatment while for bispyribac sodium and Cloransulam methyl they were taken 4 and 8 weeks after treatment, respectively. Pictures shown are representative of three plates for each herbicide group
Generation and herbicide response tests of transgenic VvALS1 in vitro plants
Constructs containing specific amino acid substitutions in VvALS1 were used in Agrobacterium-mediated transformation of Thompson Seedless’s MBs for inducing herbicide resistance (Fig. 6a). We successfully generated transgenic in vitro plants for three constructs namely P191S, P191T and W568L. Stable T-DNA integration in the transgenic plants was confirmed by genomic PCR using two pairs of transgene-specific primers, one specific to the VvALS1 transgene and the other to the selectable marker gene KanR (Fig. 6b). Both sets of primers produced positive bands for all transgenic plants as well as for the plasmid DNA, while no bands were obtained for either non-transgenic plants or negative control (Fig. 6b). All transgenic plants did not show any morphological or physiological abnormalities due to the ectopic expression of the mutant VvALS1 (Fig. S3).
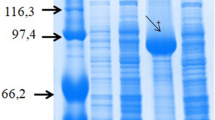
Molecular characterization of transgenic plants expressing VvALS1 gene with point mutations endowing herbicide resistance. A) Vector construct used for expression of the transgene. B) Genomic PCR confirming the insertion of the mutant VvALS1 (G) and the Kanamycin resistance (K) genes in transgenic lines. No amplification was observed for transgene-specific genes in the no DNA check or DNA samples from the non-transgenic (WT) plants. Analysis was performed with DNA extracted from young tissues of transgenic and non-transgenic Thompson Seedless. M marker (Generuler 1 kb DNA ladder-Thermo Fisher), P plasmid DNA, NT non-transgenic DNA, CK pure water, T1 to T3 transgenic events originating from independently transformed meristematic bulks. LB and RB, left and right borders of the T-DNA sequences. The double arrows in (A) show the location of the primer pairs used for genomic PCR
To determine whether transgenic plants expressing the mutant versions of VvALS1 display resistance to ALS-inhibiting herbicides, we conducted resistance tests using transgenic shoots for all three mutants under in vitro culture conditions. Three concentrations were used for this test for each of the 3 herbicides, namely imazapyr (0.5, 1 & 2 mg L−1), chlorsulfuron (10, 25 & 50 µg L−1) and bispyribac Sodium salt (22.6, 45.2 and 113 µg L−1). Cloransulam methyl was not used in this test because non-transgenic young shoots didn’t show noticeable responses to the herbicide as shown earlier. Results showed that transgenic shoots expressing the mutated versions of ALS showed variations in their response to the different types and concentrations of herbicides in the growth medium (Fig. 7). The non-transgenic control plants were all killed in under 3 weeks even at the lowest concentrations of the herbicides. P191S amino acid substitution in VvALS1 resulted in a high resistance response to up to 2 mg L−1 Imazapyr but very low resistance to chlorsulfuron. Both P191T and W568L substitutions showed a comparable level of responses to chlorsulfuron with modest resistance to up to 50 µg L−1 in the growth medium. On the other hand, W568L showed moderate resistance to up to 2 mg L−1 imazapyr while P191T substitution displayed barely any resistance to the lowest tested concentration of imazapyr (0.5 mg L−1). Transgenic shoots of ALS mutants, P191T and W568L showed no resistance responses to even the lowest concentrations of bispyribac sodium (22.6 µg L−1) tested in this study. On the other hand, ALS mutant, P191S showed slight resistance to bisbyribac concentration of only 22.6 µg L−1.
Herbicide resistance responses of transgenic and non-transgenic grapevine proliferating shoots growing on plant propagation medium containing three levels of the three herbicides: imazapyr (0.5, 1 and 2 mg L−1), chlorsulfuron (10, 25 and 50 µg L−1) and bispyribac sodium salt (22.6, 45.2 and 113 µg L−1) and the non-treated control. Pictures were taken after 30 days of exposure to the two herbicides. Pictures shown are representative of two separate tests, with each test including three plates for each genotype
Discussion
Based on the publicly available Vitis vinifera genomic data, a total of 11 VvALS homologs were identified in this study (Fig. 1 and Fig. 2). A phylogenetic analysis revealed that VvALS1 and VvALS2 had the highest amino acid sequence homology with the Arabidopsis ALS, 77 and 67%, respectively as previously reported (Li et al. 2015). Sequence analysis showed that only VvALS1 has all the eight conserved amino acid residues (A116, P191, A199, D370, R371, W568, S647 and G648) previously characterized in other plant species to impart herbicide resistance. The shorter sequence length and lack of some or most of the conserved residues in many of the other grapevine ALS homologs discovered in this study point to the fact that these VvALS genes may contain a regulatory subunit of ALS rather than a catalytic subunit that is responsible for ALS activity. Moreover, RNA expression profiles indicated that only VvALS1 showed high constitutive expression in all tissues, while all other homologs showed mild expression in only some of the tissues (Fig. 3). Taken together, VvALS1 is the most likely candidate for ALS function in grapevine, thus making it the top target for mutagenesis imparting herbicide resistance as demonstrated in this study.
Three amino acid substitutions involving proline to serine or proline to threonine substitutions at position 191 and tryptophan to leucine substitution at position 568 in VvALS1 were successfully introduced into Thompson Seedless’s MBs from which transgenic vines were produced (Fig. 6b and Fig. S3). These amino acid substitutions were selected as potential targets to evaluate in this study based on their prominence either in nature as evident in many herbicide-resistant weed biotypes or artificial mutations created to introduce herbicide resistance traits in many plant species (Powles and Yu 2010; Han & Kim 2019). We chose to use MBs as an explant for transformation in this study as part of our effort in exploring an alternative method for grapevine transformation to the widely used embryogenic-callus-based transformation. While MBs can be induced directly from in-vitro proliferated shoots and have successfully been used to generate transgenic grapevine plants via Agrobacterium-mediated transformation (Mezzetti et al. 2002, Xie et al. 2016; Sabbadini et al. 2019a, Sabbadini et al. 2019b), this method is relatively new with many parameters such as the generation and maintenance of good quality materials for longer duration and transformation efficiency yet to be optimized.
The three substitutions showed strong resistance responses to one or more of the popular ALS-inhibiting herbicides, imazapyr or chlorsulfuron (Fig. 7). These results are in agreement with the previously published results where substitutions at similar positions involving proline to serine in soybean (Li et al. 2015) and maize (Svitashev et al. 2015), proline to threonine in tomato (Veillet et al. 2019) and tryptophan to leucine in potato (Butler et al. 2016) and rice (Sun et al. 2016; li et al. 2019) shown to confer resistance to these herbicides. The use of CaMV 35S promoter to express the ALS genes in poplar plants has been shown to result in a higher level of transcript expression and enzyme activity when compared to that from the native promoter although there was hardly any difference in terms of herbicide resistance response (Brasilero et al. 1992). Moreover, ALS gene expression in tobacco from the CaMV 35S promoter was shown to be relatively higher (about 25 times) compared to that from native Arabidopsis promoter though the difference in ALS enzyme activity and herbicide resistance is much lower (~ 2 times) (Odell et al. 1990). Based on this information, similar resistance response is expected from expressing the VvALS1 genes with the native promoter. To the best of our knowledge, this is the first report on grapevine ALS mutations conferring herbicide resistance.
A herbicide resistance-based selection system in grapevine transformation requires a match of ALS mutants with the right types and suitable levels of herbicides to be used. To this end, we showed that 3 herbicides namely, imazapyr, chlorsulfuron and bispyribac sodium salt and cloransulam methyl are the best candidates as confirmed by the linear response curves to increasing concentrations of the herbicides in the medium (Figs. 4 and 5). However, the transgenic plants’ responses to these herbicides were shown to be different among the different herbicides (Fig. 7). For instance, P191S amino acid substitution in grape resulted in a higher resistance response to herbicide in IMI family but relatively a lower level of resistance to SU herbicide. This observation was in contrast with the general pattern where artificially induced mutations at proline-197 mainly imparts higher resistance to chlorsulfuron than to IMI in other species (Harms et al. 1992; Gaines et al. 2020). This difference might be due to differences in the preparation of the herbicide solution (AI vs commercial formulae that has additives) or the differences in tissue types. Based on information from the international herbicide resistance weed database, natural substitutions at proline-197 were found to impart resistance to not only herbicides in the SU family, but also IMI, PTB and TP. On the other hand, the trend in resistance response to P191T substitution fits with the reported general trend as mentioned above. Similarly, the level of resistance response observed in W568L substitution comparatively matches the general trend in published reports with a high level of resistance to chlorsulfuron and moderate resistance to up to 2 mg L−1 imazapyr (Bernasconi et al. 1995; Hattori et al. 1995). In general, these herbicides were effectively utilized in the selection of transgenic plants expressing the three mutations and hence supporting the notion that it is possible to effectively deploy a combination of the three mutations and the different herbicides for effective resistance selection.
The development of native grapevine ALS mutations incurring amino acid substitutions as a plant selectable marker provides manifold advantages. One advantage is that as a selectable marker of plant origin, it can replace markers of bacterial origin thereby facilitating the implementation of new plant breeding techniques such as cisgenesis and genome-editing using site-directed nucleases in grapes. Moreover, ALS is also an ideal target for bolstering the applicability of the newly emerging base-editing technologies due to its score-ability and larger pools of possible base changes imparting resistance. For instance, VvALS1 mutants could be utilized to enable selectable co-editing systems that can be used to enrich the desired mutations by selecting against the wild type plants. Such applications involving ALS substitution mutations are gaining attention as recently demonstrated in wheat where selection for herbicide resistance was shown to significantly enrich the rate of editing in the coupled target (Zhang et al. 2019). This technique is particularly important for grapevine genetic improvement as it can potentially be utilized for transgene-free gene-editing by using transient transformation methods to avoid stable T-DNA integration. Furthermore, the introduced herbicide resistance trait provides additional benefits as it gives protection against herbicide spray drift from herbicide application near or within the field.
In conclusion, this study confirmed VvALS1 as a functional grapevine ALS gene and demonstrated three differing amino substitutions involving P191S, P191T and W568L conferring resistance to one or more herbicides in transgenic vines. This work paved the way for adopting herbicide resistance as a selectable marker in grape genetic engineering, specifically gene-co-editing via the new base and prime editing technologies to enable transgene-free gene-editing. The observation that transgenic plants not showing any significant plant fitness costs associated with mutations conferring resistance to ALS-inhibiting herbicides highlights the great potential for the deployment of this mutation-herbicide combination for transgenic/gene-edited plant selection. As most T-DNA delivery methods are genotype-dependent and require laborious and time-consuming tissue culture steps, the use of meristematic bulk tissues producing transgenic plants demonstrated in this study provides a great promise in the realization of transgene-free gene-editing in grape via Agrobacterium-mediated transient transformation.
Data availability
All data generated or analyzed during this study are included in this published article (and its additional files). Requests for material should be made to the corresponding author.
Abbreviations
- ALS:
-
Acetolactate synthase
- IMI:
-
Imidazolinones
- PTB:
-
Pyrimidinylthiobenzoates
- SCT:
-
Sulfonylaminocarbonyltriazolinone
- SU:
-
Sulfonylureas
- TP:
-
Triazolopyrimidines
- MBs:
-
Meristematic bulks
- MPM2:
-
Maintenance and Proliferation medium 2
- PR:
-
Propagation medium
- BA:
-
Benzyl adenine
- IM:
-
Initiation medium
- IBA:
-
Indole-3-butyric acid
- AI:
-
Active ingredient
References
Bernasconi P, Woodworth AR, Rosen BA, Subramanian MV, Siehl DL (1995) A naturally occurring point mutation confers broad range tolerance to herbicides that target acetolactate synthase. J Biol Chem 270:17381–17385. https://doi.org/10.1074/jbc.270.29.17381
Brasileiro ACM, Tourneur C, Leple JC, Combes V, Jouanin L (1992) Expression of the mutant Arabidopsis thaliana acetolactate synthase gene confers chlorsulfuron resistance to transgenic poplar plants. Transgenic Res 1:133–141. https://doi.org/10.1007/BF02528778
Butler NM, Baltes NJ, Voytas DF, Douches DS (2016) Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases. Front Plant Sci 7:1045. https://doi.org/10.3389/fpls.2016.01045
Canaguier A, Grimplet J, Di Gaspero G, Scalabrin S, Duchêne E, Choisne N et al (2017) A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genomics Data 14:56–62. https://doi.org/10.1016/j.gdata.2017.09.002
Dai LM, Zhou Q, Li RM, Du YJ, He J, Wang D, Cheng SY, Zhang JX, Wang YJ (2015) Establishment of a picloram-induced somatic embryogenesis systemin Vitis vinifera cv. Chardonnay and genetic transformation of a stilbene synthase gene from wild-growing Vitis species. Plant Cell Tissue Organ Cult 121:397–412. https://doi.org/10.1007/s11240-015-0711-9
Gaines TA, Duke SO, Morran S, Rigon CAG, Tranel PJ, Küpper A, Dayan FE (2020) Mechanisms of evolved herbicide resistance. J Biol Chem 295:10307–10330. https://doi.org/10.1074/jbc.REV120.013572
Garcia MD, Nouwens A, Lonhienne TG, Guddat LW (2017) Comprehensive understanding of acetohydroxyacid synthase inhibition by different herbicide families. Proc Natl Acad Sci 114(7):1091–1100. https://doi.org/10.1073/pnas.161614211
Han YJ, Kim JI (2019) Application of CRISPR/Cas9-mediated gene editing for the development of herbicide-resistant plants. Plant Biotechnol Rep 13:447–457. https://doi.org/10.1007/s11816-019-00575-8
Harms CT, Armour SL, DiMaio JJ, Middlesteadt LA, Murray D, Negrotto DV, Thompson-Taylor H, Weymann K, Montoya AL, Shillito RD, Jen GC (1992) Herbicide resistance due to amplification of a mutant acetohydroxyacid synthase gene. Mol Gen Genet 233:427–435. https://doi.org/10.1007/BF00265440
Hattori J, Brown D, Mourad G, Labbe H, Ouellet T, Sunohara G, Rutledge R, King J, Miki B (1995) An acetohydroxy acid synthase mutant reveals a single site involved in multiple herbicide resistance. Mol Gen Genet 246:419–425. https://doi.org/10.1007/BF00290445
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467. https://doi.org/10.1038/nature06148
Légère A, Stevenson C, Beckie H, Warwick S, Johnson E, Hrynewich B (2013) Growth characterization of kochia (Kochia scoparia) with substitutions at Pro197 or Trp574 conferring resistance to acetolatate synthase inhibiting herbicides. Pest Manag Sci 61:267–276. https://doi.org/10.1614/WS-D-12-00116.1
Lee KY, Townsend J, Tepperman J, Black M, Chui CF, Mazur B, Dunsmuir P, Bedbrook J (1988) The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J 7:1241–1248. https://doi.org/10.1002/j.1460-2075.1988.tb02937.x
Li M, Yu Q, Han H, Vila-Aiub M, Powles SB (2013) ALS herbicide resistance mutations in Raphanus raphanistrum: evaluation of pleiotropic effects on vegetative growth and ALS activity. Pest Manag Sci 69:689–695. https://doi.org/10.1002/ps.3419
Li S, Li J, He Y, Xu M, Zhang J, Du W, Zhao Y, Xia L (2019) Precise gene replacement in rice by RNA transcript-templated homologous recombination. Nat Biotechnol 37:445–450. https://doi.org/10.1038/s41587-019-0065-7
Li Z, Nguyen C, Dean D, Grant T, Creech M, Das B and Gray D (2015) Mutagenesis Analysis of Acetolactate Synthase (ALS) Genes from Vitis vinifera for Use as a Selectable Marker to Facilitate Precision Breeding of Grapevine. In hortscience (Vol. 50, No. 9, pp. S146-S146). 113 S WEST ST, STE 200, ALEXANDRIA, VA 22314–2851 USA: AMER SOC HORTICULTURAL SCIENCE. Available online: https://ashs.confex.com/ashs/2015/webprogramarchives/Paper21669.html
Malabarba J, Chevreau E, Dousset N, Veillet F, Moizan J, Vergne E (2021) New strategies to overcome present CRISPR/Cas9 limitations in apple and pear: efficient dechimerization and base editing. Int J Mol Sci 22:319. https://doi.org/10.3390/ijms22010319
Mezzetti B, Pandolfini T, Navacchi O, Landi L (2002) Genetic transformation of Vitis vinifera via organogenesis. BMC Biotech 2:18. https://doi.org/10.1186/1472-6750-2-18
Mulwa R, Norton M, Farrand S, Skirvin R (2007) Agrobacterium-mediated transformation and regeneration of transgenic’Chancellor’wine grape plants expressing the tfd A gene. Vitis 46:110–115. https://doi.org/10.5073/vitis.2007.46.110-115
Odell JT, Caimi PG, Yadav NS, Mauvais CJ (1990) Comparison of increased expression of wild-type and herbicide-resistant acetolactate synthase genes in transgenic plants, and indication of posttranscriptional limitation on enzyme activity. Plant Physiol 94:1647–1654. https://doi.org/10.1104/pp.94.4.1647
Ogawa T, Kawahigashi H, Toki S, Handa H (2008) Efficient transformation of wheat by using a mutated rice acetolactate synthase gene as a selectable marker. Plant Cell Rep 27(8):1325–1331. https://doi.org/10.1007/s00299-008-0553-6
Osakabe K, Endo M, Kawai K, Nishizawa Y, Ono K, Abe K, Ishikawa Y, Nakamura H, Ichikawa H, Nishimura S, Shimizu T, Toki S (2005) The mutant form of acetolactate synthase genomic DNA from rice is an efficient selectable marker for genetic transformation. Mol Breed 16:313–320. https://doi.org/10.1007/s11032-005-0999-y
Perl A, Saad S, Sahar N et al (1995) Establishment of long-term embryogenic cultures of seedless Vitis vinifera cultivars - a synergistic effect of auxins and the role of abscisic acid. Plant Sci 104:193–200. https://doi.org/10.1016/0168-9452(94)04013-7
Powles SB, Yu Q (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61:317–347. https://doi.org/10.1146/annurev-arplant-042809-112119
Sabbadini S, Capriotti L, Limera C, Navacchi O, Tempesta G, Mezzetti B (2019a) A plant regeneration platform to apply new breeding techniques for improving disease resistance in grapevine rootstocks and cultivars. BIO Web of Conf 12:01019. https://doi.org/10.1051/bioconf/20191201019
Sabbadini S, Capriotti L, Molesini B et al (2019) Comparison of regeneration capacity and Agrobacterium-mediated cell transformation efficiency of different cultivars and rootstocks of Vitis spp. via organogenesis. Sci Rep 9:582. https://doi.org/10.1038/s41598-018-37335-7
Schnell J, Labbe H, Kovinich N, Manabe Y, Miki B (2012) Comparability of imazapyr-resistant Arabidopsis created by transgenesis and mutagenesis. Transgenic Res 21:1255–1264. https://doi.org/10.1007/s11248-012-9597-z
Shimizu M, Goto M, Hanai M, Shimizu T, Izawa N, Kanamoto H, Tomizawa K, Yokota A, Kobayashi H (2008) Selectable tolerance to herbicides by mutated acetolactate synthase genes integrated into the chloroplast genome of tobacco. Plant Physiol 147:1976–1983. https://doi.org/10.1104/pp.108.120519
Sun Y, Zhang X, Wu C, He Y, Ma Y, Hou H, Guo X, Du W, Zhao Y, Xia L (2016) Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol Plant 9:628–631. https://doi.org/10.1016/j.molp.2016.01.001
Svitashev S, Young JK, Schwartz C, Gao H, Falco SC, Cigan AM (2015) Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol 169:931–945. https://doi.org/10.1104/pp.15.00793
Tardif FJ, Rajcan I, Costea M (2006) A mutation in the herbicide target site acetohydroxyacid synthase produces morphological and structural alterations and reduces fitness in Amaranthus powellii. New Phytol 169:251–264. https://doi.org/10.1111/j.1469-8137.2005.01596.x
Tashiro RM, Philips JG, Winefield CS (2016) Identification of suitable grapevine reference genes for qRT-PCR derived from heterologous species. Mol Genet Genom 291:483–492. https://doi.org/10.1007/s00438-015-1081-z
Tian S, Jiang L, Cui X et al (2018) Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep 37:1353–1356. https://doi.org/10.1007/s00299-018-2299-0
Tranel PJ, Wright TR, and Heap IM (2022) Mutations in herbicide-resistant weeds to Inhibition of Acetolactate Synthase. Available online: http://www.weedscience.com. Accessed on 10 March 2022
Veillet F, Perrot L, Chauvin L, Kermarrec M-P, Guyon-Debast A, Chauvin J-E, Nogué F, Mazier M (2019) Transgene-free genome editing in tomato and potato plants using agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int J Mol Sci 20:402. https://doi.org/10.3390/ijms20020402
Xie X, Agüero CB, Wang Y, Walker MA (2016) Genetic transformation of grape varieties and rootstocks via organogenesis. Plant Cell Tiss Organ Cult 126:541–552. https://doi.org/10.1007/s11240-016-1023-4
Yao JL, Tomes S, Gleave AP (2013) Transformation of apple (Malus × domestica) using mutants of apple acetolactate synthase as a selectable marker and analysis of the T-DNA integration sites. Plant Cell Rep 32:703–714. https://doi.org/10.1007/s00299-013-1404-7
Yu Q, Powles SB (2014) Resistance to AHAS inhibitor herbicides: current understanding. Pest Manag Sci 70:1340–1350. https://doi.org/10.1002/ps.3710
Zhang R, Liu J, Chai Z, Chen S, Bai Y, Zong Y, Chen K, Li J, Jiang L, Gao C (2019) Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nat Plants 5:480–485. https://doi.org/10.1038/s41477-019-0405-0
Acknowledgements
We would like to thank Yingzhen Yang for providing invaluable suggestions and comments on the project, Della Cobb and Amy Szewc-McFadden of USDA-ARS Grape Genetic Research Unit for generating and maintaining embryogenic callus, and Craig A. Ledbetter of USDA-ARS San Joaquin Valley Agricultural Sciences Center for providing Chardonnay inflorescences for embryogenic callus induction.
Funding
This work was supported by the USDA Agricultural Research Service Grape Genetics Research CRIS Project 8060–21220-007-00D.
Author information
Authors and Affiliations
Contributions
WAW and GYZ: designed the study. WAW and XX: conducted the experiments. WAW: analyzed the data. WAW and GYZ: wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Communicated by Henryk Flachowsky.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wuddineh, W.A., Xu, X. & Zhong, GY. Amino acid substitutions in grapevine (Vitis vinifera) acetolactate synthase conferring herbicide resistance. Plant Cell Tiss Organ Cult 154, 75–87 (2023). https://doi.org/10.1007/s11240-023-02512-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02512-8