Abstract
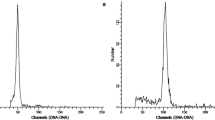
In vitro chromosome doubling was induced in octoploid (2n = 58) yacon using oryzalin and colchicine as mitotic spindle inhibitors. Nodal segments of in vitro cultured plants, 5–15 mm long, were exposed to 20, 25, or 30 μM oryzalin and 1, 3, or 5 mM colchicine for 24 or 48 h. The resulting ploidy level was determined by chromosome counting and flow cytometry. Out of 240 nodal segments, 3.33% hexadecaploid (2n = 116) plants were regenerated after the application of oryzalin. The greatest proportions of hexadecaploid plants (1.6%) were obtained after 48 h of 25 μM oryzalin treatment. With the colchicine treatment, only 0.42% hexadecaploid plants were detected and their survival rate was significantly lower in comparison with the oryzalin treatment. In hexadecaploid yacon, significantly higher levels of saccharides were detected (FOS 13.9 g/100 g FM, fructose 4.6 g/100 g FM and glucose 2.1 g/100 g FM) compared to the octoploid control (FOS 5.3 g/100 g FM, fructose 2.9 g/100 g FM and glucose 1.0 g/100 g FM). These results indicate that in vitro treatment of nodal segments with oryzalin solution could be an effective procedure for chromosome doubling and the polyploidy breeding can help to increase the FOS content in the tuberous roots.


Similar content being viewed by others
Abbreviations
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DMSO:
-
Dimethyl sulfoxide
- FOS:
-
Fructooligosaccharides
- MS:
-
Murashigue and Skoog (1962)
- RH:
-
Relative humidity
References
Allum JF, Bringloe DH, Roberts AV (2007) Chromosome doubling in a Rosa rugosa Thunb. Hybrid by exposure of in vitro nodes to oryzalin: effects of node length, oryzalin concentration and exposure time. Plant Cell Rep 26:1984–1997. doi:10.1007/s00299-007-0411-y
Awoleye F, Duren van M, Dolezel J, Novak FJ (1994) Nuclear DNA content and in vitro induced somatic polyploidization cassava (Manihot esculenta Crantz) breeding. Euphytica 76:195–202
Bouvier L, Guérif P, Djulbic M, Durel CE, Chevreau E, Lespinasse Y (2002) Chromosome doubling of pear haploid plants and homozygosity assessment using isozyme and microsatellite markers. Euphytica 123:255–262. doi:10.1023/A:1014998019674
Dolezel J, Binarová P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31:113–120. doi:10.1007/BF02907241
Duren van M, Morpurgo R, Dolezel J, Afza R (1996) Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica 88:25–34. doi:10.1007/BF00029262
Fernández CE, Kucera L (1997) Determination of number of chromosomes in select ecotypes of yacon (Polymnia sonchifolia Poeppig and Endlicher) cultivated in vitro. Agricultura tropica et subtropica 30:89–93
Frcek J, Greplová M, Rejlková M, Kopecký D, Záskodová J, Bartos J, Rychtarová J, Vagera J, Dolezel J (2002) Production of polyploids, haploids and polyhaploids for interspecific hybridization in the genus Solanum. In the GILB′02 conference “Late blight: managing the global threat”, Hamburg. Book of Abstracts: 30
Frías AM, Grau A, Lozzia E, Caro S (1997) Estudio citológico del yacón (Polymnia sonchifolia) y el yacón del campo (Polymnia macroscypha). 28 Congreso Argentino de Genética, Tucumán, Argentina
Grau A, Rea J (1997) Yacón—Smallanthus sonchifolius (Poepp. & Endl.) H. Robbinson. In: Hermann M, Heller J (eds) Andean roots and tubers: ahipa, arracacha, maca and yacon. Promoting the conservation and use of underutilized and neglected crops. 21. Institute of Plant Genetic and Crop Plant Research, Gatersleben/International Plant genetic Resources Institute, Rome, Italy, pp 199–242
Greplová M, Frcek J, Rejlková M, Kopecký D, Vagera J, Dolezel J (2003) In vitro polyploidisation of wild potato species. Scientific report. Potato Research Institute Havlíckuv Brod, Czech Republic, 14, pp 55–63
Heinz DJ, Mee GWP (1970) Colchicine-induced polyploids from cell suspension cultures of sugarcane. Crop Sci 10(6):696–699
Heiser C (1963) Numeración cromosómica de plantas ecuatorianas. Ciencia y Naturaleza 6:2–6
Hermann M, Freire I, Pazos C (1999) Compositional diversity of the yacon storage root. CIP Program Report 1997–1998, Lima, Peru, pp 425–432
Hermsen JG, Rananna MS, Roest S, Bockelmann GS (1981) Chromosome doubling through adventitious shoot formation on in vitro cultivated leaf explant from diploid interspecific potato hybrids. Euphytica 30(2):239–246. doi:10.1007/BF00033983
Ishiki K, Salgado MVX, Arellano J (1997) Revision of chromosome number and karyotype of yacon (Polymnia sonchifolia) cultivated in South America. Resúmenes del Primer taller Internacional sobre Recursos fitogenéticos del Noreste Argentino. INTA, Salta, Argentina, p 7
León J (1964) Plantas alimenticias andinas (Andean food crops). Instituto Interamericano de Ciencias Agrícolas Zona Andina. Lima–Perú. Boletín técnico 6:57–62
Leong-Skornicková J, Sída O, Jarolímová V, Sabu M, Fér T, Trávnícek P, Suda J (2007) Chromosome numbers and genome size variation in Indian species of Curcuma (Zingiberaceae). Ann Bot (Lond) 100(3):505–526. doi:10.1093/aob/mcm144
Lysák M, Dolezel J (1998) Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 81(2):123–132
Murashigue T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nieto CC (1991) Estudios agronómicos y bromatológicos en jícama (Polymnia sonchifolia Poeppig & Endlicher) (Agronomic and bromatologic studies of jícama). Instituto Nacional de Investigaciones Agropecuarias, Quito—Ecuador. Arch Latinoam Nutr 41(2):213–221
Petersen KK, Hagberg P, Kristiansen K (2003) Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult 73:137–146. doi:10.1023/A:10223854303371
Rodriguez Portela de Carvalho JF, de Carvalho CR, Campos OW (2005) In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tissue Organ Cult 80:69–75. doi:10.1007/s11240-004-8833-5
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro induction of polyploidy in japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84:263–268. doi:10.1007/s11240-005-9029-3
Talledo D, Escobar M (1996) Caracterización cariotípica de germoplasma RTA (Karyotypic characterization of germplasm of Andean root and tuber crops). In: Hermann M, Heller J (1997) (eds) Andean roots and tubers: ahipa, arracacha, maca and yacon. Promoting the conservation and use of underutilized and neglected crops. 21. Institute of Plant Genetic and Crop Plant Research, Gatersleben/International Plant genetic Resources Institute, Rome, Italy, pp 199–242
Tosca A, Pandolfi F, Citterio S, Fasoli A, Sgorbati S (1995) Determination by flow cytomery of the chromosome doubling capacity of colchicine and oryzalin in gynogenetic haploids of Gerbera. Plant Cell Rep 14:455–458. doi:10.1007/BF00234054
Väinölä A (2000) Polyploidization and early screening of Rhododendron hybrids. Euphytica 112(3):239–244. doi:10.1023/A:1003994800440
Acknowledgments
This work was supported by the Grants GI 51110/1312/513122 and GI 51110/1312/513108 of the Institute of Tropics and Subtropics, Czech University of Life Sciences Prague. The authors thank Jan Suda and Pavel Travnícek for their help in the flow-cytometric analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viehmannová, I., Cusimamani, E.F., Bechyne, M. et al. In vitro induction of polyploidy in yacon (Smallanthus sonchifolius). Plant Cell Tiss Organ Cult 97, 21–25 (2009). https://doi.org/10.1007/s11240-008-9494-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9494-6