Abstract
Citrus, and particularly sweet oranges, are very recalcitrant to anther culture. In this paper it was evaluated for the first time the response of 27 genotypes of Citrus sinensis and of one hybrid C. clementina × C. sinensis, to in vitro anther culture. Ten genotypes of sweet oranges showed embryogenic callus induction, mostly blood sweet oranges genotypes, such as Tarocco, Moro and Sanguinelli. In vitro microspore developmental switches from the gamethophytic to the sporophytic pathway were shown by DAPI staining in microspores of these responsive genotypes, after 10 months in culture. However, microsatellite marker analyses showed that these calli were heterozygous. The flow-cytometric analysis of these embryogenic calli showed the presence of two peaks, corresponding to haploid (n) and diploid (2n) genotypes. Differently, anther cultures of the hybrid C. clementina × C. sinensis produced tri-haploid (3n) embryogenic calli and the embryos obtained were homozygous when analyzed by molecular markers (sample sequence repeats), confirming the more responsive characteristic of clementine to microspore embryogenesis through anther culture.




Similar content being viewed by others
References
Aleza P, Juárez J, Hernández M, Pina JA, Ollitrault P, Navarro L (2009) Recovery and characterization of a Citrus clementina Hort. Ex Tan. ‘Clemenules’ haploid plant selected to establish the reference whole Citrus genome sequence. BMC Plant Biol 9:110. doi:10.1186/1471-2229-9-110
Bajaj YPS (1990) In vitro production of haploids and their use in cell genetics and plant breeding. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, part I. Haploids in crop improvement, vol 12. Springer, Berlin, pp 1–44
Bárány I, González-Melendi P, Fadón B, Mitykót J, Risueño MC, Testillano PS (2005) Microspore-derived embryogenesis in pepper (Capsicum annuum L.): subcellular rearrangements through development. Biol Cell 97:709–722. doi:10.1042/BC20040142
Bausher MG, Singh ND, Lee S, Jansen RK, Daniell H (2006) The complete chloroplast genome sequence of Citrus sinensis (L.) Osbeck var ‘Ridge Pineapple’: organization and phylogenetic relationships to other angiosperms. BMC Plant Biol 6:21. doi:10.1186/1471-2229-6-21
Benelli C, Germanà MA, Ganino T, Beghe D, Fabbri A (2010) Morphological and anatomical observations of abnormal somatic embryos from anther cultures of Citrus reticulate. Biol Plant 54:224–230. doi:10.1007/s10535-010-0040-0
Bohanec B, Nešković M, Vujičić R (1993) Anther culture and androgenetic plant regeneration in buckwheat (Fagopyrum esculentum Moench). Plant Cell Tiss Organ Cult 35:259–266. doi:10.1007/BF00037279
Cabral GB, Carneiro VTC, Lacerda AL, Valle CB, Martinelli AP, Dusi DMA (2011) Somatic embryogenesis and organogenesis in apomictic and sexual Brachiaria brizantha. Plant Cell Tiss Org Cult 107:271–282. doi:10.1007/s11240-011-9978-7
Cao H, Biswas MK, Lu Y, Amar MH, Tong Z, Xu Q, Xu J, Guo W, Deng X (2011) Doubled haploid callus lines of Valencia sweet orange recovered from anther culture. Plant Cell Tiss Organ Cult 104:415–423. doi:10.1007/s11240-010-9860-z
Cardoso JC, Martinelli AP, Latado RR (2012) Somatic embryogenesis from ovaries of sweet orange cv. Tobias. Plant Cell, Tiss Organ Cult 109:171–177. doi:10.1007/s11240-011-0073-x
Chen Z, Wang H, Liao H (1980) The induction of Citrus pollen plants in artificial media. Acta Genet Sin 7:189–192
Chen J, Cui L, Malik AA, Mbira KG (2011) In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tiss Organ Cult 104:311–319. doi:10.1007/s11240-010-9874-6
Chiancone B, Tassoni A, Bagni N, Germanà MA (2006) Effect of polyamines on in vitro anther cultures of Citrus clementina Hort. ex Tan. Plant Cell Tiss Organ Cult 87:145–153. doi:10.1007/s11240-006-9149-4
Chu C (1978) The N6 medium and its applications to anther culture of cereal crops. Proc of Symp Plant Tiss Cult. Science Press, Peking, pp 43–50
Cistué L, Ramos A, Castillo L (1998) Influence of anther pretreatment and culture medium composition on the production of barley doubled haploids from model and low responding cultivars. Plant Cell Tiss Org Cult 55:159–166. doi:10.1023/A:1006130028396
Deng XX, Deng ZA, Xiao SY, Zhang WC (1992) Pollen derived plantlets from anther culture of Ichang papeda hybrids No.14 and Trifoliate orange. Proc Intern Soc Citricult 1:190–192
Dunwell JM, Sunderland N (1973) Anther culture of Solanum tuberosum L. Euphytica 22:317–323
Ferrie AMR, Möllers C (2011) Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell Tiss Organ Cult 104:375–386. doi:10.1007/s11240-010-9831-4
Ferrie AMR, Irmen KI, Beattie AD, Rossnagel BG (2013) Isolated microspore culture of oat (Avena sativa L.) for the production of double haploids: effect of pre-culture and post-culture conditions. Plant Cell Tiss Organ Cult (in press). doi:10.1007/s11240-013-0385-0
Froelicher Y, Bassene JB, Jedidi-Neji E, Dambier D, Morillon R, Bernardini G, Constantino G, Ollitraut P (2007) Induced parthenogenesis in mandarin for haploid production: induction procedures and genetic analyses of plantlets. Plant Cell Rep 26:937–944. doi:10.1007/s00299-007-0314-y
Germanà MA (2006) Double haploid production in fruit crops. Plant Cell Tiss Organ Cult 86:131–146. doi:10.1007/s11240-006-9088-0
Germanà MA (2007) Haploidy. In: Khan I (ed) Citrus. Genetics, breeding and biotechnology. CABI, Wallingford, pp 167–196
Germanà MA (2009) Haploid and doubled haploids in fruit trees. In: Touraev A, Forster B, Jain M (eds) Advances in haploid production in higher plants. Springer, New York, pp 241–263
Germanà MA (2011a) Gametic embryogenesis and haploid technology as valuable support to plant breeding. INVITED REVIEW for the special issue of Plant Cell Reports entitled: “Plant Biotechnology in support of the Millenium Development Goals” 30(5):839–857. doi:10.1007/s00299-011-1061-7
Germanà MA (2011b) Anther culture for haploid and doubled haploid production. INVITED REVIEW for the special issue: “In Vitro Ploidy Manipulation in the Genomics Era”. Plant Cell Tiss Organ Cult 104:283–300. doi:10.1007/s11240-010-9852-z
Germanà MA, Chiancone B (2001) Gynogenetic haploids of Citrus after in vitro pollination with triploid pollen grains. Plant Cell Tiss Organ Cult 66:59–66. doi:10.1023/A:1010627310808
Germanà MA, Chiancone B (2003) Improvement of Citrus clementina Hort. ex Tan. microspore-derived embryoid production and regeneration. Plant Cell Rep 22:181–187. doi:10.1007/s00299-003-0669-7
Germana’ M, Aleza P, Carrera E, Chen C, Chiancone B, Costantino G, Dambier D, Deng X, Federici TC, Froelicher Y, Guo W, Ibáñez V, Juárez J, Kwok K, Luro F, Machado AM, Naranjo AM, Navarro L, Ollitrault P, Ríos G, Roose LM, Talon M, Xu Q, Gmitter Gfgermanà M, Aleza P, Carrera E, Chen C, Chiancone B, Costantino G, Dambier D, Deng X, Federici TC, Froelicher Y, Guo W, Ibáñez V, Juárez J, Kwok K, Luro F, Machado AM, Naranjo AM, Navarro L, Ollitrault P, Ríos G, Roose LM, Talon M, Xu Q, Gmitter GF (2013). Cytological and molecular characterization of three gametoclones of Citrus clementina. BMC Plant Biol 13. ISSN:1471-2229. doi:10.1186/1471-2229-13-129
Germanà MA, Wang YY, Barbagallo MG, Iannolino G, Crescimanno FG (1994) Recovery of haploid and diploid plantlets from anther culture of Citrus clementina Hort. ex Tan. and Citrus reticulata Blanco. J Hortic Sci Biotech 69(3):473–480
Germanà MA, Chiancone B, Lain O, Testolin R (2005) Anther culture in Citrus clementina: a way to regenerate tri-haploids. Aust J Agric Res 56:839–845. doi:10.1071/AR05025
Grosser JW, Gmitter FG Jr (2011) Protoplast fusion for production of tetraploids and triploids: applications for scion and rootsctock breeding in Citrus. Plant Cell Tiss Organ Cult 104(3):343–357. doi:10.1007/s11240-010-9823-4
Heberle-Bors E (1985) In vitro haploid formation from pollen: a critical review. Theor Applied Genet 71:361–374
Hidaka T (1984) Induction of Plantlets from anthers of ‘Trovita’ orange (Citrus sinensis Osbeck). J Jpn Soc Hortic Sci 53(1):1–5
Hidaka T, Yamada Y, Shichijo T (1979) In vitro differentiation of haploid plants by anther culture in Poncirus trifoliata (L.). Jpn J Breed 29:248–254
Hidaka T, Yamada Y, Shichijo T (1982) Plantlet formation by anther culture of Citrus aurantium L. Jpn J Breed 32:247–252
Höffer M (2004) In vitro androgenesis in apple—improvement of the induction phase. Plant Cell Rep 22:365–370. doi:10.1007/s00299-003-0701-y
Höffer M, Touraev A, Heberle-Bors E (1999) Induction of embryogenesis from isolated apple microspore. Plant Cell Rep 18:1012–1017. doi:10.1007/s002990050700
Johansson L (1986) Improved methods for induction of embryogenesis in anther cultures of Solanum tuberosum. Potato Res 29:179–190
Kasha KJ, Hu TC, Oro R, Simion E, Shim YS (2001) Nuclear fusion leads to chromossome doubling during mannitol pretreatment of barley (Hordeum vulgare L.) microspores. J Expt Bot 52:1227–1238. doi:10.1093/jexbot/52.359.1227
Luckett DJ, Smithard RA (1995) A comparison of several published methods for barley anther culture. Plant Cell Rep 14:763–767. doi:10.1007/BF00232918
Murashige T, Skoog F (1962) A revised médium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163(85–87):1969
Novelli VM, Cristofani M, Souza AA, Machado MA (2006) Development and characterization of polymorphic microsatellite markers for sweet orange (Citrus sinensis L. Osbeck). Genet Mol Biol 29:90–96. doi:10.1590/S1415-47572006000100018
Palmieri DA, Novelli VM, Bastianel N, Cristofani-Yali M, Astúa-Monge G, Carlos EF, Oliveira AC, Machado MA (2007) Frequency and distribution of microsatellites from ESTs of citrus. Gen Mol Biol 30:1009–1018. doi:10.1590/S1415-47572007000500029
Parra-Vega V, Renau-Morata B, Sifres A, Seguí-Simarro JM (2013) Stress treatments and in vitro culture conditions influence microspore embryogenesis and growth of callus from anther walls of sweet pepper (Capsicum annuum L.). Plant Cell Tiss Organ Cult 112:353–360. doi:10.1007/s11240-012-0242-6
Pintos P, Manzanera JA, Bueno MA (2007) Antimitotic agents increase the production of double-haploid embryos from cork oak anther culture. J Plant Physiol 164:1595–1604. doi:10.1016/j.jplph.2006.11.012
Prem D, Solís MT, Bárány I, Rodríguez-Sanz H, Risueño MC, Testillano OS (2012) A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates double-haploid plants in Brassica napus. BMC Plant Biol 12:127. doi:10.1186/1471-2229-12-127
Ramírez C, Chiancone B, Testillano P, García-Fojeda B, Germanà MA, Risueño MC (2003) First embryogenic stages of Citrus microspore-derived embryos. Acta Biol Cracov 45:53–58
Rudolf K, Bohanec B, Hansen M (1999) Microspore culture of white cabbage, Brassica oleracea var. capitata L.: genetic improvement of non-responsive cultivars and effect of genome doubling agents. Plant Breed 118:237–241. doi:10.1046/j.1439-0523.1999.118003237.x
Seguí-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol Plant 134:1–12. doi:10.1111/j.1399-3054.2008.01113.x
Seguí-Simarro JM, Corral-Martínez P, Parra-Vega V, González-García B (2011) Androgenesis in recalcitrant solanaceous crops. Plant Cell Rep 30:765–778. doi:10.1007/s00299-010-0984-8
Smykal P (2000) Pollen embryogenesis—the stress mediated switch from gametophytic to sporophytic development. Current status and future prospects. Biol Plant 43:481–489. doi:10.1023/A:1002835330799
Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996) Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex Plant Reprod 9:209–215. doi:10.1007/BF02173100
Wedzony M, Forster BP, Zur I, Golemiec M, Szechynska-Hebda E, Gotebiowska DG (2009) Progress in doubled haploid technology in higher plants. In: Touraev A, Forster BP, Jain SM (eds) Advances in haploid production in higher plants. Springer, New York, pp 1–18
Acknowledgments
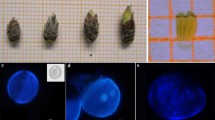
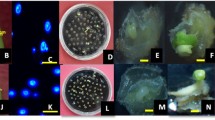
JCC and RRL thank to FAPESP for doctoral scholarship (2008/09928-8) and for financial support of the project (2008/10.203-8), and APM acknowledges CNPq (310.612/2011-0) for the research fellowship. The authors also thank the biologist Mônica Lanzoni Rossi (CENA/USP) for assistance processing the samples for anther microscopic analysis (Fig. 2b) presented in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cardoso, J.C., Martinelli, A.P., Germanà, M.A. et al. In vitro anther culture of sweet orange (Citrus sinensis L. Osbeck) genotypes and of a C. clementina × C. sinensis ‘Hamlin’ hybrid. Plant Cell Tiss Organ Cult 117, 455–464 (2014). https://doi.org/10.1007/s11240-014-0456-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0456-x