Abstract
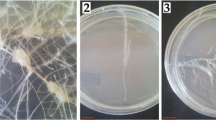
To establish cactus hairy root lines, axenically grown Escobaria chaffeyi, Ferocactus peninsulae, Mammillaria bocasana subsp. bocasana, Turbinicarpus lophophoroides, Turbinicarpus pseudopectinatus, and Turbinicarpus schmiedickeanus subsp. schwarzii explants were inoculated with an Agrobacterium rhizogenes A4 agropine-type strain carrying the ESC4 plasmid, which contains the nptII and gus genes. Cactus hairy roots showed an active branching pattern and fast growth on hormone-free medium. Twelve different basal culture media were tested for biomass production of these six species. Our results showed that medium optimization needs to be performed for each individual species, as growth responses differed among taxa. Maximum hairy root growth was obtained on Driver and Kuniyuki Walnut (DKW) medium for E. chaffeyi and T. schmiedickeanus subsp. schwarzii (3-fold weight increase over starting weight in 30 d for both species). M. bocasana subsp. bocasana responded best when cultured on WPM medium, doubling the initial inoculum weight. F. peninsulae and T. lophophoroides showed a maximum biomass increase when cultured on N6 medium (0.5- and 2-fold increases over starting weight, respectively, in 30 d). T. pseudopectinatus generated callus tissue on all basal media except for Heller medium, on which hairy root weight increased 4-fold over the starting weight in 30 d. Culture medium was also found to affect hairy root morphology of all species tested. Scaling-up of the hairy root cultures was achieved by using the temporary immersion system RITA®.





Similar content being viewed by others
References
Anderson WC (1978) Tissue culture propagation of rhododendrons. In Vitro 14:334
Anderson WC (1980) Tissue culture propagation of red and black raspberries, Rubus idaeus and R. occidentalis. Acta Hortic 112:13–20
Bárcenas Luna RT (2003) Chihuahuan Desert cacti in Mexico: an assessment of trade, management, and conservation. priorities. Part II. In: Robbins CS (ed) Prickly trade: trade and conservation of Chihuahuan Desert cacti. TRAFFIC North America, World Wildlife Fund, Washington, DC. http://www.traffic.org/species-reports/traffic_species_plants5.pdf. Cited 15 Feb 2015
Bhojwani SS, Razdan MK (1989) Plant tissue culture: theory and practice. Elsevier Science Publishers, Amsterdam
Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY, Bi FY (1975) Establishment of an efficient medium for another culture of rice through comparative experiments on the nitrogen sources. Sci Sin 18:659–668
Coke JE (1996) Basal nutrient medium for in vitro cultures of loblolly pines. US Patent 5:534,434
De la Rosa-Carrillo ML, Domínguez-Rosales MS, Pérez-Reyes ME, Pérez-Molphe-Balch E (2012) Cultivo y propagación in vitro de cactáceas amenazadas del género Turbinicarpus. Interciencia 37:114–120
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortSci 19:507–509
Dubrovsky JG (1998) Discontinuous hydration as a facultative requirement for seed germination in two cactus species of the Sonoran Desert. J Torrey Bot Soc 125:33–39
Fitz MB, Fitz MWA (2009) Mammillaria bocasana. In: IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org/. Cited 26 May 2013
Fitz MB, Fitz MWA, Sotomayor M, Smith M (2013a) Turbinicarpus pseudopectinatus. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. www.iucnredlist.org/. Cited 13 November 2013
Fitz MB, Fitz MWA, Sotomayor M, Smith M (2013b) Turbinicarpus schmiedickeanus. In IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. www.iucnredlist.org/. Cited 11 November 2013
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gamborg OL, Murashige T, Thorpe TA, Vasil IK (1976) Plant tissue culture media. In Vitro 12:473–478
George EF, Michael AH, De Klerk GJ (2008) The components of plant tissue culture media I: macro- and micro-nutrients. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture. Springer, Dordrecht, pp 65–113
Georgiev MI, Pavlov AI, Bley T (2007) Hairy root type plant in vitro systems as sources of bioactive substances. Appl Microbiol Biotechnol 74:1175–1185
Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P (2006) Harnessing the potential of hairy roots: dawn of a new era. Trends Biotechnol 24:403–409
Gupta PK, Durzan KJ (1985) Shoot multiplication from mature Douglas-fir and sugar pine. Plant Cell Rep 4:177–179
Hahlbrock K (1974) Correlation between nitrate uptake, growth and changes in metabolic activities of cultured plant cells. In: Street HE (ed) Tissue culture and plant science. Academic, London, pp 363–378
Hayashi Y, Kyozuka J, Shimamoto K (1988) Hybrids of rice (Oryza sativa L.) and wild Oryza species obtained by cell fusion. Mol Gen Genet 214:6–10
Heller R (1953) Recherches sur la nutrition minérale des tissus végétaux cultivés in vitro. Ann Sci Nat Bot Biol Veg 14:1–223
Hernández HM, Bárcenas RT (1995) Endangered cacti in the Chihuahuan Desert: I. Distribution patterns. Conserv Biol 9:1176–1188
Hernández HM, Bárcenas RT (1996) Endangered cacti in the Chihuahuan Desert: II. Biogeography and conservation. Conserv Biol 10:1200–1209
Hooykaas PJJ, Klapwjik PM, Nuti MP, Schilperoort RA, Rörsch A (1977) Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent Agrobacteria and to Rhizobium ex planta. J Gen Microbiol 98:477–484
Jain SM, Minocha SC (2000) Molecular biology of woody plants, vol 1. Kluwer Academic Publishers, Springer Science, Dordrecht
Jofre-Garfias AE, Villegas-Sepúlveda N, Cabrera-Ponce JL, Adame-Alvarez RM, Herrera-Estrella L, Simpson J (1997) Agrobacterium-mediated transformation of Amaranthus hypochondriacus: light- and tissue-specific expression of pea chlorophyll a/b-binding protein promoter. Plant Cell Rep 16:847–852
Kao KN, Michayluk MR (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3:1222–1239
Li L, Wang J, Wang W, Lu Y, Wang Y, Zhou G, Kai G (2008) Optimization of induction and culture conditions and tropane alkaloid production in hairy roots of Anisodus acutangulus. Biotechnol Bioprocess Eng 13:606–612
Lloyd G, McCown B (1981) Commercially feasible micropropagation of Mountain Laurel, Kalmia latifolia, by use of shoot tip culture. Comb Proc Int Plant Propagator Soc 30:421–427
Mano Y, Nabeshima S, Matsui C, Ohkawa H (1986) Production of tropane alkaloids by hairy root cultures of Scopolia japonica. Agric Biol Chem 50:2715–2722
Merkli A, Christen P, Kapetanidis I (1997) Production of diosgenin by hairy root cultures of Trigonella foenum-graecum L. Plant Cell Rep 16:632–636
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nas MN, Bolek Y, Sevgin N (2013) Shortcut to long-distance developing of a tissue culture medium: micropropagation of mature almond cultivars case study. Turk J Bot 37:1134–1144
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87
Nussbaumer P, Kapétanidis I, Christen P (1998) Hairy roots of Datura candida × D. aurea: effect of culture medium composition on growth and alkaloid biosynthesis. Plant Cell Rep 17:405–409
Pavlov A, Bley T (2006) Betalains biosynthesis by Beta vulgaris L. hairy root culture in a temporary immersion cultivation system. Process Biochem 41:848–852
Santos-Díaz MS, Pérez-Molphe E, Ramírez-Malagón R, Núñez-Palenius HG, Ochoa- Alejo N (2010) Mexican threatened cacti: current status and strategies for their conservation. In: Tepper GH (ed) Species diversity and extinction. Nova Science Publishers, Inc., New York, pp 1–60
Sathyanarayana BN (2007) Plant tissue culture: practices and new experimental protocols. International Publishing House, New Delhi
Sauerwein M, Shimomura K (1991) Alkaloid production in hairy roots of Hyoscyamus albus transformed with Agrobacterium rhizogenes. Phytochemistry 30:3277–3280
Secretaría del Medio Ambiente y Recursos Naturales (2010) http://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/agenda/DOFsr/DO2454.pdf. Cited 15 Feb 2015
Sivakumar G, Yu KW, Hahn EJ, Paek KY (2005) Optimization of organic nutrients for ginseng hairy roots production in large-scale bioreactors. Curr Sci 89:641–649
Smith, M, Fitz MWA, Fitz MB, Sotomayor M (2013) Turbinicarpus lophophoroides. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. www.iucnredlist.org/. Cited 13 November 2013
Srivastava S, Srivastava AK (2007) Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol 27:29–43
Starha R, Chybidziurova A, Lacny Z (1999) Alkaloids of the genus Turbinicarpus (Cactaceae). Biochem Syst Ecol 27:839–841
Stomp AM (1992) Histochemical localization of β-glucuronidase. In: Gallagher SA (ed) GUS protocols: using the GUS gene as a reporter of gene expression. Academic, San Diego, pp 103–113
Thimmaraju R, Venkatachalam L, Bhagyalakshmi N (2008) Morphometric and biochemical characterization of red beet (Beta vulgaris L.) hairy roots obtained after single and double transformations. Plant Cell Rep 27:1039–1052
Trigiano RN, Gray DJ (2011) Plant tissue culture, development, and biotechnology. CRC Press, Boca Raton
Veena V, Taylor CG (2007) Agrobacterium rhizogenes: recent developments and promising applications. In Vitro Cell Dev Biol Plant 43:383–403
White PR (1963) The cultivation of animal and plant cells, 2nd edn. Ronald Press, New York
Wilkie S (1997) Isolation of total genomic DNA. In: Clark MS (ed) Plant molecular biology: a laboratory manual. Springer, Berlin, pp 3–14
Yonemitsu LH, Shimomura K, Satake M, Mochida S, Tanaka M, Endo T, Kaji A (1990) Lobeline production by hairy root culture of Lobelia inflata. Plant Cell Rep 9:307–310
Zhao D, Fu C, Chen Y, Ma F (2004) Transformation of Saussurea medusa for hairy roots and jaceosidin production. Plant Cell Rep 23:468–474
Zhou ML, Zhu XM, Shao JR, Tang YX, Wu YM (2011) Production and metabolic engineering of bioactive substances in plant hairy root culture. Appl Microbiol Biotechnol 90:1229–1239
Acknowledgments
We thank the Consejo Nacional de Ciencia y Tecnología (CONACYT, México) for their financial support (Project: CB-2011-01000000000168997) and for the PhD grant given to APC. We thank Bernardo Pérez Zamorano for his help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Neftali Ochoa-Alejo
Rights and permissions
About this article
Cite this article
Carlín, A.P., Tafoya, F., Alpuche Solís, A.G. et al. Effects of different culture media and conditions on biomass production of hairy root cultures in six Mexican cactus species. In Vitro Cell.Dev.Biol.-Plant 51, 332–339 (2015). https://doi.org/10.1007/s11627-015-9681-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9681-1