Abstract
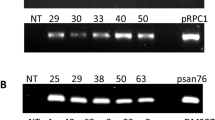
Soybean (Glycine max L.) cultivar NARC-4 was transformed with constructs carrying rolA, rolB, or rolC genes, each under the control of the Cauliflower Mosaic Virus 70S promoter. Cotyledonary nodes of soybean seeds were infected with Agrobacterium tumefaciens strain LBA4404 carrying one of the three rol genes, along with nptII in the plasmid pLBR. The efficiency of the transformation varied slightly among the three constructs, with frequencies of 6, 7, and 5% for the rolA, rolB, and rolC genes, respectively, being observed. Southern blot analysis confirmed the integration of rol genes in the soybean genome with varying numbers of copies of the transgene. All transformed plants showed enhanced rooting, but the number of adventitious roots was higher for transformants carrying the rolB transgene. rolA and rolC transformants showed dwarf phenotypes, clustered branching, and variations in leaf morphology. Furthermore, these plants flowered within a short period of time and produced lower numbers of flowers. rolB transformants showed variations in phenotype, including dwarf to semi-dwarf and shrubby growth, abnormal stem growth, short internodes, variations in leaf morphology, and greenish to yellowish-green colored leaves. These plants also flowered early, but dwarf plants produced low numbers of flowers, while shrubby plants produced high numbers of flowers, but these were mostly infertile.






Similar content being viewed by others
Abbreviations
- BA:
-
6-benzyladenine
- CaMV:
-
Cauliflower mosaic virus
- DTT:
-
Dithiothreitol
- GA3:
-
Gibberellic acid
- IBA:
-
Indol 3-butyric acid
- MES:
-
Morpholino ethanesulfonic acid
- MYA:
-
Mannitol yeast agar
- NOS:
-
Nopaline synthase
- nptII :
-
Neomycin phosphotransferase
- ORF:
-
Open reading frames
- PCR:
-
Polymerase chain reaction
- Ri:
-
Root inducing
- SE:
-
Shoot elongation
- SI:
-
Shoot induction
- T-DNA:
-
Transfer DNA
References
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223:315–328
Alpizar E, Dechamp E, Lapeyre-Montes F, Guilhaumon C, Bertrand B, Jourdan C, Lashermes P, Etienne H (2008) Agrobacterium rhizogenes-transformed roots of coffee (Coffea arabica): conditions for long-term proliferation, and morphological and molecular characterization. Ann Bot 101:929–940
Altamura MM (2004) Agrobacterium rhizogenes rolB and rolD genes: regulation and involvement in plant development. Plant Cell Tissue Organ Cult 77:89–101
Altamura MM, Tomassi M (1998) Auxin, photoperiod and putrescine affect flower neoformation in normal and rolB-transformed tobacco thin cell layers. Plant Physiol Biochem 36:441–448
Bettini P, Baraldi R, Rapparini F, Melani L, Mauro ML, Bindi D, Buiatti M (2010) The insertion of the Agrobacterium rhizogenes rolC gene in tomato (Solanum lycopersicum L.) affects plant architecture and endogenous auxin and abscisic acid levels. Sci Hortic 123:323–328
Boote KJ, Jones JW, Batchelor WD, Nafziger ED, Myers O (2003) Genetic coefficients in the CROPGRO-Soybean model: links to field performance and genomics. Agron J 95:32–51
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Bulgakov VP, Veselova MV, Tchernoded GK, Kiselev KV, Fedoreyev SA, Zhuravlev YN (2005) Inhibitory effect of the Agrobacterium rhizogenes rolC gene on rabdosiin and rosmarinic acid production in Eritrichium sericeum and Lithospermum erythrorhizon transformed cell cultures. Planta 221:471–478
Carmi N, Salts Y, Dedicova B, Shabtai S, Barg R (2003) Induction of parthenocarpy in tomato via specific expression of the rolB gene in the ovary. Planta 217:726–735
Casanova E, Valdés AE, Zuker A, Fernández B, Vainstein A, Trillas MI, Moysset L (2004) rolC-transgenic carnation plants: adventitious organogenesis and levels of endogenous auxin and cytokinins. Plant Sci 167:551–560
Casanova E, Trillas MI, Moysset L, Vainstein A (2005) Influence of rol genes in floriculture. Biotechnol Adv 23:3–39
Chriqui D, Guivarc’h A, Dewitte W, Prinsen E, van Onkelen H (1996) rol genes and root initiation and development. Plant Soil 187:47–55
Christey MC (2001) Use of Ri-mediated transformation for production of transgenic plants. In Vitro Cell Dev Biol Plant 37:687–700
Crane C, Wright E, Dixon RA, Wang ZY (2006) Transgenic Medicago truncatula plants obtained from Agrobacterium tumefaciens-transformed roots and Agrobacterium rhizogenes-transformed hairy roots. Planta 223:1344–1354
Cui M, Takayanagi K, Kamada H, Nishimura S, Handa T (2001) Efficient shoot regeneration from hairy roots of Antirrhinum majus L. transformed by the rol type MAT vector system. Plant Cell Rep 20:55–59
Dehio C, Grossmann K, Schell J, Schmülling T (1993) Phenotype and hormonal status of transgenic tobacco plants overexpressing the rolA gene of Agrobacterium rhizogenes T-DNA. Plant Mol Biol 23:1199–1210
de Klerk GJ, van der Krieken W, de Jong JC (1999) Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant 35(3):189–199
Donaldson PA, Simmonds DH (2000) Susceptibility to Agrobacterium tumefaciens and cotyledonary node transformation in short-season soybean. Plant Cell Rep 19:478–484
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Estruch JJ, Chriqui D, Grossmann K, Schell J, Spena A (1991) The plant oncogene rolC is responsible for the release of cytokinins from glucoside conjugates. EMBO J 10:2889–2895
Filippini F, Lo Schiavo F, Terzi M, Costantino P, Trovato M (1994) The plant oncogene rolB alters binding of auxin to plant cell membranes. Plant Cell Physiol 35:767–771
Filippini F, Rossi V, Marin O, Trovato M, Costantino P, Downey PM, Lo Schiavo F, Terzi M (1996) A plant oncogene as a phosphatase. Nature 379:499–500
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
García-Sogo B, Pineda B, Castelblanque L, Antón T, Medina M, Roque E, Torresi C, Beltrán JP, Moreno V, Cañas LA (2010) Efficient transformation of Kalanchoe blossfeldiana and production of male-sterile plants by engineered anther ablation. Plant Cell Rep 29:61–77
Giovannini A, Zottini M, Morreale G, Spena A, Allavena A (1999) Ornamental traits modification by rol genes in Osteospermum ecklonis transformed with Agrobacterium tumefaciens. In Vitro Cell Dev Biol 35:70–75
Godo T, Tsujii O, Ishikawa K, Mii M (1997) Fertile transgenic plants of Nierembergia scoparia Sendtner obtained by a mikimopine type strain of Agrobacterium rhizogenes. Sci Hortic 68:101–111
Hinchee MAW, Connor-Ward DV, Newell CA, McDonnell RE, Sato SJ, Gasser CS, Fischhoff DA, Re DB, Fraley RT, Horsch RB (1988) Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Nat Biotechnol 6:915–922
Kiselev KV, Dubrovina AS, Veselova MV, Bulgakov VP, Fedoreyev SA, Zhuravlev YN (2007) The rolB gene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J Biotechnol 128:681–692
Koperdáková J, Komarovská H, Košuth J, Giovannini A, Čellárová E (2009) Characterization of hairy root-phenotype in transgenic Hypericum perforatum L. clones. Acta Physiol Plant 31:351–358
Kubo T, Tsuro M, Tsukimori A, Shizukawa Y, Takemoto T, Inaba K, Shiozaki S (2006) Morphological and physiological changes in transgenic Chrysanthemum morifolium Ramat. ‘Ogura-nishiki’ with rolC. J Jpn Soc Hortic Sci 75:312–317
Kumar M, Shukla AK, Singh H, Tuli R (2009) Development of insect resistant transgenic cotton lines expressing cry1EC gene from an insect bite and wound inducible promoter. J Biotechnol 140:143–148
Liu SJ, Wei ZM, Huang JQ (2008) The effect of co-cultivation and selection parameters on Agrobacterium-mediated transformation of Chinese soybean varieties. Plant Cell Rep 27:489–498
Maghuly F, Khan MA, Fernandez EB, Druart P, Watillon B, Laimer M (2008) Stress regulated expression of the GUS-marker gene (uidA) under the control of plant calmodulin and viral 35S promoters in a model fruit tree rootstock: Prunus incisa × serrula. J Biotechnol 135:105–116
Maurel C, Barbier-Brygoo H, Spena A, Tempé J, Guern J (1991) Single rol genes from the Agrobacterium rhizogenes TL-DNA alter some of the cellular responses to auxin in Nicotiana tabacum. Plant Physiol 97:212–216
McKenzie MA, Cress WA (1992) The evaluation of South African cultivars of soybean for their susceptibility to Agrobacterium tumefaciens and the production of transgenic soybean. S Afr J Sci 88:193–196
Mishiba K, Nishihara M, Abe Y, Nakatsuka T, Kawamura H, Kodama K, Takesawa T, Abe J, Yamamura S (2006) Production of dwarf potted gentian using wild-type Agrobacterium rhizogenes. Plant Biotechnol 23:33–38
Murashige T, Skoog FA (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Olhoft PM, Flagel LE, Donovan CM, Somers DA (2003) Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216:723–735
Ouartsi A, Clérot D, Meyer A, Dessaux Y, Brevet J, Bonfill M (2004) The T-DNA ORF8 of the cucumopine-type Agrobacterium rhizogenes Ri plasmid is involved in auxin response in transgenic tobacco. Plant Sci 166:557–567
Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K (2006) Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep 25:206–213
Piispanen R, Aronen T, Chen X, Saranpää P, Häggman H (2003) Silver birch (Betula pendula) plants with aux and rol genes show consistent changes in morphology, xylem structure and chemistry. Tree Physiol 23:721–733
Rao SS, Mamadou L, McConnell M, Polisetty R, Kwanyuen P, Hildebrand D (2009) Non-antibiotic selection systems for soybean somatic embryos: the lysine analog aminoethyl-cysteine as a selection agent. BMC Biotechnol 9:94
Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12:507–518
Ross JJ, O’Neill DP, Wolbang CM, Symons GM, Reid JB (2001) Auxin-gibberellin interactions and their role in plant growth. J Plant Growth Regul 20:336–353
Schmülling T, Röhrig H, Pilz S, Walden R, Schell J (1993) Restoration of fertility by antisense RNA in genetically engineered male sterile tobacco plants. Mol Gen Genet 237:385–394
Sharma R, Mahla HR, Mohapatra T, Bhargava SC, Sharma MM (2003) Isolating plant genomic DNA without liquid nitrogen. Plant Mol Biol Report 21:43–50
Srivastava S, Srivastava AK (2007) Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol 27:29–43
Sun LY, Monneuse MO, Martin-Tanguy J, Tepfer D (1991) Changes in flowering and the accumulation of polyamines and hydroxycinnamic acid-polyamine conjugates in tobacco plants transformed by the rolA locus from the Ri TL-DNA of Agrobacterium rhizogenes. Plant Sci 80:145–156
Tanaka N, Fujikawa Y, Aly MAM, Saneoka H, Fujita K, Yamashita I (2001) Proliferation and rol gene expression in hairy root lines of Egyptian clover. Plant Cell Tissue Organ Cult 66:175–182
Tepfer D, Tempé J (1981) Production d’agropine par des raciness formees sous l’action d’Agrobacterium rhizogenes. CR Acad Sci Paris 292:153–156
Trick HN, Finer JJ (1997) SAAT: sonication-assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–336
Tsuro M, Kubo T, Shizukawa Y, Takemoto T, Inaba K (2005) Agrobacterium rhizogenes is a useful transporter for introducing T-DNA of the binary plasmid into the chrysanthemum, Dendranthema grandiflorum Kitamura, genome. Plant Cell Tissue Organ Cult 81:175–181
van der Salm TPM, Hänish ten Cate CH, Dons HJM (1996) Prospects for applications of rol genes for crop improvement. Plant Mol Biol Rep 14:207–228
van Altvorst AC, Bino RJ, van Dijk AJ, Lamers AMJ, Lindhout WH, van der Mark F, Dons JJM (1992) Effects of the introduction of Agrobacterium rhizogenes rol genes on tomato plant and flower development. Plant Sci 83:77–85
Veena V, Taylor CG (2007) Agrobacterium rhizogenes: recent developments and promising applications. In Vitro Cell Dev Biol Plant 43:383–403
von Schweinichen C, Büttner M (2005) Expression of a plant cell wall invertase in roots of Arabidopsis leads to early flowering and an increase in whole plant biomass. Plant Biol 7:469–475
Wang GL, Fang HJ (1998) Plant genetic engineering: principle and technique (in Chinese). Science Press, Beijing
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW (1985) Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164:33–44
Xue RG, Xie HF, Zhang B (2006) A multi-needle-assisted transformation of soybean cotyledonary node cells. Biotechnol Lett 28:1551–1557
Yan B, Srinivasa Reddy MS, Collins GB, Dinkins RD (2000) Agrobacterium tumefaciens-mediated transformation of soybean [Glycine max (L.) Merrill] using immature zygotic cotyledon explants. Plant Cell Rep 19:1090–1097
Yi XP, Yu DY (2006) Transformation of multiple soybean cultivars by infecting cotyledonary-node with Agrobacterium tumefaciens. Afr J Biotechnol 5:1989–1993
Zhang Z, Sun AJ, Cong Y, Sheng BC, Yao QH, Cheng ZM (2006) Agrobacterium-mediated transformation of the apple rootstock Malus micromalus Makino with the RolC gene. In Vitro Cell Dev Biol Plant 42:491–497
Zia M, Riaz-ur-Rehman, Rizvi ZF, Chaudhary MF (2010a) Agrobacterium-mediated transformation of soybean (Glycine max L.): some conditions for standardization. Pak J Bot 42 (in press)
Zia M, Rizvi ZF, Riaz-ur-Rehman, Chaudhary MF (2010b) Micropropagation of two Pakistani soybean (Glycine max L.) cultivars from mature seed cotyledonary nodes. Span J Agric Res 8:448–453
Acknowledgments
We are thankful to Dr. David Tepfer, Institut National de la Recherche Agronomique, Versailles, 78026, France, for providing the Agrobacterium tumefaciens strain LBA4404 harboring pLBR containing rol genes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zia, M., Mirza, B., Malik, S.A. et al. Expression of rol genes in transgenic soybean (Glycine max L.) leads to changes in plant phenotype, leaf morphology, and flowering time. Plant Cell Tiss Organ Cult 103, 227–236 (2010). https://doi.org/10.1007/s11240-010-9771-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9771-z