Abstract
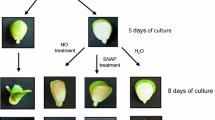
Electron transport chains (ETC) are drastically inactivated in chloroplasts of in vitro shootlets due to presence of carbon source in the growth media. This inactivation consequently creates limitations for in vitro studies on the role of chloroplasts in biotic and abiotic stresses. This research was carried out to evaluate reactivation of electron flow in chloroplasts ETC by elimination of carbon source and subsequent comparison of the effects of ETC inhibitors in the presence and absence of carbon source (i.e., sucrose) on the in vitro shootlets of apple rootstock, MM-111. All the tested ETC inhibitors, including uracil; glutaraldehyde, methyl viologen (MV) and DCMU triggered necrosis appearance on the in vitro apple shootlets exclusively on the carbon source-free media. Moreover, exposure of the in vitro shootlets to the various concentrations of ETC inhibitors demonstrated different severity of ETC inhibition by these inhibitors and their concentration-dependent effects on chloroplasts ETC. Uracil, the inhibitor of photosystem II, triggered the weakest shootlets necrosis, while DCMU exhibited no necrogenic effects at 50 mg/L and lower concentrations. MV, contrary to glutaraldehyde, did not have necrogenesis effects at 1 mg/L, but both of them caused sharp necrosis trigger on the shootlets as soon as 48–96 h after exposure to their effective concentrations. Evaluation of the shootlets H+-ATPase activity revealed that proton extrusion and pH decline in the growth media were mainly associated with the sucrose uptake by the shootlets, while no considerable pH decline observed in the presence of carbon source in the media. Finally, chloroplasts ETC activity of the shootlets was confirmed by higher percentage of O2 and less CO2 quantity in the atmospheres of the jars containing sucrose-free media, as well as by higher initial fluorescence (Fo) and maximum fluorescence (Fm) quenching parameters in the shootlets, in comparison with the sucrose-enriched condition.






Similar content being viewed by others
Abbreviations
- ETC:
-
Electron transport chain
- ROS:
-
Reactive oxygen species
- MV:
-
Methyl viologen
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- MM-111:
-
Malling-Merton 111
- QL:
-
Quoirin and Lepoivre medium
- Fo :
-
Initial fluorescence
- Fm :
-
Maximum fluorescence
References
Abdollahi H, Rugini E, Ruzzi M, Muleo R (2004) In vitro system for studying the interaction between Erwinia amylovora and genotypes of pear. Plant Cell Tissue Organ Cult 79:203–213. doi:10.1007/s11240-004-0661-0
Al-Khayri JM (2007) Date palm (Phoenix dactylifera L.) micropropagation. In: Mohan Jain S, Häggman H (eds) Protocols for micropropagation of woody trees and fruits. Springer, Dordrecht, pp 509–526
Azarabadi S, Abdollahi H, Torabi M, Salehi Z, Nasiri J (2016) ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur J Plant Pathol 147(2):279–294. doi:10.1007/s10658-016-1000-0
Balusamy SRD, Rahimi S, Sukweenadhi J, Kim YJ, Yang DC (2015) Exogenous methyl jasmonate prevents necrosis caused by mechanical wounding and increases terpenoid biosynthesis in Panax ginseng. Plant Cell Tissue Organ Cult 123(2):341–348. doi:10.1007/s11240-015-0838-8
Banerjee N, Zaitlin M (1992) Import of tobacco mosaic-virus coat protein into intact chloroplasts in vitro. Mol Plant Microbe Interact 5:466–471. doi:10.1094/MPMI-5-466
Barr R, Crane FL (2005) Inhibition or inactivation of higher plant chloroplast electron transport. In: Pessarakli M (ed) Handbook of photosynthesis. CRC Press, Boca Raton, pp 149–168
Bavaresco L, Fraschini P, Perino A (1993) Effect of the rootstock on the occurrence of lime induced chlorosis of potted Vitis vinifera L. cv. Pinot Blanc. Plant Soil 157:305–311. doi:10.1007/BF00011058
Bhattacharjee S (2011) Sites of generation and physicochemical basis of formation of reactive oxygen species in plant cell. In: Dutta Gupta S (ed) Reactive oxygen species and antioxidants in higher plants. CRC Press, Boca Raton, pp 1–30
Bhojwani SS, Dantu PK (2013) Plant tissue culture, an introductory text. Springer, New Delhi
Blanco NE, Guinea-Díaz M, Whelan J, Strand A (2014) Interaction between plastid and mitochondrial retrograde signaling pathways during changes to plastid redox status. Philos Trans R Soc Lond B 369:1–8. doi:10.1098/rstb.2013.0231
Bray L, Chriqui D, Gloux K, Le Rudulier D, Meyer M, Peduzzi J (1991) Betains and free amino acids in salt stressed vitro plants and winter resting buds of Populus trichocarpa deltoides. Physiol Plant 83:136–143. doi:10.1111/j.1399-3054.1991.tb01292.x
Capell T, Escobar C, Lui H, Burtin D, Lepri O, Christou P (1998) Overexpression of the oat arginine decarboxylase cDNA in transgenic rice (Oryza sativa L.) affects normal development patterns in vitro and results in putrescine accumulation in transgenic plants. Theor Appl Genet 97:246–254. doi:10.1007/s001220050892
Capuana M, Giannini R (1997) Micropropagation of young and adult plants of cypress (Cupressus sempervirens L.). J Hortic Sci 72:453–460. 10.1080/14620316.1997.11515533
Carvalho L, Amâncio S (2002) Effect of ex vitro conditions on growth and acquisition of autotrophic behavior during acclimatization of chestnut regenerated in vitro. Sci Hortic 95:151–164. doi:10.1016/S0304-4238(02)00037-7
Carvalho L, Esquivel MG, Amâncio S (2005) Stability and activity of Rubisco chestnut plantlets transferred to ex vitro conditions under elevated CO2. In Vitro Cell Dev Biol-Plant 41:525–531. doi:10.1079/IVP2005665
CepauskasD, Miliute I, Staniene G, Gelvonauskiene D, Stanys V, Jesaitis AJ, Baniulis D (2016) Characterization of apple NADPH oxidase genes and their expression associated with oxidative stress in shoot culture in vitro. Plant Cell Tissue Organ Cult 124:621–633. doi:10.1007/s11240-015-0920-2
Convent B, Briquet M (1978) Properties of 3-(3,4-dichlorophenyl)-1,1-dimethylurea and other inhibitors of the cytochrome bc1 segment of the mitochondrial respiratory chain in Saccharomyces cerevisiae. Eur J Biochem 82:473–481. Doi:10.1111/j.1432-1033.1978.tb12041.x
Couée I, Sulmon C, Gouesbet G, El Amrani A (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57:449–459. doi:10.1093/jxb/erj027
Dayan FE, Duke SO, Grossmann K (2010) Herbicides as probes on plant biology. Weed Sci 58:340–350. doi:10.1614/WS-09-092.1
Debergh P, Aitken-Christie J, Cohen D, Grout B, von Arnold S, Zimmerman R, Ziv M (1992) Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tissue Org Cult 30:135–140. doi:10.1007/BF00034307
Dietzel L, Steiner S, Schröter Y, Pfannschmidt T (2009) Retrograde signaling. In: Sandelius AS, Aronsson H (eds) The chloroplast, interactions with the environment. Springer, Berlin, pp 181–206
Fuentes G, Talavera C, Oropeza C, Desjardins Y, Santamaria JM (2005) Exogenous sucrose can decrease in vitro photosynthesis but improve field survival and growth of coconut (Cocos nucifera L.) in vitro plantlets. In Vitro Cell Dev Biol-Plant 41:69–76. doi:10.1079/IVP2004597
George EF, Hall MA, De Klerk GJ (2008) Plant propagation by tissue culture. Volume 1. The background, 3rd edn. Springer, Dordrecht
Gibson S (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102. doi:10.1016/j.pbi.2004.11.003
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult 126(1):1–18. doi:10.1007/s11240-016-0985-6
Gonzalez-Olmedo JL, Zaida F, Molina IA, Jihad A, Desjardins Y, Escalona M (2005) New contributions to propagation of pineapple (Ananas comosus L., Merr.) in temporary immersion bioreactors. In Vitro Cell Dev Biol-Plant 41:87–90. doi:10.1079/IVP2004603
Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou PC, Govindjee (eds) Chlorophyll a fluorescence. A signature of photosynthesis, vol 19. Advances in photosynthesis and respiration series. Springer, Dordrecht, pp 1–41
György Z, Hohtola A (2009) Production of cinnamyl glycosides in compact callus aggregate cultures of Rhodiola rosea through biotransformation of cinnamyl alcohol. In: Jain SM, Saxena PK (eds) Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants. Humana Press, Hertfordshire, pp 305–312
Hardt H, Kok B (1977) Plastocyanin as the possible site of photosynthetic electron transport inhibition by glutaraldehyde. Plant Physiol 60:225–229. doi:10.1104/pp.60.2.225
Hatzios KK, Penner D, Bell D (1980) Inhibition of photosynthetic electron transport in isolated spinach chloroplasts by two 1,3,4-thiadiazolyl derivatives. Plant Physiol 65:319–325. doi:10.1104/pp.65.2.319
Heldt HW (2005) Plant biochemistry. Elsevier Academic Press, Burlington
Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53:927–937. doi:10.1093/jexbot/53.370.927
Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J (2015) Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue Organ Cult 121:435–443. doi:10.1007/s11240-015-0714-6
Huang AHC (1987) Lipases in the biochemistry of plants: a comprehensive treatise. In: Stumpf PK (ed) Lipids: structure and function, vol 9. Academic Press, New York, pp 91–119
Huff A (1983) Nutritional control of regreening and degreening in Citrus peel segments. Plant Physiol 73:243–249
Iglesias DJ, Tadeo FR, Legaz F, Primo-Millo E, Talon M (2001) In vivo sucrose stimulation of colour change in citrus fruit epicarps: interactions between nutritional and hormonal signals. Physiol Plant 112:244–250. doi:10.1034/j.1399-3054.2001.1120213.x
Jaindl M, Popp M (2006) Cyclitols protect glutamine synthetase and malate dehydrogenase against heat induced deactivation and thermal denaturation. Biochem Biophys Res Commun 345:761–765. doi:10.1016/j.bbrc.2006.04.144
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014. doi:10.1093/emboj/19.15.4004
Kataeva NV, Alexandrova IG, Butenko RG, Dragavtceva EV (1991) Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tissue Organ Cult 27:149–154. doi:10.1007/BF00041283
Kato Y, Yamamoto Y, Murakami S, Sato F (2005) Post-translational regulation of CND41 protease activity in senescent tobacco leaves. Planta 222:643–651. doi:10.1007/s00425-005-0011-4
Komor E, Thom M, Maretzki A (1981) The mechanism of sugar uptake by sugarcane suspension cells. Planta 153:181–192. doi:10.1007/BF00384100
Kurdjian A, Guem J (1989) Intracellular pH: measurement and importance in cell activity. Ann Rev Plant Physiol Plant Mol Biol 4:271–303. doi:10.1146/annurev.pp.40.060189.001415
Lambardi M, Ozudogru EA, Mohan Jain Sh (2013) Protocols for micropropagation of selected economically-important horticultural plants. Humana Press, Hertfordshire
Leblay C, Chevreau E, Robin LM (1991) Adventitious shoot regeneration from in vitro leaves of several pear cultivar (Pyrus communis L.). Plant Cell Tissue Organ Cul 25:99–105. doi:10.1007/BF00042180
Leegood RC, Lea PJ, Adcock MD, Haeusler RD (1995) The regulation and control of photorespiration. J Exp Bot 4:397–1414
Lopez RG, Howell GS, Petrie SR (2004) Photosynthetic inhibition of ‘Seyval blanc’ grapevines with Terbacil. Acta Hortic 640:155–161. doi:10.17660/ActaHortic.2004.640.17
Martin RE, Thomas DJ, Tucker DE, Herbert SK (1997) The effects of photooxidative stress on photosystem I measured in vivo in Chlamydomonas. Plant Cell Environ 20:1451–1461. doi:10.1046/j.1365-3040.1997.d01-47.x
Masclaux C, Valadier MH, Brugiere N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211:510–518. doi:10.1007/s004250000310
Muir RM, Ibanez AM, Uratsu SL, Ingham ES, Leslie CA, McGranahan GH, Batra N, Goyal S, Joseph J, Jemmis ED, Dandekar AM (2011) Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia). Plant Mol Biol 7:555–565. doi:10.1007/s11103-011-9739-3
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998. doi:10.1093/jxb/ert208
Nasiri J, Naghavi MR, Alizadeh H, Moghadam MR, Mashouf A, Nabizadeh M (2015) Modified AHP-based decision-making model toward accurate selection of eligible maintenance media for production of taxanes in Taxus baccata callus culture. Acta Physiol Plant 37(6):110. doi:10.1007/s11738-015-1858-z
Oswald O, Martin T, Dominy PJ, Graham IA (2001) Plastid redox state and sugars: Interactive regulators of nuclear-encoded photosynthetic gene expression. Proc Natl Acad Sci USA 98:2047–2052. doi:10.1073/pnas.98.4.2047
Owen HR, Wengerd D, Miller AR (1991) Culture medium pH is influenced by basal medium, carbohydrate source, gelling agent, activated charcoal, and medium storage method. Plant Cell Rep 10:583–586. doi:10.1007/BF00232516
Perassolo M, Smith ME, Giulietti AM, Talou JR (2016) Synergistic effect of methyl jasmonate and cyclodextrins on anthraquinone accumulation in cell suspension cultures of Morinda citrifolia and Rubia tinctorum. Plant Cell Tissue Organ Cult 124(2):319–330. doi:10.1007/s11240-015-0896-y
Pérez-Tornero O, Egea J, Olmos E, Burgos L (2001) Control of hyperhydricity in micropropagated apricot cultivars. In Vitro Cell Dev Biol-Plant 37:250–254. doi:10.1007/s11627-001-0044-8
Petrouleas V, Crofts AR (2005) The iron-quinone acceptor complex. In Wydrzynski TJ, Satoh K (eds) Photosystem II, the light- driven water: plastoquinone oxidoreductase. Springer, Dordrecht, pp 177–206
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2):182. doi:10.3390/molecules21020182
Robinson SJ, Yocum CF, Ikuma H, Hayashi F (1977) Inhibition of chloroplast electron transport reactions by trifluralin and diallate. Plant Physiol 60:840–844. doi:10.1104/pp.60.6.840
Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2:198–206. doi.10.1016/S1369-5266(99)80036-3
Saltveit ME, Dilley DR (1978) Rapidly induced wound ethylene from excised segments of etiolated Pisum sativum L., cv. Alaska, I. Characterization of the response. Plant Physiol 61:447–450. doi:10.1104/pp.61.3.447
Schmitz U, Lorz H (1990) Nutrient-uptake in suspension-cultures of gramineae. 2. Suspension-cultures of rice (Oryza sativa L.). Plant Sci 66:95–111. doi:10.1016/0168-9452(90)90174-M
Tabart J, Franck T, Kevers C, Dommes J (2015) Effect of polyamines or precursors on the hyperhydricity process in micropropagated apple shoots. Plant Cell Tissue Organ Cult 120:11–18. doi:10.1007/s11240-014-0568-3
Thordal-Christensen H, Zhang Z, Wie Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194. doi:10.1046/j.1365-313X.1997.11061187.x
Tian J, Jiang F, Wu Z (2015) The apoplastic oxidative burst as a key factor of hyperhydricity in garlic plantlet in vitro. Plant Cell Tissue Organ Cult 120(2):571–584. doi:10.1007/s11240-014-0623-0
Valluri VJ (2009) Bioreactor production of secondary metabolites from cell cultures of Periwinkle and Sandalwood. In: Jain SM, Saxena PK (eds) Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants. Humana Press, Hertfordshire, pp 325–336
Van Bragt J, Pierik RLM (1971) The effect of autoclaving on the gibberellin activity of aqueous solutions containing gibberellin A3. In: van Bragt J, Mossel DAA, Pierik RLM, Veldstra H (eds) Effects of sterilization on components in nutrient media. H. Veenman & Zonen, Wageningen, pp 133–137
XiaoZ, Ji N, Zhang X, Zhang Y, Wang Y, Wu T, Xu X, Han Z (2014) The lose of juvenility elicits adventitious rooting recalcitrance in apple rootstocks. Plant Cell Tissue Organ Cult 119:51–63. doi:10.1007/s11240-014-0513-5
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58:2661–2671. doi:10.1093/jxb/erm124
Zaragoza-Martínez F, Lucho-Constantino GG, Ponce-Noyola T, Esparza-García F, Poggi-Varaldo H, Cerda-García-Rojas CM, Trejo-Tapia G, Ramos-Valdivia AC (2016) Jasmonic acid stimulates the oxidative responses and triterpene production in Jatropha curcas cell suspension cultures through mevalonate as biosynthetic precursor. Plant Cell Tissue Organ Cult 127(1):47–56. doi:10.1007/s11240-016-1028-z
Acknowledgements
This research was a collaboration project between Temperate Fruits Research Center of Horticultural Sciences Research Institute (HSRI) and Azad University of Tehran, Science and Research Unit. The authors sincerely thank all the technical stuff of fruit tissue culture and biotechnology laboratory of Temperate Fruits Research Center of Horticultural Sciences Research Institute (HSRI) for their kind supports. The funding was provided by Iranian Ministry of Agriculture, Agricultural Research Education and Extension Organization (AREEO).
Author information
Authors and Affiliations
Contributions
HA designed and supervised the study, wrote the manuscript and provided helpful discussions. KE performed experimental works of glutaraldehyde, methyl viologen (MV) and DCMU sections of the study. ZG carried out some parts of chloroplasts ETC activity by inhibitors alongside H+-ATPase activity section of the work. JN revised whole body of the paper, contributed in data analysis and provided micropropagation apples data alongside scientific information/helps about justification of activation of electron flow in chloroplast. SS participated in plant culture, chlorophyll fluorescence section, assay of O2/CO2 production and H2O2 generation. All the authors read and approved final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Henryk Flachowsky.
Rights and permissions
About this article
Cite this article
Abdollahi, H., Erfaninia, K., Ghahremani, Z. et al. Reactivation of electron flow in chloroplasts of in vitro shootlets of apple through elimination of carbon source and evaluation of its activity by inhibitors of electron transport chain and chlorophyll fluorescence quenching. Plant Cell Tiss Organ Cult 131, 377–389 (2017). https://doi.org/10.1007/s11240-017-1291-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1291-7