Abstract
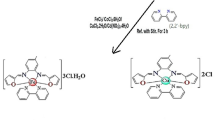
Novel dipicolinate complex of copper(II) ion, [Cu(hepy)(dpc)H2O] [hepy: 2-(2-hydroxyethyl)pyridine; dpc: dipicolinate or pyridine-2,6-dicarboxylate], was prepared and fully characterized by single crystal X-ray structure determination. [Cu(hepy)(dpc)H2O] was investigated for antimicrobial activity against a fungal strain, Gram-positive, and Gram-negative bacteria. The compound was found to be active against of all microorganisms (MIC values 512–1,024 μg mL−1). The mixed-ligand copper(II) complex was satisfactorily modeled by calculations based on following hybrid density functionals: LSDA, BPV86, B3LYP, B3PW91, MPW1PW91, PBEPBE, and HCTH. Although the supramolecular interactions have some influences on the molecular geometry in solid state phase, calculated data show that the predicted geometries can reproduce the structural parameters. The performance of these functional approaches for the calculation of electron paramagnetic resonance hyperfine coupling constant Cu2+ ion was evaluated critically by comparison with experimental data. The g values obtained from density functional theory (DFT) calculations were in compatible with the experimental results, whereas the A values were not. Electronic structure of the complex was calculated using time-dependent DFT method with the polarizable continuum model. Descriptions of frontier molecular orbitals and the relocation of the electron density of the compound were determined. Because the calculations of vibrations were carried out in gaseous phase there were shifts in vibration frequencies above 3,000 cm−1.







Similar content being viewed by others
References
Udo S (1936) J Agric Chem Soc Jpn 12:386–394
Edgecombe KE, Weaver DF, Smith VH (1994) Can J Chem 72:1388–1403
Hameka HF, Jensen JO, Jensen JL, Merrow CN, Vlahacos CP (1996) J Mol Struct (Theochem) 365:131–141
Singh RP (1987) Curr Sci 56:1232–1234
Janssen FW, Lund AJ, Anderson LE (1958) Science 127:26–27
Murakami K, Tanemura Y, Yoshino M (2003) J Nutr Biochem 14:99–103
Couper L, Mckendrick JE, Robins DJ, Chrystal EJT (1994) Bioorg Med Chem Lett 4:2267–2272
Kazuhiro Y, NorikoY, Tadayasu F (1994) Eur Patent EP0603165
Burdock GA (1996) Ancyclopedia of food and color additives, vol 3. CRC Pres, Boca Raton
Kirillova MV, Da Silva MFCG, Kirillov AM, Da Silva JJRF, Pombeiro AJL (2007) Inorg Chim Acta 360:506–512
Park H, Lough AJ, Kim JC, Jeong MH, Kang YS (2007) Inorg Chim Acta 360:2819–2823
Moghimi A, Moosavi SM, Kordestani D, Maddah B, Shamsipur M, Aghabozorg H, Ramezanipour F, Kickelbick G (2007) J Mol Struct 828:38–45
Wen YH, Cheng JK, Feng YL, Zhang J, Li ZJ, Yao YG (2005) Inorg Chim Acta 356:3347
Uçar İ, Bulut A, Büyükgüngör O (2005) Acta Crystallogr C61:m479–m482
Uçar İ, Bulut I, Karabulut B, Bulut A, Büyükgüngör O (2007) J Mol Struct 834–836:336–344
Saladino AC, Larsen SC (2005) Catal Today 105:122–133
Almeida KJ, Rinkevicius Z, Hugosson HW, Ferreira AC, Agren H (2007) Chem Phys 332:176–187
Clinical and Laboratory Standards Institute (CLSI) (2006) Methods of dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, M7-A7, 7th edn. CLSI, Wayne
National Committee for Clinical Laboratory Standards (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard M27-A2, 2nd edn. NCCLS, Wayne
Stoe & Cie X-AREA (Version 1.18) and X-RED (Version 1.04), Stoe & Cie, Dermstadt, 2002
Altomera A, Burla MC, Camalli M, Cascarano GL, Giacovazzo C, Guagliardi A, Moliterni AGG, Polidori G, Spagna R (1999) J Appl Crystallogr 32:115
Sheldric GM (1997) SHELXL97. University of Gottingen, Gottingen
Brandenburg K (2005) DIAMOND, Demonstrated Version. Crystal Impact GbR, Bonn
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, J.M. Millam JM, S.S. Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al- Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, Revision B.05. Gaussian, Inc., Pittsburgh
Dennington R II, Keith T, Milliam J (2007) GaussView Version 4.1.2. Semichem Inc., Shawnee Mission
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Schlegel HB (1982) J Comput Chem 3:214–218
Ditchfield R, Hehre WJ, Pople JA (1971) J Chem Phys 54:724–728
Lee C, Yang W, Parr RG (1988) Phys Rev B37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Peterson MR, Singh DJ, Fiolhais C (1992) Phys Rev B46:6671–6687
Perdew JP, Burke K, Wang Y (1996) Phys Rev B54:16533–16539
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1200–1211
Perdew JP (1986) Phys Rev B 33:8822–8824
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Phys Rev Lett 78:1396–1399
Burke K, Perdew JP, Wang Y (1998) In: Dobson JF, Vignale G, Das MP (eds) Electronic density functional theory: recent progress and new directions. Plenum, New York
Hamprecht FA, Cohen AJ, Tozer DJ, Handy NC (1998) J Chem Phys 109:6264–6271
Gunnarsson O, Lundqvist BI (1976) Phys Rev B 13:4274–4298
Menon AS, Radom L (2008) J Phys Chem A 112:13225–13230
Dodds JL, McWeeny R, Sadlej AJ (1980) Mol Phys 41:1419
Wolinski K, Hilton JF, Pulay P (1999) J Chem Phys 111:8251
Kim KJ, Lee JH, Lee SH, Magn J (2004) Magn Mater 279:173–177
Abada GA, Mutakainen I, Turpeinen U, Reedjik J (2002) Acta Cryst E 58:m55–m57
Uçar İ, Bulut B, Bulut A, Karadağ A (2009) Struct Chem 20:825–838
Uçar İ, Bulut A, Büyükgüngör O (2007) J Phys Chem Solids 68:2271–2277
Yenikaya C, Poyraz M, Sarı M, Demirci F, İlkimen H, Büyükgüngör O (2009) Polyhedron 28:3526–3532
Du M, Cai H, Zhao X-J (2006) Inorg Chim Acta 359:673–679
Perry JJ, McManus GJ, Zaworotko MJ (2004) J Chem Cryst 34:877–881
Mao L, Wang Y, Qi Y, Cao M, Hu C (2004) J Mol Struct 688:197–201
Lah N, Leban I (2010) Struct Chem 21:263–267
Cheng S-C, Wei H-H (2002) Inorg Chim Acta 340:105–113
Malkina OL, Vaara J, Schimmelpfenning B, Munzarova M, Malkin VG, Kaupp M (2000) J Am Chem Soc 122:9206–9218
Engstrom M, Minaev B, Vahtras O, Agren H (1998) Chem Phys 237:149–158
Neese F (2005) J Chem Phys 122:34107–34119
Gorelsky SI (2010) SWizard Program Revision 4.5, University of Ottawa, Ottawa, Canada. http://www.sg.chem.net/
Merrick JP, Moran D, Radom L (2007) J Phys Chem A 111:11683–11700
Carmona P (1980) Spectrochim Acta A 36:705–712
Van Albada GA, Gorter S, Reedijk J (1999) Polyhedron 18:1821–1824
Robinson SD, Uttley MF (1973) J Chem Soc Dalton Trans 18:1912–1920
Topacli A, Bayarı S (1999) Spectrochim Acta A 55:1389–1394
Topacli A, Akyüz S (1995) Spectrochim Acta A 51:633–641
Kolomenskii AA, Schuessler HA (2005) Spectrochim Acta A 61:647–651
McCann K, Laane J (2008) J Mol Struct 890:346–358
Gonzalez-Baro AC, Castellano EE, Piro OE, Parajon-Costa BS (2005) Polyhedron 24:49–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamer, Ö., Sarıboğa, B. & Uçar, İ. A combined crystallographic, spectroscopic, antimicrobial, and computational study of novel dipicolinate copper(II) complex with 2-(2-hydroxyethyl)pyridine. Struct Chem 23, 659–670 (2012). https://doi.org/10.1007/s11224-011-9910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9910-0