Abstract
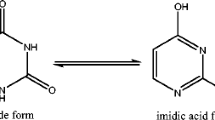
In this paper, we suggest a computational scheme for the theoretical estimation of gas-phase acidity and basicity of azulene-based uracil analogue. The proton affinities (PAs) of the two oxygen and of the two nitrogen atoms and the deprotonation energies (DPEs) of the two NH and of the two OH bonds of azulene-based uracil analogue isomers are calculated by density functional theory (DFT) using the 6-31+G(d,p) basis set and by high ab initio MO theory (CBS-QB3) method. The PAs of the oxygen and nitrogen atoms of 13 tautomers range from 197.9 to 230.4 kcal/mol and the DPEs of the OH and NH groups from 300 to 342.3 kcal/mol. The proton affinities of di-keto form AZU1 followed the order O9 (N1 site), O9 (N3 site), O11 (N3 site), and O11 (H13 site).




Similar content being viewed by others
References
Jordheim LP, Durantel D, Zoulim F, Dumontet C (2013) Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 12:447–464. https://doi.org/10.1038/nrd4010
Esposito V, Randazzo A, Piccialli G et al (2004) Effects of an 8-bromodeoxyguanosine incorporation on the parallel quadruplex structure [d(TGGGT)]4. Org Biomol Chem 2:313–318. https://doi.org/10.1039/b314672c
Denifl S, Matejcik S, Gstir B et al (2003) Electron attachment to 5-chloro uracil. J Chem Phys 118:4107–4114. https://doi.org/10.1063/1.1540108
Brovarets’ OO, Zhurakivsky RO, Hovorun DM (2014) Is the DPT tautomerization of the long A·G Watson-Crick DNA base mispair a source of the adenine and guanine mutagenic tautomers? A QM and QTAIM response to the biologically important question. J Comput Chem 35:451–466. https://doi.org/10.1002/jcc.23515
Brovarets OO, Hovorun DM (2015) The nature of the transition mismatches with Watson-Crick architecture: the G∗•T or G•T∗ DNA base mispair or both? A QM/QTAIM perspective for the biological problem. J Biomol Struct Dyn 33:925–945. https://doi.org/10.1080/07391102.2014.924879
Mahmoud S, Hasabelnaby S, Hammad SF, Sakr TM (2018). Antiviral nucleoside and nucleotide analogs: a review 547:73–88
González-Sarrías A, Tomé-Carneiro J, Bellesia A et al (2015) The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct 6:1460–1469. https://doi.org/10.1039/c5fo00120j
Smith NF, Figg WD, Sparreboom A (2004) Recent advances in pharmacogenetic approaches to anticancer drug development. Drug Dev Res 62:233–253. https://doi.org/10.1002/ddr.10361
Blicharska B, Kupka T (2002) Theoretical DFT and experimental NMR studies on uracil and 5-fluorouracil. J Mol Struct 613:153–166. 10.1016/S0022-2860(02)00171-0
Rashad AE, Shamroukh AH, Abdel-Megeid RE, El-Sayed WA (2010) Synthesis, reactions, and antimicrobial evaluation of some polycondensed thienopyrimidine derivatives. Synth Commun 40:1149–1160. https://doi.org/10.1080/00397910903050954
Rashad A, Ali M (2006) Synthesis and antiviral screening of some thieno[2,3- d]pyrimidine nucleosides. Nucleosides Nucleotides Nucleic Acids 25:17–28. https://doi.org/10.1080/15257770500377730
Simonson T, Brooks CL (1996) Charge screening and the dielectric constant of proteins: insights from molecular dynamics. J Am Chem Soc 118:8452–8458. https://doi.org/10.1021/ja960884f
Jordan F, Li H, Brown A (1999) Remarkable stabilization of zwitterionic intermediates may account for a billion-fold rate acceleration by thiamin diphosphate-dependent decarboxylases. Biochemistry 38:6369–6373. https://doi.org/10.1021/bi990373g
Tho Nguyen M, Chandra AK, Zeegers-Huyskens T (1998) Protonation and deprotonation energies of uracil implications for the uracil–water complex. J Chem Soc Faraday Trans 94:1277–1280. https://doi.org/10.1039/a708804c
Chandra AK, Nguyen MT, Uchimaru T, Zeegers-Huyskens T (1999) Protonation and deprotonation enthalpies of guanine and adenine and implications for the structure and energy of their complexes with water: comparison with uracil, and cytosine. J Phys Chem A 103:8853–8860. https://doi.org/10.1021/jp990647+
Wolken JK, Tureček F (2000) Proton affinity of uracil. A computational study of protonation sites. J Am Soc Mass Spectrom 11:1065–1071. 10.1016/S1044-0305(00)00176-8
Nguyen VQ, Tureček F (1997) Protonation sites in pyrimidine and pyrimidinamines in the gas phase. J Am Chem Soc 119:2280–2290. https://doi.org/10.1021/ja9634785
Podolyan Y, Gorb L, Leszczynski J (2000) Protonation of nucleic acid bases. A comprehensive post-Hartree−Fock study of the energetics and proton affinities . J Phys Chem A, 104 : 7346–7352. 10.1021/jp000740u
Nguyen VQ, Tureček F (1997) Gas-phase protonation of pyridine. A variable-time neutralization-reionization and ab initio study of pyridinium radicals. J Mass Spectrom 32:55–63. https://doi.org/10.1002/(SICI)1096-9888(199701)32:1<55::AID-JMS447>3.0.CO;2-M
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539. https://doi.org/10.1021/ja00905a001
Mineva T, Russo N (2010) Atomic Fukui indices and orbital hardnesses of adenine, thymine, uracil, guanine and cytosine from density functional computations. J Mole Struct: THEOCHEM 943:71–76. https://doi.org/10.1016/j.theochem.2009.10.023
Taura LS, Ndikilar CE, Muhammad A (2017) Modeling the structures and electronic properties of uracil and thymine in gas phase and water. Mod App Sci 11:36–46. https://doi.org/10.5539/mas.v11n12p36
Abdel-Mottaleb Y, Abdel-Mottaleb MSA (2016, 2016) Molecular modeling studies of some uracil and new deoxyuridine derivatives. J Chem. https://doi.org/10.1155/2016/5134732
Estrin DA, Paglieri L, Corongiu G (1994) A density functional study of tautomerism of uracil and cytosine. J Phys Chem 98:5653–5660. https://doi.org/10.1021/j100073a014
Millefiori S, Alparone A (2004) Tautomerism and polarizability in uracil: coupled cluster and density-functional theory study. Chem Phys 303:27–36. https://doi.org/10.1016/j.chemphys.2004.05.002
Pałasz A, Ciez D (2015) In search of uracil derivatives as bioactive agents. Uracils and fused uracils: synthesis, biological activity and applications. Eur J Med Chem 97:582–611. https://doi.org/10.1016/j.ejmech.2014.10.008
Jalbout AF, Trzaskowski B, Xia Y et al (2007) Structures, stabilities and tautomerizations of uracil and diphosphouracil tautomers. Chem Phys 332:152–161. https://doi.org/10.1016/j.chemphys.2006.10.026
Kurinovich MA, Lee JK (2002) The acidity of uracil and uracil analogs in the gas phase: four surprisingly acidic sites and biological implications. J Am Soc Mass Spectrom 13:985–995. https://doi.org/10.1016/S1044-0305(02)00410-5
Hu X, Li H, Liang W, Han SJ (2005). Phys Chem B 109:5935
Bakker JM, Sinha RK, Besson T et al (2008) Tautomerism of uracil probed via infrared spectroscopy of singly hydrated protonated uracil. J Phys Chem A 112:12393–12400. https://doi.org/10.1021/jp806396t
Valadbeigi Y, Farrokhpour H (2014) Effect of hydration on the stability and tautomerisms of different isomers of uracil. RSC Adv 4:61643–61651. https://doi.org/10.1039/c4ra09733e
Kryachko ES, Nguyen MT, Zeegers-Huyskens T (2001) Theoretical study of tautomeric forms of uracil. 1. Relative order of stabilities and their relation to proton affinities and deprotonation enthalpies. J Phys Chem A 105:1288–1295. https://doi.org/10.1021/jp001031j
Raczyńska ED, Zientara K, Stepniewski TM, Kolczynska K (2009) Stability, polarity, intramolecular interactions and Π-electron delocalization for all eighteen tautomers rotamers of uracil. dft studies in the gas phase. Collect Czechoslov Chem Commun 74:57–72. https://doi.org/10.1135/cccc2008149
Chandra AK, Nguyen MT, Zeegers-Huyskens T (1998) Theoretical study of the interaction between thymine and water. Protonation and deprotonation enthalpies and comparison with uracil. J Phys Chem A 102:6010–6016. https://doi.org/10.1021/jp981259v
Miller TM, Arnold ST, Viggiano AA, Stevens Miller AE (2004) Acidity of a nucleotide base: uracil. J Phys Chem A 108:3439–3446. https://doi.org/10.1021/jp037367l
Mikheev YA, Guseva LN, Ershov YA (2012) The structure of dimers and the nature of azulene chromaticity. Russ J Phys Chem A 86:85–92. https://doi.org/10.1134/S0036024412010232
Murakami A, Kobayashi T, Goldberg A, Nakamura S (2004) CASSCF and CASPT2 studies on the structures, transition energies, and dipole moments of ground and excited states for azulene. J Chem Phys 120:1245–1252. https://doi.org/10.1063/1.1631914
Rekka E, Chrysselis M, Siskou I, Kourounakis A (2002) Synthesis of new azulene derivatives and study of their effect on lipid peroxidation and lipoxygenase activity. Chem Pharm Bull (Tokyo) 50:904–907. https://doi.org/10.1248/cpb.50.904
Wang B-C, Lin Y-S, Chang J-C, Wang P-Y (2000) Theoretical studies of azulene and its derivatives. ProQuest Cent 78:224–232. https://doi.org/10.1002/chin.200024030
Ramadan M, Goeters S, Watzer B et al (2006) Chamazulene carboxylic acid and matricin: a natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J Nat Prod 69:1041–1045. https://doi.org/10.1021/np0601556
Frigola J, Torrens A, Castrillo JA, Mas J, Vano D, Berrocal JM et al (1994) 7-Azetidinylquinolones as antibacterial agents. 2. Synthesis and biological activity of 7-(2,3-disubstituted-1-azetidinyl)-4-oxoquinoline- and -1,8-naphthyridine-3-carboxylic acids. Properties and structure-activity relationships of quinolones with an azetidine moiety. J Med Chem 37(24):4195–4210. https://doi.org/10.1021/jm00050a016
Ishihara M, Wakabayashi H, Motohashi N, Sakagami H (2011) Quantitative structure-cytotoxicity relationship of newly synthesised trihaloacetylazulenes determined by a semi-empirical molecular-orbital method (PM5). Anticancer Res 31:515–520
Rejnek J, Hanus M, Kabela M, Ryja F, Hobza ek and P (2005) Correlated ab initio study of nucleic acid bases and their tautomers in the gas phase, in a microhydrated environment and in aqueous solution. Part 4. Uracil and thymine. PhysChemChemPhys 7:2006–22017
Tian SX, Zhang CF, Zhang ZJ, et al (1999) How many uracil tautomers there are? Density functional studies of stability ordering of tautomers. Chem Phys 242:217–225. 10.1016/S0301-0104(99)00009-9
Kurinovich MA, Lee JK (2000) The acidity of uracil from the gas phase to solution: the coalescence of the N1 and N3 sites and implications for biological glycosylation. J Am Chem Soc 122:6258–6262. https://doi.org/10.1021/ja000549y
Gad SF, El-Demerdash SH, El-Mehasseb IM, El-Nahas AM (2019) Structure, stability and conversions of tautomers and rotamers of azulene-based uracil analogue. J MoleStruct. https://doi.org/10.1016/j.molstruc.2019.01.020
Florián J, Leszczyński J (1996) Spontaneous DNA mutations induced by proton transfer in the guanine·cytosine base pairs: an energetic perspective. JAmeChem Soci 118:3010–3017. https://doi.org/10.1021/ja951983g
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. https://doi.org/10.1021/j100096a001
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Pokon EK, Liptak MD, Feldgus S, Shields GC (2001) Comparison of CBS-QB3, CBS-APNO, and G3 predictions of gas phase deprotonation data. J Phys Chem A 105:10483–10487. https://doi.org/10.1021/jp012920p
Nicolaides A, Rauk A, Glukhovtsev MN, Radom L (1996) Heats of formation from G2, G2 (MP2), and G2 (MP2, SVP) total energies. J Phys Chem 100:17460–17464
Glossman MD (1995) Application of density functional theory concepts to the study of the chemical reactivity of thiadiazoles. J Mol Struct THEOCHEM 330:385–388. https://doi.org/10.1016/0166-1280(94)03865-i
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L Sonnenberg, M. Hada and DJF (2009) Gaussian 09 (Gaussian, Inc., Wallingford CT
Wilson MS, McCloskey JA (1975) Chemical ionization mass spectrometry of nucleosides. Mechanisms of ion formation and estimations of proton affinity. J Am Chem Soc 97:3436–3444. https://doi.org/10.1021/ja00845a026
Hunter EPL, Lias SG (2013) Evaluated gas phase basicities and proton affinities of molecules: an update. J Phys Chem Ref Data 413:. https://doi.org/10.1063/1.556018
Löwdin P-O (1963) Proton tunneling in DNA and its biological implications. Rev Mod Phys 35:724–732. https://doi.org/10.1103/revmodphys.35.724
Fujio M, Mclver RT, Taft RW (1981) Effects on the acidities of phenols from specific substituent-solvent interactions. Inherent substituent parameters from gas-phase acidities. J Am Chem Soc 103:4017–4029. https://doi.org/10.1021/ja00404a008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Supplementary data associated with this article can be found in the online version (DOCX 646 kb)
Rights and permissions
About this article
Cite this article
El-Demerdash, S.H., Gad, S.F. A computational study of gas-phase acidity and basicity of azulene-based uracil analogue. Struct Chem 31, 319–328 (2020). https://doi.org/10.1007/s11224-019-01408-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01408-8