Abstract
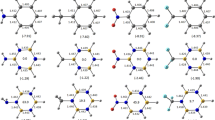
This paper presents a theoretical study of the effects of substituents (F, Cl, Br, CH3, and CN) on the aromaticity of borazine (B3N3H6), using density functional theory (DFT) and the Hartree-Fock (HF) method. The calculations to optimize the geometries, structural properties, and vibrational frequencies were performed using the same 6–311G(d,p) and 6–311++G(d,p) basis sets, comparing the methods with experimental results. In the analysis of the NICSZZ values, it was found that that replacing the hydrogen atoms by halogen atoms (F, Cl, and Br) and CH3 reduced the aromaticity of the borazine molecule, while use of the CN group resulted in NICSZZ values (0.9–2.0 Å) very close to those of borazine, presenting the following order of increasing aromaticity: B3N3H3-(Br)3 < B3N3H3-(Cl)3 < B3N3H3-(F)3 < B3N3H3-(CH3)3 < B3N3H6 ~ B3N3H3-(CN)3. All the spectra of the compounds showed only the presence of transition peaks distant from the UV region, reflecting the large energy difference between the HOMO and LUMO orbitals. After the substitution of the borazine ring, all the compounds presented an intensification of the spectrum, with a shift of the maximum absorbance toward red, indicative of a bathochromic effect. There was a direct inverse relation between the energy gap and the maximum wavelength of the compounds.






Similar content being viewed by others
References
Chen P, Lalancette RA, Jäkle F (2012) π-expanded Borazine: an Ambipolar conjugated B–π–N macrocycle. Angew Chem In Ed 51:7994–7998
Schleyer PVR, Jiao H, Hommes NJRVE, Malkin VG, Malkina OL (1997) An evaluation of the aromaticity of inorganic rings: refined evidence from magnetic properties. J Am Chem Soc 119:12669–12670
Stock A, Pohland E (1926) Borwasserstoffe, VIII. Zur Kenntnis des B2H6 und des B5H11. Ber Dtsch Chem Ges 59:2210–2215
Kesharwani MK, Suresh M, Das A, Ganguly B (2011) Borazine as a sensor for fluoride ion: a computational and experimental study. Tetrahedron Lett 52:3636–3639
Kiran B, Phukan AK, Jemmis ED (2001) Is borazine aromatic? Unusual parallel behavior between hydrocarbons and corresponding B-N analogues. Inorg Chem 40:3615–3618
Shen W, Li M, Li Y, Wang S (2007) Theoretical study of borazine and its derivatives. Inorg Chim Acta 360:619–624
Jackson KT, Rabbani MG, Reich TE, El-Kaderi HM (2011) Synthesis of highly porous borazine-linked polymers and their application to H2, CO2, and CH4 storage. Polym Chem 2:2775–2777
Harshbarger W, Lee GH, Porter RF, Bauer SH (1969) The structure of borazine. Inorg Chem 8:1683–1689
Nöth H, Lima SR, Troll A (2005) The structural chemistry of N-monolithium borazines. Eur J Inorg Chem 2005:1895–1906
Li JS, Zhang CR, Li B, Cao F, Wang SQ (2011) An investigation on the synthesis of borazine. Inorg Chim Acta 366:173–176
Paine RT, Narula CK (1990) Synthetic routes to boron nitride. Chem Rev 90:73–91
Jaschke T, Jansen MJ (2006) A new borazine-type single source precursor for Si/B/N/C ceramics. Mater Chem 16:2792–2799
Haberecht J, Nesper R, Grutzmacher H (2005) A construction kit for Si-B-C-N ceramic materials based on borazine precursors. Chem Mater 17:2340–2347
Haberecht J, Krumeich F, Grutzmacher H, Nesper R (2004) High-yield molecular borazine precursors for Si-B-N-C ceramics. Chem Mater 16:418–423
Nghiem QD, Jeon JK, Hong LY, Kim DP (2003) Polymer derived Si-C-B-N ceramics via hydroboration from borazine derivatives and trivinylcyclotrisilazane. J Organomet Chem 688:27–35
Toury B, Bernard S, Cornu D, Chassagneux F, Létoffé JM, Miele P (2003) High-performance boron nitride fibers obtained from asymmetric alkylaminoborazine. J Mater Chem 13:274–279
Duriez C, Framery E, Toury B, Toutois P, Miele P, Vaultier M, Bonnetot BJ (2002) Boron nitride thin fibres obtained from a new copolymer borazine-tri(methylamino)borazine precursor. Organomet Chem 657:107–114
Perdigon-Melon JA, Auroux A, Cornu D, Miele P, Toury B, Bonnetot B (2002) Porous boron nitride supports obtained from molecular precursors. Influence of the precursor formulation and of the thermal treatment on the properties of the BN ceramic. J Organomet Chem 657:98–106
Cornu D, Miele P, Toury B, Bonnetot B, Mongeot H, Bouix J (1999) Boron nitride matrices and coatings from boryl borazine molecular precursors. J Mater Chem 9:2605–2610
Lourie OR, Jones CR, Bartlett BM, Gibbons PC, Ruoff RS, Buhro WE (2000) CVD growth of boron nitride nanotubes. Chem Mater 12:1808–1810
Loh KP, Fan WY, Lim CW, Zhang X, Chen W, Xie XN, Xu H, Wee ATS (2003) Reactive atom beam deposition of boron nitride ultrathin films and nanoparticles using borazine. Diam Relat Mater 12:1103–1107
Volger KE, Kroke E, Gervais C, Saito T, Babonneau F, Riedel R, Iwamoto Y, Hirayama T (2003) B/C/N materials and B4C synthesized by a non-oxide sol-gel process. Chem Mater 15:755–764
Bechelany M, Bernard S, Brioude A, Cornu D, Stadelmann P, Charcosset C, Fiaty K, Miele P (2007) Synthesis of boron nitride nanotubes by a template-assisted polymer thermolysis process. J Phys Chem C 111:13378–13384
Watanabe K, Taniguchi T, Kanda H (2004) Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal. Nature 3:404–409
Narula CK, Schaeffer R, Datye A, Paine RT (1989) Synthesis of boron nitride ceramics from 2,4,6-triaminoborazine. Inorg Chem 28:4053–4055
Pauling L (1960) The nature of the chemical bond. Cornell University Press, New York
Benker D, Klapötke TM, Kuhn G, Li J, Miller C (2005) An ab initio valence bond (VB) calculation of the π delocalization energy in borazine, B3N3H6. Heteroat Chem 16:311–315
Fowler PW, Steiner E (1997) Ring currents and aromaticity of monocyclic π-electron systems C6H6, B3N3H6, B3O3H3, C3N3H3, C5H5 −, C7H7 +, C3N3F3, C6H3F3, and C6F6. J Phys Chem A 101:1409–1413
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JJA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, revision D. 01. Gaussian Inc., Wallingford
Pople JA, Gordon MH, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:5968–5975
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the colic-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Hartree DR (1928) The wave mechanics of an atom with a noncoulomb central field. Part I. Theory and methods. Proc Camb Philos Soc 24:89–110
Hartree DR (1928) The wave mechanics of an atom with a non-coulomb central field. Part II. Some results and discussion. Proc Camb Philos Soc 24:111–132
Pople JA, Gordon MH, Fox DJ, Raghavachari K, Curtiss LA (1989) Gaussian-1 theory: a general procedure for prediction of molecular energies. J Chem Phys 90:5622–5629
Curtiss LA, Raghavachari K, Trucks GW, Pople JA (1991) Gaussian-2 theory for molecular energies of first- and second-row compounds. J Chem Phys 94:7221–7230
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions. J Chem Phys 23:1833–1840
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. WIREs Comput Mol Sci 2:1–42
Boyd RJ, Choi SC, Hale CC (1984) Electronic and structural properties of borazine and related molecules. Chem Phys Lett 112:136–141
Miao R, Yang G, Zhao C, Hong J, Zhu L (2005) Theoretical study of borazine and its fluoroderivatives: aromaticity and cation–π, anion–π interaction. THEOCHEM J Mol Struct 715:91–100
Shriver DF, Atkins PW, Overton TL, Rourke JP, Weller MT, Armstrong FA (2010) Inorganic chemistry4nd edn. Oxford University Press, New York
Beyer H, Jenne H, John HB, Niedenzu K (1964) Boron-nitrogen chemistry. American Chemical Society, Washington, DC
Parker JK, Davis SR (1997) Ab initio study of the relative energies and properties of fluoroborazines. J Phys Chem A 101:9410–9414
Schleyer PVR, Maerker C, Dransfeld A, Jiao H, Hommes NJRVE (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Corminboeuf C, Heine T, Seifert G, Schleyer PVR, Weber J (2004) Induced magnetic fields in aromatic [n]-annulenes-interpretation of NICS tensor components. Phys Chem Chem Phys 6:273–276
Niedenzu K, Sawodny W, Watanabe H, Dawson JW, Totani T, Weber W (1967) The vibrational spectrum of borazine. Inorg Chem 6:1453–1461
Stratmann RE, Scuseria GE, Frisch MJ (1998) An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J Chem Phys 109:8218–8224
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473
Bauernschmitt R, Ahlrichs R (1996) Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem Phys Lett 256:454–464
Ghiasi R, Manoochehri M, Reihaneh L (2016) DFT and TD-DFT study of benzene and borazines containing chromophores for DSSC materials. Russ J Inorg Chem 61:1267–1273
Pearson RG (2005) Chemical hardness and density functional theory. J Chem Sci 117:369–377
Dewar MJS (1971) Aromaticity and pericyclic reactions. Angew Chem Int Ed 10:761–870
Willner I, Rabinovitz M (1980) Cycloocta[def]fluorene: a planar cyclooctatetraene derivative. Paratropicity of hydrocarbon and anion. J Org Chem 45:1628–1633
Minsky A, Meyer AY, Rabinovitz M (1985) Paratropicity and antiaromaticity: role of the homo-lumo energy gap. Tetrahedron 41:785–791
Cohen Y, Roelofs NH, Reinhard G, Scott LT, Rabinovitz M (1987) Novel carbocyclic dianions: NMR study of charge delocalization, paratropicity, and structure in the dianions of acephenanthrylene and aceanthrylene. J Org Chem 52:4207–4214
Budzelaar PHM, Cremer D, Wallasch M, Wurthwein EU, Schleyer PVR (1987) Dioxetenes and diazetines: nonaromatic 6π-systems in four-membered rings. J Am Chem Soc 109:6290–6299
Esrafili MD (2013) Nitrogen-doped (6, 0) carbon nanotubes: a comparative DFT study based on surface reactivity descriptors. Comput Theor Chem 1015:1–7
Acknowledgments
The authors are grateful for the financial support provided by CAPES, FINEP, CNPq, and FAPEMA.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary data associated with this article can be found in the online version at: https://link.springer.com/journal/214
ESM 1
(DOCX 1143 kb)
Rights and permissions
About this article
Cite this article
Costa, A., Costa, E.R., Silva, A.L.P. et al. Theoretical study of the effects of substituents (F, Cl, Br, CH3, and CN) on the aromaticity of borazine. J Mol Model 24, 34 (2018). https://doi.org/10.1007/s00894-017-3555-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3555-x