Abstract
The biosynthetic potential for six lignans accumulation in two lines of Taxus x media hairy roots was investigated. The cultures of KT and ATMA hairy root lines were supplemented with precursors: coniferyl alcohol (CA 1, 10 or 100 µM) and/or l-phenylalanine (100 µM PHEN) and/or methyl jasmonate (100 µM MeJa). Moreover the two-phase in vitro cultures supported with perfluorodecalin (PFD) as a gas carrier and in situ extrahent were used. The hairy root lines differed in lignan production profiles. In the control untreated cultures KT roots did not accumulate secoisolariciresinol and lariciresinol while ATMA roots did not accumulate matairesinol. In ATMA roots the treatment with CA (1 or 10 µM) resulted in the production of lariciresinol and secoisolariciresinol whereas solely lariciresinol was present after 100 µM CA application. Elicitation with 1 µM CA and MeJa yielded with hydroxymatairesinol aglyca and lariciresinol glucosides with their highest content 37.88 and 3.19 µg/g DW, respectively. The stimulatory effect of simultaneous treatment with 1 µM CA, PHEN and MeJa on lignan production was observed when the cultures were supplemented with PFD-aerated or degassed. In ATMA root cultures these applied conditions were the most favourable for matairesinol content which amounted to 199.86 and 160.25 µg/g DW in PFD-aerated and PFD-degassed supported cultures, respectively. In KT root cultures solely, hydroxymatairesinol and coniferin/CA content was enhanced with their highest yield 59.29 and 134.60 µg/g DW in PFD-aerated and PFD-degassed cultures, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxus spp. cell, tissue and organ culture systems have been developed for the production of paclitaxel and other taxanes, an important class of anticancer agents (Yared and Tkaczuk 2012), which was reviewed in detail by Zhong (2002), Tabata (2004, 2006), Maheshwari et al. (2008), Expósito et al. (2009), Sabater-Jara et al. (2010), Malik et al. (2011), Onrubia et al. (2013). Using various biotechnological methods to enhance paclitaxel production the exceptional progress has been made and finally the process was commercialized (Frense 2007; Expósito et al. 2009).
Lignans represent an abundant and widespread group of compounds demonstrating a wide range of biological activities, such as anticancer (review by Landete 2012), antimicrobial, anti-inflammatory (Küpeli et al. 2003; During et al. 2012), hypoglycemic (Banskota et al. 2006; reviews by: Erdemoglu et al. 2004; Saleem et al. 2005; Topcu and Demirkiran 2007; Dar and Arumugam 2013), antioxidant (Masuda et al. 2010), cardioprotective (Zanwar et al. 2011) and anti-osteoporotic (Yin et al. 2006). Lignan food ingredients such as secoisolariciresinol, matairesinol, lariciresinol and pinoresinol are conversed by intestinal bacteria to enterolignans: enterodiol and enterolactone and were reported to reduce the risk of some cancers and cardiovascular diseases (Milder et al. 2007; Ghisalberti 1997; Landete 2012) and exhibited estrogen activity (Cassidy et al. 2000; Aehle et al. 2011).
Apart taxanes about 50 lignans were isolated from eight Taxus species, and in the roots of T. x media the presence of hydroxymatairesinol and epi-nortrachelogenin was confirmed (Topcu and Demirkiran 2007).
Lignans are composed of two phenylpropanoid C6C3 units linked by central carbon (C8) of their propyl side chains (Umezawa 2003; Suzuki and Umezawa 2007; Topcu and Demirkiran 2007). Their biosynthesis involves the shikimate pathway providing l-phenylalanine and required multiple enzymatic steps (Vogt 2010; Umezawa 2010; Thoge et al. 2013; Wang et al. 2013). This pathway originated with a chiral coniferyl alcohol and two units of it are enantioselectively coupled by dirigent protein resulting with pinoresinol. Pinoresinol is reduced through lariciresinol to secoisolariciresinol and eventually oxidized to matairesinol (Suzuki and Umezawa 2007). Coniferyl alcohol and p-coumaryl alcohol are the major building units of conifer lignin, a heterogeneous polymer deposited during cell differentiation and secondary cell wall formation (Marjamaa et al. 2007; Wagner et al. 2012).
Plant sources of lignans are considered to be limited due to insufficient methods of cultivation, long growth and development phases, and low lignan content (Satake et al. 2015). Plant biotechnology methods offers the possibility to produce valuable plant secondary metabolites in cell and organ cultures derived from plants which are rare or considered as endangered due to their overexploitation from natural sources (Bunn et al. 2011). Lignans, such as podophyllotoxin and 5-methoxypodophyllotoxin, which are of special interest, since their derivatives etoposide and teniposide are used in cancer therapy (Bohlin and Rosén 1996), could be accumulated in considerable amounts in in vitro cultures of various species from Podophyllum and Linum genera (Petersen and Alfermann 2001; Fuss 2003; Majumder and Jha 2009). The enhancement of lignan production in in vitro culture systems of various species was achieved by optimization of culture conditions including elaboration of the most suitable for product improvement carbon source, phytohormones, culture conditions like light or dark regime, elicitor treatments (reviewed in Fuss 2003), developing of hairy root cultures (Kumar et al. 2012; Bahabadi et al. 2014; Wawrosch et al. 2014; Gabr et al. 2016, 2017; Tashackori et al. 2016; Chashimi et al. 2016) and metabolic engineering approaches (reviewed in Satake et al. 2015). There has been also one paper on in vitro cultivated Taxus x media cell suspension and shoots where pinoresinol has been determined and revealed solely in shoots (Mistrzak et al. 2015).
Our current studies were undertaken to elucidate the influence of precursors and elicitor feeding on six lignans accumulation in two lines of Taxus x media hairy roots. Moreover we have applied the two-phase culture system containing perfluorodecalin (PFD), fully synthetic, biochemically inert and immiscible with aqueous phases compound, creating in the culture vessel an auxiliary phase below the aqueous phase of the medium (Lowe et al. 2003; Davey et al. 2005).
To our best knowledge, the current research represents an original approach for an examination of the effectiveness of various biotechnological methods, applied to enhance lignan production in Taxus x media hairy root cultures. In the first stage of our investigation, carried out in ATMA root cultures, we examined the influence of various concentrations of CA added as a unique agent or in combination with MeJa and PHEN, on biomass and lignan accumulation. In the second stage the two-phase cultures created by perfluorodecalin application, aerated or not, together with the most effective elicitation regime were applied to both ATMA and KT hairy root cultures to determine their influence on root growth and lignan production.
Materials and methods
Hairy root cultures
Two lines of Taxus x media hairy roots were investigated for their potential for lignan accumulation. The first hairy root line—KT was established by transformation with wild type Agrobacterium rhizogenes LBA 9402 strain (Furmanowa and Syklowska-Baranek 2000). While the second hairy root line—ATMA was obtained as a result of direct inoculation of 10-year-old Taxus plantlets cultivated in vitro with the C58C1 strain of Agrobacterium tumefaciens carrying the plasmid RiA4 of A. rhizogenes and the binary plasmid pCAMBIA-TXS-His, harbouring the taxadiene synthase (txs) gene of T. baccata (GenBank accession: AY424738), under the control of the 35S CaMV promoter, and the hygromycin phosphotransferase gene (hptII) as a resistance marker (Sykłowska-Baranek et al. 2015a). The cultures of KT and ATMA hairy root lines were established in 1996 and 2008, respectively. These hairy root lines were subsequently subcultured into fresh hormone-free DCR-M medium every four weeks (250 ml Erlenmeyer flasks with 35 ml of medium, inoculum 0.51 ± 0.016 g FW) (Syklowska-Baranek et al. 2009). The cultures were maintained at 25 ± 1 °C in the dark on an INFORS AG TR 250 shaker (Switzerland) at 105 rpm.
Elicitor and precursor feeding experiments
The cultures of ATMA 28-day old hairy roots growing in Erlenmeyer flasks with 35 ml DCR-M were supplemented with precursors in lignan biosynthesis pathway: l-phenylalanine (PHEN, 100 µM, Sigma-Aldrich), coniferyl alcohol (CA, Sigma-Aldrich) and elicitor: methyl jasmonate (MeJa; 100 µM, Sigma-Aldrich). The following experiments were carried out: (i) medium supplementation with CA at doses of 1, 10 or 100 µM; (ii) medium supplementation with CA at doses of 1, 10 or 100 µM and MeJa 100 µM; (iii) medium supplementation with CA at doses of 1, 10 or 100 µM, each concentration of CA with PHEN 100 µM and MeJa 100 µM.
Each precursor and elicitor, according to the experiment type, was added to the 28-day old hairy root culture. The PHEN was dissolved in distilled water and filtered through Sartorius 0.20 µm PTFE filter prior to application while MeJa and CA were dissolved in EtOH (Avantor Performance Materials Poland S.A., Poland) aseptically and applied to the cultures. The amount of PHEN, CA and MeJa has been given as final concentration in medium.
The samples were harvested after 1 and 2 weeks after application of precursors and/or elicitor. At each time point, the roots were harvested, gently pressed on filter paper, and their fresh weight (FW) was recorded. After lyophilization, the dry weight (DW) of roots was determined. The fresh and dry biomass increase was expressed as the ratio of initial weight to final weight.
Perfluorodecalin phase supported experiments
The roots of both hairy root lines were cultivated in liquid hormone-free DCR-M medium for 14 days and on the 14th day PFD-degassed or PFD-aerated were added to the cultures. After the subsequent 14 days, on day 28th of culture, the mixture of 100 µM of PHEN, 1 µM of CA and 100 µM of MeJa were added to the cultivated with PFD hairy roots.
The experiments with the application of perfluorodecalin (PFD; C10F18; 98% equimolar mixture of cis-/trans- isomers; ABCR GmbH & Co. KG, Karlsruhe, Germany) were performed in ATMA and KT transformed root cultures.
The samples were harvested at three time points:(i) after 14 days of cultivation with PFD-degassed or PFD-aerated before medium supplementation with precursors (PHEN and CA) and elicitor (MeJa); (ii) one week after application of precursors and elicitor; (iii) after 2 weeks past the application of precursors and elicitor. The roots from three flasks were harvested, gently pressed on paper, and their fresh weight was recorded. After lyophilization at vacuum 0.340 mbar and initial product temperature − 20 °C (lyophilizer Christ ALPHA1-4 LSC, Germany) the dry weight (DW) of roots was determined. The fresh and dry biomass increase was expressed as the ratio of initial weight to final weight.
As a control the cultures of both line of transformed roots cultivated in the medium without any supplementations were used.
The PFD phase were prepared as described earlier (Sykłowska-Baranek et al. 2015b). In brief, PFD was autoclaved at 121 °C for 20 min to ensure aseptic conditions and degassing of PFD. To obtain the PFD-aerated, it was aseptically saturated with atmospheric air for 15 min. according to the procedure described previously (Pilarek and Szewczyk 2008). Afterwards, 20 ml of PFD, degassed or aerated, was added to 35 ml of sterile culture medium on the 14th day of culture.
Lignan determination
The determination of lignan aglyca and their glycosides was performed: (−)-hydroxymatairesinol, secoisolariciresinol (racemic), lariciresinol (racemic) manufactured by ChromaDex, (−)-matairesinol and (+)-pinoresinol manufactured by Phyto-Lab. All standards were purchased in LCG Standards (Poland). The content of CA was also determined.
For the extraction of lignans from hairy roots a modified method of Wichers et al. (1990) after Schmitt and Petersen (2002) was applied. 0.09 g DW ± 0.024 of lyophilized powdered tissue of roots was suspended in 2 ml of methanol (MeOH, Avantor Performance Materials Poland S.A., Poland) and extracted twice in an ultrasonic bath on ice for 15 min. Afterwards 8 ml of H2O (pH = 4.5) was added to the samples and next 10 ml of dichloromethane (CH2Cl2, Avantor Performance Materials Poland S.A., Poland) and mixed for 30 min. The organic phase was separated and evaporated to dryness and stored at − 20 °C till HPLC analysis. The remaining water phase (10 ml), containing lignan glycosides, was incubated with 1 mg of β-glucosidase from almonds (Sigma-Aldrich, Poland) for 3.5 h at 35 °C and then extracted with 10 ml of CH2Cl2 as described above.
Samples of water and organic phases were analyzed by the HPLC-UV-DAD method. Prior to HPLC analysis, samples were re-dissolved in 100% MeOH (1 ml) and 20 µl underwent HPLC analysis using the DIONEX (USA) system with a UVD 340S diode array detector and an automated sample injector (ASI-100). A NovaPak Phenyl column (150 × 3.9 mm) was eluted employing the following gradient: flow 1 ml min− 1, solvent A: H2O with 0.04 M of H3PO4; solvent B: acetonitrile (Sigma-Aldrich, Poland). Gradient program: 0–20 min 40% B, 20–25 min 50% B. Eluting substances were monitored by UV absorption at 230 nm (Fig. 1S).
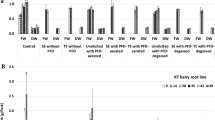
The fresh and dry biomass increase of: a ATMA hairy root line cultivated for 42 days in (i) control untreated cultures; cultures supplemented with coniferyl alcohol (CA at doses 1, 10 or 100 µM); (ii) cultures supplemented with CA (at doses 1, 10 or 100 µM) and methyl jasmonate (MeJA 100 µM) and (iii) cultures supplemented with CA (at doses 1, 10 or 100 µM) combined with l-phenylalanine (100 µM) and methyl jasmonate (100 µM) at each CA concentration; b the fresh and dry biomass increase of ATMA hairy root line and KT hairy root line cultivated for 42 days in: (i) control untreated cultures; (ii) cultures elicited without any PFD phases; (iii) cultures unelicited supplemented with PFD-aerated or PFD-degassed PFD phases and (iv) cultures elicited (with CA 100 µM, l-phenylalanine 100 µM and methyl jasmonate 100 µM) supplemented with PFD-aerated or PFD-degassed PFD phases. Asterisks (*) indicate a significant difference P < 0.05
All experiments were performed in triplicate and all results represent the mean of three repetitions ± SD. The statistical significance between means was assessed using analysis of variance (ANOVA) and Tukey’s multiple range test. For pairwise analysis Student’s t test was used. A probability of P < 0.05 was considered significant.
Results and discussion
The influence of different culture conditions on hairy roots growth
Coniferyl alcohol, l-phenylalanine and methyl jasmonate feeding experiments in ATMA hairy root line cultures
The fresh and dry biomass growth was investigated through the course of the 42-day lasting cultures. The highest growth index was achieved in control cultures where the root growth was observed till the end of the experiments—day 42th. At this time point the growth increase amounted to 1.9-fold which was over 2-times higher than in cultures treated solely with CA or CA and MeJa, regardless of the CA concentration (Fig. 1a, b). However, the growth reduction was less pronounced when culture media were supplemented with CA accompanied with MeJa and PHEN (Fig. 1a). Moreover, in the presence of 1 µM CA together with MeJa and PHEN, the root biomass increase achieved 1.45-fold and 1.52-fold in fresh and dry mass, respectively which means more than doubled values obtained in cultures treated only with 1 µM CA and MeJa, 0.58-fold and 0.71-fold on the basis of FW and DW respectively. The detrimental to biomass accumulation effect of precursor and/or elicitor treatments was statistically significant (P < 0.05) in comparison with control cultures with the exception of 1 µM CA together with MeJa and PHEN simultaneous treatment. In earlier studies of our group where we examined the influence of precursor or/and elicitor treatment on taxane content in KT root line cultures, we also observed the detrimental effect of MeJA or PHEN with MeJa (both in a dose of 100 µM) on root growth (Furmanowa and Syklowska-Baranek 2000; Syklowska-Baranek et al. 2009).
PFD-phases supported cultures of ATMA and KT hairy root lines
The fresh weight of ATMA hairy roots growing in medium supplemented with PFD-degassed or aerated (Fig. 2a–c) but without elicitors was augmented in comparison to cultures elicited (treated with mixture of 1 µM CA, 100 µM PHEN and 100 µM MeJa) but lower than in the control, however the differences were not significant at P < 0.05 (Fig. 1b). When the root dry mass was taken into consideration the biomass decrease was noted in comparison to the control and cultures elicited but without PFD phases. Such discrepancy noted in growth indexes could be attributed to the post-harvesting manipulation procedure when the medium was removed in a non sufficient manner from hairy roots. So the dry biomass growth is considered to be more appropriate to describe the influence of investigated culture conditions. The fresh root growth significantly (P < 0.05) has not been lower than in the control when elicitors were added to the medium supplemented with PFD-degassed or PFD-aerated (Fig. 1b) while the final fresh biomass increase was at the same level in elicited and PFD-phases supported elicited cultures. The comparison of growth rates obtained in PFD-supported unelicited and PFD-supported elicited cultures revealed higher fresh mass growth at the end of the culture in the first ones. However, on day 35 of cultivation, one week after elicitors addition, both fresh and dry biomass increase was higher in PFD-supported elicited cultures. This phenomena could be connected with the acting of MeJa activating primary and secondary metabolism which was discussed on the proteome level (Bhattacharyya et al. 2012).
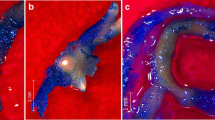
Taxus x media hairy roots cultivated under various conditions: a ATMA root line in control untreated culture on the 28th day of cultivation; b ATMA root line on the 14th day of cultivation after PFD-degassed application; c ATMA root line on the 42th day of cultivation in presence of PFD-degassed and elicitors; d KT root line in control untreated culture on the 28th day of cultivation; e KT root line on the 14th day of cultivation after PFD-degassed application; f KT root line on the 42th day of cultivation in presence of PFD-degassed and elicitors. (Photo I. Rudnicki)
The highest fresh and dry biomass increase of KT line roots was achieved in control cultures and amounted to 4.50-fold and 3.32-fold, respectively over their initial weight. It was 2.36-fold and 1.88-fold more than the fresh and dry growth rates of ATMA roots, respectively in control cultures (Fig. 1b). The addition of precursors and MeJa to the medium caused over twofold and 1.5-fold decrease in fresh and dry root biomass growth, respectively, in relation to the control. The application of PFD phases with elicitors, did not hamper significantly (P < 0.05) the root growth on the basis of their fresh and dry mass in comparison to elicited cultures without and PFD-phases (Fig. 2d–f). In the previous investigation of our group performed in ATMA root line cultures the diminished biomass growth was noted as a result of PFD-aerated or degassed applied alone or in combination with 100 µM MeJa with a marked tendency of more pronounced growth suppression in PFD-aerated supported cultures (Sykłowska-Baranek et al. 2015a).
Up to now, published research on Taxus spp. cell, tissue and organ cultures reported diversified responses of cells to MeJa elicitation (review in Tabata 2004, Vongpaseuth and Roberts 2007, Ramirez-Estrada et al. 2016), although the decrease in cell growth is mainly described. Exposito et al. (2010) in suspension cultures of T. x media, comparing the MeJa effect on the growth of two transformed cell lines, one of them was carrying additional txs gene and rol genes another only rol genes, observed a slight growth suppression in comparison to the control, however the cell line with txs gene was more sensitive to MeJa treatment. The authors reported, in the first stage of cultures carried out in a medium advantageous for cell growth, the significantly higher biomass increase in rol genes carrying cell suspension cultures than in the cultures of cells carrying txs and rol genes. These results corroborate with our observation of current experiments (Fig. 1). The growth decline under MeJa treatment was further reported in cell suspension cultures of T. x. media carrying the additional txs gene by Onrubia et al. (2012). The results of studies on the exploration of the protein profile of MeJa elicited cell suspension cultures of Podophyllum hexandrum, revealed the up-accumulation of defense/stress—related protein, proteins engaged in secondary metabolites production and signal transduction (Bhattacharyya et al. 2012). However, the authors also noted the down-regulation of some proteins involved in primary cell metabolism, for example cyclin A protein connected with the cell division cycle which could be correlated with cell growth suppression caused by MeJa. Recently, in Taxus cuspidata suspension cultures the growth suppression caused by MeJa was reported. The authors demonstrated that growth depletion was connected with the inhibition of cell cycle progression rather than cell death and impaired the G1/S transition as observed by an increase in G0/G1 phase cells, and decreased the number of actively dividing cells (Patil et al. 2013).
The lignan accumulation under various culture conditions
The content of six lignans was examined in hairy root tissues, aqueous and PFD phases during six weeks of cultivation of two Taxus x media hairy root lines. The root cultures were supplemented with precursors and elicitor on the 28th day of cultivation while PFD aerated or degassed phases were added to appropriate culture variants on day 14.
Lignans were detected neither in aqueous nor PFD phases in any of the cultures variants (Fig. 2S d, e).
Lignan accumulation in control cultures of ATMA and KT hairy root lines
The chemical investigation of ATMA line roots growing in control cultures revealed the presence of secoisolariciresinol as lignan aglyca. Its content amounted to 2.35 ± 0.36 µg/g DW, 2.03 ± 0.19 µg/g DW and 1.56 ± 0.44 µg/g DW on the 28th, 35th and 42nd day of culture, respectively. While in the form of glucoside only lariciresinol was present and detected in a concentration of 1.43 ± 0.31 µg/g DW, 1.44 ± 0.21 µg/g DW and 1.28 ± 0.27 µg/g DW on the 28th, 35th and 42nd day of culture, respectively. HMR and pinoresinol was determined as glucosides and aglyca (Table 1; Fig. 2S). The content of HMR glucoside determined on the 28th day was over threefold higher than that of its aglyca. After 14 subsequent days of cultivation HMR was detected only as aglyca and its yield increased over 13-fold, from 0.2 ± 0.01 µg/g DW up to 2.61 ± 0.09 µg/g DW. Pinoresinol was detected only on the 14th day of culture and the ratio of glucoside to its aglyca was 1.8. In transformed roots of ATMA line, after β-glucosidase hydrolysis, 63.20 ± 2.80 µg/g DW of CA in root tissues growing for 28 days was also noted.
In untreated control roots of KT line pinoresinol and matairesinol were present predominantly in the form of glucosides and determined only on the 14th day of cultivation (Table 1). The most abundant was matairesinol and its content was 75.40 ± 3.15 µg/g DW while 9.70 ± 1.01 µg/g DW of pinoresinol was noted. Starting from the 28th day HMR was found as glucoside and aglyca (Fig. 3S a–c), with its highest total yield on the 42nd day. Although the major form of HMR was its aglyca (Table 1) which exceeded the level of HMR glucoside almost ninefold, 20.40 ± 2.32 µg/g DW and 2.30 ± 0.23 µg/g DW, respectively.
In the control cultures of KT root line CA was detected, also after hydrolysis and in this case probably in the form of coniferin which is considered to be a storage or transported form of CA (Savidge 1989; Whetten and Sederoff 1995) hydrolyzed by specific β-glucosidase to CA (Steevens at al. 2001). The CA/coniferin was noted in roots from the 14th till the 42nd day of cultivation, however simultaneously with its aglyca – CA only till the 35th day (Table 1). While on the 14th day 5.60 ± 0.82 µg/g DW of CA was solely determined. On day 28 of the culture coniferin/CA and CA levels were comparable, 22.40 ± 2.07 µg/g DW and 20.50 ± 5.26 µg/g DW, respectively. On day 35 the ratio coniferin/CA to CA was changed in favour of coniferin/CA and finally on day 42 only 30.40 ± 3.77 µg/g DW of coniferin/CA was detected.
The two investigated Taxus x media hairy root lines differed in the lignan production profiles (Table 1). Roots of KT line did not accumulate secoisolariciresinol and lariciresinol while ATMA roots did but were unable to accumulate matairesinol. Similar observations on the differences in biosynthetic potential of Taxus x media cell suspension cultures derived from callus tissues, one carried rol and txs genes and the second only rol genes, was reported by Exposito et al. (2010), who noted significant variations in the percentage participation of five determined taxanes depending on the cell line. In our study the divergence in coniferin/CA accumulation followed by the higher abundance of lignan content in KT root line cultures in comparison to ATMA roots could be also explained by KT roots more intensive biomass increase as a result of prolific cell divisions (Fig. 1b). It was previously demonstrated that in Pinus spp. cambium CA content began to increase immediately before the initiation of cell-division activity in late winter (Savidge and Förster 2001) while the coniferin was found in the cambial zone only during cambial growth and became undetectable at the beginning of cambial dormancy (reviewed by Steevens at al. 2001). Moreover, the activity of UDPG: coniferyl alcohol glucosylotransferase, an enzyme that catalyses the transfer of glucose from UDPG to coniferyl alcohol in cambium and developing xylem, is closely coupled with cambium activity (Savidge and Förster 1998).
Up to now there has been one report on lignan production in shoot and cell suspension cultures of T. x media where only pinoresinol was detected in cultivated in vitro shoots (Mistrzak et al. 2015). The pinoresinol reported yield ranged from 0.51 to 0.69 mg/g DW of total content, depending on culture conditions and was detected mainly as its glucoside the amount of which exceeded its aglyca by about 3-times.
Lignan accumulation in supplemented with precursors and elicitor cultures of ATMA hairy root line
The medium supplementation with CA at dose of 1 µM used as a single biosynthesis stimulating factor, resulted in the accumulation solely of lariciresinol after one week of treatment. Lariciresinol was present mainly as a free lignan at the level of 4.41 ± 0.10 µg/g DW and after hydrolysis at the concentration of 2.74 ± 0.28 µg/g DW. However, after one week of 1 µM CA treatment 3.07 ± 1.07 µg/g DW of lariciresinol glucoside was noted. After two weeks of precursor treatment secoisolariciresinol was detected under the influence of 1 and 10 µM CA, 4.72 ± 1.25 and 3.79 ± 0.31 µg/g DW, respectively. While 100 µM of CA caused the production of 2.09 ± 0.61 µg/g DW and 1.60 ± 0.61 µg/g DW of lariciresinol aglyca and glucoside, respectively. The yield of lariciresinol under the influence of 1 or 10 µM CA increased about twofold and over twofold compared to the control after 1 and 2 weeks of treatment, respectively (Table 2). Moreover its aglycone was revealed that was undetectable in the control. The content of secoisolariciresinol was enhanced over threefold and 2.4-fold under the same culture conditions, respectively.
The application simultaneously of MeJa and CA at doses of 10 or 100 µM brought about the biosynthesis of hydroxymatairesinol (HMR) (Table 2). None of the other lignans were detected under the described conditions while after hydrolysis only 3.19 ± 0.28 and 1.49 ± 0.27 µg/g DW of lariciresinol was revealed on 35th and 42nd day, respectively. Simultaneous treatments with CA and MeJa caused exclusively the production of HMR aglyca (Table 2). The yield of HMR was considerably enhanced by precursor and elicitor addition in a dose dependent manner in comparison to control. The most pronounced HMR content increase, over 14-times, was noted under 1 µM CA and MeJa two week treatment. While 10 or 100 µM CA and MeJa two week treatments resulted in over 9 and twofold HMR content augmentation, respectively. Hydrolysis of extracts showed solely the presence of lariciresinol, 1.49 ± 0.27 µg/g DW and its amount was not affected by culture elicitation.
The investigation of the effect of the combined two precursors: PHEN and CA together with MeJa resulted in the accumulation of HMR and lariciresinol as free lignans and lariciresinol in the form of glucoside (Table 2). The simultaneous application of two lignan biosynthesis precursors and elicitor resulted in higher HMR and lariciresinol accumulation than in the control, however the influence of theses culture conditions was less pronounced in comparison to CA and MeJa treatments. HMR yield augmentation was also dose dependent, although it was only 5.5-fold, 4.4-fold higher than in the control when CA at a concentration of 1 or 10 µM were applied. Moreover HMR content was even lower than in the control in the presence of 100 µM CA, PHEN and MeJa. The opposite effect was exerted by the mentioned precursors and elicitor on lariciresinol content which increased up to 1.93 ± 0.34 µg/g DW and represents over a 1.3-fold increase in relation to the control. We observed variability in respect to lignan content between flasks that was earlier reported and investigating in details for paclitaxel production in suspension cultures of T. cuspidata by Kim et al. (2004). The authors’ results indicated that there may be other factors affecting paclitaxel accumulation than MeJa like protein signal compounds.
The pinoresinol and matairesinol content increase after 100 µM MeJa or 3 mM CA treatment was described by Schmitt and Petersen (2002) in Forsythia x intermedia suspension cultures. Lignan yield was enhanced 3- and sevenfold, respectively by MeJa while CA enhanced only the pinoresinol content by over ninefold. The authors reported also the presence of coniferin/CA in cell extracts obtained from CA treated cultures whereas in our studies coniferin/CA was not detected in root cultures subjected to CA or CA, PHEN and MeJa treatments. Phenylpropanoids were reported to play an important role in plant response to abiotic (Cabane et al. 2012) and biotic stresses (Dixon et al. 2002; Naoumkina et al. 2010) and the biosynthesis of CA was found to be induced as a result of wounding (Savidge and Förster 2001). Anterola et al. (2002) reported that CA and p-coumaryl alcohol biosynthesis were enhanced with providing cell cultures of Pinus taeda with saturating amounts of PHEN and Cabane et al. (2012) concluded that effective stimulation of phenylpropanoid pathway takes place when PHEN availability from the shikimate pathway is sufficient.
Lignan accumulation in cultures of ATMA and KT hairy root lines cultivated in two-phase cultures supplemented with PFD-phases
The results obtained in previous research performed by our group indicated that direct application of PFD to the culture system constitutes simple and efficient conditions for the enhancement of secondary metabolites production (Sykłowska-Baranek et al. 2014, 2015a). In cultures of KT root line supplemented with PFD-aerated or degassed but without elicitors no lignan content was detected (Table 4). ATMA roots in cultures supported with PFD-degassed accumulated CA/coniferin 3.06 ± 0.87 µg/g DW (Table 3). Also aglycone of HMR and glycoside of pinoresinol were present, 0.24 ± 0.11 µg/g DW on the 35th day and 2.22 ± 0.43 µg/g DW on the 28th day, respectively (Table 3). When PFD-aerated was added to the cultures of ATMA hairy root line and after subsequent elicitor feeding a significant increase in lignan content was noted (Table 3). The highest CA/coniferin content was stated in KT hairy root line cultures, 72.77 ± 0.34 µg/g DW and 134.60 ± 12.13 µg/g DW on day 35 and 42 respectively. At the same time points, in ATMA line cultures CA/coniferin concentration amounted to 23.81 ± 1.49 and 15.50 ± 2.57 µg/g DW, respectively. Both ATMA and KT hairy root cultures accumulated HMR mainly as its aglyca (Tables 3, 4). KT roots produced also lariciresinol in the form of its aglycone (0.59 ± 0.02 µg/g DW and 0.68 ± 0.10 µg/g DW) on day 35 and 42, respectively. In ATMA roots cultivated in the presence of PFD-aerated and elicitors the most spectacular was the occurrence of matairesinol aglyca which at the same time proved to be the most abundant among all investigated lignans (Table 3). The content of matairesinol aglyca amounted to 159.79 ± 10.91 µg/g DW and 199.86 ± 22.55 µg/g DW, on day 35 and 42 respectively. In KT roots matairesinol was determined only in elicited cultures without any PFD phases (Table 4). ATMA roots cultivated in the medium supported with PFD-degassed and elicited accumulated significantly lower levels of matairesinol aglyca (P < 0.05), 27.86 ± 3.86 µg/g DW and 160.25 ± 32.33 µg/g DW, on day 35 and 42, respectively. Matairesinol glucosides were detected only on day 42, 4.47 ± 1.10 µg/g DW and 31.83 ± 2.89 µg/g DW during cultivation in elicited cultures supported with PFD-aerated or degassed, respectively. In suppoered with PFD-degassed elicited cultures, CA/coniferin 25.71 ± 4.67 µg/g DW was found in KT roots on the 42nd day (Table 4) while it was not detected in ATMA roots (Table 3). Under these culture conditions HMR, solely in the form of its aglyca, was more intensively produced by ATMA roots than KT ones, 2.30 ± 0.12 and 19.06 ± 5.14 µg/g DW versus 48.04 ± 8.92 and 59.29 ± 0.95 µg/g DW, on the 35th and 42nd day, respectively. In ATMA roots also aglyca of pinoresinol (31.06 ± 7.71 µg/g DW) was found on the 42nd day of cultivation (Table 3). Whereas in KT root line cultures pinoresinol was noted only in elicited cultures without PFD phases (Table 4).
For the first time our group developed PFD-supported cultures systems for plant cell and organ cultures in the simultaneous function of gas carrier and in situ extrahent. PFD-aerated applied to the cell suspension cultures of Arnebia euchroma allowed to enhance naphthoquinone yield about 50% in comparison to control cultures (Sykłowska-Baranek et al. 2014). The influence of PFD application at two time points: the day of inoculation (day 0) and the 14th day of culture together with elicitation (100 µM PHEN and MeJa) in ATMA transformed root cultures was studied (Sykłowska-Baranek et al. 2015a). It was demonstrated that the most suitable time point for PFD addition is the 14th day of culture and the highest total (i.e. intracellular + extracellular: both in aqueous and PFD phases) yield of paclitaxel was obtained in PFD-degassed carrying cultures after two weeks of elicitation. Under the conditions of the current study we also observed in most of the cases higher lignan contents after two week elicitation, however, the PFD-aerated supported elicited cultures seemed to be more favourable for lignan accumulation (Tables 3, 4) likely due to generated to a higher extent than in PFD-degassed supported cultures oxidative stress which is believed to affect plant secondary metabolism (Shilpa et al. 2010; Kolewe et al. 2010). PFD application in the function of in situ extrahent could also contribute to the lower metabolite feedback inhibition and diminished cytotoxic metabolites accumulation in aqueous phases of the medium which seemed to be often the reason of limited secondary metabolites production (Malik et al. 2013; Kolewe et al. 2010).
Taxus hairy root cultures were used for lignan production for the first time under conditions of current investigations. The applied various elicitation strategies combined with PFD-phases application caused mostly dose- and treatment dependent enhancement of lignan accumulation over the control in T. x media hairy root cultures, however their level were lower than those reported up to now in hairy root cultures, dealing mainly with species of Linum genus. In hairy root cultures of Linum album lignan accumulation was successfully enlarged by fungal elicitor usage, and lariciresinol content rose threefold over control up to 260 µg/g DW (Bahabadi et al. 2014). Similary Tashackori et al. (2016), examining various lignan production in L. album hairy roots elicited with mycelium extract of Piriformospora indica, reported an 1.6-fold increase (up to 157.3 µg/g DW) over control in lariciresinol content. Chashimi et al. (2016) demonstrated that coniferyl aldehyde (2 mM) significantly stimulated larici- and pinoresinol production up to 14.8- and 8.7-fold, respectively. Gabr et al. (2017) demonstrated that the total lignan content (secoisolariciresinol diglucoside, secoisolariciresinol and matairesinol) was 55% higher in hairy root cultures of L. usitatissimum than in non-transformed roots.
Under conditions of reported herein experiments, the potential of two Taxus x media hairy root lines for lignan production have been demonstrated, however for the lignan production enhancement further biotechnological investigations are needed.
Summary
Two yew hairy root lines were examined for their potential to lignan accumulation. It was demonstrated that the hairy root lines subjected to the investigations differed in lignan production profiles. Among six lignans, in the control untreated cultures, roots of KT line did not accumulate secoisolariciresinol and lariciresinol while ATMA roots were unable to accumulate matairesinol. The elicitation of ATMA root line cultures with CA at different doses also resulted solely in the production of lariciresinol and secoisolariciresinol while under simultaneous CA and MeJA treatment HMR was also accumulated. The addition of PHEN together with CA and MeJa neither changed the accumulated lignan profile nor enhanced their production. The most beneficial conditions for lignan production under the conditions of the current experiments, were created by two-phase cultures supplemented with PFD-aerated or degassed. In ATMA hairy roots HMR, pinoresinol and matairesinol were present predominantly as their aglyca while in KT hairy roots pinoresinol and matairesinol were mainly detected as glucosides. In both root lines CA was found only in its glucosylated form.
References
Aehle E, Müller U, Eklund PC, Willför SM, Sippl W, Dräger B (2011) Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry 72:2396–2405. https://doi.org/10.1016/j.phytochem.2011.08.013
Anterola AM, Jeon J-H, Davin LB, Lewis NG (2002) Transcriptional control of monolignol biosynthesis in Pinus taeda. Factors affecting monolignol ratios and carbon allocation in phenylpropanoid metabolism. J Biol Chem 277:18272–18280. https://doi.org/10.1074/jbc.M112051200
Bahabadi SE, Sharifi M, Chashimi NA, Murata J, Satake H (2014) Significant enhancement of lignan accumulation in hairy root cultures of Linum album using biotic elicitors. Acta Physiol Plant (2014) 36:3325–3331. https://doi.org/10.1007/s11738-014-1700-z
Banskota AH, Nguyen NT, Tezuka Y, Nobukawa T, Kadota S (2006) Hypoglycemic effects of the wood of Taxus yunnanensis on streptozotocin-induced diabetic rats and its active components. Phytomedicine 13:109–114. https://doi.org/10.1016/j.phymed.2004.01.015
Bhattacharyya D, Sinha R, Ghanta S, Chakraborty A, Hazra S, Chattopadhyay S (2012) Proteins differentially expressed in elicited cell suspension culture of Podophyllum hexandrum with enhanced podophyllotoxin content. Proteome Sci 10:34. https://doi.org/10.1186/1477-5956-10-34
Bohlin L, Rosén B (1996) Podophyllotoxin derivatives: drug discovery and development. DDT 8:343–351
Bunn E, Turner SR, Dixon KW (2011) Biotechnology for saving rare and threatened flora in a biodiversity hotspot. Vitr Cell Dev Biol - Plant 47:188–200. https://doi.org/10.1007/s11627-011-9340-0
Cabane M, Afif D, Hawkins S (2012) Lignins and abiotic stress. Adv Bot Res 61:219–262. https://doi.org/10.1016/B978-0-12-416023-1.00007-0
Cassidy A, Hanley B, Lamuela-Raventos RM (2000) Isoflavones, lignans and stilbenes–origins,metabolism and potential importance to human health. J Sci Food Agric 80:1044–1062. https://doi.org/10.1002/(SICI)1097-0010(20000515)80:7
Chashimi NA, Sharifi M, Behmanesh M (2016) Lignan enhancement in hairy root cultures of Linum album using coniferaldehyde and methylenedioxycinnamic acid. Prep Biochem Biotechnol 46:454–460. https://doi.org/10.1080/10826068.2015.1068802
Dar AA, Arumugam N (2013) Lignans of sesame: purification methods, biological activities and biosynthesis - a review. Bioorg Chem 50:1–10. https://doi.org/10.1016/j.bioorg.2013.06.009
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplast technology: current status. Acta Physiol Plant 27:117–129. https://doi.org/10.1007/s11738-005-0044-0
Dixon R, Achnine L, Kota P, Liu C-J, Srinivasa Reddy MS, Wang L (2002) The phenylpropanoid pathway and plant defence-a genomics perspective. Mol Plant Pathol 3:371–390. https://doi.org/10.1046/j.1364-3703.2002.00131.x
During A, Debouche C, Raas T, Larondelle Y (2012) Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J Nutr 142:1795–1798. https://doi.org/10.3945/jn.112.162453
Erdemoglu N, Sener B, Choudhary MI (2004) Bioactivity of Lignans from Taxus baccata. Z Naturforsch C 59:494–498
Exposito O, Syklowska-Baranek K, Moyano E, Onrubia M, Bonfill M, Palazon J, Cusido RM (2010) Metabolic responses of Taxus media transformed cell cultures to the addition of methyl jasmonate. Biotechnol Prog 26:1145–1153. https://doi.org/10.1002/btpr.424
Expósito O, Bonfill M, Moyano E, Onrubia M, Mirjalili MH, Cusidó R, Palazón J (2009) Biotechnological production of taxol and related taxoids: current state and prospects. Anti-cancer Agents Med Chem 9:109–121
Frense D (2007) Taxanes: perspectives for biotechnological production. Appl Microbiol Biotech 73:1233–1240. https://doi.org/10.1007/s00253-006-0711-0
Furmanowa M, Syklowska-Baranek K (2000) Hairy root cultures of Taxus × media var. Hicksii Rehd. as a new source of paclitaxel and 10-deacetylbaccatin III. Biotechnol Lett 22:683–686. https://doi.org/10.1023/A:1005683619355
Fuss E (2003) Lignans in plant cell and organ cultures: An overview. Phytochem Rev 2:307–320. https://doi.org/10.1023/B:PHYT.0000045500.56476.f5
Gabr AMM, Mabrok HB, Kadry ZG, Blaut M, Smetanska I (2016) Lignan accumulation in callus and Agrobacterium rhizogenes mediated hairy root cultures of flax (Linum usitatissimum). Plant Cell Tiss Organ Cult (2016) 126:226–255. https://doi.org/10.1007/s11240-016-0995-4
Gabr AMM, Mabrok HB, Abdel-Rahim EA, El-Bahr MK, Smetanska I (2017) Determination of lignans, phenolic acids and antioxidant capacity in transformed hairy root culture of Linum usitatissimum. Nat Prod Res Nov 20:0. https://doi.org/10.1080/14786419.2017.1405405
Ghisalberti EL (1997) Cardiovascular activity of naturally occurring lignans. Phytomedicine 4:151–166. https://doi.org/10.1016/S0944-7113(97)80063-3
Kim BJ, Gibson DM, Shuler ML (2004) Effect of subculture and elicitation on instability of taxol production in Taxus sp. suspension cultures. Biotechnol Prog 20:1666–1673. https://doi.org/10.1021/bp034274c
Kolewe ME, Henson MA, Roberts SC (2010) Characterization of aggregate size in Taxus suspension cell culture. Plant Cell Rep 29:485–494. https://doi.org/10.1007/s00299-010-0837-5
Kumar V, Rajauria G, Sahai V, Bisaria VS (2012) Culture filtrate of root endophytic fungus Piriformospora indica promotes the growth and lignan production of Linum album hairy root cultures. Process Biochem 47:901–907. https://doi.org/10.1016/j.procbio.2011.06.01
Küpeli E, Erdemoğlu N, Yeşilada E, Şener B (2003) Anti-inflammatory and antinociceptive activity of taxoids and lignans from the heartwood of Taxus baccata L. J Ethnopharmacol 89:265–270. https://doi.org/10.1016/j.jep.2003.09.005
Landete JM (2012) Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res Int 46:410–424. https://doi.org/10.1016/j.foodres.2011.12.023
Lowe KC, Anthony P, Power JB, Davey MR (2003) Novel approaches for regulating gas supply to plant systems in vitro: application and benefits of artificial gas carriers. In Vitro Cell Dev Biol- Plant 39:557–566. https://doi.org/10.1079/IVP2003469
Maheshwari P, Garg S, Kumar A (2008) Taxoids: biosynthesis and in vitro production. Mol Biol 3:71–87
Majumder A, Jha S (2009) Biotechnological approaches for the production of potential anticancer leads podophyllotoxin and paclitaxel: an overview. J Biol Sci 1:46–69
Malik S, Cusidó R, Mirjalili MH, Moyano E, Palazón J, Bonfill M, Hossein M (2011) Production of the anticancer drug taxol in Taxus baccata suspension cultures: a review. Process Biochem 46:23–34. https://doi.org/10.1016/j.procbio.2010.09.004
Malik S, Mirjalili MH, Fett-Neto AG, Mazzafera P, Bonfill M (2013) Living between two worlds: two-phase culture systems for producing plant secondary metabolites. Critical Rev Biotechnol 33:1–22. https://doi.org/10.3109/07388551.2012.659173
Marjamaa K, Kukkola EM, Fagerstedt KV (2007) Lignification in development. Int J Plant Dev 1:160–169
Masuda T, Akiyama J, Fujimoto A, Yamauchi S, Maekawa T, Sone Y (2010) Antioxidation reaction mechanism studies of phenolic lignans, identification of antioxidation products of secoisolariciresinol from lipid oxidation. Food Chem 123:442–450. https://doi.org/10.1016/j.foodchem.2010.04.065
Milder IEJ, Arts ICW, van de Putte B, Venema DP, Hollman PCH (2007) Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr 93:393. https://doi.org/10.1079/BJN20051371
Mistrzak P, Celejewska-Marciniak H, Szypuła WJ, Olszowska O, Kiss AK (2015) Identification and quantitative determination of pinoresinol in Taxus xmedia Rehder needles, cell suspension and shoot cultures. Acta Soc Bot Pol 84:125–132. https://doi.org/10.5586/asbp.2014.038
Naoumkina MA, Zhao Q, Gallego-Giraldo L, Dai X, Zhao PX, Dixon RA (2010) Genome-wide analysis of phenylpropanoid defence pathways. Mol Plant Pathol 11:829–846. https://doi.org/10.1111/J.1364-3703.2010.00648.X
Onrubia M, Moyano E, Bonfill M, Cusidó RM, Goosens A, Palazón J (2012) Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate. J Plant Physiol 170:129–211. https://doi.org/10.1016/j.jplph.2012.09.004
Onrubia M, Cusidó RM, Ramirez K, Hernández-Vázquez L, Moyano E, Bonfill M, Palazon J (2013) Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives. Curr Med Chem 20:880–891
Patil RA, Kolewe ME, Roberts SC (2013) Cellular aggregation is a key parameter associated with long term variability in paclitaxel accumulation in Taxus suspension cultures. Plant Cell Tiss Organ Cult 112:303–310. https://doi.org/10.1007/s11240-012-0237-3
Petersen M, Alfermann AW (2001) The production of cytotoxic lignans by plant cell cultures. Appl Microbiol Biotechnol 55:135–142. https://doi.org/10.1007/s002530000510
Pilarek M, Szewczyk KW (2008) Effects of perfluorinated oxygen carrier application in yeast, fungi and plant cell suspension cultures. Biochem Eng J 41:38–42
Sabater-Jara AB, Tudela LR, López-Pérez AJ (2010) In vitro culture of Taxus sp.: strategies to increase cell growth and taxoid production. Phytochem Rev 9:343–356. https://doi.org/10.1007/s11101-013-9275-7
Saleem M, Kim HJ, Ali MS, Lee YS (2005) An update on bioactive plant lignans. Nat Prod Rep 22:696–716. https://doi.org/10.1039/B514045P
Satake H, Koyama T, Bahabadi SE, Matsumoto E, Ono E, Murata J (2015) Essences in metabolic engineering of lignan biosynthesis. Metabolites 5:270–290. https://doi.org/10.3390/metabo5020270
Savidge RA (1989) Coniferin, a biochemical indicator of commitment to tracheid differentiation in conifers. Can J Bot 67:2663–2668. https://doi.org/10.1139/b89-343
Savidge RA, Förster H (1998) Seasonal activity of uridine 5′-diphosphoglucose:coniferyl alcohol glucosyltransferase in relation to cambial growth and dormancy in conifers. Can J Bot: 76:486–493. https://doi.org/10.1139/b98-015
Savidge RA, Förster H (2001) Coniferyl alcohol metabolism in conifers—II. Coniferyl alcohol and dihydroconiferyl alcohol biosynthesis. Phytochem 57:1095–1103
Schmitt J, Petersen M (2002) Pinoresinol and matairesinol accumulation in a Forsythia × intermedia cell suspension culture. Plant Cell Tiss Organ Cult 68:91–98. https://doi.org/10.1007/s11101-010-9167-z
Shilpa K, Varun K, Lakshmi BS (2010) An alternative method of natural drug production: eliciting secondary metabolite production using plant cell culture. J Plant Sci 5:222–227. https://doi.org/10.3923/jps.2010.222.247
Steevens V, Förster H, Pommer U, Savidge R (2001) Coniferyl alcohol metabolism in conifers—I. Glucosidic turnover of cinnamyl aldehydes by UDPG : coniferyl alcohol glucosyltransferase from pine cambium. Phytochem 57:1085–1093
Suzuki S, Umezawa T (2007) Biosynthesis of lignans and norlignans. J Wood Sci 53:273–284. https://doi.org/10.1007/s10086-007-0892-x
Syklowska-Baranek K, Pietrosiuk A, Kokoszka A, Furmanowa M (2009) Enhancement of taxane production in hairy root culture of Taxus x media var. Hicksii. J Plant Physiol 166:1950–1954. https://doi.org/10.1016/j.jplph.2009.05.001
Sykłowska-Baranek K, Pilarek M, Cichosz M, Pietrosiuk A (2014) Liquid perfluorodecalin application for in situ extraction and enhanced naphthoquinones production in Arnebia euchroma cell suspension cultures. Appl Biochem Biotechnol 172:2618–2627. https://doi.org/10.1007/s12010-013-0701-5
Sykłowska-Baranek K, Pilarek M, Bonfill M, Kafel K, Pietrosiuk A (2015a) Perfluorodecalin-supported system enhances taxane production in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene. Plant Cell Tiss Org 120:1051–1059. https://doi.org/10.1007/s11240-014-0659-1
Sykłowska-Baranek K, Grech-Baran M, Naliwajski MR, Bonfill M, Pietrosiuk A (2015b) Paclitaxel production and PAL activity in hairy root cultures of Taxus x media var. Hicksii carrying a taxadiene synthase transgene elicited with nitric oxide and methyl jasmonate. Acta Physiol Plant 10:1–9. https://doi.org/10.1007/s11738-015-1949-x
Tabata H (2004) Paclitaxel production by plant-cell-culture technology. Adv Biochem Engin/Biotechnol 7:1–23. https://doi.org/10.1007/b13538
Tabata H (2006) Production of paclitaxel and the related taxanes by cell suspension cultures of Taxus species. Curr Drug Targets 7: 453–461. https://doi.org/10.2174/138945006776359368
Tashackori H, Sharifi M, Chashmi NA, Safaie N, Behmanesh M (2016) Induced-differential changes on lignan and phenolic acid compounds in Linum album hairy roots by fungal extract of Piriformospora indica. Plant Cell Tiss Organ Cult 127:187–194. https://doi.org/10.1007/s11240-016-1041-2
Thoge T, Watanabe M, Hoefgen R, Femie AR (2013) Shikimate and phenylalanine biosynthesis in the green lineage. Frontiers Plant Sci 4:62. https://doi.org/10.3389/fpls.2013.00062
Topcu G, Demirkiran O (2007) Lignans from Taxus species. In: Khan MTH (ed) Bioactive Heterocycles V, vol 11. Springer-verlag, Berlin, pp 103–144. https://doi.org/10.1007/7081_2007_082
Umezawa T (2003) Diversity in lignan biosynthesis. Phytochem Rev 2:371–390. https://doi.org/10.1023/B:PHYT.0000045487.02836.32
Umezawa T (2010) The cinnamate/monolignol pathway. Phytochem Rev 9:1–17. https://doi.org/10.1007/s11101-009-9155-3
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3:2–20. https://doi.org/10.1093/mp/ssp106
Vongpaseuth K, Roberts SC (2007) Advancements in the understanding of paclitaxel metabolism in tissue culture. Curr Pharm Biotechnol 8:219–236. https://doi.org/10.2174/138920107781387393
Wagner A, Donaldson L, Ralph J (2012) Lignification and lignin manipulations in conifers. Adv Bot Res 61:37–76. https://doi.org/10.1016/B978-0-12-416023-1.00002-1
Wang Y, Chantreau M, Sibout R, Hawkins S (2013) Plant cell wall lignification and monolignol metabolism. Front Plant Sci 4:220. https://doi.org/10.3389/fpls.2013.00220
Wawrosch C, Schwaiger S, Stuppner H, Kopp B (2014) Lignan formation in hairy root cultures of Edelweiss (Leontopodium nivale ssp. alpinum. (Cass.) Greuter) Fitoterapia 97:219–223. https://doi.org/10.1016/j.fitote.2014.06.008
Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7:1001–1013. https://doi.org/10.1105/tpc.7.7.1001
Wichers HJ, Harkes MP, Arroo RRJ (1990) Occurrence of 5-methoxypodophyllotoxin in plants, cell cultures and regenerated plants of Linum flavum. Plant Cell Tiss Org 23:93–100. https://doi.org/10.1007/BF00035828
Yared JA, Tkaczuk KHR (2012) Update on taxane development: new analogs and new formulations. Drug Des Devel Ther 6:371–384. https://doi.org/10.2147/DDDT.S28997
Yin J, Tezuka Y, Shi L, Nobukawa M, Nobukawa T, Kadota S (2006) In vivo anti-osteoporotic activity of isotaxiresinol, a lignan from wood of Taxus yunnanensis. Phytomedicine 13:37–42. https://doi.org/10.1016/j.phymed.2004.06.017
Zanwar AA, Hegde MV, Bodhankar SL (2011) Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip Toxicol 4:90–97. https://doi.org/10.2478/v10102-011-0016-8
Zhong J-J (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94:591–599
Acknowledgements
This study was supported by research Grant No. N N405 362537 from the Polish Ministry of Science and Higher Education and this work was supported by the Statutory Budget [FW21/N/2017] of the Medical University of Warsaw. The authors are grateful to Alina Łukasiewicz and Bożenna Sztyber for their technical assistance. We are also grateful to Paulina Mistrzak for the inspiring talk which led us to apply in our investigations methods developed by Prof. Petersen and Dr. Schmitt for examinations of lignan production in Forsythia x intermedia in vitro cultures.
Author information
Authors and Affiliations
Contributions
KSB planning of the investigations, perfluorodecalin application, implementation of HPLC-UV-DAD analysis, elaboration of the results and preparation of the manuscript. KŁ carrying out in vitro cultures of KT root line and sample preparations. MJ carrying out statistical analysis of results and manuscript preparation. AW carrying out in vitro precursors and elicitor feeding cultures of ATMA root line and sample preparations. MG carrying out in vitro cultures in two-phase in vitro cultures of ATMA root line and sample preparations. AP results elaboration and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest
Additional information
Communicated by Sergio J. Ochatt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sykłowska-Baranek, K., Łysik, K., Jeziorek, M. et al. Lignan accumulation in two-phase cultures of Taxus x media hairy roots. Plant Cell Tiss Organ Cult 133, 371–384 (2018). https://doi.org/10.1007/s11240-018-1390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-018-1390-0