Abstract
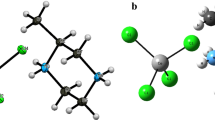
The crystal and molecular structure of [Cu(ampf)(ClO4)(MeOH)2]ClO4, (1), ampf = N,N′-bis(4-acetyl-5-methylpyrazol-3-yl)formamidine, determined by X-ray crystallography is described and compared with the structurally related copper(II) complexes, formed under similar experimental conditions, using CuII salts with different anions. The complex formation is discussed in view of the structures of cobalt(II) and nickel(II) complexes with the same organic ligand and different anions, also formed under similar reaction conditions. Solvent molecules coordinated to the central atom play an important role in biologic systems. To get a better insight into the desolvation mechanism, in this study the desolvation pattern of 1 is presented. As in literature little attention is paid to the desolvation mechanism of solvate complexes, the desolvation mechanism of three, potentially biologically active isostructural pairs of octahedral NiII and CoII compounds with ampf and dmpc (3,5-dimethyl-1H-pyrazole-1-carboxamidine) ligands are evaluated and compared with the desolvation of 1. The results of the thermal data are discussed on the basis of structural features of the compounds. The minor differences in structures of the related compounds cannot be straightforwardly connected with the different solvent evaporation mechanism. To explain the differences found in desolvation pattern in isostructural CoII and NiII complexes the Jahn-Teller effect is proposed.





Similar content being viewed by others
Notes
The repeatability of the measurements was also tested. Caution: Most of the perchlorates belong to explosives therefore special attention should be paid to protective measures. In the case of thermal measurements, the sample mass (<1 mg) and the heating rate (<20 °C min−1) should be kept low.
References
Quirante J, Ruiz D, Gonzalez A, López C, Cascante M, Cortés R, Messeguer R, Calvis C, Baldomà L, Pascual A, Guérardel Y, Pradines B, Font-Bardía M, Calvet T, Biot J (2011) J Inorg Biochem 105:1720–1728
Kaushik D, Kumar R, Ahmed Khan S, Chawla G (2012) Med Chem Res 21:3646–3655
Mutti FG, Gullotti M, Casella L, Santagostini L, Pagliarin R, Andersson KK, Iozzie MF, Zoppellaro G (2011) Dalton Trans 40:5436–5457
Kulkarni NV, Kamath A, Budagumpi S, Revankar VK (2011) J Mol Struct 1006:580–588
Zheng Ch-y, Wang D-j, Fan L, Zheng J (2013) Struct Chem 24:705–711
Ke F, Yuan Y-P, Qiu L-G, Shen Y-H, Xie A-J, Zhu J-F, Tian X-Y, Zhang L-D (2011) J Mater Chem 21:3843–3848
Morley AD, Cook A, King S, Roberts B, Lever V, Weaver R, MacDonald C, Unitt J, Fagura M, Phillips V, Lewis R, Wenlock M (2011) Bioorg Med Chem Lett 21:6456–6460
Singh UP, Kashyap S, Butcher RJ, Singh HJ, Mishra BK (2011) Struct Chem 22:931–941
Gentschev P, Lüken M, Möller N, Rompel A, Krebs B (2001) Inorg Chem Commun 4:752–756
Guo X, He D, Huang L, Liu L, Liu L, Yang H (2012) J Theor Comput Chem 995:17–23
Solomon EI, Penfield WK, Wilcox DE (1983) Struct Bond 53:1–54
Richardson DE, Reem RC, Solomon EI (1983) J Am Chem Soc 105:1781–7780
Dołęga A (2010) Coord Chem Rev 254:916–937
Rezaei Behbehani G, Saboury AA, Fallah Baghery A, Abedini A (2008) J Therm Anal Calorim 93:479–483
Kandeel M, Nabih M, Kitade Y (2013) J Therm Anal Calorim 111:1737–1741
Ebrahimi KH, Hagedoorn P-L, Hagen WR (2012) PLoS One 7:e40287
Behbehani GR, Saboury AA, Taherkhani A, Barzegar L, Mollaagazade A (2011) J Therm Anal Calorim 105:1081–1086
Wyrzykowski D, Zarzeczańska D, Jacewicz D, Chmurzyński L (2011) J Therm Anal Calorim 105:1043–1047
Behbehani GR, Saboury AA, Barzegar L, Zarean O, Abedini J, Payehghdr M (2011) Therm Anal Calorim 101:379–384
Łyszczeka R, Rzączyńska Z, Kula A, Gładysz-Płaska A (2011) J Anal Appl Pyrolysis 92:347–354
Fernandes RL, Takahashi PM, Frem RCG, Netto AVG, Mauro AE, Matos JR (2009) J Therm Anal Calorim 97:153–156
Sun Sh-J, Wang J-F, Ren N, Zhang J-J, Ye H-M, Wang Sh-P (2012) Struct Chem 23:79–89
Łyszczek R (2012) J Therm Anal Calorim 108:1101–1110
Ponikvar-Svet M, Liebman JF (2011) Struct Chem 22:717–740
Ponikvar-Svet M, Zieger DN, Liebman JF (2012) Struct Chem 23:1267–1280
Que L (ed) (2000) Physical methods in bioinorganic chemistry: spectroscopy and magnetism. University Science Books, Sausalito
Holló B, Leovac VM, Bombicz P, Kovács A, Jovanović LS, Bogdanović G, Kojić V, Divjaković V, Joksović MD, Mészáros Szécsényi K (2010) Aust J Chem 63:1557–1564
Holló B, Jašo V, Leovac VM, Divjaković V, Kovács A, Mészáros Szécsényi K (2013) J Coord Chem 66:453–463
Neela YI, Mahadevi AS, Sastry GN (2013) Struct Chem 24:637–650
Agilent Technologies (2011) CrysAlisPro Software system, Version 1.171.34.36
Altomare A, Cascarano G, Giacovazzo C, Gualardi A (1993) J Appl Cryst 26:343
Sheldrick GM (2008) Acta Cryst A64:112
Hübschle CB, Sheldrick GM, Dittrich B (2011) J Appl Cryst 44:1281
Farrugia LJ (1997) J Appl Cryst 30:565
Spek AL (2009) Acta Cryst D65:148
Leovac VM, Tomić ZD, Kovács A, Joksović MD, Jovanović LS, Mészáros Szécsényi K (2008) J Organomet Chem 693:77–86
Mészáros Szécsényi K, Leovac VM, Petković R, Jaćimović ŽK, Pokol G (2007) J Therm Anal Calorim 90:899–902
Jaćimović ŽJ, Leovac V, Mészáros Szécsényi K, Howard JAK, Radosavljević Evans I (2004) Acta Cryst C60:m467–m470
Hargittai I (2011) Struct Chem 22:3–10
Tamer Ö, Sarıboğa B, Uçar I (2012) Struct Chem 23:659–670
Boča M, Jameson RF, Linert W (2011) Coord Chem Rev 255:290–317
Içbudak H, Adiyaman E, Çetin N (2006) Transit Metal Chem 31:666–672
Içbudak H, Bulut A, Çetin N, Kazak C (2005) Acta Cryst C61:m1–m3
Valle-Bourrouet G, Pineda LW, Falvello LR, Lusar R, Weyhermueller T (2007) Polyhedron 26:4470–4478
Tsukerblat B, Klokishner S, Palii A (2009) In: Köppel H, Yarkony DR, Barenzen H (eds) The Jahn-Teller effect: fundamentals and implications for physics and chemistry. Springer, Berlin
Begum Y, Wright AJ (2012) J Mater Chem 22:21110–21116
Shevchenko VA (2012) Struct Chem 23:1089–1101
Acknowledgments
The authors thank to the Ministry of Education, Science and Technological Development of the Republic of Serbia for financial support (Projects No. ON172014 and III45022) and Secretariat for Science and Technological Development, Autonomous Province of Vojvodina, Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
CCDC 922242 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge at www.ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Holló, B., Rodić, M.V., Bera, O. et al. Cation- and/or anion-directed reaction routes. Could the desolvation pattern of isostructural coordination compounds be related to their molecular structure?. Struct Chem 24, 2193–2201 (2013). https://doi.org/10.1007/s11224-013-0270-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0270-9