Abstract
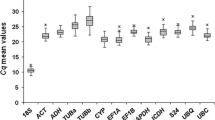
Identification and validation of suitable reference genes that exhibit robust transcriptional stability across many sample types is an absolute requirement of all qRT-PCR experiments. Often, however, only small numbers of reference genes, validated across limited sample types, are available for non-model species. This points to a clear need to assess and validate a wider range of potential reference genes than is currently available. We therefore looked to test and validate a large number of potential reference genes across a wide range of tissue types and treatments to determine the applicability of these reference genes for use in grapevine and other non-model plant species. Potential reference genes were selected based on stability of gene transcription in the model plant species Arabidopsis or due to their common use in the grapevine community. The selected reference genes were analyzed across two datasets consisting of a range of either ‘Sauvignon blanc’ or ‘Pinot noir’ tissues. A total of 11 potential reference genes were screened across the two datasets. Gene stability was analyzed by GeNorm, a widely used Excel application, or an ANOVA-based method developed in red clover. Both analysis methods showed that all 11 potential reference genes are stably expressed in the datasets tested, but the rankings of gene stability differed based on the datasets and analysis method used. Furthermore, the transcript stability of these genes, initially identified in Arabidopsis and now validated in grapevine, suggests applicability across a wide range of non-model plant species in addition to their utility in grapevine.



Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Burland TG (2000) DNASTAR’s Lasergene sequence analysis software. Methods Mol Biol (Clifton, N.J.) 132:71–91
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE Guidelines: minimum Information for publication of quantitative Real-Time PCR Experiments. Clin Chem 55:611–622
Coombe BG (1995) Growth stages of the grapevine: adoption of a system for identifying grapevine growth stages. Australian J Grape Wine Res 1:104–110
Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W-R (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Gamm M, Heloir M-C, Kelloniemi J, Poinssot B, Wendehenne D, Adrian M (2011) Identification of reference genes suitable for qRT-PCR in grapevine and application for the study of the expression of genes involved in pterostilbene synthesis. Mol Genet Genom 285:273–285
Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre J-F, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C, Van Wuytswinkel O (2008) The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotech J 6:609–618
Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
Khanlou KM, Van Bockstaele E (2012) A critique of widely used normalization software tools and an alternative method to identify reliable reference genes in red clover (Trifolium pratense L.). Planta 236:1381–1393
McDonald JH (2009) Handbook of Biological Statistics, 2nd edn. Sparky House Publishing, Baltimore
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: bestKeeper—Excel-based tool using pair-wise correlations. Biotech Lett 26:509–515
Podolyan A, White J, Jordan B, Winefield C (2010) Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13-lipoxygenases expressed in grape berries of ‘Sauvignon Blanc’. Funct Plant Biol 37:767–784
Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol 6:27. doi:10.1186/1471-2229-6-27
Sabel J, Gunstream S, Menezes A, Owens B, Rose S (2011) PrimeTime qPCR Application Guide. Integrated DNA Technologies. http://www.idtdna.com/92ED7514-1E93-487F-8F4C-317E52BF2F5E/FinalDownload/DownloadId-B4AE99B29BDEA8121FE30A3C387C6E5C/92ED7514-1E93-487F-8F4C-317E52BF2F5E/pages/docs/default-source/user-guides-and-protocols/primetime-qpcr-application-guide-3rded-.pdf?sfvrsn=20
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. CSHL press, New York
Selim M, Legay S, Berkelmann-Loehnertz B, Langen G, Kogel KH, Evers D (2012) Identification of suitable reference genes for real-time RT-PCR normalization in the grapevine-downy mildew pathosystem. Plant Cell Rep 31:205–216
Selvey S, Thompson EW, Matthaei K, Lea RA, Irving MG, Griffiths LR (2001) beta-actin—an unsuitable internal control for RT-PCR. Mol Cell Probes 15:307–311
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034. doi:10.1186/gb-2002-3-7-research0034
Wang AM, Doyle MV, Mark DF (1989) Quantitation of messenger-RNA By the polymerase chain-reaction. Proc Natl Acad Sci USA 86:9717–9721
Acknowledgments
This work is part of the New Zealand Grape and Wine Research programme, a joint investment by Plant and Food Research (PFR) and NZ Winegrowers. R.T. would like to acknowledge financial support from the Lincoln University Doctoral Scholarship scheme. We would also like to thank our colleagues at Lincoln University and PFR who have provide support throughout. In particular we would like to thank Dr. M. Trought (PFR) for guidance and intellectual support throughout this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All ethical standards applicable to this work were met or exceeded.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tashiro, R.M., Philips, J.G. & Winefield, C.S. Identification of suitable grapevine reference genes for qRT-PCR derived from heterologous species. Mol Genet Genomics 291, 483–492 (2016). https://doi.org/10.1007/s00438-015-1081-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-015-1081-z