Abstract
The problem of aromaticity in heterocyclic rings of uracil and its 5-halogenoderivatives (5XU) was analyzed theoretically by calculating modified harmonic oscillator model of aromaticity (HOMA) for Heterocycle Electron Delocalization (HOMHED), nucleus-independent chemical shift parameters (NICS) and the so-called scan experiments, using helium-3 atom as a magnetic probe. The impact of halogen electronegativity on C5 atom’s NBO charges was also investigated. Water, as a polar environment, has a negligible impact on 5XU aromaticity. The most stable diketo tautomer shows a very low aromaticity while the “rare” dihydroxy form (tautomer No 6) is aromatic and resembles benzene. This is in agreement with traditional drawing of chemical formula of uracil’s six-membered ring, directly showing three alternating single and double bonds in its tautomer No 6. No good correlation between magnetic and geometric indexes of aromaticity for the studied 5XU tautomers was found. Linear correlation between the magnitude of NICS minimum, as well as the distance of the minimum above uracil ring plane center from 3He NMR chemical shift scan plot with respect to halogen electronegativity were observed. A strong linear dependence of magnetic index of aromaticity and the electronegativity of 5X substituent was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uracil is an important component of living systems and belongs to small molecules which are responsible for information transfer [1]. Its structure could be derived from pyrimidine (1,3-diazine). Detection of the occurrence of uracil and pyrimidine in space [2] indirectly supports the fact that such molecules, containing two nitrogen atoms in a six-membered ring are stable and present in both extraterrestrial space matter and in living systems. For over 50 years, its 5-halogeno derivatives have been studied [3, 4]. Among them, 5-fluorouracil has been widely used as an anticancer drug [3, 4].
The stability of uracil and its derivatives reflect their specific structure. Recently, on the basis of theoretical modeling using density functional theory (DFT at B3LYP-D3/aug-cc-pVQZ level of theory), it was postulated [5] that mutual arrangement of local C=O and N-H functional groups in uracil, as well as in 1,3-diazines, influences the electron density and local dipole moments distribution in such a way that it is energetically more favorable than in both 1,2- and 1,4-diazines.
Stability of many organic molecules is often related to aromaticity [6,7,8,9,10]. Among them are cyclic organic compounds, with double bonds and heteroatoms with lone electron pairs included in their structure, which could be aromatic. The concept of aromaticity is based on equilibration of planar ring bond lengths due to the presence of shared and delocalized electrons. The number of delocalized electrons could be determined according to Hückel’s formula 4n + 2, where n = 1 for benzene. There are two commonly used theoretical indexes of aromaticity, the geometric one (HOMA, and closely related parameters, derived from structural dimensions [11,12,13,14]) and magnetic [7, 9, 15, 16] (NICS). For benzene, HOMA is close to 1. HOMHED is a reparametrized version of HOMA and is dedicated to heterocyclic molecules. Magnetic indexes of aromaticity are usually calculated in the middle of ring as NICS(0), 1 Å above that point, NICS(1), or its zz-component, NICS(1)zz. Negative values of NICS are calculated for aromatic compounds while positive for anti-aromatic molecular systems. Another magnetic probe of aromaticity is nuclear shielding and chemical shift of helium-3 atom [17,18,19,20,21]. Studies of aromaticity using 3He are not so popular but they possess one advantage: in principle the predicted 3He NMR chemical shifts could be verified by experiment [17, 18, 22].
For example, a good agreement was observed between a position of a narrow resonance signal in a 3He NMR spectra of He@C60, as well as for other fullerenes and theoretically predicted isotropic value of helium-3 nuclear magnetic shieldings, converted to chemical shift [20, 21, 23,24,25]. In many experimental studies, the fullerene cage size, symmetry, and aromaticity in the presence of the encapsulated 3He atom [20, 21, 23, 24] was studied by noble gas NMR technique. In such works, the 3He atom was treated as another, independent magnetic probe of aromaticity.
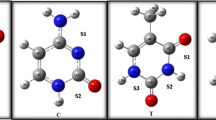
In Fig. 1 are presented six optimized tautomeric structures of 5-fluorouracil, which is the most important 5-halogeno derivative of uracil. The structures are labeled with the commonly used abbreviations [5]. From the six potential uracil and its 5XU tautomers [26,27,28,29,30,31,32], the diketo form (tautomer No 1 in Fig. 1) is the most stable, as verified by experiment and theoretical calculations. On the other hand, it is possible to guess that tautomer No 6 should be the most aromatic one [5]. This tautomer is formed by three alternating single and double bonds, respectively. However, it is difficult “to guess” quantitatively the amount of aromaticity for tautomers 1 and 6 from the formulas, presented in Fig. 1, only. Besides, the nature of 5X substituent should also alter the aromaticity of the corresponding uracil derivatives. Moreover, the magnitude of magnetic shielding in the vicinity of uracil plane is related to the degree of electron delocalization and it should change perpendicularly to the plane with the distance R from the ring center. These phenomena could be modeled by so-called scanning experiment based on calculation of NICS(R) value as function of varied distance R [16, 33, 34] or by the change of 3He isotropic nuclear magnetic shielding σR, often converted to chemical shift δR, when moving the helium probe by a distance R above the ring center [17, 18].
Recently, the aromaticity of acenes was correlated with the HOMO-LUMO energy gap Eg [22]. In case of 5XU, by changing the electron-donating properties of X substituent, the magnitude of Eg and electron delocalization within the uracil plane should also vary [5]. Thus, by changing the halogen substituent one could expect a direct impact on Eg, as well as on the aromaticity of 5-halogenouracils. However, the above mentioned problems were not discussed explicitly in the literature [5] or used to explain some structural and spectroscopic properties of 5XU molecules.
The aim of the current study is to characterize the aromaticity of uracil and its 5X-derivatives using geometric index of aromaticity HOMHED [35]. Additionally, a profile of magnetic field above the uracil ring will be modeled in separate scan experiments for NICS indexes and helium-3 chemical shift. The electron-donating or electron-accepting nature of X substituent could also influence the magnitude of charge at C5 atom, as well as ring aromaticity. Thus, the changes of charge at C5 and X atoms will be studied using natural bond orbital [36] concept (NBO). Finally, we will look for same correlations between uracil HOMO/LUMO gap, electronegativity of halogen substituent and aromaticity. Since solvent affects aromaticity indexes very little, we will model our compounds in vacuum, and only in some cases compare the results to those, obtained with the presence of water, incorporated by polarized continuum model (PCM) [37].
Computational part
All calculations were performed with Gaussian 16 program [38]. In this study, we selected a popular and fairly reliable B3LYP [39,40,41] hybrid density functional improved by inclusion of dispersion interactions via added Grimme’s GD3 term [42], for modeling of uracil and its four 5-halogeno derivatives in vacuum and in the condense phase. The latter calculations were conducted using a polar surrounding (water) approximately described by PCM model [37]. The presence of all positive harmonic frequencies confirmed that the calculated structures were the true energy minima. Large Dunning-type correlation-consistent valence basis set of quadruple–zeta quality, augmented with a diffuse function (aug-cc-pVQZ) [43] was selected for better characterization of multiple bonds and lone electron pairs. Due to the problems with SCF convergence and optimization of 5-iodouracil tautomers, calculated with larger basis sets, we tested several combinations of available basis sets. Finally, all tautomers of 5 IU were optimized using 6-31 + G(d) for C, N, O, H, and 6-311G for I. We also managed to optimize the 5 IU1 and 5 IU6 tautomers with two larger basis sets, aug-cc-pVQZ for C, H, N, and O, and aug-cc-pVDZ-PP for I. The latter basis set was downloaded from EMSL basis set exchange [44, 45]. Magnetic index of aromaticity NICS [7, 9] was used to determine the aromaticity of the studied tautomers and monohalogenoderivatives of benzene as reference. In addition, geometric indexes of aromaticity, HOMA [12,13,14] and HOMHED [35], were calculated. NBO charges [36] were calculated at optimized geometries. Gauge including atomic orbital [46, 47] (GIAO) NMR calculations of NICS parameters and 3He isotropic nuclear magnetic shieldings at the ring center and above, at distance of 1 Å and R, from tautomers 1 and 6 of uracil and 5XU were also performed at the B3LYP/aug-cc-pVQZ level of theory (only for 5 IU, a previously mentioned 6-31 + G(d) for C, N, O, H, and 6-311G for I basis sets were used). The distance R from the middle of rings was varied from 0 to 2 Å with steps of 0.05 Å, from 2 to 5 Å with step of 0.5 Å and from 5 to 10 Å with steps of 1 Å. In particular, in agreement with our earlier studies [17, 18], the theoretical chemical shifts of 3He in the gas phase (δHe) were obtained using the isotropic value of free probe atom nuclear magnetic shielding (σo) as reference and calculating its shielding σR at a distance R from the middle of ring, according to the following formula
At that point, we want to stress that the geometry of uracil and 5XU derivatives was optimized at B3LYPD3/aug-cc-pVQZ level of theory, but the subsequent GIAO NMR calculations were conducted without Grimme correction term since the results obtained with, and without dispersion corrections, were the same.
For graphical correlations, both linear and nonlinear least square fits were performed and their quality assessed from the corresponding R2 parameter. The latter one used second order polynomial formula, abbreviated as polynomial_2.
Results and discussion
HOMA, HOMHED, and NICS parameters of uracil and 5XU
The calculated HOMHED parameters for the studied structures are gathered in Table 1. It is apparent from Table 1 that all tautomers of uracil and its 5-halogeno derivatives are to some extent aromatic, as reflected by their fairly high HOMHED index (between 0.7 and 1). Following the suggestion of an anonymous reviewer, we also checked the aromaticity of 5FU6 tautomer with OH group at C4 position rotated and directed toward the X atom. This molecule was only negligibly less aromatic than the one with opposite direction of hydroxyl substituent (HOMHED of 0.984 vs. 0.987 for 5FU6). However, comparing the magnitudes of calculated aromaticity indexes, there is a clear difference between the most stable tautomer 1 and 6. Thus, lower values of HOMEHED (about 0.76 and 0.72) are calculated for 5XU1 and 5XU4 tautomers. On the other hand, these indexes of aromaticity are about 0.99 for tautomers 6 and are close to the value calculated for benzene (HOMEHED ~ 1). The same order of aromaticity for the studied tautomers was earlier reflected by the calculated NICS and HOMA values in ref. [5]
In order to test a potential correlation between geometric parameters of aromaticity and the magnetic ones, in Fig. 2 we plotted the HOMA and HOMHED values for all tautomers of uracil and 5FU vs. the calculated NICS(1) values. The HOMHED parameters were calculated in the current study and both NICS and HOMA ones were taken from our earlier work [5].
Interestingly, it is apparent from Fig. 2 that no good correlation between geometric HOMA, HOMHED, and magnetic index of aromaticity NICS(1) exists. However, the corresponding R2 parameter for a dependence between NICS(1) calculated for all studied 5XU tautomers and geometric indexes is higher for HOMHED than for HOMA (0.82 vs. 0.67). Probably, this also illustrates the multidimensional character of aromaticity derived by using various parameters and points out to some advantage of using HOMHED combined with NICS(1) for characterization of heterocyclic compounds instead of traditional HOMA descriptor.
NICS scan above molecular planes of uracil and 5XU
A more clear picture of aromaticity profile above 5FU ring is shown in Fig. 3A and it somehow represents a “magnitude” of ring current at different distances from the molecular plane, e.g., above the center of the ring. As result of “scan experiment [16,17,18, 33, 34]” it is possible to show a characteristic profile of NICS(R) dependence on the distance R above the ring center of tautomers 1 and 6 of uracil, as well as benzene, taken as a model compound with a NICS showing a minimum at Rmin distance, markedly below 1 Å. The “scan” curve resembles a “Morse curve” and is related to the aromaticity profile above (and below) the benzene ring [10, 16,17,18, 34]. However, in case of the diketo tautomer of uracil (U1), the minimum is very shallow what indicates a very small aromaticity. Interestingly, on the contrary to tautomer 1, tautomer 6 shows a scan curve closely resembling benzene. Thus, it is apparent that the significant depth of scan minimum, NICS(Rmin) of about − 7.5 ppm for tautomer 6 indicates its high aromaticity while tautomer 1 is significantly less aromatic (NICS(Rmin) is about − 1 ppm). Evidently, the curve depth of about − 11 ppm and pattern shape depends on the π-electron current density, being larger in benzene (NICS(Rmin) than in tautomer 6 of uracil. In Fig. 3B, the results of similar “scan” experiments for tautomer 1 of all the studied 5XU1 compounds are shown. Analogous NICS(R) curve shapes with minimum in the range of R = 0.5 and 1 Å are observed for all 5XU except the tautomer 1 of 5-fluorouracil (Fig. 3B). In the latter case the minimum is observed at R = 0 Å. The same situation was observed for pyrrole [18]. Besides, in that study we demonstrated that the position (Rmin) and magnitude of NICS(Rmin) could be roughly correlated with the aromaticity degree of ring compounds [18]. Analyzing curve patterns from Fig. 3B, one could observe some systematic changes of Rmin and NICS(Rmin) parameters with the type of X substituent. Thus, the dashed red line, placed in the figure for the reader’s convenience, starts at Rmin = 0 Å and goes nicely through all the curves minima.
At this point, we also tested the impact of water, modeled by PCM, on the aromaticity of uracil’s tautomer 1 and 6. The scan experiment for R changing from 0 to 2 Å was performed in vacuum and in water (see Fig. S1A and B in the supplementary material). The NICS(R) scan curves calculated in the presence of water nearly coincide with those, obtained in the gas phase. Thus, the subsequent calculations were performed in the gas phase only since the used model indicates a lack of effect of polar solvent on the aromaticity of uracil.
One could also expect a direct correlation between electronegativity of halogen substituent and the density of π-electron ring current, as reflected in “NICS scan experiments”. In fact, nice linear (R2 > 0.9) dependences of Rmin and NICS(Rmin) vs. Pauling’s electronegativity of X substituent are observed in Fig. 4A and B. The increase of X substituent electronegativity results in decreasing Rmin and a small increase of ring aromaticity (see Fig. 4B), as reflected by more negative value of NICS(Rmin).
To support our data for 5XU molecular systems we also checked the impact of halogen electronegativity on monohalogenobenzenes C6H5X, where X = H, I, Br, Cl, and F. The NICS(R) scan experiment produced similar curve profiles to those, presented in Fig. 3B (see Fig. S2 in the Supplementary Material). However, the minima for halogenobenzenes were significantly lower (more negative) than for 5XU1 indicating a higher degree of aromaticity for these compounds (NICS(Rmin) from about − 9 to − 11 ppm). In addition, for C6H5F, the calculated Rmin ~ 0.7 Å. Besides, strong linear correlations between Rmin, NICS(Rmin) and electronegativity were observed for monohalogenobenzenes (Fig. S3 in the supplementary material).
Electronegativity of X substituent and NBO charge at C5 atom
It is also important to check the impact of X substituent electronegativity on NBO charge at C5 atom in the most stable tautomer of 5XU1 molecules. It is apparent from Fig. 5 that changes of NBO charge of X substituent is reflected in formation of almost equal but opposite sign charge at carbon No 5. The observed changes of charge are very regular and point to the electronegativity as the main factor controlling the electronic impact on the halogeno-substituted uracil ring. The difference of charges (X – C5) also decreases in a regular, nonlinear way. Here, we want to stress, that in this case, no linear dependence exist. Instead, a second-order polynomial nicely fits the calculated points (R2 > 0.99).
The observed patterns of NBO charge variations at C5 are similar to those for benzene ring carbon atom, directly bonded to halogen (see Fig. S4 in the supplementary material).
Our results are also in agreement with earlier studies on electronic effect of X substituent, in particular its donating and accepting properties, changing only to a little extend the aromaticity of benzene ring [48,49,50,51]. On the other hand, olefins are significantly more sensitive to such effects, including “push-pull cases”) [52].
Therefore, the above data strongly point out to halogen electronegativity (and fluorine as an extreme case), reflected as an inductive effect (I), being responsible for small changes of aromaticity in 5-halogenouracils. On the other hand, halogen atoms also produce a resonance (mezomeric) effect (R) which is absent for X = H. The order of this effect is F > Cl > Br > I. Thus, each substituent shows a competing I and R effects and fluorine is an extreme example. It is also worth mentioning that in case of borazine derivatives, the fluorine substituent also resulted in NICS(R) scan curve with a minimum at R = 0 Å [53] (the reported curve patterns were similar to that in Fig. 3B). The behavior of F-substituent somehow differs from the other halogens probably because of its extremely low electronegativity. However, the fluorine anomaly is well known in the literature (see its anomalous reactivity in ref. [54]).
3He atom as a magnetic probe of uracil and 5XU aromaticity
Despite general acceptance of aromaticity as a very important description of structural, energetic and spectroscopic properties of chemical compounds and their reactivity, there exists many controversies in this field [10, 51]. Aromaticity is often regarded as a multidimensional property. Thus, sometimes no direct correlation between the individual indexes of aromaticity could be seen. In this regard, in the current study we were interested to see, if there is some correlation between the geometric indexes of aromaticity for uracil and its 5-halogeno derivatives and their two, apparently not related magnetic indexes—NICS and helium-3 nuclear shieldings.
At this stage of our study, the helium-3 atom has been used as another magnetic probe of aromaticity. Isotropic values of 3He nuclear magnetic shielding constants for the helium atom located 1 Å above the ring center for uracil and its tautomers, as well as for the remaining 5XU molecules were calculated. For brevity, in Table S1 in the supporting material are shown 3He isotropic nuclear magnetic shieldings and chemical shifts of helium-3 atom 1 Å above the ring center (calculated with respect to isolated probe in the gas phase). In order to test a correlation between two magnetic indexes of aromaticity, δ(3He) and the NICS(1) for all tautomers of uracil and 5XU, in Fig. 6 were plotted the calculated parameters and fitted using a linear least square (LSQ) procedure. As result, a very good linear correlation between the two magnetic parameters of aromaticity, calculated in vacuum was produced (R2 = 0.98888). This result suggests that in the future carefully designed experiments, 3He NMR spectroscopy could be used to determine experimentally the “magnitude of aromaticity” of the studied molecular systems. In other words, we postulate to use helium-3 not only as a computational but also an experimental magnetic probe of aromaticity.
On the contrary, a significantly worse linear correlation between the chemical shift of the 3He atom 1 Å above the middle of ring and the geometric index of aromaticity, HOMHED is apparent from Fig. 7A (R2 = 0.84). This correlation is even worse for HOMA (not shown, R2 = 0.71).
HOMO-LUMO energy gap and aromaticity of uracil and 5XU1
Recently, we reported on exponential correlation between Eg and NICS index calculated for a series of acenes [22]. In the current studies, we also expected a similar correlation between Eg and aromaticity indexes of uracil tautomers and their derivatives. In Table S2 are gathered B3LYP-D3/aug-cc-pVQZ calculated HOMO, LUMO, and Eg energies for uracil tautomers, as well as their derivatives in the gas phase, and in Fig. 7B, a dependence between NICS(1) and Eg is plotted. The results presented in Fig. 7B are very scattered and once more demonstrate the fact that aromaticity is a very complicated and multidimensional phenomenon [10]. Contrary to the earlier work on regularly growing size of conjugated acenes [22], no correlation could be observed between Eg and the calculated indexes of aromaticity for all tautomers of uracil and 5-halogenuracils (an attempt of the linear fit produced RMS of only 0.14304). However, by analyzing Eg and NICS(1) of tautomer 1 only a nice linear correlation is observed with R2 of 0.92 (See Fig. S5 in the supplementary material).
Conclusions
DFT modeling of molecular structure of six tautomers of uracil and its 5-halogeno derivatives was performed at DFT (B3LYPD3/aug-cc-pVQZ) level of theory. The aromaticity of the studied heterocyclic compounds was determined using geometric indexes of aromaticity, HOMA, and HOMHED. The most stable diketo tautomer 5XU1 showed very small aromaticity (HOMA and HOMHED about 0.5 and 0.76, respectively) while the “rare” U6 one was fairly aromatic (HOMA and HOMHED close to 1). GIAO NMR calculations of magnetic indexes of aromaticity, NICS(0), NICS(1), NICS(1)zz, and NICS(R), for R = 0 to 10 Å with step of 0.05 Å were also conducted on the previously optimized structures at B3LYP/aug-cc-pVQZ level of theory, e.g., without dispersion effects included. Electronegativity of X substituent and its induction effect was the main factor controlling the charge at C5 atom in 5XU tautomers. Regular changes of NBO charges at C5 (nicely fitted with second order polynomial formula), with respect to electronegativity of X substituent were observed for tautomer 1 of 5XU structures.
The last magnetic probe of aromaticity used was isotope of helium-3. Its nuclear shielding at R = 1 Å, as well as scans up to 10 Å were also calculated and converted to helium chemical shifts.
Both the geometric indexes of aromaticity and the magnetic ones reflected low aromaticity of tautomer 1 and high, comparable to benzene for tautomer 5XU6. High aromaticity of tautomer 6 confirms the suitability of its traditional formula, presented with three alternated single and double bonds. A linear increase of 5XU aromaticity, expressed by Rmin and NICS(Rmin), obtained from scan experiments, with the electronegativity of halogen substituent was observed (R2 about 0.92 and 0.93).
Linear correlations were observed for δ(3He) and NICS(1) with R2 close to 1 (R2 = 0.99). However, the linear correlation between δ(3He) and HOMHED with R2 of 0.83 was less efficient (correlation for HOMA was even worse, with R2 = 0.71). On the contrary, no direct correlation between NICS(1) for all tautomers and Eg was observed (R2 about 0.14). On the contrary, a strong linear correlation between these parameters was observed for tautomer 1.
It was also observed that the presence of a polar environment (water modeled by PCM) increased only negligible the aromaticity of 5XU.
Data availability
From corresponding authors upon request.
References
Krokan HE, Drabløs F, Slupphaug G (2002) Uracil in DNA – occurrence, consequences and repair. Oncogene 21(58):8935–8948. https://doi.org/10.1038/sj.onc.1205996
Martins Z, Botta O, Fogel ML, Sephton MA, Glavin DP, Watson JS, Dworkin JP, Schwartz AW, Ehrenfreund P (2008) Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet Sci Lett 270(1):130–136. https://doi.org/10.1016/j.epsl.2008.03.026
Duschinsky R, Pleven E, Heidelberger C (1957) The synthesis of 5-fluoropyrimidines. J Am Chem Soc 79(16):4559–4560. https://doi.org/10.1021/ja01573a087
Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J (1957) Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 179(4561):663–666
Rzepiela K, Buczek A, Kupka T, Broda MA (2020) Factors governing the chemical stability and NMR parameters of uracil tautomers and its 5-halogen derivatives. Molecules 25:3931
McMurry J (2007) Organic chemistry7th edn Brooks-Cole.
Chen Z, Wannere CS, Corminboeuf C, Puchta R, Schleyer PR (2005) Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem Rev 105(10):3842–3888. https://doi.org/10.1021/cr030088+
Cyrański MK, Krygowski TM, Wisiorowski M, van Eikema Hommes NJR, Von Rague SP (1998) Global and local aromaticity in porphyrins: an analysis based on molecular geometries and nucleus-independent chemical shifts. Angew Chem Int Ed 37:177–180
Schleyer PvR, Maerker C, Dransfeld A, Jiao H, Hommes NJRvE (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318. doi:https://doi.org/10.1021/ja960582d
Solà M (2017) Why aromaticity is a suspicious concept? Why? Front Chem 5 (MAR). https://doi.org/10.3389/fchem.2017.00022
Raczyńska ED (2019) Application of the extended HOMED (harmonic oscillator model of aromaticity) index to simple and tautomeric five-membered heteroaromatic cycles with C, N, O, P, and S atoms. Symmetry 11 (2). doi:https://doi.org/10.3390/sym11020146
Dobrowolski JC (2019) Three queries about the HOMA index. ACS Omega 4(20):18699–18710. https://doi.org/10.1021/acsomega.9b02628
Kruszewski J, Krygowski TM (1972) Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett 13:3839–3842
Frizzo C, Martins M (2011) Aromaticity in heterocycles: new HOMA index parametrization. Struct Chem 23. https://doi.org/10.1007/s11224-011-9883-z
Fallah-Bagher-Shaidaei H, Wannere CS, Corminboeuf C, Puchta R, Schleyer PVR (2006) Which NICS aromaticity index for planar π rings is best? Org Lett 8(5):863–866. https://doi.org/10.1021/ol0529546
Stanger A (2006) Nucleus-independent chemical shifts (NICS): distance dependence and revised criteria for aromaticity and antiaromaticity. J Organomet Chem 71(3):883–893. https://doi.org/10.1021/jo051746o
Gajda Ł, Kupka T, Broda MA, Leszczyńska M, Ejsmont K (2018) Method and basis set dependence of the NICS indexes of aromaticity for benzene. Magn Reson Chem 56:265–275
Kupka T, Gajda Ł, Stobiński L, Kołodziej Ł, Mnich A, Buczek A, Broda MA (2019) Local aromaticity mapping in the vicinity of planar and nonplanar molecules. Magn Reson Chem 57:359–372
Radula-Janik K, Kupka T (2015) 3He NMR studies on helium–pyrrole, helium–indole, and helium–carbazole systems: a new tool for following chemistry of heterocyclic compounds. Magn Reson Chem 53:103–109
Saunders M, Jiménez-Vázquez HA, Cross RJ, Mroczkowski S, Freedberg DI, Anet FAL (1994) Probing the interior of fullerenes by 3He NMR spectroscopy of endohedral 3He@C60 and 3He@C70. Nature 367(6460):256–258. https://doi.org/10.1038/367256a0
Sternfeld T, Saunders M, Cross RJ, Rabinovitz M (2003) The inside story of fullerene anions: a 3He NMR aromaticity probe. Angew Chem Int Ed 42:3136–3139
Gajda M, Gajda Ł, Kupka T, Kar T (2020) Local aromaticity in polyacenes manifested by individual proton and carbon shieldings: DFT mapping of aromaticity. Magn Reson Chem 51:463-468. https://doi.org/10.1002/mrc.4967
Saunders M, Cross RJ, Jiménez-Vázquez HA, Shimshi R, Khong A (1996) Noble gas atoms inside fullerenes. Science 271:1693–1697
Saunders M, Jimenez-Vazquez HA, Bangerter BW, Cross RJ, Mroczkowski S, Freedberg DI, Anet FAL (1994) 3He NMR, a powerful new tool for following fullerene chemistry. J Am Chem Soc 116:3621–3622
Kupka T, Stachów M, Stobiński L, Kaminský J (2013) 3He NMR: from free gas to its encapsulation in fullerene. Magnetic Resonance in Chemistry : MRC 51:463–468
Hanus M, Kabeláč M, Nachtigallová D, Hobza P (2005) Mutagenic properties of 5-halogenuracils: correlated quantum chemical ab initio study. Biochemistry 44(5):1701–1707. https://doi.org/10.1021/bi048112g
Colasurdo DD, Pila MN, Iglesias DA, Laurella SL, Ruiz DL (2017) Tautomerism of uracil and related compounds: a mass spectrometry study. Eur J Mass Spectrom 24(2):214–224. https://doi.org/10.1177/1469066717712461
Leszczynski J (1992) Tautomerism of uracil: the final chapter? Fourth-order electron correlation contributions to the relative energies of tautomers. J Phys Chem 96(4):1649–1653. https://doi.org/10.1021/j100183a029
Markova N, Enchev V, Timtcheva I (2005) Oxo−hydroxy tautomerism of 5-fluorouracil: water-assisted proton transfer. J Phys Chem A 109(9):1981–1988. https://doi.org/10.1021/jp046132m
Muñoz Freán S, Alcolea Palafox M, Rastogi VK (2013) Effect of the microhydration on the tautomerism in the anticarcinogenic drug 5-fluorouracil and relationships with other 5-haloderivatives. J Mol Struct 1054-1055:32–45. https://doi.org/10.1016/j.molstruc.2013.09.008
Rastogi VK, Palafox MA (2011) Vibrational spectra, tautomerism and thermodynamics of anticarcinogenic drug: 5-fluorouracil. Spectrochim Acta Part A: Mol Biomol Spectrosc 79(5):970–977. https://doi.org/10.1016/j.saa.2011.04.008
Yekeler H, Özbakır D (2001) Concerning the solvent effect in the tautomerism of uracil, 5-fluorouracil, and thymine by density-functional theory and ab initio calculations. J Mol Model 7(4):103–111. https://doi.org/10.1007/s008940100015
Acke G, Van Damme S, Havenith RWA, Bultinck P (2019) Quantifying the conceptual problems associated with the isotropic NICS through analyses of its underlying density. Phys Chem Chem Phys 21(6):3145–3153. https://doi.org/10.1039/c8cp07343k
Gershoni-Poranne R, Stanger A (2015) Magnetic criteria of aromaticity. Chem Soc Rev 44(18):6597–6615. https://doi.org/10.1039/c5cs00114e
Frizzo CP, Martins MAP (2012) Aromaticity in heterocycles: new HOMA index parametrization. Struct Chem 23(2):375–380. https://doi.org/10.1007/s11224-011-9883-z
Weinhold F, Landis CR (2001) Natural bond orbitals and extensions of localized bonding concepts. Chem Educ Res Pract 2(2):91–104. https://doi.org/10.1039/B1RP90011K
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105(8):2999–3094. https://doi.org/10.1021/cr9904009
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Rev. C.01. Gaussian 16 Rev. C.01. Wallingford, CT
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang, W., and Parr, R. G. (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37 (2):785–789
Miehlich B, Savin A, Stoll H, Preuss H (1989) Results obtained with the correlation-energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett 157:200–206
Grimme S, Antony J, Ehrlich S, Krieg S (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J Chem Phys 132:154104
Kendall RA, Dunning Jr TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
EMSL. EMSL basis set exchange. https://bse.pnl.gov.bse/portal. Accessed many times in 2019 and 2020
Schuchardt KL, Didier BT, Elsethagen T, Sun L, Gurumoorthi V, Chase J, Li J, Windus TL (2007) Basis set exchange: a community database for computational sciences. J Chem Inf Model 47:1045–1052
Ditchfield R (1974) Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for N.M.R. chemical shifts. Mol Phys 27:789–807
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112(23):8251–8260
Manassir M, Pakiari AH (2019) An electronic properties investigation to interpret the substituent constants of monosubstituted benzene derivatives. J Mol Graphic Model 92:201–207. https://doi.org/10.1016/j.jmgm.2019.07.017
Krygowski TM, Ejsmont K, Stepień BT, Cyrański MK, Poater J, Solà M (2004) Relation between the substituent effect and aromaticity. J Organomet Chem 69:6634–6640
Zarate X, MacLeod-Carey D, Muñoz-Castro A, Schott E (2019) Understanding the aromaticity of C6X6 (X = H, F, Cl, Br, I). Insights from different theoretical criteria. Chem Phys Lett 720:52–57. https://doi.org/10.1016/j.cplett.2019.01.058
Shishkin OV, Omelchenko IV, Krasovska MV, Zubatyuk RI, Gorb L, Leszczynski J (2006) Aromaticity of monosubstituted derivatives of benzene. The application of out-of-plane ring deformation energy for a quantitative description of aromaticity. J Mol Struct 791(1):158–164. https://doi.org/10.1016/j.molstruc.2006.01.019
Siodla T, Szatylowicz H, Varaksin KS, Krygowski TM (2016) Difference in pi-electron delocalization for monosubstituted olefinic and aromatic systems. RSC Adv 6(99):96527–96530. https://doi.org/10.1039/C6RA20163F
Costa A, Costa ER, Silva ALP, Tanaka AA, de Jesus GJ (2018) Theoretical study of the effects of substituents (F, Cl, Br, CH3, and CN) on the aromaticity of borazine. J Mol Model 24(1):34. https://doi.org/10.1007/s00894-017-3555-x
Rosenthal J, Schuster DI (2003) The anomalous reactivity of fluorobenzene in electrophilic aromatic substitution and related phenomena. J Chem Educ 80(6):679. https://doi.org/10.1021/ed080p679
Acknowledgments
Calculations have been carried out using resources provided by the Wrocław Centre for Networking and Supercomputing (http://wcss.pl).
Code availability
Gaussian 16 at WCSS Wroclaw.
Author information
Authors and Affiliations
Contributions
K. R.—conducting research and partial analysis of results; A. B.—conducting research and partial analysis of results; T. K.—planning research, analysis results, and preparing a manuscript; M. A. B.—planning research, analysis results, and preparing a manuscript.
Corresponding authors
Ethics declarations
Not applicable. The ethical standards have been met.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The following materials, consisting of additional tables and figures, are available online at http/ …
ESM 1
(DOCX 357 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rzepiela, K., Buczek, A., Kupka, T. et al. On the aromaticity of uracil and its 5-halogeno derivatives as revealed by theoretically derived geometric and magnetic indexes. Struct Chem 32, 275–283 (2021). https://doi.org/10.1007/s11224-020-01682-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01682-x