Abstract
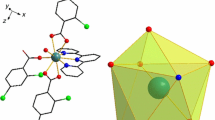
Some binuclear lanthanide complexes with the general formula [Ln(2,3-DClBA)3bipy]2 (Ln = Sm(1), Eu(2), Tb(3), Dy(4), and Ho(5); 2,3-DClBA = 2,3-dichlorobenzoate; bipy = 2,2′-bipyridine) were synthesized and characterized by elemental analysis, molar conductance, infrared, ultraviolet, luminescent spectroscopy, thermogravimetry, and different thermogravimetry (TG–DTG) techniques. The single crystals of the complexes have been obtained except the complex 2 and their structures have been determined by single-crystal X-ray diffraction. The four complexes are isostructural and the rare earth ions are all nine coordinated. The two rare earth ions in each complex are linked by two bridging bidentate and two chelating-bridging tridentate carboxylate groups. Under ultraviolet light excitation, the europium and terbium complexes exhibited characteristic red fluorescence of Eu3+ ion and green fluorescence of Tb3+ ion at room temperature. The non-isothermal kinetics was investigated by using the integral isoconversional non-linear (NL-INT) and the Popescu methods. The mechanism functions of the first decomposition step of the complexes 3–5 were determined. Meanwhile, the thermodynamic parameters (ΔG ≠ , ΔH ≠, and ΔS ≠) at DTG peak temperatures were also calculated.











Similar content being viewed by others
References
Edwards Y, Claude C, Sokolik I, Chu T, Okamoto Y, Dorsinville R (1997) J Appl Phys 82:1841–1846
Li Y, Zheng FK, Liu X, Zou WQ, Guo GC, Lu CZ, Huang JS (2006) Inorg Chem 45(16):6308–6316
Li X, Zhang ZY, Zou YQ (2005) Eur J Inorg Chem 14:2909–2918
Ye HM, Ren N, Zhang JJ, Sun SJ, Wang JF (2010) New J Chem 34(3):533–540
Su Q (1993) Chemistry of rare earths. Henan Scientific Press, Zhengzhou, p 99
Wang M, Xu ZD, Feng DZ (1999) Spectrosc Spect Anal 19:484–486
Okamoto Y, Kido J (1992) Mater Res Soc Symp Proc 277:65–74
Liu P, Huang MS, Pan WZ, Zhang YM, Hu JH, Deng WJ (2006) J Lumin 121:109–112
Wolff NE, Ressley RJ (1963) J Appl Phys Lett 2:152–154
Chen Y, Chen Q, Song L, Li HP, Hou FZ (2009) Microporous Mesoporous Mater 122:7–12
Li X, Zhang ZY, Zou YQ (2005) Eur J Inorg Chem 14:2909–2918
Ferenc W, Bocian B (2001) J Therm Anal Calorim 63:309–316
Han H, Yang HJ, Ge QS, Wang SP, Wang RF (2010) Chin J Lumin 31:908–913
Wan YH, Zhang LP, Jin LP, Gao S, Lu SZ (2003) Inorg Chem 42:4985–4994
Zhang HY, Ren N, Tian L, Zhang JJ (2009) J Therm Anal Calorim 98:401–408
Ye HM, Ren N, Zhang JJ, Sun SJ, Wang JF (2010) J Struct Chem 21:165–173
Popescu C (1996) Thermochim Acta 285:309–323
Vyazovkin S, Dollimore D (1996) J Chem Inf Comput Sci 36:42–45
Geary W (1971) J Coord Chem Rev 7:81–122
Wang RF, Jin LP, Wang MZ, Huang SH, Chen XT (1995) Acta Chim Sin 53:39–45
Shi YZ, Sun XZ, Jiang YH (1988) Spectra and chemical identification of organic compounds. Science and Technology Press, Nanjing, p 98
Xu CJ, Xie F, Guo XZ, Yang H (2005) Spectrochim Acta A 61:2005–2008
An BL, Gong ML, Li MX, Zhang JM (2004) J Mol Struct 687:1–6
Li Y, Zheng FK, Liu X, Zou WQ, Guo GC, Lu CZ, Huang JS (2006) Inorg Chem 45:6308–6316
Zhang RH, Shen PW (1987) Rare earth element chemistry. Tianjin Science and Technology Press, Tianjin, p 70
Yin MC, Ai CC, Yuan LJ, Wang CW, Sun JT (2004) J Mol Struct 691:33–37
Janiak C (2000) Dalton Trans 3885–3896
Lu ZR, Ding YC, Xu Y, Li BL, Zhang Y (2005) Chin J Inorg Chem 21:181–185
Hu RZ, Gao SL, Zhao FQ, Shi QZ, Zhang TL, Zhang JJ (2008) Thermal analysis kinetics, 2nd edn. Science Press, Beijing, p 151
Straszko J, Olstak-Humienik M, Mozejko J (1997) Thermochim Acta 292:145–150
Olstak-Humienik M, Mozejko J (2000) Thermochim Acta 344:73–79
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Nos. 21073053 and 20773034).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, SJ., Wang, JF., Ren, N. et al. Crystal structures, luminescent properties and thermal decomposition kinetics of some binuclear lanthanide complexes with 2,3-dichlorobenzoic acid anion and 2,2′-bipyridine. Struct Chem 23, 79–89 (2012). https://doi.org/10.1007/s11224-011-9847-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9847-3